Introduction
Parkinson's disease (PD) is a neurological disorder
with complex pathogenesis implicating both environmental and
genetic factors. Epidemiological evidence suggests that chronic
exposure to heavy metals such as iron, lead, manganese and their
combinations can be associated with an increased risk of developing
PD, since they could accumulate in the substantia nigra and
generate oxidative stress (1).
Iron is an important microelement implicated in
normal neuronal functioning as well as in several metabolic
processes. Iron is first introduced in the body through various
food products as iron salts or haemoglobin and it is then absorbed
in the intestinal mucosa. Once in the enterocytes, iron is then
excreted through transporter proteins (ferroportin and haephestin)
in the bloodstream where it circulates attached to transferrin
(2). After passing through the
blood-brain barrier, iron enters the brain.
Non-physiological accumulation of iron in specific
regions of the brain has been associated primarily with a
heterogeneous group of diseases known as neurodegeneration with
brain iron accumulation (NBIA) (3), but it has also been associated with
Alzheimer's disease (4),
amyotrohpic lateral sclerosis (5)
and PD (6). Iron accumulation may
exerts its pathogenic activity through the increase of reactive
oxygen species (ROS) that then cause a wide array of damage to
intracellular proteins, but there is also evidence of other
mechanisms unrelated to ROS production, such as the promotion of
apoptotic processes and the interaction with pathological protein
aggregates found in these diseases (7).
PD is associated with extensive involvement of iron,
with most extensive deposition in substantia nigra and lateral
globus pallidus, as well as in dopaminergic neurons. The sources of
increased iron should be searched in one or all of the following:
homeostatic dysregulation, dysregulation of molecules involved in
the intra and extracellular distribution of iron and aging
(8).
Limited data are available concerning the levels of
iron in serum/blood in PD and their relationship with the
pathological process. It is not clear whether the alterations in
metal homeostasis may be a cause or consequence in the pathology of
the disease. However, whether metals are primary risk factors or
their imbalances are consequences of pathological mechanisms,
changes in metal ion concentration may upset the whole element
homeostasis, resulting in significant imbalances in element levels
in the whole system (serum, cerebrospinal fluid and brain)
(9). Even more, the effect of
heavy metals, including iron, derived from environmental sources
such as contaminated atmosphere or food/drinking water on the
pathogenesis of PD and their relationship with their serum levels
remain still difficult to investigate (10).
Few epidemiological studies have evaluated the
possible association between serum/plasma levels of iron and PD and
controversial results have been reported, probably due to
methodological limitations. On this ground, we conducted a
systematic review and a meta-analysis of the literature to better
evaluate the possible relationship between iron and PD.
We evaluated a possible significant association
between iron serum levels, expression of metal exposure or result
of pathological processes related to the disease, and PD as
compared to controls, using a meta-analytic approach of available
case-control studies.
Materials and methods
Literature search
A systematic MEDLINE search was conducted by a
medical investigator without time or language restriction, to
identify published observational, case-control studies dealing with
the association between iron blood levels and PD. Combined text
words and Medical Subject Headings (MeSH) terminology were used.
Specifically, to detect available study evaluating serum trace
elements in PD including iron, the following search key words and
boolean operators were entered in PubMed as search strategy:
(metal* OR element* OR iron OR silicium OR nickel OR copper OR
selenium OR zincum OR manganese OR chromium OR mercury) AND (blood
or serum or plasma) AND Parkinson AND control*. Titles were
scanned for relevance, identifying papers requiring further
consideration. For the systematic research, a period up to
September 2016 was considered.
Study selection
For the study selection, the following eligibility
criteria were used considering PD as the outcome and iron blood
level as exposure: i) the presence of a control group; ii) average
values per group of iron blood levels together with information
about methods used for the metal detection in the blood/serum and
unit of measure adopted; iii) the sample sizes adopted. As
additional criteria: iv) information about methods and criteria
used for case-finding and control selection; v) information on
demographical and geographical covariates; vi) the presence of a
group-matching method by age and/or gender adopted in the selected
case-control studies (if not directly available, it was
subsequently tested using proper statistics); and vii) the presence
of clinical information for cases characterization (i.e., disease
stage or duration, motor status severity). The search results were
independently assessed by a second reviewer. Disagreements were
resolved through consensus among reviewers.
Data extraction and collection
The following data were extracted to be recorded in
an ad hoc created collecting form: author, year of publication,
measure unit adopted for the iron blood level, geographical
information, setting, sample size, clinical-demographical
characteristics and iron blood levels. Continuous variables were
expressed as mean ± standard deviation (SD) and min-max range,
categorical variables as frequency and percent values.
Specifically, iron blood levels in both groups, since continuous
data, were extracted as means and SDs to calculate the standardized
mean differences (SMDs) with 95% confidence intervals (CIs).
Synthesis
Heterogeneity of selected studies was investigated
using the forest plot as standardized method to display
meta-analysis results and the I2 statistic (11). To estimate the association between
iron blood levels and PD as compared to controls, we then performed
a meta-analysis applying a random effects model as more
conservative approach than its fixed effect counterpart, assuming
that, in addition to sampling variation, the true effect varies
between studies. In this case, the conclusion estimate is
considered as the mean effect assuming that the true study effects
vary (12). Possible causes of
bias were also examined. Presence of publication bias or, in the
presence of small studies, or bias due to low methodological
quality (12), was tested
graphically using the funnel plot and implemented by the fail-safe
N calculation using the Rosenthal approach, in order to estimate
the number of additional ‘negative’ studies that would be needed to
increase the P-value for the meta-analysis to ~0.05 (13). A meta-regression analysis was also
conducted to investigate whether associations varied according to
specified confounding factors, in particular demographic,
geographical and clinical covariates, representing possible source
of heterogeneity in observational studies (12). In the case of analysis based on one
covariate, either the Z-test or the Q-test (equal to Z2)
was used to assess its relationship with effect size and thus
regression model validity (14).
