Introduction
Hepatic fibrosis is a continuous wound-healing
process that is activated in response to chronic liver injury of
various etiologies, including chronic hepatitis B and C infection,
alcohol abuse, non-alcoholic steatohepatitis and autoimmune
hepatitis (1). Hepatic
fibrogenesis is characterized by the aberrant deposition of
extracellular matrix (ECM) components in the liver (1,2).
Activated hepatic stellate cells (HSCs) are the primary source of
excess ECM components, and thus serve a crucial role in the
progression of hepatic fibrogenesis (3). During chronic liver injury, HSCs have
been reported to transdifferentiate from quiescent vitamin
A-storing cells into proliferative myofibroblast-like cells with
enhanced secretory capabilities, which causes an imbalance between
ECM synthesis and degradation (1).
The detailed molecular mechanisms underlying HSC
transdifferentiation are complex and have yet to be fully
elucidated; various cytokines and signaling pathways have reported
to be involved in HSC activation. The transforming growth factor
(TGF)-β/mothers against decapentaplegic homolog (SMAD) pathway has
been identified as an essential signaling cascade during HSC
activation (4). SMAD proteins are
intracellular mediators of TGF-β signaling (5) and have been reported to modulate the
biogenesis of microRNAs (miRNAs) in several pathophysiological
processes (6).
miRNAs are endogenous, short (~22 nucleotides-long),
non-coding RNA molecules that are involved in the
post-transcriptional regulation of gene expression in plants and
animals through binding to the 3′-untranslated regions (UTRs) of
target mRNAs, which results in mRNA degradation or the suppression
of their translation (7,8). Numerous miRNAs have been identified
as pivotal regulators in several physiological processes, including
immune responses and cellular differentiation and proliferation
(9). Previous studies have
reported on the regulatory functions for miRNAs during hepatic
fibrogenesis (10,11), and specific miRNAs have been
revealed to mediate fibrosis by targeting SMAD3 (12,13).
Notably, miR-203 has been demonstrated to negatively regulate
hepatic tumorigenesis through various signal transduction pathways
(14–16). miR-203 downregulation has been
reported in rat and human fibrotic liver tissue, as well as in
activated rat HSCs, whereas miR-203 upregulation was demonstrated
to prevent HSC activation and proliferation by targeting transient
receptor potential vanilloid 4 (TRPV4) channels (17). However, the effects of miR-203
expression on collagen synthesis in HSCs, as well as its
interaction with SMAD3, have yet to be elucidated.
The present study aimed to explore the putative
regulatory roles of miR-203 during the development of hepatic
fibrosis. Bioinformatics analysis was used to predict SMAD3
as a target gene for miR-203. In addition, the effects of altered
miR-203 expression on HSC-T6 proliferation and on the expression of
collagen-related genes were investigated. Furthermore, the putative
interaction between miR-203 and SMAD3 was confirmed,
revealing SMAD3 as a direct target gene for miR-203 in
hepatocytes.
Materials and methods
Cell culture and transfection
HSC-T6 (18), rat
hepatic stellate cells were purchased from Yingrun Biotechnologies,
Inc. (Hunan, China). HSC-T6 cells were seeded (3×105 cells/well) in
six-well plates and cultured with high-glucose Dulbecco's modified
Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), supplemented with 10% fetal bovine serum (FBS;
Gibco; Thermo Fisher Scientific, Inc.). Cells were maintained at
37°C in a 5% CO2 humidified atmosphere. Following a 24-h
incubation, cells were cultured in serum-free DMEM for ~12 h and
then transfected with a miR-203 mimic, which is an artificial miRNA
that enhances the function of miR-203, a miR-203 inhibitor, which
is an antisense oligonucleotide for miR-203, or a negative control
scramble miRNA. All miRNAs were purchased from Shanghai GenePharma
Co., Ltd., (Shanghai, China). Lipofectamine® 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.) was used as the transfection
reagent, according to the manufacturer's protocol. The
concentration of miR-203 mimic, miR-203 inhibitor and scramble
miRNA that were used for transfection was 0.02 µM, and each step
was completed in 3 min at room temperature. The culture medium was
replaced 4–6 h post-transfection, the cells were cultured for an
additional 48 h and then used for subsequent experiments. The
sequences of the miRNAs that were used were as follows: miR-203
mimic, forward 5′-GUGAAAUGUUUAGGACCACUAG-3′, reverse
5′-AGUGGUCCUAAACAUUUCACUU-3′; miR-203 antisense oligonucleotide,
5′-CUAGUGGUCCUAAACAUUUCAC-3′; and scramble miRNA,
5′-CAGUACUUUUGUGUAGUACAA-3′.
Transfection efficiency
assessment
The efficiency of HSC-T6 cell transfection was
assessed in order to optimize the concentrations of miRNAs and
transfection reagent that were used in the present experiments,
using transient transfection with scramble miRNAs, including
fluorescein amidite (FAM)-labeled and unlabeled miRNAs. FAM-labeled
miRNAs emit fluorescence following excitation at 420–485 nm, which
can be observed using fluorescence microscopy, whereas unlabeled
miRNAs do not. Culture and transfection conditions were similar to
the aforementioned.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from HSC-T6 cells (~2×106)
using TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. miR-203 was reverse
transcribed using the following stem-loop RT
primer:5′-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACGTTGAA-3′.
