Introduction
Colorectal cancer (CRC) is a commonly occurring
cancer, with approximately one million new cases diagnosed annually
worldwide (1). In its early
stages, the disease is curable with surgery, however 50–60% of
patients diagnosed with CRC will develop metastases (2). In order to facilitate the development
of more effective treatments for patients with CRC, prognostic and
predictive markers need to be identified. Currently, tumor staging
at the time of diagnosis and determination of histological grade,
such as the tumor node metastasis (TNM) and the Duke's staging
systems, are the two most important techniques.
Previous studies have identified several biomarkers
in multiple tumor types that are associated with disease
progression and clinical outcome. Among these established
biomarkers, 4E-binding protein 1 (4E-BP1) is associated with cell
signaling and downstream regulation of the mitogen-activated
protein kinase (MAPK) signaling pathway and the
phosphatidylinositol 3-kinase (PI3K)/protein kinase B
(AKT)/mammalian target of rapamycin (mTOR) signaling pathway
(3). 4E-BP1 is activated when
phosphorylated by AKT and ribosomal protein S6 kinase B1, and
serves a critical role in RNA translation and in the regulation of
cell growth (4,5). Previous studies have established that
phosphorylated 4E-BP1 (p-4E-BP1) is involved in the initiation and
progression of cancer. Therefore, p-4E-BP1 may be a suitable tumor
biomarker. Certain types of tumor, including those of the
esophagus, stomach, breast, ovary, uterine cervix and endometrium
exhibit high expression levels of p-4E-BP1, which has been
demonstrated to be associated with poor prognosis (6–11).
However, the prognostic value of p-4E-BP1 in CRC remains unclear.
The aim of the present study was to assess the status of p-4E-BP1
in CRC specimens, and to establish its clinical significance. As
phosphatase and tensin homolog (PTEN) is an important member of the
PI3K/AKT signaling pathway, the association between p-4E-BP1 and
PTEN expression was also examined.
Materials and methods
Study population
The present study enrolled 89 patients (48 men, 41
women; age range, 40–81 years; median age, 58 years) with primary
CRC that had undergone surgical resection during the period from
February 2008 to June 2010 in Yancheng First People's Hospital
(Yancheng, China). No patients had received preoperative treatment,
such as radiation or chemotherapy. Tumor stage was assessed using
the 2010 version of the TNM classification system (12), issued by the American Joint
Committee on Cancer (AJCC). A total of 8 patients were at stage I,
30 at stage II, 45 at stage III and 6 at stage IV. Cellular
differentiation was graded using the World Health Organization
grading system (13). Clinical
follow-up data was obtained for all patients. The study was
approved by the ethical committee of Yancheng First People's
Hospital, and all patients provided informed consent prior to
sample examination.
Immunohistochemical analysis
A total of 89 pairs of 10% formalin-fixed,
paraffin-embedded sections (thickness, 4 µm) of cancerous and
adjacent non-cancerous tissues were prepared for
immunohistochemical analysis. Serial tissue sections were
deparaffinized with xylene, then rehydrated through grade alcohols
and subjected to autoclave antigen retrieval in
ethylenediaminetetraacetic acid buffer (pH 8.0) at 100°C for 5 min.
Endogenous peroxidase activity was blocked by 3% hydrogen peroxide
for 10 min. Next, tissue sections were incubated at 4°C overnight
with a rabbit monoclonal anti-p-4E-BP1 antibody (Thr 37/46, 236B4;
dilution, 1:250; cat. no. 2855; Cell Signaling Technology, Inc.,
Danvers, MA, USA) or a rabbit anti-human PTEN monoclonal antibody
(dilution, 1:250; cat. no. 9188; Cell Signaling Technology, Inc.).