Results
Of 155 studies detected by the research strategy, a
total of 23 case-control studies with full available data were
selected based on the adopted criteria (period, 1992–2016)
(Table I).
 | Table I.Case-control studies on blood/serum
iron levels in PD and controls (CTR). Iron detection methods and
iron levels among groups (N=23). |
Table I.
Case-control studies on blood/serum
iron levels in PD and controls (CTR). Iron detection methods and
iron levels among groups (N=23).
| Study, year
(ref.) | Detection method | Measure unit | PD (N) | PD iron level (mean ±
SD) | CTR (N) | CTR iron level (mean
± SD) |
|---|
| Chen and Shih, 1992
(21) | Unknown | µg/dl | 15 | 95.53±33.5 | 30 | 102.5±32.5 |
| Takahashi et
al, 1994 (22) | PIXE | µg/ml | 13 | 1.69±0.61 | 14 | 1.64±0.72 |
| Logroscino et
al, 1997 (23) | Atomic absorption
graphite furnace micromethod | µg/dl | 104 | 28.3±11.6 | 352 | 33.9±15.2 |
| Jiménez-Jiménez et
al, 1998 (24) | AAS | mg/l | 37 | 1.01±0.33 | 37 | 0.95±0.3 |
| Tórsdóttir et
al, 1999 (25) | Colorimetric test
with ferrozine ascorbic acid | µmol/l | 33 | 16±4.25 | 33 | 16±4.5 |
| Forte et al,
2004 (26) | ICP-AES | µg/l | 26 | 1318±481 | 13 | 1136±393 |
| Hedge et al,
2004 (9) | ICP-AES | µmol/ml | 27 | 0.02±0.004 | 25 | 0.023±0.009 |
| Qureshi et
al, 2006 (27) | AAS | mg/ml | 17 | 1.02±0.11 | 21 | 1.16±0.05 |
| Annanmaki et
al, 2007 (28) | Spectrophotometric
reaction with ferrozine | µmol/l | 40 | 18.2±5.5 | 29 | 20.5±6.3 |
| Squitti et
al, 2007 (29) | AAS | unknown | 65 | 91.1±18.1 | 52 | 84.5±18.1 |
| Gellein et
al, 2008 (30) | HR-ICP-MS | µg/l | 33 | 1275±551 | 99 | 1146±463 |
| Ahmed and Santosh,
2010 (31) | ICP-AES and
ICP-MS | µg/dl | 45 | 110.4±0.6 | 42 | 123±8 |
| Fukushima et
al, 2010 (10) | ICP-AES | µg/ml | 82 | 2±0.83 | 82 | 1.5±0.78 |
| Fukushima et
al, 2011 (32) | ICP-AES | µg/ml | 71 | 1.95±0.85 | 71 | 1.44±0.77 |
| Madenci et
al, 2012 (33) | Unknown | unknown | 60 | 74.6±29.3 | 42 | 74.8±27.1 |
| Farhoudi et
al, 2012 (34) | Unspecified
biochemical methods | mg/dl | 50 | 70.22±25.18 | 50 | 67.62±39.53 |
| Fukushima et
al, 2013 (35) | ICP-AES | µg/ml | 58 | 2.1±0.84 | 81 | 1.51±0.78 |
| Zhao et al,
2013 (36) | Fast sequential
atomic absorption spectroscopy | µg/l | 238 | 1656±749 | 302 | 1470±648 |
| Kumudini et
al, 2014 (37) | ICP-MS | ng/ml | 150 | 554.4±123.8 | 175 | 421.7±126.1 |
| Hu et al,
2015 (38) | ELISA | nmol/ml | 102 | 4.124±1.064 | 31 | 4.192±1.054 |
| Costa-Mallen et
al, 2015 (39) | Unknown | µg/100 ml | 128 | 83.28±29.46 | 226 | 94±34.14 |
| Madeiros et
al, 2016 (40) | Standard methods on
cobas mira | µg/dl | 40 | 67.5±18.89 | 46 | 78±18.15 |
| Mariani et
al, 2016 (41) | Ferene | µg/dl | 92 | 79±34 | 112 | 86.1±34.9 |
The studies were carried out in different countries
[12 (52.2%) studies in Asiatic countries, of which five in China;
eight (34.8%) studies in Europe, of which three in Italy and four
in North-European countries; three (13%) studies in the American
continent, of which two in the USA].
In 21 (91.3%) of the selected studies, cases
selection was hospital-based, while in the other two studies it was
community-based or based on registries. Adopted sample sizes for
the cases group varied among studies, with a broad range of
variation (mean ± SD, 66±52; min-max range, 13–238). The percentage
of men in PD samples was on average 57.5±11.5 (min-max range,
37.8–92.3), with an average male-to-female ratio of 1.8±2.4
(min-max range, 0.5–12). Only 15 studies (65.2%) provided
information on the adopted standardized diagnostic criteria for the
cases selection (UK PD Society Brain Bank Clinical diagnostic
criteria) (15).
Clinical information of cases were only partially
provided by the adopted studies. Information on PD patients'
disease duration were available in 11 (47.8%) studies, with an
average value per group of 5.3±2.8 (min-max range, 2-9.5 years).
Disease stage using standardized tool (the Hoehn and Yahr scale)
(16) was provided in three (13%)
studies (average value per group of 2±1; min-max range, 1-2.9).
Standardized information on patients' motor status using the
Unified PD Rating Scale (UPDRS) (17) total score was provided in seven
(30.4%) studies, with an average value per group of 32.8±11.4
(min-max range, 18.8–45.1) (Table
II).
 | Table II.Case-control studies on blood/serum
iron levels in PD and controls (CTR). PD group characteristics. |
Table II.