Thermocycling conditions were as follows: denaturation at 42°C for
60 min, extension at 70°C for 10 min, storage at 4°C. qPCR was
performed on cDNA using SYBR Green Realtime PCR Master Mix-Plus
(Toyobo Co., Ltd., Osaka, Japan) and miR-203-specific qPCR primers
(Table I; Shanghai GenePharma Co.,
Ltd.). The reaction volume was 20 µl [7.2 µl diethyl
pyrocarbonate-treated water, 10 µl SYBR Green mix, 0.4 µl (10 µM)
of each primer and 2 µl cDNA]. Thermocycling conditions were as
follows: Initial 1 cycle at 95°C for 180 sec, followed by 40 cycles
at 95°C for 12 sec and at 62°C for 40 sec.
 | Table I.Gene-specific primers for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Gene-specific primers for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Sequence
(5′→3′) |
|---|
| β-actin | F:
CGTAAAGACCTCTATGCCAACA |
|
| R:
-GGAGGAGCAATGATCTTGATCT |
| α-SMA | F:
GTGCTGTCCCTCTATGCCTCTGG |
|
| R:
GGCACGTTGTGAGTCACACCATC |
| COL1A1 | F:
GTACATCAGCCCAAACCCCAAG |
|
| R:
CGGAACCTTCGCTTCCATACTC |
| COL3A1 | F:
GACTGCCCCAACCCAGAGATC |
|
| R:
TACCATCAGGAATGACAGGAGCAG′ |
| SMAD3 | F:
CGATGTCCCCAGCACACAATAAC |
|
| R:
TAGTAGGAGATGGAGCACCAAAAGG |
| miR-203 | F:
CGATGCTGTGAAATGTTTAGGGAC |
|
| R:
TATGGTTTTGACGACTGTGTGAT |
| U6 | F:
ATTGGAACGATACAGAGAAGATT |
| | R:
GGAACGCTTCACGAATTTG |
To detect the mRNA expression of collagen type 1, α1
(COL1A1), COL3A1, α-smooth muscle actin
(α-SMA) and SMAD3, total RNA was reverse transcribed
into cDNA using the RevertAid First Strand cDNA Synthesis kit
(Thermo Fisher Scientific, Inc.), according to the manufacturer's
protocol. qPCR was performed on the cDNA using specific primers
(Table I; Shanghai GenePharma Co.,
Ltd.) and SYBR Green Realtime PCR Master Mix-Plus (Toyobo Co.,
Ltd.). The reaction volume was 20 µl [7.2 µl diethyl
pyrocarbonate-treated water, 10 µl SYBR Green mix, 0.4 µl (10 µM)
of each primer and 2 µl cDNA]. Thermocycling conditions were as
follows: Initial 1 cycle at 95°C for 180 sec, followed by 40 cycles
at 95°C for 12 sec and at 62°C for 40 sec. Relative gene expression
was quantified using the 2−ΔΔCq method (19); miR-203 expression was normalized to
the expression of U6, whereas COL1A1, COL3A1,
α-SMA and SMAD3 expression was normalized to
β-actin. Experiments were performed in triplicate. GraphPad
Prism software version 5.01 (GraphPad Software, Inc., La Jolla, CA,
USA) was used for analysis.
Western blot analysis
HSC-T6 cells (~2×106) were lysed using RIPA lysis
buffer (Beyotime Institute of Biotechnology, Haimen, China) at 4°C
for 30 min and total protein concentration was measured using a
bicinchoninic acid protein assay kit (Beyotime Institute of
Biotechnology). Protein samples were denatured at 100°C for 5 min,
and equal amounts of extracted protein samples (20 µg) were
separated by 8–10% SDS-PAGE and transferred onto polyvinylidene
difluoride membranes (EMD Millipore, Billerica, MA, USA). Following
blocking against non-specific protein binding using 5% bovine serum
albumin (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) for 60 min
at room temperature, membranes were incubated with the following
primary antibodies at 4°C overnight: rabbit monoclonal anti-α-SMA
(1:1,000; cat no. ab32575; Abcam, Cambridge, MA, USA), rabbit
monoclonal anti-Smad3 (1:1,000; cat no. C67H9; Cell Signaling
Technology, Inc., Danvers, MA, USA), mouse monoclonal anti-COL1A1
(1:500; cat no. ab6308; Abcam) and anti-COL3A1 (1:500; cat no.
ab6310; Abcam), and rabbit polyclonal anti-GAPDH (1:500; cat no.
ab8245; Abcam). Membranes were then washed with 1X TBS containing
0.1% Tween-20 (TBST) 3 times for 10 min and incubated with the
following horseradish peroxidase-conjugated AffiniPure secondary
antibodies: Goat anti-rabbit (1:8,000; cat no. 111-035-003; Jackson
ImmunoResearch Laboratories, Inc., West Grove, PA, USA) and goat
anti-mouse (1:2,000; cat no. 115-035-003; Jackson ImmunoResearch
Laboratories, Inc.) for 1 h at room temperature. The membranes were
washed with TBST 3 times for 10 min and the protein bands were
visualized by enhanced chemiluminescence (ECL) using SuperSignal™
West Femto Maximum Sensitivity Substrate for ECL (Thermo Fisher
Scientific, Inc.). GAPDH was used as the loading control. Blots
were semi-quantified by densitometric analysis using the Image Lab
software version 4.1 (Bio-Rad Laboratories, Inc., Hercules, CA,
USA).
Cellular proliferation assay
HSC-T6 cells were seeded (~1×104 cells/well) in
96-well plates and transfected with miR-203 mimic, miR-203
inhibitor or scramble miRNA for 48 h, using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.). Cellular
proliferation was measured using an MTT assay (Nanjing KeyGen
Biotech Co., Ltd., Nanjing, China). Following transfection, 0.5%
MTT (20 µl) was added in each well and cells were incubated at 37°C
for 4 h. Subsequently, 150 µmol DMSO were added to each well to
dissolve the formazan crystals. The absorbance was measured at 490
nm and the optical density (OD) of the samples was calculated.
