Introduction
Glucosamine (GlcN), a naturally occurring amino
monosaccharide, is present in the connective and cartilage tissues
and contributes to maintaining their strength, flexibility and
elasticity. GlcN has been widely used as a supplement for
alleviating the symptoms of osteoarthritis (OA). According to
previous biochemical and pharmacological findings, the
administration of GlcN normalized cartilage metabolism by
inhibiting degradation (1) and
stimulating the synthesis of proteoglycans (2,3), and
restores articular functions. GlcN has also been reported to exert
an anti-inflammatory action by inhibiting the production of
inflammatory mediators and inflammatory cytokines, including nitric
oxide (NO), prostaglandin (PG) E2 and interleukin (IL)-8
via the suppression of the activation of the p38 mitogen activated
protein kinase (MAPK) signaling pathway and nuclear factor (NF)-κB
signaling (4–9).
Furthermore, other functional food materials are
understood to exhibit anti-inflammatory actions. Chondroitin
sulfate (CS) inhibits the activation and nuclear translocation of
NF-κB in chondrocytes and synoviocytes (10). Methylsulfonylmethane (MSM) and
agaro-oligosaccharide (AO) are reported to inhibit
lipopolysaccharide (LPS)-induced production of NO in the RAW264.7
mouse macrophage-like cell line (11,12).
In addition, AO, CS and MSM reduce the symptoms of rheumatoid
arthritis models (12–14). Similarly, L-methionine (Met)
reduces the symptoms of rheumatoid arthritis in model animals
(15), and undenatured type II
collagen (UC-II) improves the symptoms of human OA (16). Thus, these functional food
materials are expected to enhance the alleviation of the symptoms
of OA by combination with GlcN. However, it remains to be
elucidated whether the combination of these materials with GlcN
cooperatively exhibits the anti-inflammatory actions.
The present study therefore aimed to determine the
cooperative and anti-inflammatory actions of functional food
materials, and evaluated the production of IL-8 and phosphorylation
of p38 MAPK and NF-κB in IL-1β-activated synovial cells, incubated
with the combination of GlcN and various functional food materials,
containing Met, UC-II, CS, MSM, AO and other substances with
anti-inflammatory or anti-arthritic action (proteoglycans,
hyaluronic acid, collagen peptides, olive leaf extract, red ginger
extract, Boswellia serrata extract, devil's claw and
curcumin).
Materials and methods
Reagents
GlcN and Met were supplied by Protein Chemical Co.,
Ltd. (Tokyo, Japan), UC-II by Ryusendo Co., Ltd. (Tokyo, Japan), CS
by Yaegaki Bio-industry, Inc. (Himeji, Japan), MSM by CIC Frontier
Co., Ltd. (Tokyo, Japan) and AO by Takara Bio Inc. (Otsu, Japan).
IL-1β was purchased from Peprotech EC Ltd. (London, UK). In
addition, the effects of the following substances, which are known
to exhibit anti-inflammatory or anti-arthritic action (17–25),
were evaluated: Proteoglycan extracted from salmon nasal cartilage
(17) was supplied by Ichimaru
Pharcos Co., Ltd. (Gifu, Japan), hyaluronic acid (18) by N.C. Corporation (Kagawa, Japan),
collagen peptides (19,20) by Nitta Gelatin Inc. (Osaka, Japan),
olive leaf extract (21) by Eisai
Food & Chemical Co., Ltd. (Tokyo, Japan), red ginger extract
(22) from Oryza Oil & Fat
Chemical Co., Ltd. (Aichi, Japan), Boswellia serrata extract
(23) from Nippon Shinyaku Co.,
Ltd., devil's claw extract (24)
from Ask Intercity Co., Ltd. (Chiba, Japan) and curcumin (25) from Indena Japan Co., Ltd. (Tokyo,
Japan).
Cell culture
MH7A human synovial cells (RCB1512) were purchased
from RIKEN Cell Bank (Tsukuba, Japan) and were maintained in
RPMI-1640 medium (Nacalai Tesque, Kyoto, Japan) supplemented with
10% fetal bovine serum (cat. no. 171012; Nichirei Biosciences Inc.,
Tokyo, Japan), penicillin and streptomycin at 37°C in 5%
CO2.
Measurement of IL-8 production
MH7A cells (0.35×105 cells/well) were
seeded and cultured into 24-well plates overnight. The cells were
then incubated with Met (50–100 µg/ml), UC-II (50–100 µg/ml), CS
(50–100 µg/ml), MSM (2,500-5,000 µg/ml), AO (50–100 µg/ml),
proteoglycan (25–100 µg/ml), hyaluronic acid (250–1,000 µg/ml),
collagen peptides (100–400 µg/ml), olive leaf extract (5–20 µg/ml),
red ginger extract (10–40 µg/ml), devil's claw extract (25–100
µg/ml), curcumin (5–20 µg/ml) and Boswellia serrata extract
(12.5 µg/ml) or GlcN (1 mM), and a combination of GlcN and other
materials for 3 h at 37°C. Following this, cells were stimulated
with 50 pg/ml IL-1β in a total volume of 0.5 ml RPMI-1640 medium
for 24 h at 37°C (4).
