Introduction
B lymphocytes, also known as B cells, are well known
for their ability to mediate the humoral immune response by
differentiating into antibody-secreting cells. In previous years, a
novel subset of B cells, which exert immunomodulatory effects
through the production of interleukin (IL)-10 and transforming
growth factor-β (TGF-β) have been identified and classified as
regulatory B cells (Bregs). To date, Bregs have been described in a
number of reports (1–3), some of which refer to Bregs as
IL-10-producing B cells or B10/Br1 cells (4,5). The
role of IL-10-producing B cells was first demonstrated in a murine
model of chronic intestinal inflammation (6), however, Bregs have also been reported
to function in autoimmune diseases (7), allergic diseases (8,9),
graft-versus-host disease (10,11)
and cancer (12) through
antigen-specific and non-specific immunoregulatory mechanisms.
Previously, a novel subset of Bregs possessing a plasma cell
phenotype, which expresses CD138 and produces anti-inflammatory
IL-10 and IL-35 cytokines during Salmonella infections and
experimental autoimmune encephalomyelitis (EAE) was reported.
Accordingly, studies investigating Bregs have become more extensive
(13,14).
A previous report described a naturally occurring
subset of Bregs, which facilitated the maintenance of homeostasis
within adipose tissue and suggested that Breg cell dysfunction may
be pivotal in the progression of adipose tissue inflammation in
obesity (14). However,
corresponding data on the role of Bregs during infection,
particularly their functions during intracellular infections, are
limited. The present review aimed to discuss the role of Bregs in
various infections and attempt to uncover potential markers or
valuable targets for the development of therapeutic interventions
for the treatment of infectious diseases.
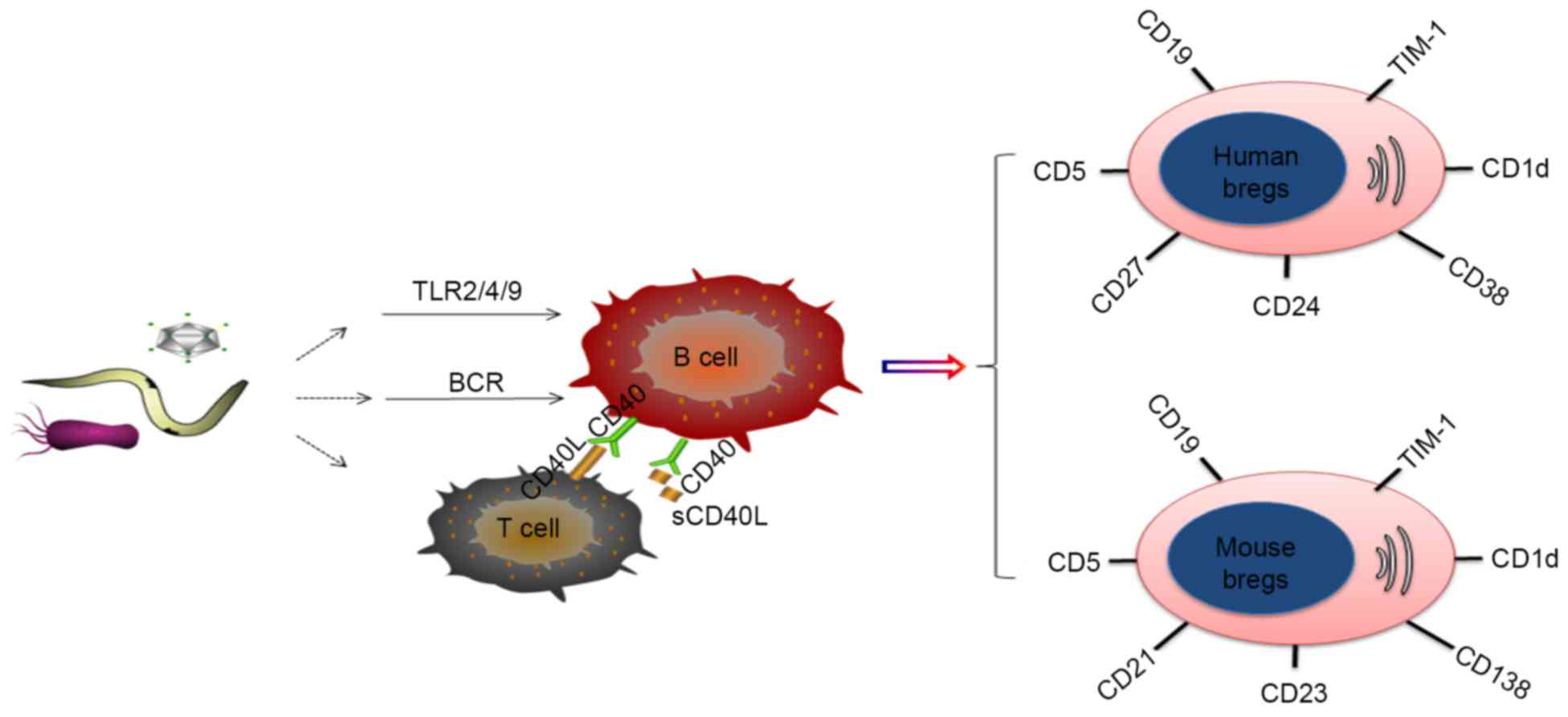
Breg phenotypes
Although several potential markers for Bregs have
been described, the unequivocal identification of Bregs in
infectious disease has not been achieved. In mice, the most widely
accepted Breg subsets are
CD19+CD5+CD1dhi Bregs (5,15,16)
and CD19+CD21+CD23+ Bregs
(15,17). However, controversy remains over
the identification of Bregs in mice. A subset of Bregs possessing a
CD138+ plasma cell phenotype were previously reported to
produce IL-10 and IL-35 during Salmonella infection in mice
(18). In addition, Wilson et
al reported the identification of a population of Bregs
expressing high levels of CD23 in the absence of CD5 and CD1d in
the mesenteric lymph nodes of helminth-infected mice (19).
