Introduction
Diabetic nephropathy (DN) is a leading cause of
end-stage renal disease (ESRD). The worldwide occurrence of DN is
increasing, and this disease is associated with increased morbidity
and mortality in patients with type 1 and type 2 diabetes (1). DN is characterized by the
accumulation of extracellular matrix (ECM), resulting in
progressive kidney fibrosis that leads to kidney function decline
and irreversible loss of tissue (2). The increasing prevalence of DN has
resulted in a growing research focus on this disease. Therefore,
the identification of a novel therapeutic target in the management
of DN is required.
Autophagy is emerging as a key cellular stress
response that is involved in a variety of disease states, including
DN (3). Autophagy is a highly
conserved ‘self-feeding’ pathway involved in degrading and
recycling macromolecules and damaged organelles, to maintain
intracellular homeostasis (4).
This cellular function serves important roles in human health and
disease. Generally, autophagy serves a dual purpose; it may perform
a cytoprotective or deleterious role in the body, depending on the
type and severity of the inducing injury (5). A certain degree of autophagic
activity serves a critical role in promoting tissue homeostasis and
cell survival. However, excessive autophagic activity may also
contribute to type II programmed cell death and, in certain
circumstances, promote the development of DN (6).
Hydrogen sulfide (H2S) is an endogenously
produced gaseous molecule with important roles in cellular
signaling. This chemical has been implicated in the regulation of
inflammatory responses, cardiovascular functions, renal functioning
and the gastrointestinal system. Furthermore, H2S has
been shown to exert potent cytoprotective abilities against tissue
injury, including organ fibroses such as myocardial and renal
fibrosis (7). However, the role of
H2S and autophagy in the pathogenesis of DN, and the
relationship between H2S and the dysregulation of
autophagy remains unclear. Therefore, further studies are required
to clarify these mechanisms in detail. The present study
established a streptozotocin (STZ)-induced diabetic rat model to
investigate the role of H2S and autophagy in the
pathogenesis of DN, and the protective effects of H2S
against DN.
Materials and methods
Animals and reagents
Adult male Sprague-Dawley rats (n=52; weight, 280±40
g) were obtained from the animal experiment center of South China
University (Henyang, China). The study was approved by the Ethics
Committee of the Department of Laboratory Animals of South China
University [animal qualified number: SYXK (Hunan), 2015–0006]. All
rats were maintained in a climate-controlled room with a 12-h
light/dark cycle, at constant temperature (23±1°C) and humidity
(40–50%) with free access to food and water. Antibodies against
collagen IV (cat. no. BA3585-2), GAPDH (cat. no. PB0141), matrix
metalloproteinase 9 (MMP9; cat. no. BA0573), MMP7 (cat. no.
BA2110), tissue inhibitor of metalloproteinase 1 (TIMP1; cat. no.
BA3727), superoxide dismutase (SOD; cat. no. BA3836-2), AKT (cat.
no. BA0631), transforming growth factor (TGF)-β1 (cat. no. BA0290)
and nuclear factor (NF)-κB (cat. no. BA1872-2) were purchased from
Wuhan Boster Biological Technology, Ltd. (Wuhan, China). Antibodies
against microtubule associated protein light chain 3 (LC3; cat. no.
12741), autophagy related (Atg)-3 (cat. no. 3415), Atg5 (cat. no.
12994), Atg7 (cat. no. 8558), Atg12 (cat. no. 4180) and Atg16 (cat.
no. 8089) were purchased from Cell Signaling Technology, Inc.
(Danvers, MA, USA). The antibody against cystathionine β synthase
(CBS; cat. no. RS-4193R) was obtained from Shanghai Ruiqi
Biological Technology Co., Ltd. The goat anti-rabbit horseradish
peroxidase-conjugated IgG secondary antibody (cat. no. 074-1506)
was purchased from SeraCare Life Sciences (Milford, MA, USA).