Subsequently, IL-8 in the culture supernatants was quantified by a
sandwich enzyme-linked immunosorbent assay (ELISA) using the DuoSet
ELISA Development kit (R&D Systems, Inc., Minneapolis, MN,
USA). Microtiter plates (96-well half area flat bottom; Corning,
Acton, MA, USA) were coated with 4 µg/ml mouse anti-human IL-8
antibody (cat. no. #890805; 180-fold dilution, 25 µl/well; R&D
Systems, Inc.) diluted in phosphate-buffered saline (PBS; 137 mM
NaCl, 2.7 mM KCl, 8.1 mM Na2HPO4 and 1.5 mM
KH2PO4; pH7.4) overnight at 4°C. Following
washing with PBS and 0.05% Tween-20, plates were blocked with PBS
containing 1% bovine serum albumin (BSA) and 0.05% sodium azide for
1 h at room temperature. The plates were subsequently washed three
times with PBS containing 0.05% Tween-20, 25 µl/well culture
supernatants or standards (15–2,000 pg/ml) were added, and plates
were incubated for 2 h at room temperature. Following washing three
times with PBS containing 0.05% Tween-20, the plates were incubated
with 3.6 µg/ml of biotinylated goat anti-human IL-8 antibody
(180-fold dilution; cat. no. #890805, R&D Systems, Inc.) for 2
h at room temperature, then washed and incubated with
streptavidin-horseradish peroxidase (cat. no. #890803; 200-fold
dilution, 25 µl/well; R&D Systems, Inc.) at room temperature
for 10 min. The plates were washed again and incubated with 25 µl
tetramethyl benzidine liquid substrate. The reaction was terminated
with 1 M H2SO4, and the plates were read
immediately at a wavelength of 450 nm with a microplate reader
(Model 680; Bio-Rad, Hercules, CA, USA). The detection limit was
<15 pg/ml.
Phosphorylation of p38MAPK and
NF-κB
MH7A cells (9×105/well) were seeded and
cultured in 35 mm tissue culture dishes overnight. Thereafter, the
cells were incubated with 1 mM GlcN, 100 µg/ml Met, 50 µg/ml UC-II,
50 µg/ml CS or 2,500 µg/ml MSM, 100 µg/ml AO and a combination of
GlcN and other materials for 3 h, and stimulated with 200 pg/ml
IL-1β for 10 min at 37°C (8,9).
Following washing with ice-cold PBS containing 100 mM
Na3VO4, the cells were harvested in 1.2 ml
lysis buffer (1% Triton X-100, 0.5% Nonidet P-40, 10 mM Tris-HCl
and 150 mM NaCl; pH 7.4) containing 1/25 v/v Complete™ (Roche
Diagnostics GmbH, Mannheim, Germany) and 1/100 v/v Phosphatase
Inhibitor Cocktail™ (Nacalai Tesque, Kyoto, Japan).
Following sonication, the lysates were centrifuged at 4°C at 12,000
× g for 10 min and the supernatants were recovered. The protein
concentrations of the supernatants were determined with a
Bicinchoninic Acid protein assay kit (Pierce; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) using BSA as a standard. The
supernatants were mixed with 6X SDS-PAGE sample buffer and boiled
for 3 min. Samples (10 µg protein/lane) were separated by 12%
SDS-PAGE and electrophoretically transferred onto an Immobilon-P
polyvinylidene difluoride membrane (EMD Millipore, Billerica, MA,
USA). To detect phosphorylated p38 MAPK and NF-κB levels, the
membranes were blocked in Blocking One solution (Nacalai Tesque,
Inc., Kyoto, Japan), and probed with a mouse anti-phosphorylated
(p)-p38 MAPK monoclonal antibody (pT180/pY182; 1,000-fold dilution;
cat. no. #612168; BD Bioscience Pharmingen, San Diego, CA, USA) or
a rabbit anti-phosphorylated NF-κB p65 monoclonal antibody (Ser
536; 250-fold dilution; cat. no. #3033; Cell Signaling Technology,
Inc., Danvers, MA, USA). Horseradish peroxidase (HRP)-conjugated
goat anti-mouse immunoglobulin (Ig)G/IgM (5,000-fold dilution; cat.
no. #115-035-044; Jackson ImmunoResearch Laboratories, Inc., West
Grove, PA, USA) or HRP-conjugated goat anti-rabbit IgG (5,000-fold
dilution; cat. no. #115-035-144; Jackson ImmunoResearch
Laboratories, Inc.) served as secondary antibodies.
The membranes were stripped by incubating in Restore
Western Stripping Buffer (Pierce; Thermo Fisher Scientific, Inc.)
at 37°C for 15 min. The p38 MAPK and NF-κB proteins contained in
each sample were detected by reprobing with mouse anti-p38MAPK
(2,000-fold dilution; SAPK2a; cat. no. #612168; BD Bioscience
Pharmingen) or rabbit anti-NF-κB (2,000-fold dilution; D14E12; cat.
no. #8242; Cell Signaling Technology, Inc.) primary antibodies
followed by the HRP-conjugated goat anti-mouse (5,000-fold
dilution) or anti-rabbit (5,000-fold dilution) IgG secondary
antibodies. Signals were detected with SuperSignal West Pico
Chemiluminescent Substrate (Pierce; Thermo Fisher Scientific,
Inc.), and quantified using a LAS-3000 luminescent image analyzer
and Multi Gauge version 3.0 (both from Fujifilm Corporation,
Tokyo).