The samples were washed 3 times in PBS, and then treated for 2 h at
24°C with an EnVision peroxidase-labeled polymer antibody (Dako;
cat. no. k4011, ready-to-use; Agilent Technologies, Inc., Santa
Clara, CA, USA). The slides were developed for 8 min with
3,3′-diaminobenzidine (DAB)/H2O2 chromogen
and counterstained with hematoxylin for 5 min. Omission of the
primary antibody served as a control. A BX41 microscope (Olympus
Corporation, Tokyo, Japan) was used to identify positive staining
in the cytoplasm and was semi-quantitatively graded using the
following three categories: 1+, 1–30% of the tumor cells were
positive; 2+, ≥30 to <60% of the tumor cells were positive; 3+,
≥60% of the tumor cells were positive. All assays were performed at
least in triplicate. Immunohistochemical results were determined by
two independent pathologists.
Statistical analysis
Statistical tests were performed using SPSS software
(version 16.0; SPSS Inc., Chicago, IL, USA). Differences in protein
expression between groups were analyzed using Student's t-test. The
chi-squared test was used to identify differences in frequency.
Correlation between p-4E-BP1 and PTEN expression was determined by
Pearson analysis. Overall survival (OS) was calculated using the
Kaplan-Meier method, and OS values were compared using Mantell-Cox
log-rank testing. The multivariate Cox proportional hazards model
was used to establish the prognostic significance of each specific
parameter. P<0.05 was considered to indicate a statistically
significant difference.
Results
p-4E-BP1 protein expression profiles
in CRC
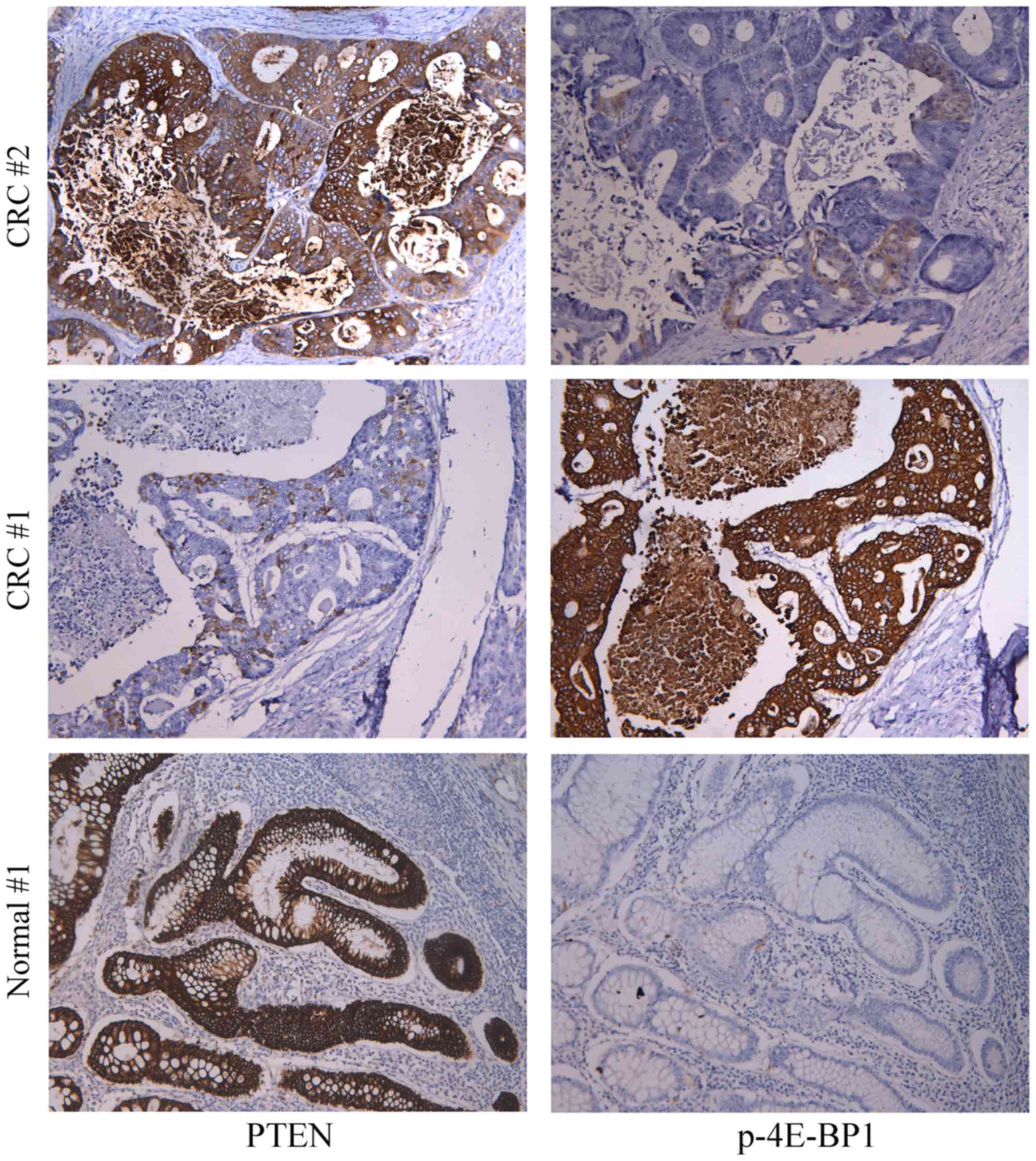
Fig. 1 presents
representative immunohistochemical results of p-4E-BP1 and PTEN
staining in CRC and adjacent normal tissue samples. A total of 65
CRC cases (73.0%) demonstrated positive expression of the p-4E-BP1
protein, where 53 cases (59.6%) exhibited moderate to high
expression (grade 2+ and 3+). By contrast, p-4E-BP1 exhibited
little or no expression in adjacent normal tissues. PTEN exhibited
significantly lower expression in CRC samples when compared with