Results were averaged from at least three independent
experiments.
Dual-luciferase reporter assay
Bioinformatics analysis was used to predict
potential target genes for miR-203; TargetScan (www.targetscan.org/vert_71/) (20), PicTar (pictar.mdc-berlin.de/)
(21) and miRanda (www.microrna.org/microrna/home.do)
(22) software were used. Based on
the results of the bioinformatics analysis, oligonucleotides (62
bp) of wild-type and mutant SMAD3 3′-untranslated region
(UTR), containing the putative miR-203 binding sites, were
synthesized by Shanghai GenePharma Co., Ltd. and cloned into the
pmirGLO Dual-Luciferase miRNA Target Expression Vector (Promega
Corporation, Madison, WI, USA) by double digestion with
SacI/XhoI restriction enzymes by Shanghai GenePharma
Co., Ltd. The dual-luciferase reporter assay was performed in 293T
human embryonic kidney cells obtained from the Central Laboratory
of the First Affiliated Hospital of Wenzhou Medical University
(Wenzhou, China). The 293T cell line is characterized by high
transfection efficiency, with no influence on the expression of
target genes; therefore, 293T cells were used for this experiment.
Briefly, 293T cells were seeded in 24-well plates at a density of
3×104 cells/well, and incubated for 24 h at 37°C in a 5%
CO2 humidified atmosphere. Subsequently, cells were
co-transfected with either miR-203 mimics (20 pmol) or scramble
miRNA negative control (20 pmol) and a reporter plasmid (50 ng)
containing either wild-type or mutant SMAD3-3′UTR sequences,
or a blank reporter plasmid, using Lipofectamine® 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.) as the transfection reagent.
Following 48 h transfection at 37°C in a 5% CO2
humidified atmosphere, luciferase activity was measured using the
Dual-Luciferase Reporter Assay System (Promega Corporation), and
the signal was recorded using the Smart Line TL Tube Luminometer
(Berthold Detection Systems GmbH, Pforzheim, Germany).
Renilla luciferase was used as the internal control.
Statistical analysis
The statistical significance of the differences
between groups was assessed using a Student's t-test for pair-wise
comparisons and a one-way analysis of variance followed by a post
hoc Dunnett's test for multiple comparisons. Data are expressed as
the mean ± standard deviation of at least three independent
experiments. P<0.05 was considered to indicate a statistically
significant difference. Statistical analysis was performed using
SPSS software version 19.0 (IBM Corp., Armonk, NY, USA).
Results
miR-203 suppresses COL1A1, COL3A1 and
α-SMA expression in HSCs
To investigate the putative roles of miR-203 in the
development of hepatic fibrosis, the expression levels of genes
associated with hepatic fibrogenesis were assessed. During chronic
liver injury, activated stellate cells have been reported to
remodel the ECM and enrich the fibril-forming collagen contents,
particularly collagen types I and III (23), whereas α-SMA has been identified as
a marker of hepatic fibrogenesis (24). In the present study, HSC-T6 cells
were transfected with a miR-203 mimic, a miR-203 inhibitor or with
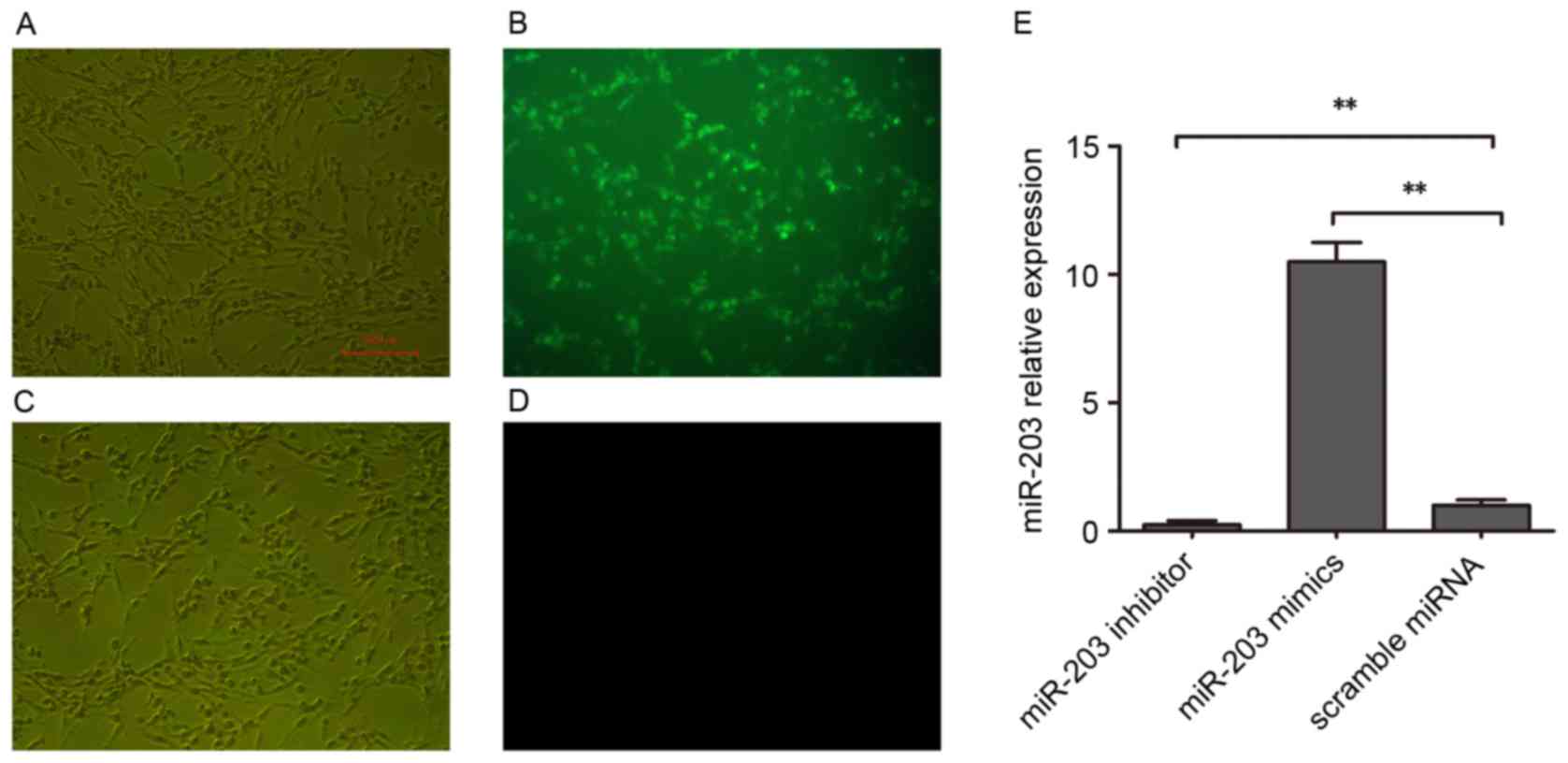
scramble miRNA. Transfection efficiency was assessed using
FAM-labeled miRNAs (Fig. 1A-D).