Statistical analysis
Data are expressed as the mean ± standard deviation,
and were analyzed using one-way analysis of variance followed by a
post hoc Bonferroni test for multiple comparisons. Statistical
analysis was performed using GraphPad Prism software version 5
(GraphPad Software, Inc., La Jolla, CA, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Effects of Met, UC-II, CS, MSM, AO and
GlcN on IL-1β-stimulated IL-8 production by MH7A cells
To evaluate the effects of functional food materials
and GlcN on IL-8 production, MH7A was stimulated with IL-1β in the
presence of Met, UC-II, CS, MSM, AO or GlcN, and the combination of
GlcN and other materials. Met (50 and 100 µg/ml), UC-II (50 and 100
µg/ml), CS (50 and 100 µg/ml), MSM (2,500 and 5,000 µg/ml) and AO
(50 and 100 µg/ml) slightly or moderately suppressed the
IL-1β-stimulated IL-8 production by MH7A cells (Fig. 1A-E, respectively), although GlcN (1
mM) suppressed IL-8 production more potently compared with other
materials (Fig. 1). The
combination of GlcN and these functional food materials on IL-8
production was evaluated. Met (100 µg/ml), UC-II (50 µg/ml), CS (50
µg/ml), MSM (2,500 and 5,000 µg/ml, P<0.05) and AO (100 µg/ml,
P<0.01) further decreased the IL-8 level lowered by GlcN
(Fig. 1A-E, respectively). In
contrast, proteoglycan (25–100 µg/ml), hyaluronic acid (250–1,000
µg/ml), collagen peptides (100–400 µg/ml), olive leaf extract (5–20
µg/ml), red ginger extract (10–40 µg/ml), devil's claw extract
(25–100 µg/ml), curcumin (5–20 µg/ml) and Boswellia serrata
extract (12.5 µg/ml) neither suppressed the IL-1β-stimulated IL-8
production by MH7A cells nor affected the GlcN-induced suppression
of IL-8 production (data not shown).
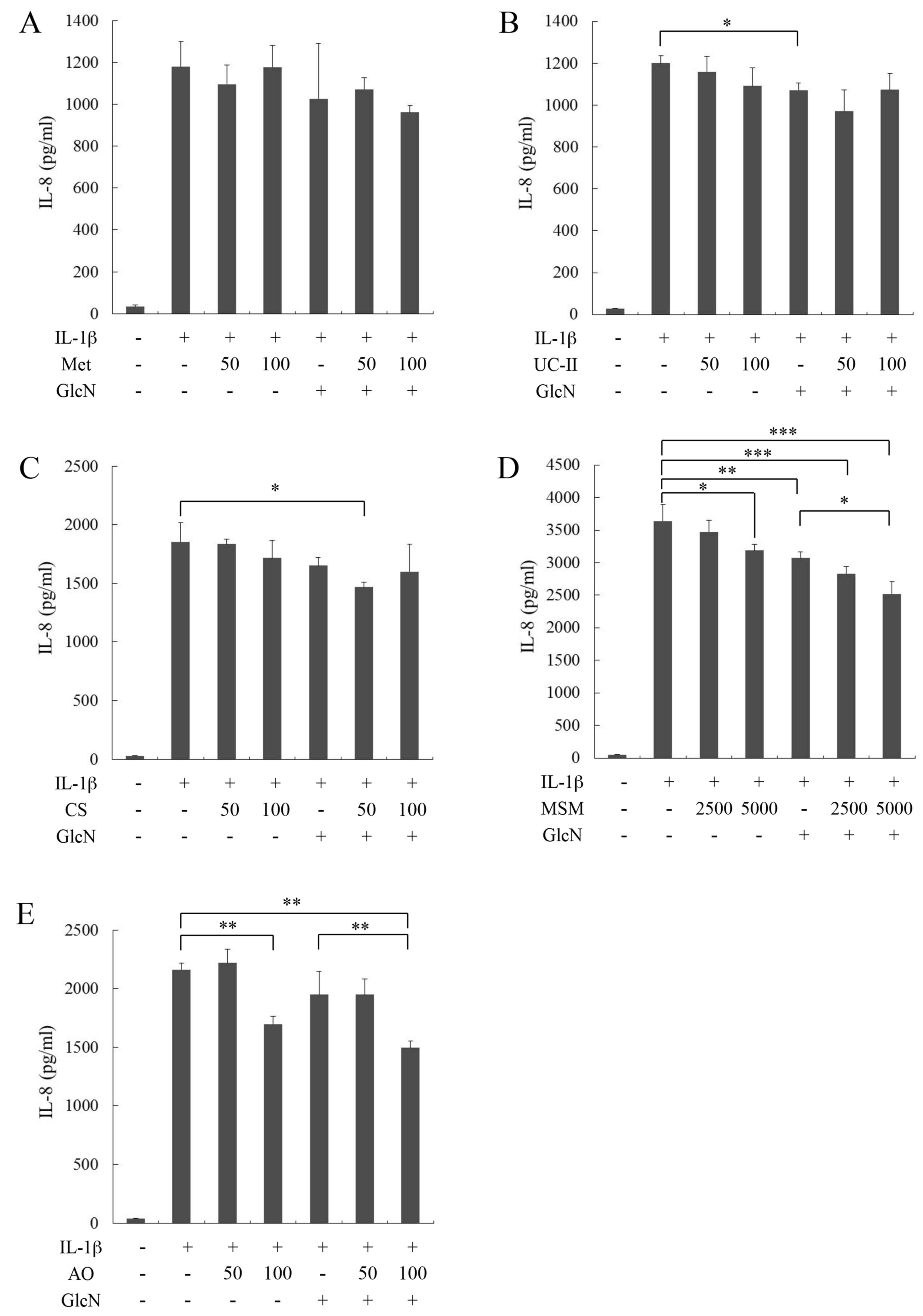 | Figure 1.Effect of functional food materials
and glucosamine (GlcN) on IL-1β-stimulated IL-8 production by MH7A
cells. MH7A cells were incubated in the absence (−) or presence (+)
of (A) Met (B) UC-II, (C) CS, (D) MSM and (E) AO, in with or
without or GlcN and IL-1β. Data are presented as the mean ±
standard deviation of three separate experiments. The level of
IL-1β-stimulated IL-8 production was compared between cells with
and without functional food materials or glucosamine, and between
cells treated with glucosamine alone and those treated with a
combination of functional food materials and glucosamine.