Case-control studies on blood/serum
iron levels in PD and controls (CTR). PD group characteristics.
| Study, year
(ref.) | PD (N) | PD source | Diagnostic
criteria | PD men (N, %) | PD M/F ratio | PD age (mean ±
SD) |
|---|
| Chen and Shih, 1992
(21) | 15 | Hospital | Not specified | Not specified | Not specified | Not specified |
| Takahashi et
al, 1994 (22) | 13 | Hospital | Not specified | Not specified | Not specified | 61.2±10.3 |
| Logroscino et
al, 1997 (23) | 104 | Community | UK Brain Bank | Not specified | Not specified | Not specified |
| Jiménez-Jiménez
et al, 1998 (24) | 37 | Hospital | UK Brain Bank | 14 (37.8) | 0.61 | 65.7±8.8 |
| Tórsdóttir et
al, 1999 (25) | 33 | Hospital | Not specified | 18 (54.5) | 1.2 | 67±8.5 |
| Forte et al,
2004 (26) | 26 | Hospital | UK Brain Bank | 24 (92.3) | 12 | 64.9±10.8 |
| Hedge et al,
2004 (9) | 27 | Hospital | Not specified | 14 (51.8) | 1.08 | 57.1±5.2 |
| Qureshi et
al, 2006 (27) | 17 | Hospital | UK Brain Bank | 10 (58.8) | 1.43 | 70±15 |
| Annanmaki et
al, 2007 (28) | 40 | Hospital | UK Brain Bank | 23 (58) | 1.35 | 60.8±6.5 |
| Squitti et
al, 2007 (29) | 65 | Hospital | Not specified | 34 (52.3) | 1.1 | 67.9±7.1 |
| Gellein et
al, 2008 (30) | 33 | Hospital | UK Brain Bank | 16 (48.5) | 0.94 | 61.1±9.1 |
| Ahmed and Santosh,
2010 (31) | 45 | Hospital | Not specified | 26 (57.8) | 1.37 | 57.6±9.1 |
| Fukushima et
al, 2010 (10) | 82 | Hospital | UK Brain Bank | 47 (57.3) | 1 | 63.9±9.4 |
| Fukushima et
al, 2011 (32) | 71 | Hospital | UK Brain Bank | 41 (57.7) | 1.37 | 63.7±9.7 |
| Madenci et
al, 2012 (33) | 60 | Hospital | UK Brain Bank | 33 (55) | 1.22 | 68.5±9.2 |
| Farhoudi et
al, 2012 (34) | 50 | Hospital | Not specified | 28 (56) | 1.27 | 64.5±10.2 |
| Fukushima et
al, 2013 (35) | 58 | Hospital | UK Brain Bank | 36 (62.1) | 1.64 | 64.3±9.4 |
| Zhao et al,
2013 (36) | 238 | Hospital | UK Brain Bank | 121 (50.8) | 1.03 | 66.6±11.3 |
| Kumudini et
al, 2014 (37) | 150 | Hospital | Not specified | 107 (71.3) | 2.49 | 55.7±10.6 |
| Hu et al,
2015 (38) | 102 | Hospital | UK Brain Bank | 47 (46.1) | 0.46 | 56.3±13.4 |
| Costa-Mallen et
al, 2015 (39) | 128 | Registry | UK Brain Bank | 88 (68.7) | 2.2 | 69 |
| Madeiros et
al, 2016 (40) | 40 | Hospital | UK Brain Bank | 18 (45) | 0.82 | 69.95±12.3 |
| Mariani et
al, 2016 (41) | 92 | Hospital | UK Brain Bank | 62 (67.4) | 2.07 | 70±11.25 |
Concerning the control groups, selection was
hospital-based in 19 (82.6%) studies, while in the other three
studies it was community-based and in a single study based on
registries. An age and gender group-matching strategy was adopted
in 16 (69.6%) studies, while a group-matching only by age was
adopted in five (21.7%) studies. One study did not adopt any
group-matching strategy. No data were provided in one study
(Table III).
 | Table III.Case-control studies on blood/serum
iron levels in PD and controls (CTR). CTR group
characteristics. |
Table III.
Case-control studies on blood/serum
iron levels in PD and controls (CTR). CTR group
characteristics.