normal tissues (moderate/high expression CRC, 16/89 vs. normal,
49/89; P<0.001). Upregulation of p-4E-BP1 protein expression was
associated with downregulated PTEN (r=−0.731; Fig. 2A).
Association between p-4E-BP1 protein
expression and clinicopathological features
No correlations were observed between p-4E-BP1
protein expression and patient age, gender, tumor location, tumor
diameter, local invasion (T stage) or clinical stage (Table I). Although the upregulation of
p-4E-BP1 was more prevalent in patients with lymph node metastasis
and poor differentiation, no statistical significance was observed
(Table I).
 | Table I.Association between p-4E-BP1
expression in colorectal cancer tissues and clinicopathological
features. |
Table I.
Association between p-4E-BP1
expression in colorectal cancer tissues and clinicopathological
features.
| Characteristic | n | No. with
high/moderate p-4E-BP1 expression (%) | P-value |
|---|
| Gender |
|
| 0.492 |
| Male | 48 | 27 (56.3) |
|
|
Female | 41 | 26 (63.4) |
|
| Age |
|
| 0.579 |
|
<55 | 34 | 19 (55.9) |
|
| ≥55 | 55 | 34 (61.8) |
|
| Tumor location |
|
| 0.558 |
|
Proximal | 21 | 11 (52.4) |
|
|
Distal | 30 | 17 (56.7) |
|
|
Rectum | 38 | 25 (65.8) |
|
| Tumor diameter
(cm) |
|
| 0.594 |
|
<5 | 39 | 22 (56.4) |
|
| ≥5 | 50 | 31 (62.0) |
|
| Differentiation |
|
| 0.161 |
| Well | 20 | 10 (50.0) |
|
|
Moderate | 39 | 21 (53.8) |
|
| Poor | 30 | 22 (73.3) |
|
| Local invasion |
|
| 0.251 |
| T1-2 | 38 | 20 (52.6) |
|
| T3-4 | 51 | 33 (64.7) |
|
| Lymph metastasis |
|
| 0.130 |
| No | 36 | 18 (50.0) |
|
| Yes | 53 | 35 (66.0) |
|
| TNM stage |
|
| 0.209 |
| I/II | 35 | 18 (51.4) |
|
|
III/IV | 54 | 35 (64.8) |
|
Association between p-4E-BP1 protein
expression and OS
Prior to the follow-up deadline, 42 patients did not
survive 5 years following surgery. Univariate survival analysis
indicated that CRC patients with moderate to high expression of the
p-4E-BP1 protein demonstrated significantly shorter OS (mean 37.5
months, 95% CI: 32.693–42.307) when compared with patients
exhibiting little or no p-4E-BP1 expression (mean 47.8 months, 95%
CI: 42.564–53.112; P=0.08; Fig.