*P<0.05, **P<0.01 and ***P<0.001. IL, interleukin. Met,
methionine; UC-II, undenatured type II collagen; CS, chondroitin
sulfate; MSM, methysulfonylmethane; AO, agaro-oligosaccharide;
GlcN, glucosamine; IL-1β, interleukin-1β. |
Effects of Met, UC-II, CS, MSM, AO and
GlcN on phosphorylation of p38 MAPK and NF-κB
To determine whether the suppressive action of Met,
UC-II, CS, MSM, AO or GlcN, and the combination of GlcN and other
materials on the synovial cell activation is mediated by the
actions of p38 MAPK and NF-κB signaling, the effects of these
substances on the phosphorylation of p38 MAPK and NF-κB p65 was
investigated. Met (100 µg/ml), UC-II (50 µg/ml), CS (50 µg/ml), MSM
(2,500 µg/ml) and AO (100 µg/ml) slightly or moderately suppressed
the IL-1β-stimulated phosphorylation of p38 MAPK (Fig. 2A-E, respectively), although GlcN (1
mM) substantially suppressed the IL-1β-induced phosphorylation of
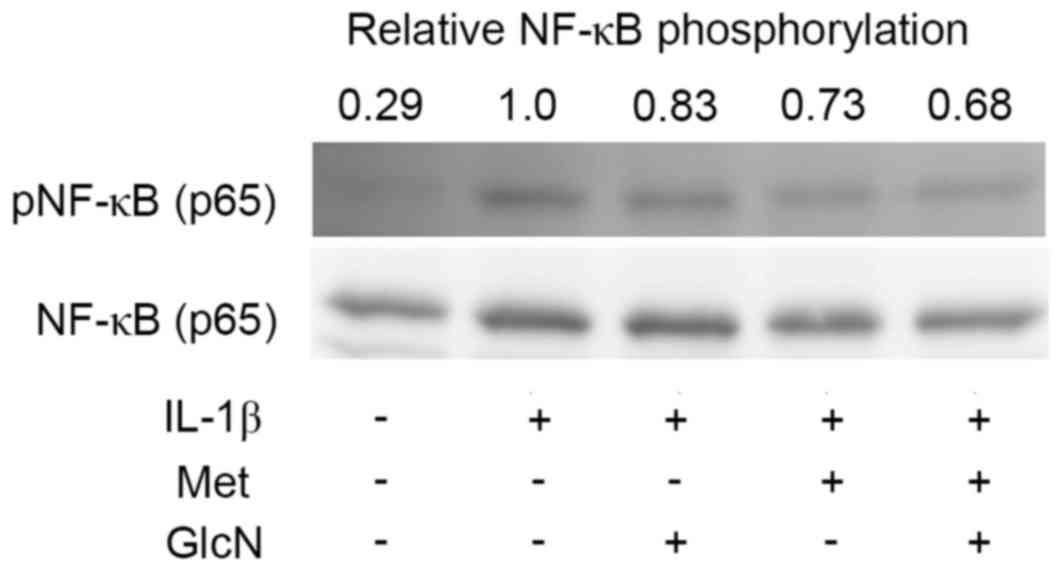
p38 MAPK (Fig. 2). Notably, Met,
UC-II, CS, MSM and AO slightly or moderately decreased the
phosphorylation level of p38 MAPK lowered by GlcN. In addition, Met
further reduced the phosphorylation level of NF-κB p65 lowered by
GlcN (Fig. 3), although the
suppressive effects of UC-II, CS, MSM and AO on the phosphorylation
of NF-κB p65 were not clearly observed (data not shown). However,
the suppressive effects of Met, UC-II, CS, MSM, AO and GlcN on the
phosphorylation levels of p38 MAPK and NF-κB p65 were not
statistically significant (data not shown).