RT-qPCR was used to confirm that miR-203 expression was
significantly increased in HSC-T6 cells following miR-203 mimic
transfection, whereas it was significantly suppressed following
transfection with the miR-203 inhibitor (P<0.01 vs. scramble
miRNA; Fig. 1E).
RT-qPCR and western blot analyses were used to
investigate mRNA and protein expression levels of COL1A1, COL3A1
and α-SMA (Fig. 2). Notably, mRNA
expression levels of the fibrosis-associated genes COL1A1,
COL3A1 and α-SMA were significantly upregulated in
HSC-T6 cells transfected with the miR-203 inhibitor, whereas they
were significantly downregulated in miR-203 mimic-transfected cells
compared with the scramble group (P<0.01; Fig. 2A and B). Similarly, COL1A1, COL3A1
and α-SMA protein expression levels were increased following
transfection with the miR-203 inhibitor and decreased following
transfection with mimics, compared with the scramble miRNA control
group (P<0.01 and P<0.01, respectively; Fig. 2C and D).
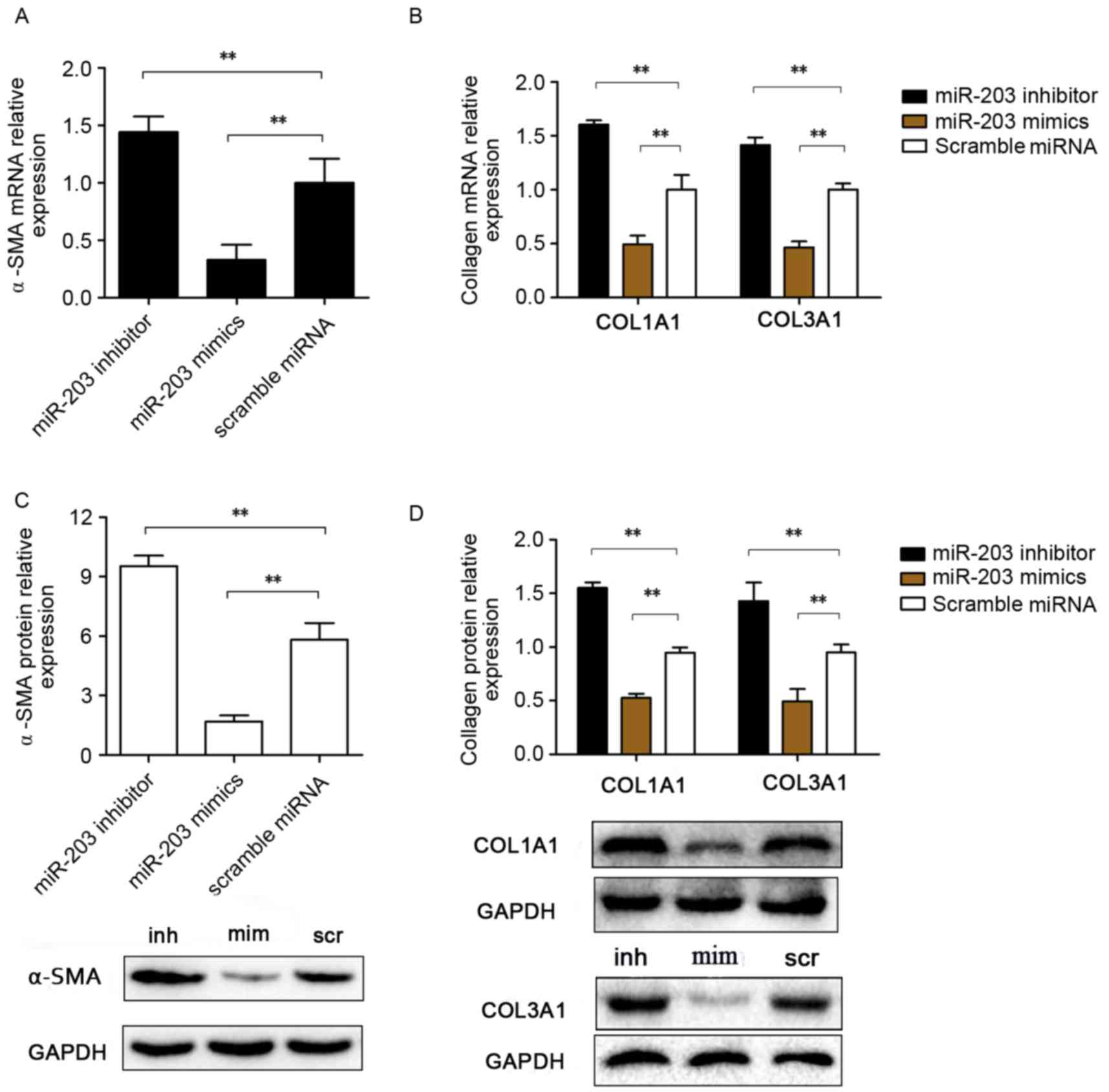 | Figure 2.Effects of miR-203 on COL1A1, COL3A1
and α-SMA expression in rat HSCs. Rat HSC-T6 cells were transfected
with a miR-203 mimic, a miR-203 inhibitor or scramble miRNA. mRNA
expression levels for (A) α-SMA, (B) COL1A1 and
COL3A1 were assessed using reverse
transcription-quantitative polymerase chain reaction. (C) α-SMA,
(D) COL1A1 and COL3A1 protein expression levels were assessed by
western blot analysis. Data are expressed as the mean ± standard
deviation of at least three independent experiments. **P<0.01.