In humans, reports of the identification of Bregs
are more diverse. Generally, the established Breg subsets in humans
include CD19+CD24+CD38+Bregs
(1,2,20,21)
and CD19+CD24+CD27+Bregs (22), although reports have also included
the identification of
CD19+CD5+CD1d+ Bregs (23,24)
and CD19+TIM-1+Bregs (17) in humans. However, certain
phenotypes are not supported by evidence in human immunodeficiency
virus (HIV)-infected individuals, which suggests human ‘Bregs’
lacking the expression of CD1d may not be Bregs.
Multiple Breg cell surface markers have been
reported, a number of which have been identified in humans and mice
(Fig. 1). However, discrepancies
in the phenotypes reported across studies are apparent. These
differences are most likely due to the use of an imperfect panel of
markers to characterize the B cell subsets, different disease
models and organic sources, and the use of different induction
methods, including Toll-like receptor (TLR) ligands or CD40 and B
cell receptor (BCR) agonists, all of which can affect phenotype
(Table I).
 | Table I.Overview of the phenotypic
characteristics and mechanisms of action of different Breg subsets
during infection. |
Table I.
Overview of the phenotypic
characteristics and mechanisms of action of different Breg subsets
during infection.
| Author (date) | Source | Organ | Breg cell
phenotype | Mechanism of
action | Disease model | Stimuli | (Refs.) |
|---|
| Garner-Spitzer
et al (2013) | Human | PBMC |
CD19+CD24+CD38hi |
IL-10/contact-mediated interaction; Treg
cell induction | CHB | Unknown | (1) |
| Das et al
(2012) | Human | PBMC |
CD19+CD24+CD27+ | IL-10;
CD8+ T cell suppression | CHB | TLR9 | (22) |
| Gong et al
(2014) | Human | PBMC |
CD19+IL-10 |
IL-10/contact-mediated interaction; Treg
cell induction/Th1 suppression | CHB | Unknown | (25) |
| Jiao et al
(2014) | Human | PBMC |
CD19lowCD5+CD38+CD24+ | IL-10;
CD4+ T cell suppression | HIV | Unknown | (20) |
| Siewe et al
(2013) | Human | PBMC |
CD19+CD24hiCD38+ | IL-10/PD-1;
CD8+ T cell suppression | HIV | TLR2, TLR9 and
CD40 | (2) |
| Kaltenmeier et
al (2015) | Human | PBMC |
CD5+CD43+CD86+CD147+ | granzyme B; T cell
suppression | HIV | IL-21 | (36) |
| Ronet et al
(2010) | Mouse | Spleen cells/lymph
node |
CD1d+CD5+CD21lowCD23low | IL-10; Th2
induction/DC suppression | Leishmania
major | Unknown | (15) |
| Jeong et al
(2012) | Mouse | Spleen cells |
CD1dhiCD5+ | IL-10; Treg cell
interaction | Babesia | Unknown | (16) |
| van der Vlugt et
al (2014) | Human | PBMC |
CD1dhi | IL-10;
CD4+ T cell suppression |
Schistosoma | Unknown | (3) |
| Horikawa et
al (2013) | Mouse | Spleen cells |
CD1dhiCD5+ | IL-10/CD22;
macrophage/CD4+ T cell suppression |
Listeria | Unknown | (5) |
| Zhang et al
(2014) | Human | PBMC |
CD19+CD1d+CD5+ | contact-mediated
interaction; Th22/CD4+ T cell suppression | Mycobacterium
tuberculosis | Unknown | (23) |
| Shen et al
(2014) | Mouse | Spleen cells |
IgM+CD138hiTACI+
CXCR4+CD1dintTim1int | IL-10/IL-35;
macrophage/inflammatory T cell suppression |
Salmonella | CD40 and TLR4 | (18) |
Immunomodulation by Bregs
Although the regulatory mechanism of Bregs in
infectious disease remains to be fully elucidated, data from
multiple studies have indicated that a diverse array of
immunomodulatory cytokines follow the emergence of Bregs, of which
IL-10 is the most frequently investigated. Although IL-10 is
produced by various cell types and exhibits several pleiotropic
effects, the expression of IL-10 by human and mouse Bregs is
central to their negative regulation of adaptive and innate immune
responses (1–3,5,15,20,22,25).
The differentiation of T cells into T helper cells
(Th), including Th1, Th2 and Th17 cells, all of which possess a
protective capacity during infection, can be inhibited by IL-10
(23–27), as shown in Fig. 2. In other circumstances, IL-10 can
suppress the secretion of IL-22, IL-17 and interferon-γ (IFN-γ) by
CD4+ T cells (3,21,23,24,28).
In addition, IL-10 can attenuate the differentiation of
CD8+ T cells into cytotoxic T lymphocytes (CTLs) under
certain stimuli. Breg depletion in vitro leads to enhanced
CTL activity in CD8 +T cells, whereas activated Bregs
may contribute to the attenuation of CTL functions (2,22,29)
(Fig. 2). Other reports have
indicated that increased numbers of Bregs potentially contribute to
elevated levels of IL-10 and induce forkhead box P3 (FOXP3)
+ regulatory T cells (Tregs), which exhibit broader
suppressive functions (1,16).