2B).
Following adjustment for potential confounding
cofactors, multiple Cox regression analysis indicated that high to
moderate expression of the p-4E-BP1 protein was an independent
factor for predicting adverse OS in patients, apart from lymph
metastasis (Table II).
 | Table II.Multivariate analysis of
clinicopathological features and OS of 89 patients with colorectal
cancer. |
Table II.
Multivariate analysis of
clinicopathological features and OS of 89 patients with colorectal
cancer.
|
| OS |
|---|
|
|
|
|---|
| Variable | RR (95% CI) | P-value |
|---|
| Tumor
differentiation (poor vs. moderate vs. well) | 1.778
(0.732–4.321) | 0.202 |
| Local invasion
(T3-4 vs. T1-2) | 1.725
(0.736–4.043) | 0.208 |
| Lymph node
metastasis (yes vs. no) | 2.609
(1.082–6.292) | 0.031 |
| TNM stage (III/IV
vs. I/II) | 1.963
(0.823–4.684) | 0.126 |
| p-4E-BP1 expression
(high/moderate vs. low/negative) | 2.816
(1.123–6.535) | 0.025 |
Discussion
CRC is the third most common cause of
cancer-associated mortality worldwide, accounting for 8% of all
cancer-associated deaths (1). The
AJCC staging system is the current standard used for determining
the prognosis of patients with cancer. Typically, patients with
stage II and stage III disease, which are at risk of locoregional
or distant relapse are treated using chemotherapy, whereas patient
with stage I disease are treated using surgery alone (14). However, in patients undergoing
surgery for localized CRC, pathological staging is unable to
predict recurrence accurately, due to the highly heterogeneous
phenotype of CRC (15). Cancer
recurs in 10–20% of patients with stage II disease and in 30–40% of
patients with stage III disease (16). Thus, molecular biomarkers have been
extensively investigated with respect to the characterization and
prognosis of CRC. The CpG island methylator phenotype,
microsatellite instability, chromosomal instability, KRAS and BRAF
mutations have been demonstrated to constitute an important
prognostic system for CRC (17–19).
Gene expression profiling has previously demonstrated considerable
promise in predicting prognosis in individual patients with cancer.
As a result, several gene expression signatures have been developed
to classify specific prognostic groups beyond the
clinicopathological features of CRC (20).
4E-BP1 binds eukaryotic initiation factor 4E (eIF4E)
and serves a critical role in the control of protein synthesis,
cell survival and growth (21,22).
Alterations in the PI3K/AKT/mTOR and Ras-Raf-extracellular
signal-regulated kinase (ERK) signaling cascade pathways are
frequently detected in tumors (23). The cap-dependent mRNA translation
initiation complex is a final effector of these signaling cascades,
and 4E-BP1 negatively regulates this complex. 4E-BP1 promotes the
expression of growth factors and survival factors. eIF4E binds to
the mRNA cap structure during cap-dependent translation and
promotes both ribosome binding and the formation of the eIF4F
initiation complex. When active, non-phosphorylated 4E-BP1 binds to
eIF4E, formation of the initiation complex is prevented. Therefore,
translation is inhibited and apoptosis is initiated. However, when
4E-BP1 is phosphorylated, its binding affinity is reduced and eIF4E
is released, thus initiating cap-dependent translation (24). Therefore, p-4E-BP1 expression in
tumor cells may reflect their oncogenic potential. In several human
cancers, p-4E-BP1 expression was identified as being associated
with poor prognosis. These included carcinomas of the esophagus,
stomach, breast, ovary, cervix and endometrium, and in childhood
rhabdomyosarcoma, hilar cholangiocarcinoma and melanoma (6–11,25,26).