COL, collagen; HSC, hepatic stellate cell; miR, microRNA; SMA,
smooth muscle actin; inh, miR-203 inhibitor; scr, scramble miRNA;
mim, miR-203 mimic. |
miR-203 inhibits HSC
proliferation
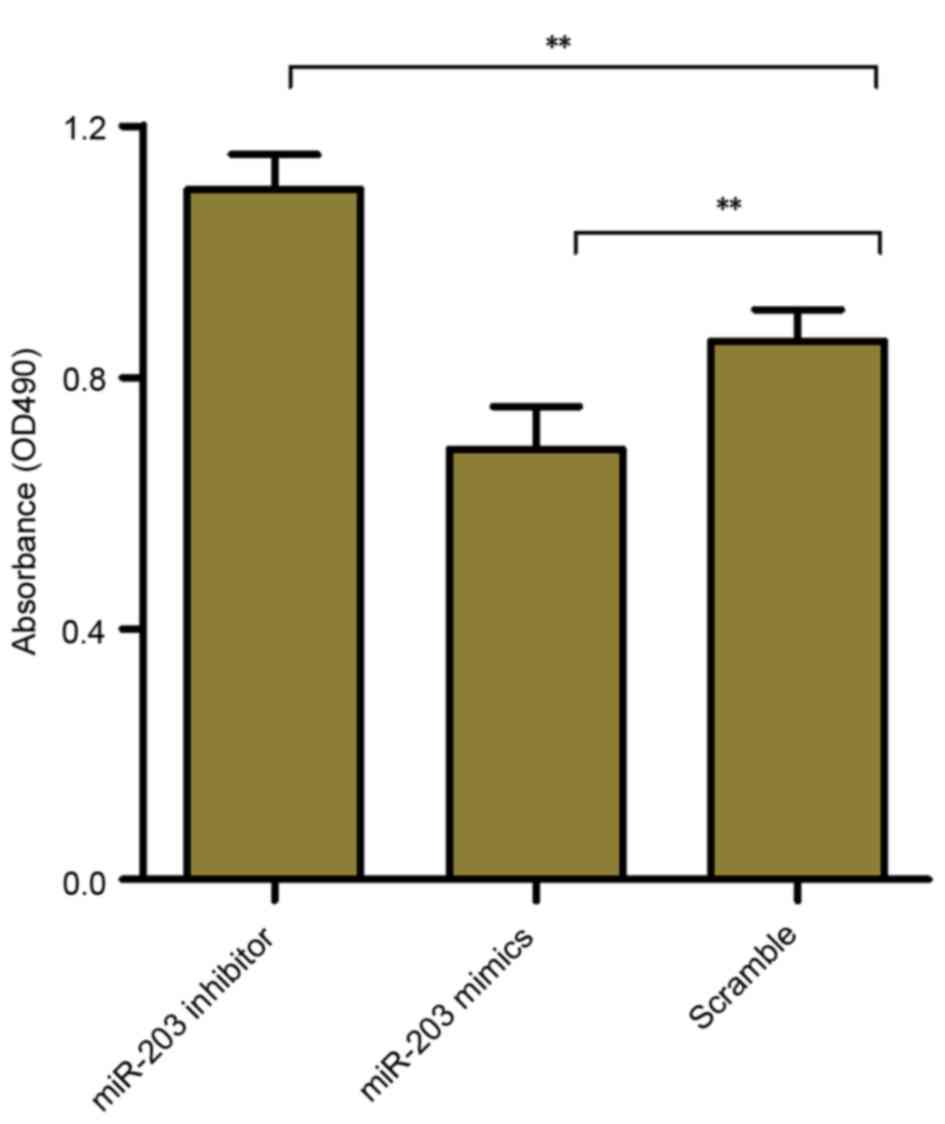
To investigate whether miR-203 may be involved in
the regulation of HSC proliferation, the MTT assay was used to
assess the effects of miR-203 inhibition and upregulation on the
proliferative capabilities of rat HSCs. The results demonstrated
that HSC-T6 cell proliferation was significantly increased
following transfection with the miR-203 inhibitor (P<0.01),
whereas it was significantly suppressed in cells transfected with
the miR-203 mimic (P<0.01) compared with the scramble group
(Fig. 3). These results suggested
that miR-203 may serve a role as a modulator of HSC
proliferation.