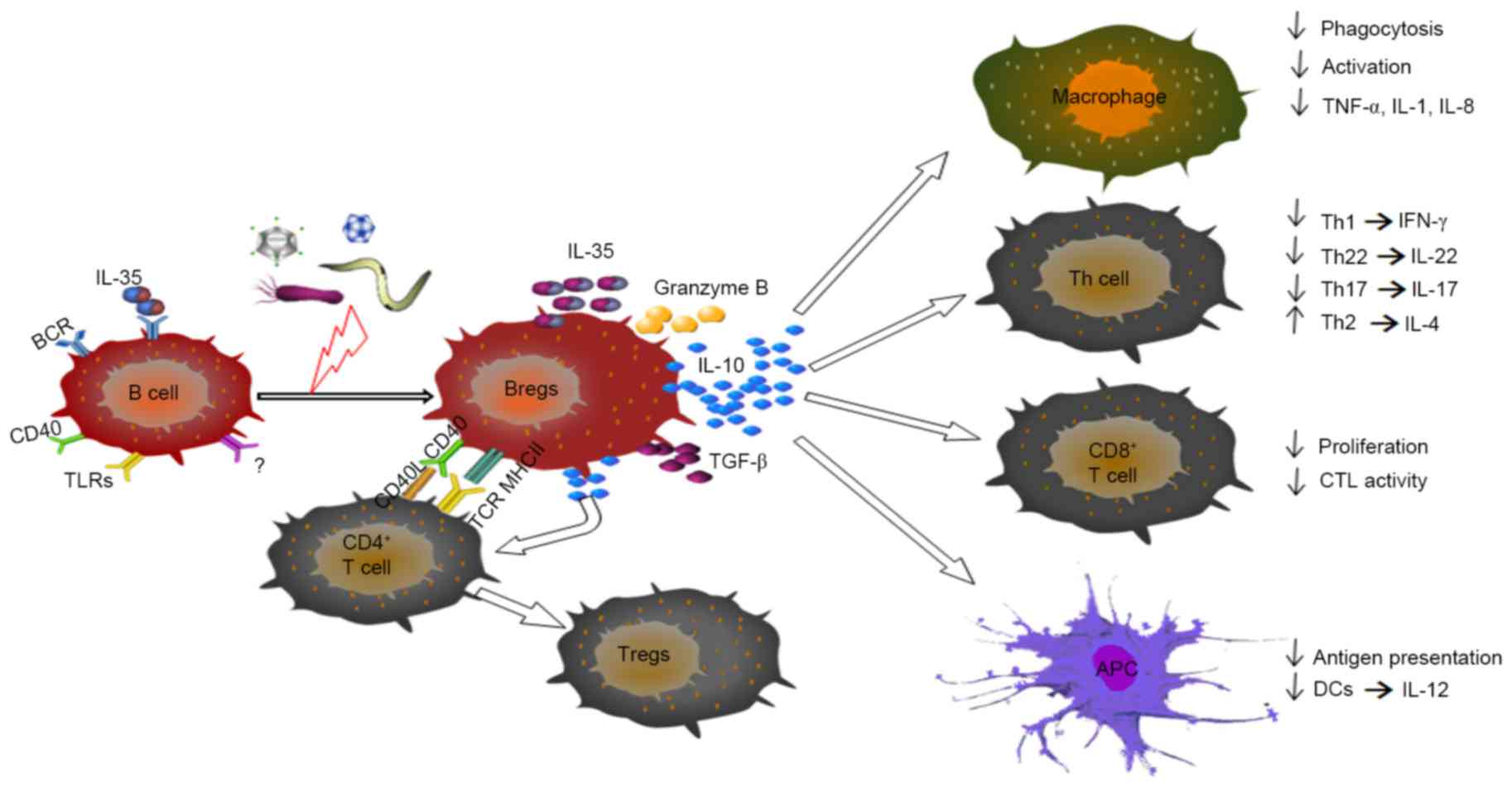 | Figure 2.Immunomodulatory properties of Bregs
during infectious disease. Through the contact-mediated interaction
and/or secretion of IL-10, TGF-β, IL-35 or granzyme B, Bregs can
suppress the activation and production of cytokines by
pro-inflammatory lymphocytes, including macrophage, certain Th
cells, CD8+ T cells and DCs. Bregs can also induce the
differentiation of Th2 cells and Treg cells, primarily through
IL-10. Breg, regulatory B cell; Treg, regulatory T cell; Th, T
helper; DCs, dendritic cells; APC antigen-presenting cell; BCR, B
cell receptor; CTL, cytotoxic T lymphocyte; IL, interleukin; TGF-β,
transforming growth factor-β; TLR, Toll-like receptor. |
Following TLR activation, innate CD5+
Bregs are able to suppress dendritic cell (DC) IL-12 secretion by
producing high levels of IL-10 (15,30).
In a separate study, Qian et al demonstrated that regulatory
DCs can induce splenic B cells to differentiate into a distinct
subtype of IL-10-producing Bregs with a unique
CD19hiFcγIIbhi phenotype (31). Other reports have indicated that
Bregs produce the immunomodulatory cytokine, TGF-β, in allergic
(9,32,33)
and infectious (3,34) diseases. Although the potential
co-expression of TGF-β and IL-10 remains to be fully elucidated,
the expression of TGF-β and IL-10 by different Breg subsets, and
the presence of an increased percentage of
CD24hiCD27+ and CD1dhi B cells in
Schistosoma-infected mice expressing TGF-β and IL-10,
respectively, has been determined (3). However, the distinct role of human
TGF-β-expressing Bregs in infectious diseases requires
elucidation.
A previous study revealed that B cells activated by
TLR4 and CD40 can upregulate the expression of Epstein-Barr
virus-induced gene 3 (Ebi3), also known as IL-27β, and p35, also
known as IL12a, which dimerize to generate IL-35 (18) (Table
I). The development of Salmonella infection in mice
lacking either the Ebi3 or the p35 subunit in B cells was found to
be exacerbated, which indicated that IL-35 was essential for the
suppressive functions of B cells. Wang et al (35) reported that IL-35 induced B cells
and promoted their conversion into Breg subsets, which produced
IL-35 and IL-10 by activating signal transducer and activator of
transcription 1 (STAT1) and STAT3. However, whether the
differential expression of IL-35 and IL-10 by these subsets was due
to instructive or stochastic mechanisms remains to be elucidated.
The increased expression of serine protease granzyme B (GraB) by a
subset of Bregs, termed GraB cells, is another regulatory B cell
mechanism, which weakens T cell responses (36).