| Study, year
(ref.) | CTR (N) | CTR source | CTR men (N, %) | CTR M/F ratio | CTR age (mean ±
SD) | Age matching | Gender
matching |
|---|
| Chen and Shih, 1992
(21) | 30 | Hospital | Not specified | Not specified | Not specified | Not specified | Not specified |
| Takahashi et
al, 1994 (22) | 14 | Hospital | Not specified | Not specified | 60.8±8.9 | Yes | Not specified |
| Logroscino et
al, 1997 (23) | 352 | Community | Not specified | Not specified | Not specified | Yes | No |
| Jiménez-Jiménez
et al, 1998 (24) | 37 | Hospital | 16 (43.2) | 0.76 | 62.4±17.8 | Yes | Yes |
| Tórsdóttir et
al, 1999 (25) | 33 | Hospital | Not specified | Not specified | Not specified | Yes | Yes |
| Forte et al,
2004 (26) | 13 | Hospital | 6 (46.1) | 0.86 | 63.8±13.7 | Yes | No |
| Hedge et al,
2004 (9) | 25 | Hospital | 13 (52) | 1.08 | 55.4±6.4 | Yes | Yes |
| Qureshi et
al, 2006 (27) | 21 | Hospital | 13 (61.9) | 1.63 | 62±11 | Yes | Yes |
| Annanmaki et
al, 2007 (28) | 29 | Hospital | 13 (45) | 0.81 | 60.2±5.1 | Yes | Yes |
| Squitti et
al, 2007 (29) | 52 | Hospital | 32 (61.5) | 1.6 | 71.1±8.5 | Yes | Yes |
| Gellein et
al, 2008 (30) | 99 | Community | 48 (48.5) | 0.94 | Not specified | Yes | Yes |
| Ahmed and Santosh,
2010 (31) | 42 | Hospital | 25 (59.9) | 1.47 | 55.6±3.2 | Yes | Yes |
| Fukushima et
al, 2010 (10) | 82 | Hospital | 47 (57.3) | 1 | 63.6±9.3 | Yes | Yes |
| Fukushima et
al, 2011 (32) | 71 | Hospital | 41 (57.7) | 1.37 | 63.4±9.7 | Yes | Yes |
| Madenci et
al, 2012 (33) | 42 | Hospital | 22 (52.4) | 1.1 | 66.9±8.3 | Yes | Yes |
| Farhoudi et
al, 2012 (34) | 50 | Hospital | 25 (50) | 1 | 63.5±9.8 | Yes | Yes |
| Fukushima et
al, 2013 (35) | 81 | Hospital | 47 (58) | 1.38 | 63.7±9.4 | Yes | Yes |
| Zhao et al,
2013 (36) | 302 | Hospital | 153 (50.7) | 1.03 | 65.6±12.2 | Yes | Yes |
| Kumudini et
al, 2014 (37) | 175 | Hospital | 120 (70.6) | 2.18 | 53.7±10.9 | Yes | Yes |
| Hu et al,
2015 (38) | 31 | Hospital | Not specified | Not specified | Not specified | Yes | Not specified |
| Costa-Mallen et
al, 2015 (39) | 226 | Registry | 104 (46) | 0.85 | 62.6 | Yes | Not specified |
| Madeiros et
al, 2016 (40) | 46 | Community | 19 (41) | 0.7 | 62.3±10.2 | Yes | Yes |
| Mariani et
al, 2016 (41) | 112 | Hospital | 40 (35.8) | 0.56 | 62±14 | No | No |
Iron serum levels (using different units of measure)
in both groups and the different methods of detection adopted are
shown in Table I. A meta-analysis
was performed on all the 23 studies included. Results are
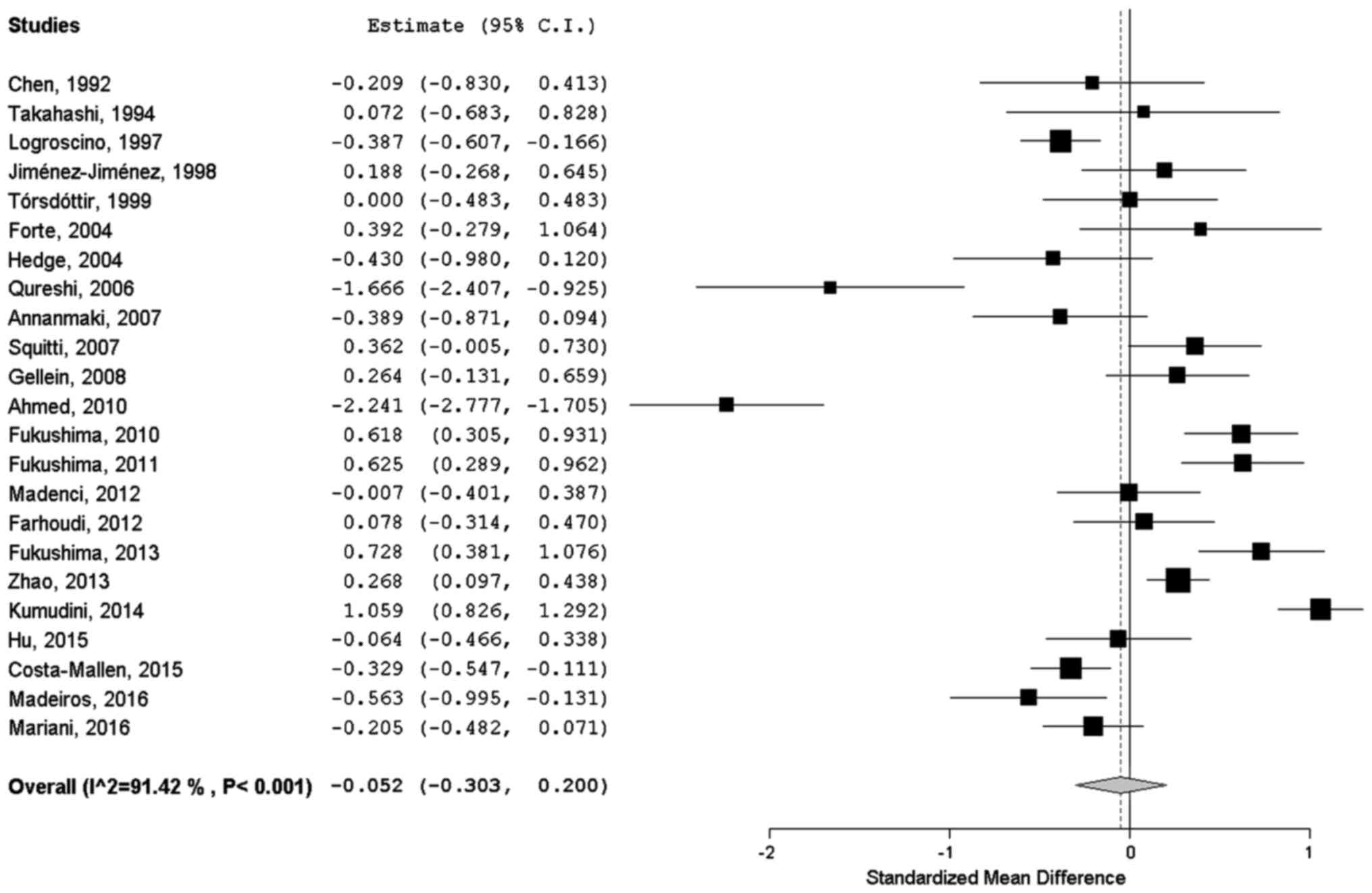
summarized as forest plot in Fig.