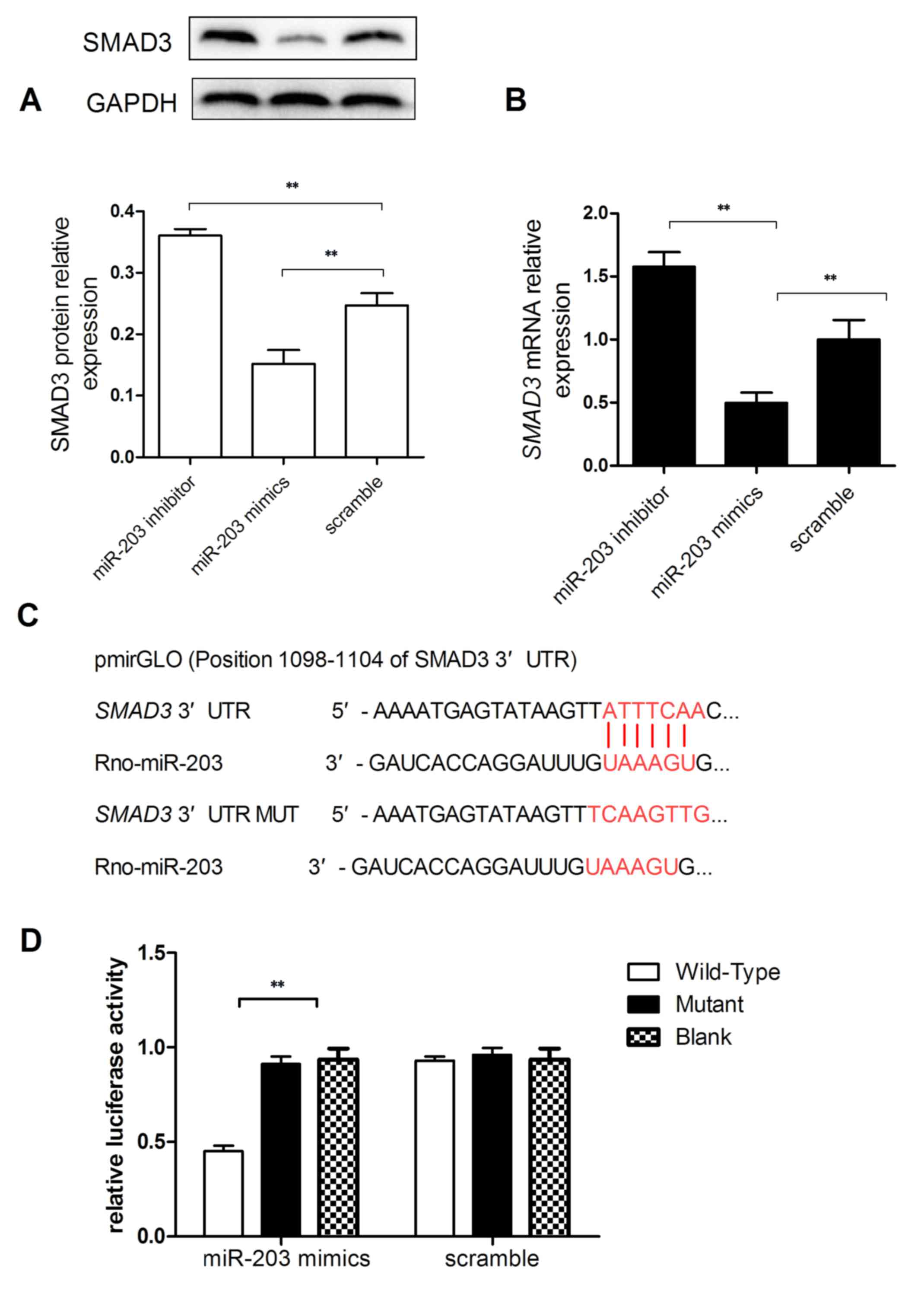
SMAD3 is a target of miR-203
To investigate the potential mechanisms underlying
the implication of miR-203 in HSC activation and proliferation,
bioinformatics analysis was performed to identify potential target
genes of miR-203. TRPV4 has previously been reported as a
direct target gene of miR-203 during HSC activation (17), and the TGF-β/Smad signaling pathway
has been demonstrated to serve an important role in hepatic
fibrosis (25). Therefore, the
present study investigated whether SMAD3 may be a direct
target gene for miR-203. RT-qPCR and western blot analyses were
used to evaluate SMAD3 mRNA and protein expression levels, and
revealed that SMAD3 mRNA and protein expressions in miR-203
mimic-transfected cells were significantly reduced compared with
the scramble group (Fig. 4A and
B). Conversely, SMAD3 mRNA and protein expression levels were
significantly increased in HSC-T6 cells transfected with the
miR-203 inhibitor (P<0.01; Fig. 4A
and B).
To investigate whether SMAD3 may be a target
of miR-203, the 3′-UTR of the SMAD3 mRNA was cloned into a
pmirGLO vector (Fig. 4C). A
dual-luciferase reporter assay demonstrated that cells
co-transfected with the plasmid containing the wild-type
SMAD3 3′-UTR and the miR-203 mimic exhibited the lowest
luciferase activity (P<0.01 vs. mutant SMAD3; Fig. 4D). These results suggested that
SMAD3 may be a direct target gene of miR-203.