Previous studies have indicated that 4E-BP1 is essential for cell
transformation. Transferring mutant 4E-BP1 phosphorylation sites
into breast carcinoma cells was found to suppress their
tumorigenicity (27). In a recent
study, the expression levels of eIF4E increased gradually as CRC
progressed from benign dysplasia to adenocarcinoma. However, total
4E-BP1 protein expression increased only during the premalignant
state of the disease, and then decreased or ceased entirely upon
malignancy (28). Therefore,
4E-BP1 demonstrates a biphasic pattern of expression during CRC
carcinogenesis, and is expressed only in hyperplasic or dysplastic
tissues as an endogenous tumor suppressor molecule.
The aim of the present study was to investigate the
status of p-4E-BP1 expression in CRC and to establish its clinical
significance. The results indicated that p-4E-BP1 was expressed at
significantly lower levels in CRC tissue samples compared with
adjacent normal tissues. Increased expression of p-4E-BP1 was
demonstrated to be predominant in patients with regional lymph node
metastases and in poorly differentiated tumors, and was
significantly associated with reduced OS. As demonstrated in
previous studies of gastric and breast cancers, the results of the
present study further confirmed that p-4E-BP1 may be useful in
predicting the prognosis of CRC.
A number of cancers demonstrated activation of the
PI3K/AKT and RAS/RAF/MEK/ERK signaling pathways. In addition, they
frequently display mutations in genes that encode components of
these pathways. In a number of human tumors, the AKT and ERK
signaling pathways are activated concurrently by separate mutations
(29). During tumorigenesis,
4E-BP1 is an effector for the oncogenic roles of ERK and AKT
signaling pathways (30). In an
experimental model of CRC, involving involves KRAS and PIK3CA
mutations, little or no 4E-BP1 phosphorylation in response to
inhibition of either ERK or AKT was observed (30). Additional studies have demonstrated
that active 4E-BP1 inhibits tumorigenesis in PTEN-mutant breast
cancer (31). PTEN is a tumor
suppressor gene that is frequently mutated or deleted in tumor cell
lines and human cancers. Results from previous studies have
demonstrated that overexpression of PTEN in breast cancer cells
impairs insulin-induced phosphorylation of MAPK (32). These data are consistent with the
results of the current study, which demonstrated that
downregulation of the PTEN protein was associated with p-4E-BP1
upregulation in CRC samples. Together, these data suggest that
4E-BP1 phosphorylation may the result of different oncogenic events
associated with biochemical pathways, including those associated
with growth factor receptors, loss of function mutations or
mutations in p53, PTEN, RAS and PI3K, and additional mechanisms
associated with cellular oncogenic activation. As there are
numerous genetic alterations that affect 4E-BP1, the phosphorylated
form of 4E-BP1 may function as an inhibitor of the transforming
signals, channeling the oncogenic proliferative signal
independently of any upstream-specific oncogenic alterations.
Further studies are required to identify the mechanisms by which
4E-BP1 affects the development and progression of CRC.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Saif MW and Chu E: Biology of colorectal
cancer. Cancer J. 16:196–201. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Armengol G, Rojo F, Castellvi J, Iglesias
C, Cuatrecasas M, Pons B, Baselga J and Cajal Ramón y S: 4E-binding
protein 1: A key molecular ‘funnel factor’ in human cancer with
clinical implications. Cancer Res. 67:7551–7555. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Magagnin MG, van den Beucken T, Sergeant
K, Lambin P, Koritzinsky M, Devreese B and Wouters BG: The mTOR
target 4E-BP1 contributes to differential protein expression during
normoxia and hypoxia through changes in mRNA translation
efficiency. Proteomics. 8:1019–1028. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Barnhart BC, Lam JC, Young RM, Houghton
PJ, Keith B and Simon MC: Effects of 4E-BP1 expression on hypoxic
cell cycle inhibition and tumor cell proliferation and survival.