In addition to the aforementioned secretory factors,
Bregs are involved in pathophysiological processes by directly
inhibiting autoreactive T cells and innate immune cells. Bregs can
make contact with effector T cells through CD40/CD40L, which
accelerates the process of T cell apoptosis (5). Previously, enhanced bacterial
clearance in mice lacking IL-10-producing Bregs (B10 cells) was
found to correspond with improved bacterial phagocytosis by
macrophages, and to significant increases in the ex vivo
production of IFN-γ, TNF-α and nitric oxide by macrophages
(5,23). The expression of major
histocompatibility complex (MHC) class II molecules and CD21R by
B10 cells supports the argument that B10 cells require cognate
interactions with CD4+ T cells to induce their
regulatory effector functions during infection. Therefore,
macrophage function is likely to be regulated downstream of B10
cell interactions with CD4+ T cells (5,25)
(Fig. 2). Bregs have also been
shown to represent a significant source of serum immunoglobulin
(Ig)M and IgG during adoptive transfer experiments, and produce
antigen-specific, polyreactive and autoreactive antibody (Ab)
specificities (30). However,
their antibody-mediated immune responses in infectious diseases
remain to be fully elucidated.
Bregs in infections
Role of Bregs in viral infections
The effects of B cell-mediated humoral immunity on
the clearance of intracellular infection are limited. Only a few
studies have focused on the role of Bregs in intracellular
infections, and investigation in viral infections has focused
predominantly on the HIV and hepatitis B virus (HBV). The
involvement of Bregs in viral infections is primarily through
IL-10-mediated immunological effects. Das et al (22) provided the first demonstration that
Bregs regulate antigen-specific CD8+ T cells in HBV
infection. It was found that IL-10-producing B cells (25) and serum levels of IL-10 correlated
with spontaneous flare-ups of liver disease in patients with
chronic HBV infection (CHB), but not in patients with acute HBV
infection or in healthy subjects. In vitro, the inhibition
of IL-10 may restore HBV-specific CD8+ T cell
polyfunctionality (22). In
addition Bregs contribute to increased levels of IL-10 in HVB
non-responders and leads to an induction of suppressive
FOXP3+ Tregs, which exert broader suppressive functions
(1,25) (Table
I). In the absence of stimulation, IL-10-producing B cells
predominantly exhibit an immature phenotype; however, these are the
only B cell subset to correlate with hepatic flares (22) (Table
I).
Previous studies have demonstrated that Bregs
contribute to immune dysfunction associated with HIV infection
through T cell impairment, specifically by the expression of IL-10
and possibly programmed death (PD)-L1, a member of the B7-H1
family. The suppressive properties of Bregs in HIV infection are
associated with a systemic prevalence of TLR ligands and CD40L, as
demonstrated by an in vitro experiment in which TLR2-, TLR9-
and CD40L-costimulated Bregs from healthy controls led to increased
expression of PD-L1 and a higher frequency of IL-10-positive cells
(2). Although the percentage of
B10 cells in the CD19+CD24hiCD38hi
B cell population is increased following ex vivo
stimulation, a reduction in
CD19+CD24hiCD38hi B cell frequency
has been reported in HIV-infected individuals. This may be due to
the suppression of CTL functions by activated Bregs, which lead to
viral persistence (2) (Table I). Ex vivo, the frequency of
IL-10-producing B cells has been negatively associated with
contemporaneous T cell responses, which supports the role of B10
cells in HIV-1-specific CD8+ T cell dysfunction
(17).
A previous report indicated that B cells from
untreated patients with HIV differentiated into Bregs
overexpressing GraB when cultured with autologous
IL-21+CD40L−Th cells. These GraB cells are
distinct from traditional B10 cells, and exhibit increased
expression levels of CD5, CD86, CD43 and CD147, but do not produce
IL-10 (36) (Table I). A feedback mechanism has been
identified in HIV-infected children whereby the increased
circulating Bregs induced by robust humoral and cell-mediated
immunity following vaccination may contribute to poor vaccine
responses (37).
Compared with other diseases, CD1d is not a marker
for Bregs in HIV infection (20),
despite its apparent downregulation during HIV-1 infection
(38). These observations may be
explained by differences in the immune systems of infected
individuals, or by the effects of HIV on Breg phenotypes. In
addition, CD19+CD1dhiCD5+ B cells
have been reported to infiltrate the brain and control
neuroinflammation in a chronic infection model, as evidenced by
reduced CD8+T cells/microglial responses and enhanced
accumulation of Tregs (29).
Although IL-10 likely originates from different cell
types including T cells (39),
macrophages (40), and natural
killer cells (41), several
reports have indicated that Bregs may be a primary source of IL-10
and that the production of IL-10 begins to increase in the early
stage of infection in tandem with viral load (22,25)
(Table I). However, whether or not
Bregs are in the primary source of IL-10 during host response to
infection remains to be elucidated. Accordingly, current data are
based on a ‘relative depletion’ model of IL-10-expressing cells,
which means a certain level remains present in the system.
Therefore, the possibility exists that other cell types are
similarly responsible for the production of IL-10.
Role of Bregs in parasitic
infections
Studies of allergic inflammation have demonstrated
that chronic parasitic (protozoa and helminth) infections are often
associated with a reduced prevalence of hyper-inflammatory
responses, including allergies (42,43).
The prevalence of autoimmune disease inversely correlates with
parasitic infections, and increasing evidence has shown that
parasitic worm infection may protect against autoimmune conditions
(44). ES-62, a molecule secreted
by the parasitic filarial nematode Acanthocheilonema viteae,
had therapeutic potential in the treatment of rheumatoid arthritis
due to its properties associated with the restoration of
IL-10-producing Bregs and reduced plasma cell infiltration in the
joints (45). The induction of
immune regulatory cells and soluble cytokines upon helminthic
infection is important for understanding the control of
autoimmunity and allergic inflammation. Until now, the majority of
studies investigating Bregs in helminthic infection have involved
adoptive transfer experiments and used alleviated disease symptoms
and/or protection against disease development as read-outs for Breg
activity (43).
The shift in Th1/Th2 balance triggered by infection
induces the Th2 response, and likely leads to a concomitant
suppression of the Th1 involved in different stages of parasite
infections (15,27,46).