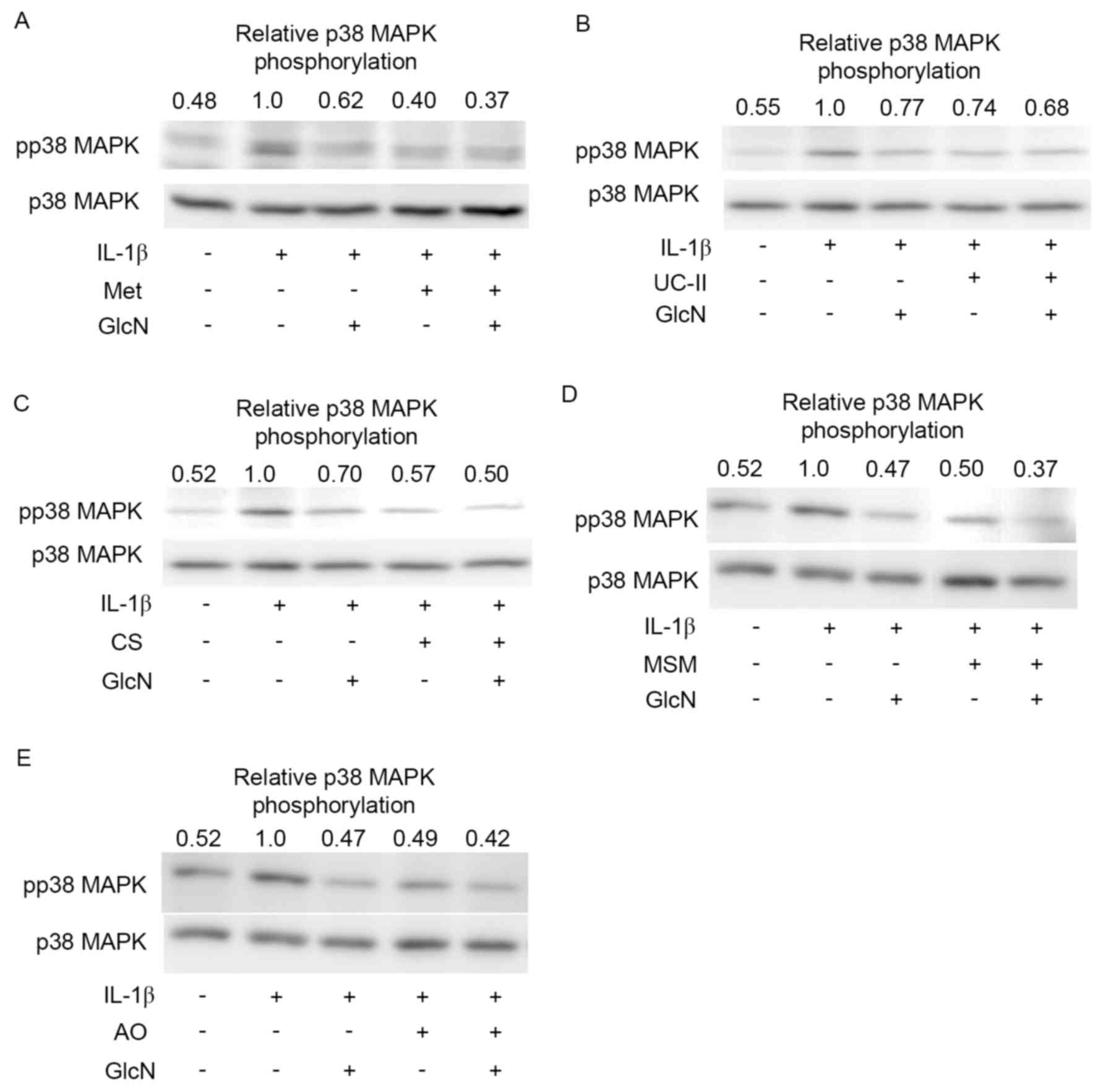 | Figure 2.Effect of functional food materials
and glucosamine on the phosphorylation of p38 MAPK. Representative
western blot images of MH7A cells incubated in the absence (−) or
presence (+) of (A) Met, (B) UC-II, (C) CS, (D) MSM, (E) AO, with
or without or GlcN and IL-1β. Images and quantified data are
representative of three separate experiments. MAPK,
mitogen-activated protein kinase; Met, methionine; UC-II,
undenatured type II collagen; CS, chondroitin sulfate; MSM,
methysulfonylmethane; AO, agaro-oligosaccharide; GlcN, glucosamine;
IL-1β, interleukin-1β; pp38, phosphorylated p38. |
Discussion
The anti-inflammatory actions of GlcN in arthritic
disorders involve the suppression of inflammatory mediator
production from synovial cells and chondrocytes (4–6). In
addition, GlcN has been reported to inhibit activation of the p38
MAPK NF-κB signaling pathways (4,6–9). The
present study examined the anti-inflammatory actions of other
functional food materials; the effects of Met, UC-II, CS, MSM and
AO were evaluated using the MH7A human synovial cell line, and IL-8
as an inflammatory cytokine/chemokine. The results indicated that
Met, UC-II, CS, MSM and AO slightly or moderately suppressed
IL-1β-stimulated IL-8 production by MH7A cells. Notably, Met,
UC-II, CS, MSM and AO further decreased the IL-8 levels lowered by
GlcN. Similarly, Met, UC-II, CS, MSM and AO slightly or moderately
suppressed the phosphorylation levels of p38 MAPK and further
reduced the phosphorylation level lowered by GlcN (statistical data
not shown). These observations suggested a possibility that these
functional food materials exerted an anti-inflammatory action
(inhibition of IL-8 production) in combination with GlcN by
cooperatively suppressing p38 MAPK signaling (phosphorylation).