Cancer Biol Ther. 7:1441–1449. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rojo F, Najera L, Lirola J, Jiménez J,
Guzmán M, Sabadell MD, Baselga J and Cajal Ramon y S: 4E-binding
protein 1, a cell signaling hallmark in breast cancer that
correlates with pathologic grade and prognosis. Clin Cancer Res.
13:81–89. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Castellvi J, Garcia A, Rojo F,
Ruiz-Marcellan C, Gil A, Baselga J and Cajal Ramon y S:
Phosphorylated 4E binding protein 1: A hallmark of cell signaling
that correlates with survival in ovarian cancer. Cancer.
107:1801–1811. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Benavente S, Vergés R, Hermosilla E,
Fumanal V, Casanova N, García A, Ramón Y, Cajal S and Giralt J:
Overexpression of phosphorylated 4E-BP1 predicts for tumor
recurrence and reduced survival in cervical carcinoma treated with
postoperative radiotherapy. Int J Radiat Oncol Biol Phys.
75:1316–1322. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Castellvi J, Garcia A, Ruiz-Marcellan C,
Hernández-Losa J, Peg V, Salcedo M, Gil-Moreno A and Cajal Ramon y
S: Cell signaling in endometrial carcinoma: Phosphorylated
4E-binding protein-1 expression in endometrial cancer correlates
with aggressive tumors and prognosis. Hum Pathol. 40:1418–1426.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yeh CJ, Chuang WY, Chao YK, Liu YH, Chang
YS, Kuo SY, Tseng CK, Chang HK and Hsueh C: High expression of
phosphorylated 4E-binding protein 1 is an adverse prognostic factor
in esophageal squamous cell carcinoma. Virchows Arch. 458:171–178.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jiao X, Pan J, Qian J, Luo T, Wang Z, Yu G
and Wang J: Overexpression of p-4ebp1 in Chinese gastric cancer
patients and its correlation with prognosis.
Hepatogastroenterology. 60:921–926. 2013.PubMed/NCBI
|
|
12
|
Edge SB, Byrd DR, Compton CC, Fritz AG,
Greene FL and Trotti A: AJCC cancer staging manual. 7th. New York:
Springer; 2010
|
|
13
|
Ueno H, Kajiwara Y, Shimazaki H, Shinto E,
Hashiguchi Y, Nakanishi K, Maekawa K, Katsurada Y, Nakamura T,
Mochizuki H, et al: New criteria for histologic grading of
colorectal cancer. Am J Surg Pathol. 36:193–201. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rousseau B, Chibaudel B, Bachet JB, Larsen
AK, Tournigand C, Louvet C, André T and de Gramont A: GERCOR
(French Oncology Research Group): Stage II and stage III colon
cancer: Treatment advances and future directions. Cancer J.
16:202–209. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
De Sousa E, Melo F, Vermeulen L, Fessler E
and Medema JP: Cancer heterogeneity-a multifaceted view. EMBO Rep.
14:686–695. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Walker AS, Johnson EK, Maykel JA,
Stojadinovic A, Nissan A, Brucher B, Champagne BJ and Steele SR:
Future directions for the early detection of colorectal cancer
recurrence. J Cancer. 5:272–280. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sinicrope FA and Sargent DJ: Molecular
pathways: Microsatellite instability in colorectal cancer:
Prognostic, predictive, and therapeutic implications. Clin Cancer
Res. 18:1506–1512. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Roth AD, Tejpar S, Delorenzi M, Yan P,
Fiocca R, Klingbiel D, Dietrich D, Biesmans B, Bodoky G, Barone C,
et al: Prognostic role of KRAS and BRAF in stage II and III
resected colon cancer: Results of the translational study on the
PETACC-3, EORTC 40993, SAKK 60–00 trial. J Clin Oncol. 28:466–474.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Watanabe T, Kobunai T, Yamamoto Y, Matsuda
K, Ishihara S, Nozawa K, Yamada H, Hayama T, Inoue E, Tamura J, et
al: Chromosomal instability (CIN) phenotype, CIN high or CIN low,
predicts survival for colorectal cancer. J Clin Oncol.