Th1 responses were found to be exaggerated in
Schistosoma-infected B-cell-deficient mice, indicating that
Schistosoma-induced B cells suppressed Th1 cells in
wild-type mice (27). A previous
study (15) also revealed that
IL-10 produced by Bregs is required for susceptibility to
Leishmania major LV39 infection in BALB/c mice and for
polarization of the Th cell response toward the Th2 phenotype. This
is consistent with a previous report that B cells from
Schistosoma-infected mice secrete IL-10 and promote
development of the Th2 immune response (46). In a separate study, B cells from
patients with visceral Leishmaniasis exhibited enhanced ability to
differentiate into B10 cells, which potently inhibited the
activation, proliferation and cytokine secretion of
CD4+T cells, compared with healthy controls (21). Following stimulation by
Leishmania major, the IL-10 secreted by B cells also stems
the production of IL-12 by DCs (15) (Table
I). These IL-10-producing Bregs may be derived from existing
circulating B cells or from progenitor Bregs following stimulation
with CD40 ligand and/or BCR ligation for 2 days (47,48).
van der Vlugt et al found that, in
Schistosoma-infected individuals, IL-10 and membrane-bound
latency-associated peptide (LAP)/TGF-β were predominantly expressed
by the Breg subsets CD1dhi B cells and
CD24hiCD27+ B cells, respectively (3). However, the role of LAP/TGF-β is not
addressed in this review.
In addition to the production of secreted factors,
Bregs are able to induce other suppressive cell types by
contact-mediated interactions. When co-cultured with Breg cells
from Schistosoma-infected individuals, CD4+ T
cells produced lower levels of IFN-γ, IL-4 and IL-17. By contrast,
the conversion to CD25hiFoxP3+ and
IL-10+ T cells was enhanced (3,28)
(Table I).
Unlike trematode parasites, including
Schistosoma, Babesia microti is a common protozoan
parasite, which invades and replicates within erythrocytes. A
previous study demonstrated that the adoptive transfer of Bregs
enhanced susceptibility to protozoan parasite infection and
suppressed allergic disease by inducing
CD4+CD25+FoxP3+ Tregs. These
suppressive Tregs were not induced in the absence of
CD1dhiCD5+ Bregs (16). This was the first report on the
expansion of Bregs following infection, suggesting that Babesia
microti infection in mice may serve as a suitable model for
investigating Bregs in helminthic infections (16). Although the involvement of Tregs in
helminthic infections has been investigated extensively (42), only a limited number of studies
have investigated the role of Bregs in helminthic infections. Thus,
several questions remain, including whether or not the induction of
Bregs and Tregs persists in chronic infections or following
clearance of the parasite, and whether or not any other molecules
secreted by the parasite have therapeutic potential in autoimmunity
and allergic diseases.
Role of Bregs in bacterial
infections
Pathogens utilize multiple mechanisms to manipulate
Breg functions in order to modulate immune responses and evade host
defenses. During intracellular Listeria infection (5), B10-mediated inhibition of bacterial
clearance depends on the expression of IL-10, MHC class II
molecules and CD21-R. This finding suggested that B10 cells require
cognate interactions with CD4+ T cells to exert their
regulatory functions, but not CD8+ T cells. By contrast,
the depletion of Bregs using CD22 mAb substantially accelerated
ex vivo bacterial clearance (92–97% higher, compared with
wild-type mice) as a result of significantly reduced
CD4+ T cell expansion, enhanced macrophage phagocytosis,
and enhanced production of IFN-γ, TNF-α and nitric oxide (Table I).
An earlier report (34) showed that B cell-deficient mice
exhibited markedly enhanced resistance to the intracellular
bacterium Brucella abortus, reflecting the importance of
non-antibody-mediated B cell effector mechanisms. Similarly, the
depletion of Bregs by CD20 mAb during disease initiation has been
shown to enhance the severity of EAE (49). Previously, it was reported that
antibiotic treatment induces a population of CD5+ Bregs
in mice (50). However, another
murine study demonstrated that intestinal dysbacteriosis imposed by
antibiotic treatment inhibited the production of IL-1β and IL-6 in
DCs and macrophages, and reduced the number and function of Bregs
(51).
In Mycobacterium tuberculosis infections,
active tuberculosis (TB) has been directly associated with high
frequencies of CD19+CD1d+CD5+
Bregs, which exhibited higher suppressive activity and selectively
inhibited Th17 cell activation by direct cell contact in the
absence of IL-10. The inhibition of CD4+ T cells by B
cells did not involve the induction of Tregs (24). Subsequent investigations by Zhang
et al (23) revealed that
successful anti-TB treatment in human TB induced an enhanced IL-22
response, an important cytokine in the immune response to
Mycobacterium tuberculosis infection, by reducing the
frequencies of CD19+CD5+CD1d+
Bregs. Of note, it was found that patients with cavitary TB, which
is more severe than TB without cavitation, had significantly higher
frequencies of CD19+CD1d+CD5+ B
cells. This finding suggested that Bregs impaired protective
immunity and increased disease severity. In addition, the results
of our previous study indicated that CD19+ B cells in
patients with active TB expressed Ebi3 and IL-12p35, the subunits
of IL-35 (data not published). Similar to IL-10, IL-35 is well
known for its suppressive function in a several diseases (18,35).
According to a report by Shen et al, mice with a
B-cell-restricted deficiency in p35 or Ebi3 exhibited higher
survival rates, which were associated with increased macrophage and
CD4+ T cell activation (18). However, further investigations are
warranted to determine whether or not Bregs are a subset of
IL-35-secreting B cells and to determine their exact role in immune
regulation.
Conclusion
Bregs have emerged in the field of immune regulation
and are critical in immune system balance (4). However, their indefinable phenotype,
and the marked differences between humans and mice is a subject of
debate. The identification and investigation of Bregs is complex as
they do not demonstrate regulatory activity in situ, and can
only be investigated following stimulation ex vivo. In
addition, the concentration of Bregs is only ~1–2% in PBMCs or the
mouse spleen (52,53). Despite this, Bregs are reported to
have a distinctive role in infectious disease.
As previously described, increases in Bregs are
positively correlated with viral and bacterial load, and can
contribute to poor vaccine responses (1,37).
Bregs can also facilitate pathogen survival, particularly in early
stages of infection, and subsequently cause increased disease
severity by inhibiting inflammatory T cell and macrophage
activation, primarily through the production of IL-10 (5,22,25,34).