1. A small, around zero, overall SMD of −0.052 (95% CI,
−0.303–0.2) was estimated, indicating no substantial differences
between groups among selected studies. I2 statistic
revealed high heterogeneity among studies (I2=91.42%;
p<0.001); it means that ~91% of the observed variance comes from
real differences between studies and can potentially be explained
by study-level covariates.
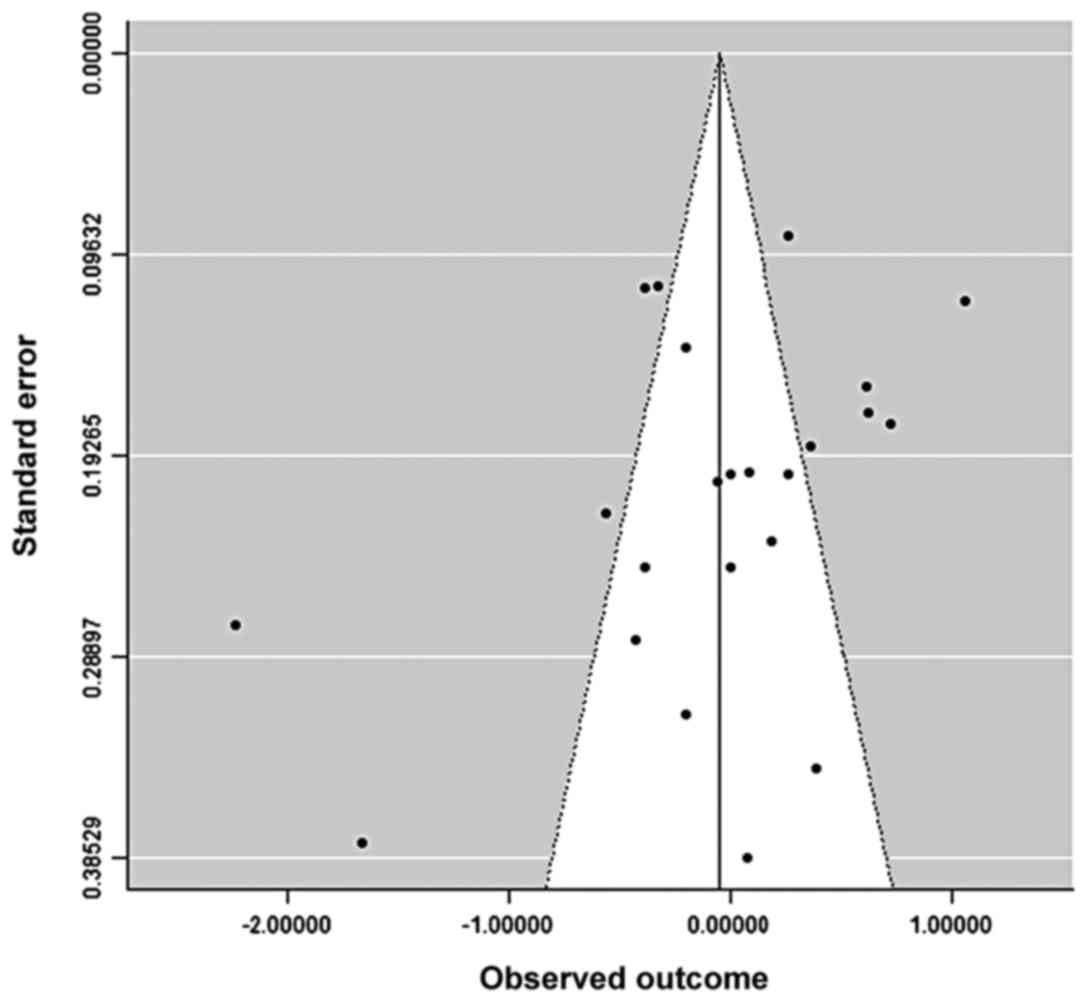
Asymmetry in funnel plot was consistent with the
presence of publication bias in favor of positive results, showing
also that studies were principally allocated in bottom of the graph
indicating a broad range of standard error of the effect measures
among studies due to their small sample size (Fig. 2). The fail-safe N calculation was
equal to zero (observed significance level, 0.319; target
significance level, 0.05).