30:2256–2264. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
O'Connell MJ, Lavery I, Yothers G, Paik S,
Clark-Langone KM, Lopatin M, Watson D, Baehner FL, Shak S, Baker J,
et al: Relationship between tumor gene expression and recurrence in
four independent studies of patients with stage II/III colon cancer
treated with surgery alone or surgery plus adjuvant fluorouracil
plus leucovorin. J Clin Oncol. 28:3937–3944. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Heesom KJ, Gampel A, Mellor H and Denton
RM: Cell cycle-dependent phosphorylation of the translational
repressor eIF-4E binding protein-1 (4E-BP1). Curr Biol.
11:1374–1379. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Topisirovic I, Ruiz-Gutierrez M and Borden
KL: Phosphorylation of the eukaryotic translation initiation factor
eIF4E contributes to its transformation and mRNA transport
activities. Cancer Res. 64:8639–8642. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Halilovic E, She QB, Ye Q, Pagliarini R,
Sellers WR, Solit DB and Rosen N: PIK3CA mutation uncouples tumor
growth and cyclin D1 regulation from MEK/ERK and mutant KRAS
signaling. Cancer Res. 70:6804–6814. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Averous J and Proud CG: When translation
meets transforma-tion: The mTOR story. Oncogene. 25:6423–6435.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Petricoin EF III, Espina V, Araujo RP,
Midura B, Yeung C, Wan X, Eichler GS, Johann DJ Jr, Qualman S,
Tsokos M, et al: Phosphoprotein pathway mapping: Akt/mammalian
target of rapamycin activation is negatively associated with
childhood rhabdomyosarcoma survival. Cancer Res. 67:3431–3440.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
O'Reilly KE, Warycha M, Davies MA, Rodrik
V, Zhou XK, Yee H, Polsky D, Pavlick AC, Rosen N, Bhardwaj N, et
al: Phosphorylated 4E-BP1 is associated with poor survival in
melanoma. Clin Cancer Res. 15:2872–2878. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Avdulov S, Li S, Michalek V, Burrichter D,
Peterson M, Perlman DM, Manivel JC, Sonenberg N, Yee D, Bitterman
PB and Polunovsky VA: Activation of translation complex eIF4F is
essential for the genesis and maintenance of the malignant
phenotype in human mammary epithelial cells. Cancer Cell.
5:553–563. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Diab-Assaf M, Abou-Khouzam R,
Saadallah-Zeidan N, Habib K, Bitar N, Karam W, Liagre B, Harakeh S
and Azar R: Expression of eukaryotic initiation factor 4E and 4E
binding protein 1 in colorectal carcinogenesis. Int J Clin Exp
Pathol. 8:404–413. 2015.PubMed/NCBI
|
|
29
|
Simi L, Pratesi N, Vignoli M, Sestini R,
Cianchi F, Valanzano R, Nobili S, Mini E, Pazzagli M and Orlando C:
High-resolution melting analysis for rapid detection of KRAS, BRAF,
and PIK3CA gene mutations in colorectal cancer. Am J Clin Pathol.
130:247–253. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
She QB, Halilovic E, Ye Q, Zhen W,
Shirasawa S, Sasazuki T, Solit DB and Rosen N: 4E-BP1 is a key
effector of the oncogenic activation of the AKT and ERK signaling
pathways that integrates their function in tumors. Cancer Cell.
18:39–51. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hsieh AC, Costa M, Zollo O, Davis C,
Feldman ME, Testa JR, Meyuhas O, Shokat KM and Ruggero D: Genetic
dissection of the oncogenic mTOR pathway reveals druggable
addiction to translational control via 4EBP-eIF4E. Cancer Cell.
17:249–261. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Weng LP, Smith WM, Brown JL and Eng C:
PTEN inhibits insulin-stimulated MEK/MAPK activation and cell
growth by blocking IRS-1 phosphorylation and IRS-1/Grb-2/Sos
complex formation in a breast cancer model. Hum Mol Genet.
10:605–616. 2001. View Article : Google Scholar : PubMed/NCBI
|