In addition, Bregs afford protection against the hyper-inflammatory
response in parasitic infections (16,43,54),
and an inverse correlation between the induction of B10 cells by
parasites and the prevalence of autoimmune disease has been
reported (44,55).
In conclusion, the current review describes the
role of Bregs in infectious disease in animal and human
investigations. However, a comprehensive understanding of the
regulatory mechanisms exerted by Bregs is required prior to
exploiting novel therapies. It is possible that the inhibition or
depletion of Bregs may assist in the clearance of intracellular
pathogens and improve the efficiency of vaccines in chronic
infection states. Following confirmation of Breg phenotypes and
elucidation of their associated suppressive mechanisms, cell
therapies aimed at treating allergic and autoimmune disease using
autologous Bregs induced by in vitro parasite antigens may
be possible in the future.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant nos. 81273237, 81570009 and
30972779), the Natural Science Foundation of Guangdong Province
(grant no. 2015A030313513), the Key Project of Science and
Technology Innovation from Education Department of Guangdong
Province (grant no. 2012KJCX0059), the Science and Technology
Project of Dongguan (grant no. 201450715200503), the Science and
Technology Project of Zhanjiang (grant no. 2013C03012) and the
Science and Technology Innovation Fund of GDMC (grant nos.
STIF201110 and B2012078).
Glossary
Abbreviations
Abbreviations:
|
IL
|
interleukin
|
|
TGF-β
|
transforming growth factor-β
|
|
B10
|
IL-10-producing B cell
|
|
Breg
|
regulatory B cell
|
|
Treg
|
regulatory T cell
|
|
DC
|
dendritic cell
|
|
EAE
|
experimental autoimmune
encephalomyelitis
|
|
PBMC
|
peripheral blood mononuclear cell
|
|
TLR
|
Toll-like receptor
|
|
BCR
|
B cell receptor
|
|
Th
|
T helper cell
|
|
IFN
|
interferon
|
|
CTL
|
cytotoxic T lymphocyte
|
|
Ebi3
|
Epstein-Barr virus-induced gene 3
|
|
STAT
|
signal transducer and activator of
transcription
|
|
GraB
|
granzyme B-expressing B cell
|
|
MHC
|
major histocompatibility complex
|
|
HIV
|
human immunodeficiency virus
|
|
PD
|
programmed death
|
|
LAP
|
latency-associated peptide
|
|
TB
|
tuberculosis
|
|
Ig
|
immunoglobulin
|
References
|
1
|
Garner-Spitzer E, Wagner A, Paulke-Korinek
M, Kollaritsch H, Heinz FX, Redlberger-Fritz M, Stiasny K, Fischer
GF, Kundi M and Wiedermann U: Tick-borne encephalitis (TBE) and
hepatitis B nonresponders feature different immunologic mechanisms
in response to TBE and influenza vaccination with involvement of
regulatory T and B cells and IL-10. J Immunol. 191:2426–2436. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siewe B, Stapleton JT, Martinson J,
Keshavarzian A, Kazmi N, Demarais PM, French AL and Landay A:
Regulatory B cell frequency correlates with markers of HIV disease
progression and attenuates anti-HIV CD8+ T cell function
in vitro. J Leukoc Biol. 93:811–818. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
van der Vlugt LE, Zinsou JF,
Ozir-Fazalalikhan A, Kremsner PG, Yazdanbakhsh M, Adegnika AA and
Smits HH: Interleukin 10 (IL-10)-producing CD1dhi regulatory B
cells from Schistosoma haematobium-infected individuals
induce IL-10-positive T cells and suppress effector T-cell
cytokines. J Infect Dis. 210:1207–1216. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Berthelot JM, Jamin C, Amrouche K, Le Goff
B, Maugars Y and Youinou P: Regulatory B cells play a key role in
immune system balance. Joint Bone Spine. 80:18–22. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Horikawa M, Weimer ET, DiLillo DJ, Venturi
GM, Spolski R, Leonard WJ, Heise MT and Tedder TF: Regulatory B
cell (B10 Cell) expansion during Listeria infection governs innate
and cellular immune responses in mice. J Immunol. 190:1158–1168.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mizoguchi A, Mizoguchi E, Takedatsu H,
Blumberg RS and Bhan AK: Chronic intestinal inflammatory condition
generates IL-10-producing regulatory B cell subset characterized by
CD1d upregulation. Immunity. 16:219–230. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Saussine A, Tazi A, Feuillet S, Rybojad M,
Juillard C, Bergeron A, Dessirier V, Bouhidel F, Janin A, Bensussan
A, et al: Active chronic sarcoidosis is characterized by increased
transitional blood B cells, increased IL-10-producing regulatory B
cells and high BAFF levels. PLoS One. 7:e435882012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee SJ, Noh G and Lee JH: In vitro
induction of allergen-specific interleukin-10-producing regulatory
B cell responses by interferon-γ in non-immunoglobulin E-mediated
milk allergy. Allergy Asthma Immunol Res. 5:48–54. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Natarajan P, Singh A, McNamara JT, Secor
ER Jr, Guernsey LA, Thrall RS and Schramm CM: Regulatory B cells
from hilar lymph nodes of tolerant mice in a murine model of
allergic airway disease are CD5+, express TGF-β, and co-localize
with CD4+Foxp3+ T cells. Mucosal Immunol. 5:691–701. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Furuzawa-Carballeda J, Lima G, Simancas P,
Ramos-Bello D, Simancas M, Bostock IC, Vilatobá M, Gabilondo B,
Granados J, Morales-Buenrostro L, et al: Peripheral regulatory
cells immunophenotyping in kidney transplant recipients with
different clinical profiles: A cross-sectional study. J Transplant.