Discussion
Hepatic fibrosis is a common pathophysiological
process that underlies chronic liver disease, regardless of
etiology. Hepatic fibrogenesis may progress to liver cirrhosis,
hepatic failure or even hepatocellular carcinoma (1). Currently, no effective antifibrotic
therapeutic strategies are available for clinical use; therefore,
the elucidation of the pathophysiological mechanisms underlying
fibrogenesis and the development of novel therapeutic strategies
are imperative for the treatment of patients with hepatic fibrosis
(26). HSC activation has been
identified among the primary critical events in the development of
hepatic fibrosis, and activated HSCs are the major source of ECM
components (3,27). Altered ECM composition has been
demonstrated in the fibrotic liver, with increased prevalence of
fibrillar types of collagen, such as type I and type III.
Furthermore, activated HSCs have been reported to express myogenic
markers, including α-SMA (28).
Previous studies have suggested that the prevention of the
synthesis and deposition of ECM components may have potential as an
effective antifibrotic therapeutic strategy (28,29).
Song et al (17) reported
decreased miR-203 expression levels in human and rodent fibrotic
liver tissue, and in TGF-β1-treated HSC-T6 cells. However, the
roles of miR-203 in collagen synthesis and HSC proliferation have
yet to be elucidated. The present study aimed to investigate the
roles of miR-203 in collagen synthesis and HSC proliferation, as
well as to explore the molecular mechanisms underlying its actions.
Following transfection of the HSC-T6 rat hepatic stellate cell line
with a miR-203 inhibitor, the mRNA and protein expression levels of
the fibrosis-related proteins COL1A1, COL3A1 and α-SMA were
significantly upregulated; whereas transfection with a miR-203
mimic resulted in a significant decrease in the mRNA and protein
expression levels. In addition, HSC proliferation was significantly
enhanced following miR-203 inhibition, and suppressed following
miR-203 potentiation.
Proinflammatory cytokines, including
platelet-derived growth factor, TGF-β, connective tissue growth
factor and endothelin-1, have been implicated in the processes of
HSC activation (4,30–32).
Among these cytokines, the TGF-β signaling pathway has been
revealed as an important regulator of HSC activation (4). SMAD proteins, which are intracellular
mediators belonging to the TGF-β family, are classified into three
groups: receptor-regulated Smads (R-Smads), common mediator Smads
(Co-Smads) and inhibitor Smads (30). R-Smads (SMADs 1–3, 5 and 8) are
phosphorylated and activated through the TGF-β receptor I kinase,
and form heteromeric complexes with Co-Smads (Smad4). These
complexes can translocate into the nucleus and associate with other
co-mediators to regulate the expression of target genes (33). Smads have previously been
identified as miRNA targets in various diseases: miR-203 has been
reported to inhibit heat-denatured fibroblast proliferation and
migration through the regulation of SMAD3, whereas miR-454
has been demonstrated to inhibit HSC activation by directly
targeting SMAD4 (34).
Furthermore, SMAD3 has been demonstrated to modulate E-cadherin
expression through a miR-200-dependent mechanism (12). These results suggested that SMAD
proteins may act as miRNA targets during the modulation of gene
expression. In the present study, miR-203 target genes were
predicted using bioinformatics analysis, and SMAD3 was
identified among ~937 other targets. SMAD3 mRNA and protein
expression levels were demonstrated to be significantly upregulated
in HSCs transfected with a miR-203 inhibitor, whereas they were
downregulated in cells transfected with a miR-203 mimic. In
addition, a dual-luciferase reporter assay validated SMAD3
as a direct target gene of miR-203. These results suggested that
miR-203 may silence the expression of SMAD3 and thus prevent
the activation of the TGF-β/Smad signaling pathway and the
activation of HSCs, therefore suppressing the synthesis and
secretion of ECM components, including, COL1A1, COL3A1 and α-SMA.
However, further studies are required to investigate the specific
molecular mechanisms underlying the regulatory effects of miR-203
on the synthesis and deposition of ECM components, and on HSC
proliferation.
In conclusion, the results of the present study
suggested that miR-203 may inhibit HSC proliferation and suppress
the expression of collagen-related genes. Furthermore, SMAD3
was identified as a direct target gene of miR-203, thus suggesting
that miR-203-mediated modulation of SMAD3 signaling may be
implicated in HSC activation. Further studies are required in order
to elucidate the specific roles of miR-203 in the complex molecular
mechanisms underlying hepatic fibrogenesis.
Acknowledgements
The present study was supported by The Natural
Science Foundation of Zhejiang Province (grant no.
LY14H030010).
Glossary
Abbreviations
Abbreviations:
|
HSC
|
hepatic stellate cell
|
|
α-SMA
|
α-smooth muscle actin
|
|
SMAD3
|
mothers against decapentaplegic
homolog 3
|
|
ECM
|
extracellular matrix
|
|
COL1A1
|
collagen 1A1
|
|
COL3A1
|
collagen 3A1
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
|
UTR
|
untranslated region
|
|
miRNA
|
microRNA
|
|
TGF
|
transforming growth factor
|
|
DMEM
|
Dulbecco's modified Eagle's medium
|
|
FBS
|
fetal bovine serum
|
|
GAPDH
|
glyceraldehyde-3-phosphate
dehydrogenase
|
|
MTT
|
methyl thiazolyl trazolium
|
References
|
1
|
Friedman SL: Mechanisms of hepatic
fibrogenesis. Gastroenterology. 134:1655–1669. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Friedman SL: Molecular mechanisms of
hepatic fibrosis and principles of therapy. J Gastroenterol.
32:424–430. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li D and Friedman SL: Liver fibrogenesis
and the role of hepatic stellate cells: New insights and prospects
for therapy. J Gastroenterol Hepatol. 14:618–633. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bissell DM, Roulot D and George J:
Transforming growth factor beta and the liver. Hepatology.