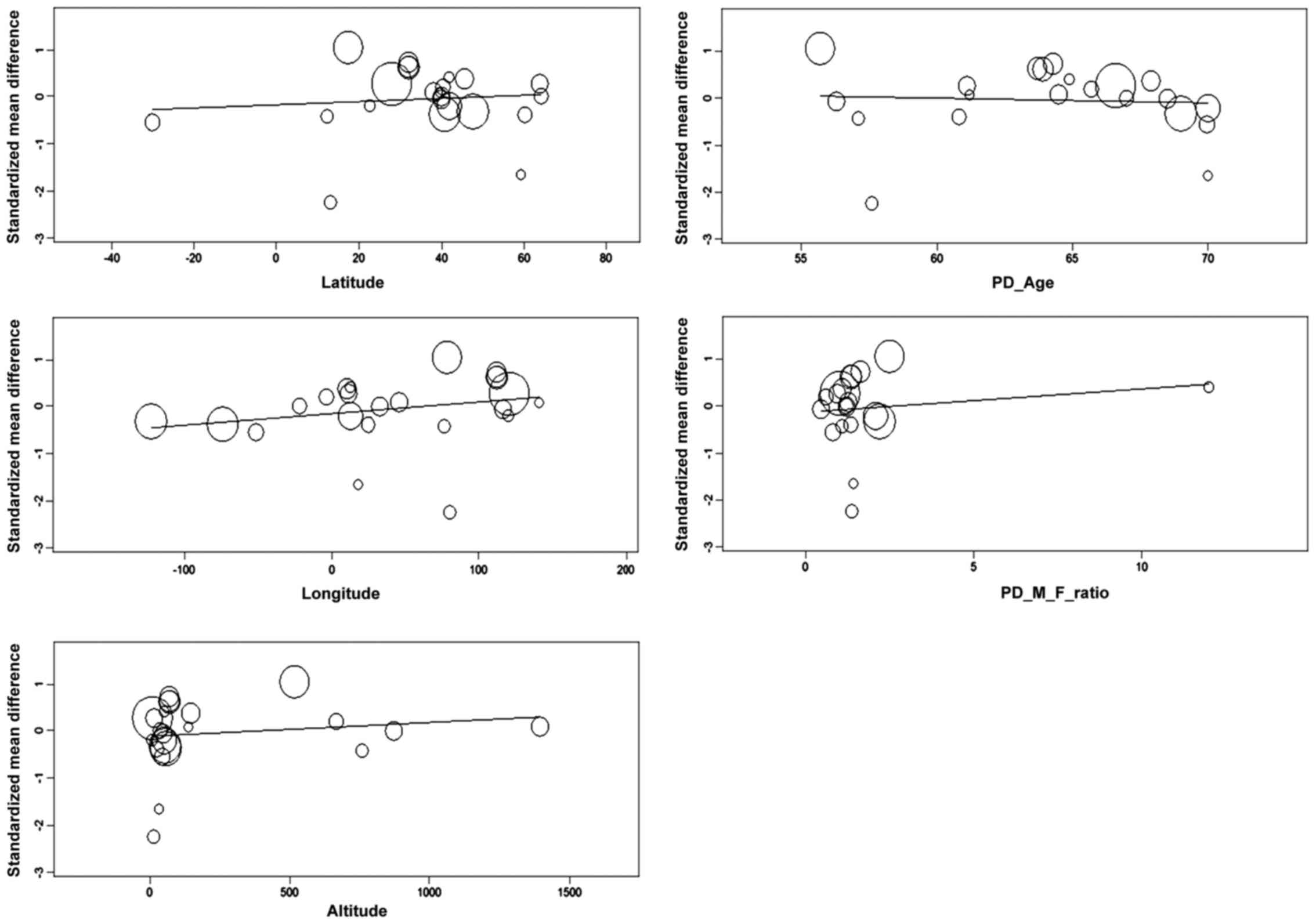
A meta-regression analysis was then performed
considering single demographic, geographical and clinical
covariates using a random-effects model. The regression coefficient
for latitude was 0.003, which means that every one degree of
latitude corresponds to an increase of 0.003 units in effect size,
even if this was not statistically significant (Z=0.457, Q=0.209,
p=0.648). Regression coefficients for longitude and altitude were
respectively 0.002 (Z=1.175, Q=1.38, p=0.240) and 0 (Z=0.721,
Q=0.52, p=0.471), both not statistically significant (Fig. 3).
Demographical covariates, based on the assumption
that most of studies were group-matched by age and gender, the
regression coefficient for PD age was −0.01 (N=21, Z=−0.269,
Q=0.072, p=0.788) while for the PD male-to-female ratio was 0.049
(N=20, Z=0.66, Q=0.436, p=0.509), both not statistically
significant (Fig. 3).
When we looked at the clinical characteristics of
selected groups of cases, we found a regression coefficient for
disease duration of 0.035 (N=11, Z=0.607, Q=0.368, p=0.544) and for
the UPDRS total score of 0.017 (N=7, Z=1.28, Q=1.64, p=0.201), both
not statistically significant.
Discussion
Iron and its deregulated homeostasis have been
proposed to have a role in the pathogenesis of PD because of its
pro-oxidants characteristics that may lead to ROS generation via
Fenton and Haber-Weiss reactions. However, epidemiological evidence
concerning the possible association between iron and PD remains
still controversial.
In this systematic revision and meta-analysis, we
searched for a possible association between serum iron levels and
PD, as compared to controls. Based on selected case-control
studies, we did not find a significant pooled mean difference
between groups. Our results are in agreement with a previous
meta-analysis by Mariani et al (18) demonstrating no variation of metal
concentrations in serum between PD patients and healthy controls
(SMD, −0.45; 95% CI, −0.98–0.08), probably due to the high
heterogeneity among evaluated studies (I2=93.4%;
p<0.001). Evaluation of a second meta-analysis based on 11
selected studies showed instead overall higher iron serum levels in
PD patients when compared to controls (SMD, 0.97; 95% CI,
0.18–0.37), even though a significant heterogeneity was also found
among studies (I2=96.5%; p<0.001) (19).
We confirmed a high level of heterogeneity among
evaluated studies, as expression of the overall small-sampled,
methodologically-limited studies available in literature which are
insufficient to provide practical evidence; for instance, most of
the selected studies had a hospital-based design, which does not
permit to exclude a possible selection bias. Moreover, the presence
of publication bias could have lead to an underestimation of
possible negative results.
Heterogeneity among studies can be related to
several issues: the different approaches in finding and selection
of cases and controls; the lack of an adequate matching strategy
between groups; the use of an inadequate sample size for obtaining
statistically-relevant results; the different methods used for iron
detection in blood samples; the lack of corrected analysis for
possible confounders including comorbidities affecting iron serum
levels. These issues justified the choice of a random-effects
approach for meta-analysis of data.
We performed a meta-regression analysis considering
single available demographic, geographical and clinical covariates,
all potential confounders affecting our pooled results. However, no
significant association was detected.