2012:2569602012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee KM, Kim JI, Stott R, Soohoo J,
O'Connor MR, Yeh H, Zhao G, Eliades P, Fox C, Cheng N, et al:
Anti-CD45RB/anti-TIM-1-induced tolerance requires regulatory B
cells. Am J Transplant. 12:2072–2078. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen T, Song D, Min Z, Wang X, Gu Y, Wei
B, Yao J, Chen K, Jiang Z, Xie H, et al: Perioperative dynamic
alterations in peripheral regulatory T and B cells in patients with
hepatocellular carcinoma. J Transl Med. 10:142012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Giannoukakis N and Trucco M: A role for
tolerogenic dendritic cell-induced B-regulatory cells in type 1
diabetes mellitus. Curr Opin Endocrinol Diabetes Obes. 19:279–287.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nishimura S, Manabe I, Takaki S, Nagasaki
M, Otsu M, Yamashita H, Sugita J, Yoshimura K, Eto K, Komuro I, et
al: Adipose natural regulatory B cells negatively control adipose
tissue inflammation. Cell Metab. Oct 22–2013.(Epub ahead of print).
View Article : Google Scholar
|
|
15
|
Ronet C, Torre Hauyon-La Y, Revaz-Breton
M, Mastelic B, Tacchini-Cottier F, Louis J and Launois P:
Regulatory B cells shape the development of Th2 immune responses in
BALB/c mice infected with Leishmania major through IL-10
production. J Immunol. 184:886–894. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jeong YI, Hong SH, Cho SH, Lee WJ and Lee
SE: Induction of IL-10-producing CD1dhighCD5+ regulatory B cells
following Babesia microti-infection. PLoS One. 7:e465532012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu J, Zhan W, Kim CJ, Clayton K, Zhao H,
Lee E, Cao JC, Ziegler B, Gregor A, Yue FY, et al: IL-10-producing
B cells are induced early in HIV-1 infection and suppress
HIV-1-specific T cell responses. PLoS One. 9:e892362014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shen P, Roch T, Lampropoulou V, O'Connor
RA, Stervbo U, Hilgenberg E, Ries S, Dang VD, Jaimes Y, Daridon C,
et al: IL-35-producing B cells are critical regulators of immunity
during autoimmune and infectious diseases. Nature. 507:366–370.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wilson MS, Taylor MD, O'Gorman MT, Balic
A, Barr TA, Filbey K, Anderton SM and Maizels RM: Helminth-induced
CD19+CD23hi B cells modulate experimental allergic and autoimmune
inflammation. Eur J Immunol. 40:1682–1696. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jiao Y, Wang X, Zhang T, Sun L, Wang R, Li
W, Ji Y, Wu H and Liu C: Regulatory B cells correlate with HIV
disease progression. Microbiol Immunol. 58:449–455. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Andreani G, Ouellet M, Menasria R, Gomez
AM, Barat C and Tremblay MJ: Leishmania infantum amastigotes
trigger a subpopulation of human B cells with an immunoregulatory
phenotype. PLoS Negl Trop Dis. 9:e00035432015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Das A, Ellis G, Pallant C, Lopes AR,
Khanna P, Peppa D, Chen A, Blair P, Dusheiko G, Gill U, et al:
IL-10-producing regulatory B cells in the pathogenesis of chronic
hepatitis B virus infection. J Immunol. 189:3925–3935. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang M, Zeng G, Yang Q, Zhang J, Zhu X,
Chen Q, Suthakaran P, Zhang Y, Deng Q, Liu H, et al:
Anti-tuberculosis treatment enhances the production of IL-22
through reducing the frequencies of regulatory B cell. Tuberculosis
(Edinb). 94:238–244. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang M, Zheng X, Zhang J, Zhu Y, Zhu X,
Liu H, Zeng M, Graner MW, Zhou B and Chen X: CD19(+)CD1d(+)CD5(+) B
cell frequencies are increased in patients with tuberculosis and
suppress Th17 responses. Cell Immunol. 274:89–97. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gong Y, Zhao C, Zhao P, Wang M, Zhou G,
Han F, Cui Y, Qian J, Zhang H, Xiong H, et al: Role of
IL-10-producing regulatory B cells in chronic hepatitis B virus
infection. Dig Dis Sci. 60:1308–1314. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Flores-Borja F, Bosma A, Ng D, Reddy V,
Ehrenstein MR, Isenberg DA and Mauri C: CD19+CD24hiCD38hi B cells
maintain regulatory T cells while limiting TH1 and TH17
differentiation. Sci Transl Med. 5:173ra1232013. View Article : Google Scholar
|
|
27
|
Amu S, Saunders SP, Kronenberg M, Mangan
NE, Atzberger A and Fallon PG: Regulatory B cells prevent and
reverse allergic airway inflammation via FoxP3-positive T
regulatory cells in a murine model. J Allergy Clin Immunol.
125:1114–1124.e8. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tian F, Hu X, Xian K, Zong D, Liu H, Wei
H, Yang W and Qian L: B10 cells induced by Schistosoma
japonicum soluble egg antigens modulated regulatory T cells and
cytokine production of T cells. Parasitol Res. 114:3827–3834. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mutnal MB, Hu S, Schachtele SJ and
Lokensgard JR: Infiltrating regulatory B cells control
neuroinflammation following viral brain infection. J Immunol.
193:6070–6080. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lo-Man R: Regulatory B cells control
dendritic cell functions. Immunotherapy. 3:(4 Suppl). S19–S20.
2014. View Article : Google Scholar
|
|
31
|
Qian L, Qian C, Chen Y, Bai Y, Bao Y, Lu L
and Cao X: Regulatory dendritic cells program B cells to
differentiate into CD19hiFcγIIbhi regulatory B cells through IFN-β
and CD40L. Blood. 120:581–591. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lee JH, Noh J, Noh G, Choi WS, Cho S and
Lee SS: Allergen-specific transforming growth factor-β-producing
CD19+CD5+ regulatory B-cell (Br3) responses in human late
eczematous allergic reactions to cow's milk. J Interferon Cytokine
Res. 31:441–449. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kessel A, Haj T, Peri R, Snir A, Melamed
D, Sabo E and Toubi E: Human CD19(+)CD25(high) B regulatory cells
suppress proliferation of CD4(+) T cells and enhance Foxp3 and
CTLA-4 expression in T-regulatory cells. Autoimmun Rev. 11:670–677.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Goenka R, Parent MA, Elzer PH and Baldwin
CL: B cell-deficient mice display markedly enhanced resistance to
the intracellular bacterium Brucella abortus. J Infect Dis.