The functional food materials evaluated in the
present study are included in various foods (http://www.naturaldatabase.com/): GlcN is contained in
the shells of crustaceans, including shrimps and crabs; Met is
contained mainly in meat, vegetables and nuts; UC-II and CS are
contained in cartilage; MSM is present in milk, tea and vegetables;
and, AO is contained in agar. GlcN has been reported to suppress
the progression of adjuvant arthritis by inhibiting synovial
hyperplasia, cartilage destruction, inflammatory cell infiltration
and the production of inflammatory mediators (NO and
PGE2) (26).
Furthermore, GlcN suppressed the progression of an experimental OA
model by inhibiting type II collagen degradation and enhancing type
II collagen synthesis (27). Among
the functional food materials, Met is reported to suppress the
progression of adjuvant arthritis by inhibiting the synovial
hyperplasia and production of inflammatory mediators (NO and
PGE2) (15). CS exerts
an anti-inflammatory action by reducing NF-κB nuclear translocation
in synoviocytes and chondrocytes (10). AO suppresses the elevated levels of
NO, PGE2, and pro-inflammatory cytokines including tumor
necrosis factor (TNF)-α, IL-1β and IL-6 by inducing heme
oxygenase-1 in LPS-stimulated monocytes and macrophages (28). MSM inhibits the LPS-induced release
of inflammatory mediators (NO, PGE2, IL-6 and TNF-α) in
murine macrophages through the downregulation of NF-κB signaling
(11). UC-II suppresses
collagen-induced arthritis by inducing
CD4+CD25+ regulatory T cells (29). Met reduces the symptoms of
rheumatoid arthritis in model animals (15). AO, CS and MSM reduce the symptoms
of rheumatoid arthritis models (12–14).
UC-II improves the symptoms of human OA (16). The present study revealed that
these functional food materials slightly inhibit the activation of
synovial cells by suppressing IL-8 production and p38 MAPK
signaling by themselves. Furthermore, the combinations of these
functional food materials and GlcN cooperatively inhibit the
synovial cell activation (IL-8 production) and p38 MAPK signaling.
Therefore, the combinations of these materials and GlcN are
expected to exhibit the chondroprotective action on
cartilage-degenerative diseases including OA and rheumatoid
arthritis by suppressing p38 MAPK signaling.
IL-1β activates a variety of signaling cascades
(including p38MAPK and NF-κB pathways), which lead to the induction
of inflammatory response (i.e., the production of inflammatory
cytokines and mediators) (30,31).
The present study demonstrated that GlcN and other functional food
materials (mentioned above) cooperatively suppress the
IL-1β-induced IL-8 production and the phosphorylation of p38MAPK
(Figs. 2 and 3) (data not shown). These observations
suggest that GlcN and other functional food materials inhibit the
IL-1β-induced synovial cell activation (i.e., IL-8 production)
possibly via the suppression of intracellular signaling including
p38MAPK.
It is now recognized that the addition of
O-linked N-acetylGlcN (O-GlcNAc) to target proteins
may modulate cellular functions, including nuclear transport,
transcription, translation, cell signaling, apoptosis and cell
shape (32,33). We previously suggested (8) that GlcN suppressed the TNF-α-induced
intercellular adhesion molecule (ICAM-1) and monocyte
chemoattractant protein-1 (MCP-1) expression in endothelial cells,
possibly by downregulating the phosphorylation of p38 MAPK and
NF-κB via O-GlcNAc modification, using an O-GlcNAc
transferase (OGT) inhibitor, alloxan. Thus, it is reasonable to
hypothesize that GlcN inhibits the IL-1β-induced synovial cell
activation (i.e., IL-8 production), possibly by downregulating the
phosphorylation of p38 MAPK and NF-κB via O-GlcNAc
modification. However, it remains unclear whether O-GlcNAc
modification is involved in the suppression of p38 MAPK and NF-κB
phosphorylation by other functional food materials evaluated in
this study.
By contrast, proteoglycan, hyaluronic acid, collagen
peptides, olive leaf extract, red ginger extract, Boswellia
serrata extract, devil's claw extract and curcumin did not
suppress the IL-1β-stimulated IL-8 production by MH7A cells under
the experimental conditions of the present study (data not shown).
Proteoglycan is reported to attenuate the progression of
collagen-induced arthritis by modulating immune response of
splenocytes to collagen stimulation (17). Hyaluronic acid exerts the
anti-inflammatory action by inhibiting bradykinin-induced
arachidonic acid release from synovial fibroblasts in
osteoarthritic inflamed joints (18). Collagen peptides exert a
chondroprotective action on OA by inhibiting matrix
metalloproteinase-13 expression and type II collagen degeneration
(19). Olive leaf extract exhibits
the anti-arthritis effect by preventing the destruction of
cartilage and limiting peri-articular soft tissue inflammation in
kaolin- and carrageenan-induced arthritis (21). An active substance of red ginger
extract, 6-Gingerol, exerts its anti-inflammatory action by
reducing the inducible nitric oxide synthase and TNF-α expression
through the blocking of NF-κB and protein kinase c signaling in
LPS-stimulated macrophages (22).