Limits of the present meta-analysis are the same as
those related to other meta-analysis performed using observational,
case-control studies as target. In particular, the appropriate
control of confounding factors is of fundamental importance in the
analysis and interpretation of observational studies. The presence
of other types of bias, for example recall bias, should represent
additional concerns (12). Even
more, we focused our selection on studies evaluating serum iron
levels and not other biological fluids (i.e., urine or
cerebrospinal fluid) or tissues (i.e., hair), limiting results
interpretation. Furthermore, it should be underlined that in
case-control studies evaluating a possible association between
metals and PD by detecting levels of metals in biological fluids,
these measurements often show just the actual iron homeostasis,
lacking a clear history of exposure to high levels of this metal
(20). Finally, even if a
systematical approach has been used for the search strategy, we
cannot exclude that some data were missed affecting the study
results.
In conclusion, based on our systematic review and
meta-analysis of available case-control studies, we can state there
are still not sufficient evidence supporting higher or lower serum
levels of iron in PD patients as compared to controls, assuming
this may be related to metal exposure or pathological processes in
such subjects. Principal reasons should be sought in the elevated
methodological heterogeneity we found among available studies. A
particular attention should be paid on bias and confounding effects
to limit heterogeneity among studies and to facilitate the summary
of the results.
References
|
1
|
Chin-Chan M, Navarro-Yepes J and
Quintanilla-Vega B: Environmental pollutants as risk factors for
neurodegenerative disorders: alzheimer and Parkinson diseases.
Front Cell Neurosci. 9:1242015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fuqua BK, Vulpe CD and Anderson GJ:
Intestinal iron absorption. J Trace Elem Med Biol. 26:115–119.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schneider SA, Hardy J and Bhatia KP:
Syndromes of neurodegeneration with brain iron accumulation (NBIA):
an update on clinical presentations, histological and genetic
underpinnings, and treatment considerations. Mov Disord. 27:42–53.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Smith MA, Zhu X, Tabaton M, Liu G, McKeel
DW Jr, Cohen ML, Wang X, Siedlak SL, Dwyer BE, Hayashi T, et al:
Increased iron and free radical generation in preclinical alzheimer
disease and mild cognitive impairment. J Alzheimers Dis.
19:363–372. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kasarskis EJ, Tandon L, Lovell MA and
Ehmann WD: Aluminum, calcium, and iron in the spinal cord of
patients with sporadic amyotrophic lateral sclerosis using laser
microprobe mass spectroscopy: a preliminary study. J Neurol Sci.
130:203–208. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dexter DT, Wells FR, Agid F, Agid Y, Lees
AJ, Jenner P and Marsden CD: Increased nigral iron content in
postmortem Parkinsonian brain. Lancet. 2:1219–1220. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ward RJ, Zucca FA, Duyn JH, Crichton RR
and Zecca L: The role of iron in brain ageing and neurodegenerative
disorders. Lancet Neurol. 13:1045–1060. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jellinger KA: The relevance of metals in
the pathophysiology of neurodegeneration, pathological
considerations. Int Rev Neurobiol. 110:1–47. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hegde ML, Shanmugavelu P, Vengamma B, Rao
TS, Menon RB, Rao RV and Rao KS: Serum trace element levels and the
complexity of inter-element relations in patients with Parkinsons
disease. J Trace Elem Med Biol. 18:163–171. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fukushima T, Tan X, Luo Y and Kanda H:
Relationship between blood levels of heavy metals and Parkinsons
disease in China. Neuroepidemiology. 34:18–24. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Higgins JP, Thompson SG, Deeks JJ and
Altman DG: Measuring inconsistency in meta-analyses. BMJ.
327:557–560. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kirkwood BR and Sterne JAC: Essential
Medical Statistics. 2nd. Blackwell Publishing; Oxford: 2003
|
|
13
|
Rosenthal R: The ‘file drawer problem’ and
tolerance for null results. Psychol Bull. 86:638–641. 1979.
View Article : Google Scholar
|
|
14
|
Borenstein M, Hedges LV, Higgins JPT and
Rothstein HR: Introduction to Meta-Analysis. Wiley; Chichester, UK:
2009, View Article : Google Scholar
|
|
15
|
Hughes AJ, Daniel SE, Kilford L and Lees
AJ: Accuracy of clinical diagnosis of idiopathic Parkinsons
disease: a clinico-pathological study of 100 cases. J Neurol
Neurosurg Psychiatry. 55:181–184. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hoehn MM and Yahr MD: Parkinsonism: onset,
progression and mortality. Neurology. 17:427–442. 1967. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fahn S and Elton RL: Unified Parkinsons
disease rating scaleRecent Developments in Parkinsons Disease. Fahn
S, Marsden CD, Calne DB and Goldstein M: 2. Macmillan; Florham
Park, NJ: pp. 153–163. 1987
|
|
18
|
Mariani S, Ventriglia M, Simonelli I,
Donno S, Bucossi S, Vernieri F, Melgari JM, Pasqualetti P, Rossini
PM and Squitti R: Fe and Cu do not differ in Parkinsons disease: a
replication study plus meta-analysis. Neurobiol Aging. 34:632–633.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jiao J, Guo H, He Y, Wang J, Yuan J and Hu
W: Meta-analysis of the association between serum iron levels and
Parkinson's disease: evidence from 11 publications. Brain Res.
1646:490–493. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sahin C, Pala C, Kaynar L, Torun YA, Cetin
A, Kurnaz F, Sivgin S and Sahin FS: Measurement of hair iron
concentration as a marker of body iron content. Biomed Rep.
3:383–387. 2015.PubMed/NCBI
|
|
21
|
Chen WH and Shih PY: The serum
ferrokinetics in Parkinsons disease. Gaoxiong Yi Xue Ke Xue Za Zhi.