203:1136–1146. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang RX, Yu CR, Dambuza IM, Mahdi RM,
Dolinska MB, Sergeev YV, Wingfield PT, Kim SH and Egwuagu CE:
Interleukin-35 induces regulatory B cells that suppress autoimmune
disease. Nat Med. 20:633–641. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kaltenmeier C, Gawanbacht A, Beyer T,
Lindner S, Trzaska T, van der Merwe JA, Härter G, Grüner B,
Fabricius D, Lotfi R, et al: CD4+ T cell-derived IL-21 and
deprivation of CD40 signaling favor the in vivo development of
granzyme B-expressing regulatory B cells in HIV patients. J
Immunol. 194:3768–3777. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Weinberg A, Muresan P, Fenton T,
Richardson K, Dominguez T, Bloom A, Petzold E, Anthony P,
Cunningham CK, Spector SA, et al: High proportions of regulatory B
and T cells are associated with decreased cellular responses to
pH1N1 influenza vaccine in HIV-infected children and youth (IMPAACT
P1088). Hum Vaccin Immunother. 9:957–968. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Leonard JA, Filzen T, Carter CC, Schaefer
M and Collins KL: HIV-1 Nef disrupts intracellular trafficking of
major histocompatibility complex class I, CD4, CD8, and CD28 by
distinct pathways that share common elements. J Virol.
85:6867–6881. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Belkaid Y, Hoffmann KF, Mendez S, Kamhawi
S, Udey MC, Wynn TA and Sacks DL: The role of interleukin (IL)-10
in the persistence of Leishmania major in the skin after
healing and the therapeutic potential of anti-IL-10 receptor
antibody for sterile cure. J Exp Med. 194:1497–1506. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Pistoia V: Production of cytokines by
human B cells in health and disease. Immunol Today. 18:343–350.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Maroof A, Beattie L, Zubairi S, Svensson
M, Stager S and Kaye PM: Posttranscriptional regulation of II10
gene expression allows natural killer cells to express
immunoregulatory function. Immunity. 29:295–305. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Jeong YI, Kim SH, Ju JW, Cho SH, Lee WJ,
Park JW, Park YM and Lee SE: Clonorchis sinensis-derived total
protein attenuates airway inflammation in murine asthma model by
inducing regulatory T cells and modulating dendritic cell
functions. Biochem Biophys Res Commun. 407:793–800. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hussaarts L, van der Vlugt LE,
Yazdanbakhsh M and Smits HH: Regulatory B-cell induction by
helminths: Implications for allergic disease. J Allergy Clin
Immunol. 128:733–739. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Rodgers DT, Pineda MA, McGrath MA,
Al-Riyami L, Harnett W and Harnett MM: Protection against
collagen-induced arthritis in mice afforded by the parasitic worm
product, ES-62, is associated with restoration of the levels of
interleukin-10-producing B cells and reduced plasma cell
infiltration of the joints. Immunology. 141:457–466. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Pineda MA, McGrath MA, Smith PC, Al-Riyami
L, Rzepecka J, Gracie JA, Harnett W and Harnett MM: The parasitic
helminth product ES-62 suppresses pathogenesis in collagen-induced
arthritis by targeting of the interleukin-17-producing cellular
network at multiple sites. Arthritis Rheum. 64:3168–3178. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hernandez HJ, Wang Y and Stadecker MJ: In
infection with Schistosoma mansoni, B cells are required for
T helper type 2 cell responses but not for granuloma formation. J
Immunol. 158:4832–4837. 1997.PubMed/NCBI
|
|
47
|
Iwata Y, Matsushita T, Horikawa M, Dilillo
DJ, Yanaba K, Venturi GM, Szabolcs PM, Bernstein SH, Magro CM,
Williams AD, et al: Characterization of a rare IL-10-competent
B-cell subset in humans that parallels mouse regulatory B10 cells.
Blood. 117:530–541. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
DiLillo DJ, Matsushita T and Tedder TF:
B10 cells and regulatory B cells balance immune responses during
inflammation, autoimmunity, and cancer. Ann N Y Acad Sci.
1183:38–57. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Matsushita T, Horikawa M, Iwata Y and
Tedder TF: Regulatory B cells (B10 cells) and regulatory T cells
have independent roles in controlling experimental autoimmune
encephalomyelitis initiation and late-phase immunopathogenesis. J
Immunol. 185:2240–2252. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ochoa-Reparaz J, Mielcarz DW, Haque-Begum
S and Kasper LH: Induction of a regulatory B cell population in
experimental allergic encephalomyelitis by alteration of the gut
commensal microflora. Gut Microbes. 1:103–108. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Rosser EC, Oleinika K, Tonon S, Doyle R,
Bosma A, Carter NA, Harris KA, Jones SA, Klein N and Mauri C:
Regulatory B cells are induced by gut microbiota-driven
interleukin-1β and interleukin-6 production. Nat Med. 20:1334–1339.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Yanaba K, Bouaziz JD, Matsushita T, Magro
CM, St Clair EW and Tedder TF: B-lymphocyte contributions to human
autoimmune disease. Immunol Rev. 223:284–299. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Yanaba K, Bouaziz JD, Haas KM, Poe JC,
Fujimoto M and Tedder TF: A regulatory B cell subset with a unique
CD1dhiCD5+ phenotype controls T cell-dependent inflammatory
responses. Immunity. 28:639–650. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Tang H, Ming Z, Liu R, Xiong T, Grevelding
CG, Dong H and Jiang M: Development of adult worms and
granulomatous pathology are collectively regulated by T- and
B-cells in mice infected with Schistosoma japonicum. PLoS
One. 8:e544322013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Panda AK, Ravindran B and Das BK:
Rheumatoid arthritis patients are free of filarial infection in an
area where filariasis is endemic: Comment on the article by Pineda
et al. Arthritis Rheum. 65:1402–1403. 2013. View Article : Google Scholar : PubMed/NCBI
|