Boswellia serrata exhibits an anti-inflammatory action on
colonic epithelial cells by suppressing cytokine- and hydrogen
peroxide-induced activation of NF-κB (23). Harpagophytum procumbens
(devil's claw) suppresses the inflammatory reaction of
LPS-stimulated human monocytes by inhibiting the release of
cytokines and PGE2, and the expression of COX-2, IL-6
and TNF-α mRNA, without affecting the NF-κB and MAPK signaling
pathways (24). Curcumin reduces
LPS-stimulated inflammatory responses (NO, PGE2 and
cytokine production) in the lungs of diabetic rats by suppressing
NF-κB activation (25). Thus, all
the functional food materials described above are unlikely to
exhibit anti-inflammatory actions on IL-1β-induced production of
IL-8 by synovial cells evaluated in the present study, although
they suppress the inflammatory reactions assessed under other
experimental conditions.
In conclusion, the present study demonstrated that a
combination of functional food materials (Met, UC-II, CS, MSM and
AO) and GlcN resulted in a chondroprotective action on
cartilage-degenerative diseases. However, it is for future studies
to elucidate whether the combinations of these functional food
materials and GlcN exerts a therapeutic action in vivo,
using animal models of OA and rheumatoid arthritis.
Acknowledgements
The present study was supported in part by a grant
from the Strategic Research Foundation Grant-aided Project for
Private Universities from the Ministry of Education, Culture,
Sport, Science and Technology (Tokyo, Japan; 2014 to 2018; grant
no. S1411007). The authors would like to thank Dr. K Miyazawa
(Kissei Pharmaceutical Co., Ltd., Nagano, Japan) for the
establishment of MH7A cells, and the researchers of Department of
Host Defense and Biochemical Research, Juntendo University,
Graduate School of Medicine, for technical assistance and helpful
discussions.
References
|
1
|
Fenton JI, Chlebec-Brown KA, Peters TL,
Caron JP and Orth MW: Glucosamine HCl reduces equine articular
cartilage degradation in explants culture. Osteoarthritis
Cartilage. 8:258–265. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Oegema TR Jr, Deloria LB, Sandy JD and
Hart DA: Effect of oral glucosamine on cartilage and meniscus in
normal and chymopapain-injected knees of young rabbits. Arthritis
Rheum. 46:2495–2503. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gouze JN, Bordji K, Gulberti S, Terlain B,
Netter P, Magdalou J, Fournel-Giqleux S and Ouzzine M:
Interleukin-1beta down-regulates the expression of
glucuronosyltransferase I, a key enzyme priming glycosaminoglycan
biosynthesis: Influence of glucosamine on
Interleukin-1beta-mediated effects in rat chondrocytes. Arthritis
Rheum. 44:351–360. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hua J, Sakamoto K, Kikukawa T, Abe C,
Kurosawa H and Nagaoka I: Evaluation of the suppressive actions of
glucosamine on the interleukin-1beta-mediated activation of
synoviocytes. Inflamm Res. 56:432–438. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nagaoka I: Recent aspects of the
chondroprotective and anti-inflammatory actions of glucosamine, a
functional food. Juntendo Medical J. 60:580–587. 2014. View Article : Google Scholar
|
|
6
|
Largo R, Alvarez-Soria MA, Díez-Ortego I,
Sánchez-Pernatute O, Eqido J and Herrero-Beaumont G: Glucosamine
inhibits IL-1beta-induced NFkappaB activation in human
osteoarthritic chondrocyte. Osteoarthritis Cartilage. 11:290–298.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rafi MM, Yadav PN and Rossi AO:
Glucosamine inhibits LPS-induced COX-2 and iNOS expression in mouse
macrophage cells (RAW 264.7) by inhibition of p38-MAP kinase and
transcription factor NF-kappaB. Mol Nutr Food Res. 51:587–593.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ju Y, Hua J, Sakamoto K, Ogawa H and
Nagaoka I: Modulaion of TNF-α-induced endothelial cell activation
by glucosamine, a naturally occurring amino monosaccharide. Int J
Mol Med. 22:809–815. 2008.PubMed/NCBI
|
|
9
|
Yomogida S, Hua J, Sakamoto K and Nagaoka
I: Glucosamine suppresses interleukin-8 production and
ICAM-1expression by TNF-α-stimulated human colonic epithelial HT-29
cells. Int J Mol Med. 22:205–211. 2008.PubMed/NCBI
|
|
10
|
Iouv M, Dumais G and du Souich P:
Anti-inflammatory activity of chondroitin sulfate. Osteoarthritis
Cartilage. 16 Suppl 3:S14–S18. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim YH, Kim DH, Lim H, Baek DY, Shin HK
and Kim JK: The anti-inflammatory effects of methylsulfonylmethane
on lipopolysaccharide-induced inflammatory responses in murine
macrophages. Biol Pharm Bull. 32:651–656. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ohnogi H, Kudo Y, Hayami S, Mizutani S and
Enoki T: Synergistic anti-inflammatory effects of
agaro-oligosaccharides and glucosamine in vivo and in vitro.