8:581–584. 1992.(In Chinese). PubMed/NCBI
|
|
22
|
Takahashi S, Takahashi J, Osawa N, Abe T,
Yonezawa H, Sera K and Tohgi H: Trace elements analysis of serum
and cerebrospinal fluid with PIXE-effect of age and changes in
Parkinsonian patients. Nippon Ronen Igakkai Zasshi. 31:865–871.
1994.(In Japanese). View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Logroscino G, Marder K, Graziano J, Freyer
G, Slavkovich V, LoIacono N, Cote L and Mayeux R: Altered systemic
iron metabolism in Parkinsons disease. Neurology. 49:714–717. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jiménez-Jiménez FJ, Molina JA, Aguilar MV,
Meseguer I, Mateos-Vega CJ, González-Muñoz MJ, de Bustos F,
Martínez-Salio A, Ortí-Pareja M, Zurdo M, et al: Cerebrospinal
fluid levels of transition metals in patients with Parkinsons
disease. J Neural Transm Vienna. 105:497–505. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tórsdóttir G, Kristinsson J,
Sveinbjörnsdóttir S, Snaedal J and Jóhannesson T: Copper,
ceruloplasmin, superoxide dismutase and iron parameters in
Parkinsons disease. Pharmacol Toxicol. 85:239–243. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Forte G, Bocca B, Senofonte O, Petrucci F,
Brusa L, Stanzione P, Zannino S, Violante N, Alimonti A and
Sancesario G: Trace and major elements in whole blood, serum,
cerebrospinal fluid and urine of patients with Parkinsons disease.
J Neural Transm Vienna. 111:1031–1040. 2004.PubMed/NCBI
|
|
27
|
Qureshi GA, Qureshi AA, Memon SA and
Parvez SH: Impact of selenium, iron, copper and zinc in on/off
Parkinson's patients on L-dopa therapy. J Neural Transm (Suppl).
71:229–236. 2006. View Article : Google Scholar
|
|
28
|
Annanmaki T, Muuronen A and Murros K: Low
plasma uric acid level in Parkinsons disease. Mov Disord.
22:1133–1137. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Squitti R, Gorgone G, Binetti G, Ghidoni
R, Pasqualetti P, Draicchio F, Albini E, Benedetti L, Lucchini R
and Rossini PM: Metals and oxidative stress in Parkinsons disease
from industrial areas with exposition to environmental toxins or
metal pollution. G Ital Med Lav Ergon. 29:(Suppl 3). 294–296.
2007.(In Italian). PubMed/NCBI
|
|
30
|
Gellein K, Syversen T, Steinnes E, Nilsen
TI, Dahl OP, Mitrovic S, Duraj D and Flaten TP: Trace elements in
serum from patients with Parkinsons disease: a prospective
case-control study: the Nord-Trøndelag health study (HUNT). Brain
Res. 1219:111–115. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ahmed SS and Santosh W: Metallomic
profiling and linkage map analysis of early Parkinsons disease: a
new insight to aluminum marker for the possible diagnosis. PLoS
One. 5:e112522010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fukushima T, Tan X, Luo Y and Kanda H:
Serum vitamins and heavy metals in blood and urine, and the
correlations among them in Parkinsons disease patients in China.
Neuroepidemiology. 36:240–244. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Madenci G, Bilen S, Arli B, Saka M and Ak
F: Serum iron, vitamin B12 and folic acid levels in Parkinson's
disease. Neurochem Res. 37:1436–1441. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Farhoudi M, Taheraghdam A, Farid GA,
Talebi M, Pashapou A, Majidi J and Goldust M: Serum iron and
ferritin level in idiopathic Parkinson. Pak J Biol Sci.
15:1094–1097. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fukushima T, Tan X, Luo Y, Wang P, Song J,
Kanda H, Hayakawa T, Kumagai T, Kakamu T, Tsuji M, et al: Heavy
metals in blood and urine and its relation to depressive symptoms
in Parkinsons disease patients. Fukushima J Med Sci. 59:76–80.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhao HW, Lin J, Wang XB, Cheng X, Wang JY,
Hu BL, Zhang Y, Zhang X and Zhu JH: Assessing plasma levels of
selenium, copper, iron and zinc in patients of Parkinsons disease.
PLoS One. 8:e830602013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kumudini N, Uma A, Devi YP, Naushad SM,
Mridula R, Borgohain R and Kutala VK: Association of Parkinsons
disease with altered serum levels of lead and transition metals
among south Indian subjects. Indian J Biochem Biophys. 51:121–126.
2014.PubMed/NCBI
|
|
38
|
Hu Y, Yu SY, Zuo LJ, Piao YS, Cao CJ, Wang
F, Chen ZJ, Du Y, Lian TH, Liu GF, et al: Investigation on abnormal
iron metabolism and related inflammation in Parkinson disease
patients with probable RBD. PLoS One. 10:e01389972015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Costa-Mallen P, Zabetian CP, Agarwal P, Hu
SC, Yearout D, Samii A, Leverenz JB, Roberts JW and Checkoway H:
Haptoglobin phenotype modifies serum iron levels and the effect of
smoking on Parkinson disease risk. Parkinsonism Relat Disord.
21:1087–1092. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Medeiros MS, Schumacher-Schuh A, Cardoso
AM, Bochi GV, Baldissarelli J, Kegler A, Santana D, Chaves CM,
Schetinger MR, Moresco RN, et al: Iron and oxidative stress in
Parkinsons disease: an observational study of injury biomarkers.
PLoS One. 11:e01461292016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Mariani S, Ventriglia M, Simonelli I,
Bucossi S, Siotto M, Donno S, Vernieri F and Squitti R: Association
between sex, systemic iron variation and probability of Parkinsons
disease. Int J Neurosci. 126:354–360. 2016. View Article : Google Scholar : PubMed/NCBI
|