34:859–867. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Attisano L and Wrana JL: Signal
transduction by the TGF-beta superfamily. Science. 296:1646–1647.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Blahna MT and Hata A: Smad-mediated
regulation of microRNA biosynthesis. FEBS Lett. 586:1906–1912.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Valencia-Sanchez MA, Liu J, Hannon GJ and
Parker R: Control of translation and mRNA degradation by miRNAs and
siRNAs. Genes Dev. 20:515–524. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schickel R, Boyerinas B, Park SM and Peter
ME: MicroRNAs: Key players in the immune system, differentiation,
tumorigenesis and cell death. Oncogene. 27:5959–5974. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang XW, Heegaard NH and Orum H: MicroRNAs
in liver disease. Gastroenterology. 142:1431–1443. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yu F, Guo Y, Chen B, Dong P and Zheng J:
MicroRNA-17-5p activates hepatic stellate cells through targeting
of Smad7. Lab Invest. 95:781–789. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ahn SM, Cha JY, Kim J, Kim D, Trang HT,
Kim YM, Cho YH, Park D and Hong S: Smad3 regulates E-cadherin via
miRNA-200 pathway. Oncogene. 31:3051–3059. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhong X, Chung AC, Chen HY, Meng XM and
Lan HY: Smad3-mediated upregulation of miR-21 promotes renal
fibrosis. J Am Soc Nephrol. 22:1668–1681. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang F, Yang Z, Cao M, Xu Y, Li J, Chen
X, Gao Z, Xin J, Zhou S, Zhou Z, et al: MiR-203 suppresses tumor
growth and invasion and down-regulates MiR-21 expression through
repressing Ran in esophageal cancer. Cancer Lett. 342:121–129.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang C, Wang X, Liang H, Wang T, Yan X,
Cao M, Wang N, Zhang S, Zen K, Zhang C and Chen X: miR-203 inhibits
cell proliferation and migration of lung cancer cells by targeting
PKCα. PLoS One. 8:e739852013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu Y, Ren F, Rong M, Luo Y, Dang Y and
Chen G: Association between underexpression of microrna-203 and
clinicopathological significance in hepatocellular carcinoma
tissues. Cancer Cell Int. 15:622015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Song Y, Zhan L, Yu M, Huang C, Meng X, Ma
T, Zhang L and Li J: TRPV4 channel inhibits TGF-β1-induced
proliferation of hepatic stellate cells. PLoS One. 9:e1011792014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Vogel S, Piantedosi R, Frank J, Lalazar A,
Rockey DC, Friedman SL and Blaner WS: An immortalized rat liver
stellate cell line (HSC-T6): A new cell model for the study of
retinoid metabolism in vitro. J Lipid Res. 41:882–893.
2000.PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lewis BP, Burge CB and Bartel DP:
Conserved seed pairing, often flanked by adenosines, indicates that
thousands of human genes are microRNA targets. Cell. 120:15–20.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen K and Rajewsky N: Natural selection
on human microRNA binding sites inferred from SNP data. Nat Genet.
38:1452–1456. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Betel D, Koppal A, Agius P, Sander C and
Leslie C: Comprehensive modeling of microRNA targets predicts
functional non-conserved and non-canonical sites. Genome Biol.
11:R902010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gressner AM: Cytokines and cellular
crosstalk involved in the activation of fat-storing cells. J
Hepatol. 22 Suppl 2:S28–S36. 1995.
|
|
24
|
Nouchi T, Tanaka Y, Tsukada T, Sato C and
Marumo F: Appearance of alpha-smooth-muscle-actin-positive cells in
hepatic fibrosis. Liver. 11:100–105. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wells RG: Fibrogenesis. V. TGF-beta
signaling pathways. Am J Physiol Gastrointest Liver Physiol.
279:G845–G850. 2000.PubMed/NCBI
|
|
26
|
Friedman SL: Evolving challenges in
hepatic fibrosis. Nat Rev Gastroenterol Hepatol. 7:425–436. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Safadi R and Friedman SL: Hepatic
fibrosis-role of hepatic stellate cell activation. Med Gen Med.
4:272002.
|
|
28
|
Bataller R and Brenner DA: Liver fibrosis.
J Clin Invest. 115:209–218. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ghiassi-Nejad Z and Friedman SL: Advances
in antifibrotic therapy. Expert Rev Gastroenterol Hepatol.
2:803–816. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Borkham-Kamphorst E, van Roeyen CR,
Ostendorf T, Floege J, Gressner AM and Weiskirchen R:
Pro-fibrogenic potential of PDGF-D in liver fibrosis. J Hepatol.
46:1064–1074. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Paradis V, Dargere D, Vidaud M, de
Gouville AC, Huet S, Martinez V, Gauthier JM, Ba N, Sobesky R,
Ratziu V and Bedossa P: Expression of connective tissue growth
factor in experimental rat and human liver fibrosis. Hepatology.
30:968–976. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rockey DC, Fouassier L, Chung JJ, Carayon
A, Vallee P, Rey C and Housset C: Cellular localization of
endothelin-1 and increased production in liver injury in the rat:
Potential for autocrine and paracrine effects on stellate cells.
Hepatology. 27:472–480. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shi Y and Massagué J: Mechanisms of
TGF-beta signaling from cell membrane to the nucleus. Cell.
113:685–700. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhu D, He X, Duan Y, Chen J, Wang J, Sun
X, Qian H, Feng J, Sun W, Xu F and Zhang L: Expression of
microRNA-454 in TGF-β1-stimulated hepatic stellate cells and in
mouse livers infected with Schistosoma japonicum. Parasit Vectors.
7:1482014. View Article : Google Scholar : PubMed/NCBI
|