Glucosamine Res. 8:51–56. 2012.(In Japanese).
|
|
13
|
Volpi N: Anti-inflammatory activity of
chondroitin sulphate: New functions from an old natural
macromolecule. Inflammopharmacology. 19:299–306. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hasegawa T, Ueno S and Kumamoto S:
Suppressive effect of methylsulphonylmethane (MSM) on type II
collagen-induced arthritis in DBA/1J mice. Jpn Pharmacol Ther.
32:421–427. 2004.
|
|
15
|
Yamagishi Y, Igarashi M, Suzuki A, Suguro
S, Hirano SI and Nagaoka I: Evaluation of the effect of methionine
and glucosamine on adjuvant arthritis in rats. Exp Ther Med.
4:640–644. 2012.PubMed/NCBI
|
|
16
|
Lugo JP, Saiyed ZM and Lane NE: Efficacy
and tolerability of an undenatured type II collagen supplement in
modulating knee osteoarthritis symptoms: A multicenter randomized,
double-blind, placebo-controlled study. Nutr J. 15:142016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yoshimura S, Asano K and Nakane A:
Attenuation of collagen-induced arthritis in mice by salmon
proteoglycan. Biomed Res Int. 2014:4064532014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tobetto K, Yasui T, Ando T, Hayashi M,
Motohashi N, Shinogi M and Mori I: Inhibitort effects of hyaluronan
on [14C]arachidonic acid release from labeled human synovial
fibroblast. Jap J Pharmacol. 60:79–84. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Isaka S, Someya A, Nakamura S, Naito K,
Nozawa M, Inoue N, Sugihara F, Nagaoka I and Kaneko K: Evaluation
of the effect of oral administration of collagen peptides on an
experimental rat osteoarthritis model. Exp Ther Med. (In press).
PubMed/NCBI
|
|
20
|
Ohara H, Matsumoto H, Ito K, Iwai K and
Sato K: Comparison of quantity and structures of
Hydroxyproline-containing peptides in human blood after oral
ingestion of gelatin hydrolysates from different sources. J Agric
Food Chem. 55:1532–1535. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gong D, Geng C, Jiang L, Wang L, Yoshimura
H and Zhong L: Mechanisms of olive leaf extract-ameliorated rat
arthritis caused by kaolin and carrageenan. Phytother Res.
26:397–402. 2012.PubMed/NCBI
|
|
22
|
Lee TY, Lee KC, Chen SY and Chang HH:
6-Gingerol inhibits ROS and iNOS through the suppression of
PKC-alpha and NF-kappaB pathways in lipopolysaccharide-stimulated
mouse macrophages. Biochem Biophys Res Commun. 382:134–139. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Catanzaro D, Rancan S, Orso G, Dall'Acqua
S, Brun P, Giron MC, Carrara M, Castagliuolo I, Ragazzi E,
Caparrotta L and Montopoli M: Boswellia serrata Preserves
Intestinal Epithelial Barrier from Oxidative and Inflammatory
Damage. PLoS One. 10:e01253752015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fiebich BL, Muñoz E, Rose T, Weiss G and
McGregor GP: Molecular targets of the antiinflammatory
Harpagophytum procumbens (devil's claw): Inhibition of TNFα and
COX-2 gene expression by preventing activation of AP-1. Phytother
Res. 26:806–811. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang F, Yang F, Zhao H and An Y: Curcumin
alleviates lung injury in diabetic rats by inhibiting NF-κB
pathway. Clin Exp Pharmacol Physiol. 2015.(Epub ahead of print).
View Article : Google Scholar
|
|
26
|
Hua J, Suguro S, Sakamoto K and Nagaoka I:
Preventive actions of a high dose of glucosamine on adjuvant
arthritis in rats. Inflamm Res. 54:127–132. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Naito K, Watari T, Furuhata A, Yomogida S,
Sakamoto K, Kurosawa H, Kaneko K and Nagaoka I: Evaluation of the
effect of glucosamine on an experimental rat osteoarthritis model.
Life Sci. 86:538–543. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Enoki T, Okuda S, Kudo Y, Takshima F,
Sagawa H and Kato I: Oligosaccharides from agar inhibit
pro-inflammatory mediator release by inducing heme oxygenase 1.
Biosci Biotechnol Biochem. 74:766–770. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yoshinari O, Shiojima Y, Moriyama H,
Shinozaki J, Nakane T, Masuda K and Bagchi M: Water-soluble
undenaturated type II collagen ameliorates collagen-induced
arthritis in mice. J Med Food. 16:1039–1045. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Parhar K, Ray A, Steinbrecher U, Nelson C
and Salh B: The p38 mitogen-activated protein kinase regulates
interleukin-1beta-induced IL-8 expression via an effect on the IL-8
promoter in intestinal epithelial cells. Immunology. 108:502–512.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hu DN, Chen M, Zhang DY, Ye F, McCormick
SA and Chan CC: Interleukin-1beta increases baseline expression and
secretion of interleukin-6 by human uveal melanocytes in vitro via
the p38 MAPK/NF-kappaB pathway. Invest Ophthalmol Vis Sci.
52:3767–3774. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wells L, Vosseller K and Hart GW:
Glycosylation of nucleocytoplasmic proteins: Signal transduction
and O-GlcNAc. Science. 291:2376–2378. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hanover JA: Glycan-dependent signaling:
O-linled N-acetylglucosamine. FASEB J. 15:1865–1876. 2001.
View Article : Google Scholar : PubMed/NCBI
|