Sodium hydrogen sulfide (NaHS), an exogenous donor of
H2S, was purchased from Sigma-Aldrich (Merck KGaA,
Darmstadt, Germany). The bicinchoninic acid (BCA) protein
quantitative kit and the cell lysis solution were purchased from
Beyotime Institute of Biotechnology (Guangzhou, China).
Animal model and grouping
All rats had free access to food and water and,
following 7 days of acclimatization, the rats were randomly divided
into four equal groups (n=13/group): Control group, STZ group, STZ
+ H2S group and H2S group. The STZ and STZ +
H2S groups received intraperitoneal injections of 40
mg/kg body weight STZ, 5% glucose water was provided for
STZ-treated rats within 24 h to prevent hypoglycemic shock, and
blood sugar levels were tested 72 h after injection. The diabetic
animal model was considered successful if blood glucose levels rose
to >16.7 mmol/l-1. Following animal model set-up, the
H2S and STZ + H2S groups were
intraperitoneally administered the H2S donor NaHS after
the models were established successfully, at a dose of 100
µmol/kg/day for a further 8 weeks. The first dose of NaHS was
administered 24 h after models were successfully established.
Control and STZ groups were treated with an equivalent volume of
intraperitoneal 0.9% saline for 8 weeks. During this 8-week period
a total of 12 rats died (control, n=2; STZ, n=4; STZ +
H2S, n=3; H2S, n=3); the overall survival
rate was 77%. Diabetic rats were observed to be unresponsive and
lackluster, and displayed various degrees of polydipsia,
polyphagia, diuresis and moved slowly.
Specimen collection and
processing
At the end of the 8 weeks, the 24 h proteinuria
levels were determined by using a UP ELISA kit (cat. no. SBJ-H1384;
Nanjing Senbeijia Biological Technology Co., Ltd. Nanjing, China),
and the rats were sacrificed following chloral hydrate anesthesia.
Both kidneys were removed; the right kidney was immersed in 10%
formalin and embedded in paraffin. The left kidney was frozen
(−70°C) and later used for western blot analysis.
Histopathological examinations
Paraffin-embedded specimens were cut into 5 µm thick
sections and stained with Masson's trichrome. Five random
microscopic fields per well were quantified at a magnification of
×400 using a BH-2 light microscope (Olympus Corporation, Tokyo,
Japan).
Immunohistochemistry
Paraffin-embedded specimens were cut into 4 µm thick
sections, dewaxed, rehydrated and boiled. Sections were washed
three times for 5 min each in PBST [PBS (pH 7.4), 0.05% Tween-20],
blocked with 5% bovine serum albumin (Wuhan Boster Biological
Technology, Ltd.) for 10 min at room temperature and then incubated
with the collagen IV primary antibody (diluted 1:50) overnight at
4°C. The following day, sections were washed three times for 5 min
each in PBST, incubated with horseradish peroxidase-conjugated
secondary antibodies (1:2,000) for 30 min at 37°C, washed in PBST
and visualized using a DAB staining kit (Nanjing Senbeijia
Biological Technology Co., Ltd.). Sections were counterstained with
hematoxylin and collagen IV deposition was observed at a
magnification of ×400 using a digital microscope (Olympus
Corporation).
Periodic acid-Schiff (PAS)
staining
Paraffin-embedded kidney specimens were cut into 2–3
µm thick sections, dewaxed, rehydrated, and oxidized with 5%
periodate for 6–8 min at 15°C. Sections were subsequently washed
with distilled water for 2 min, placed into colorless Schiff's
reagent and incubated in the dark for 20 min at 15°C. The samples
were then droplet washed twice for 1 min with sodium sulfite
solution (0.5%), rinsed with running water for 2 min,
counterstained with hematoxylin for 1 min at 18°C, washed in
running water and differentiated with 1% hydrochloric acid alcohol
at 18°C. Samples were then repeatedly rinsed with running water,
the blue stain was developed with warm water and sections were
dehydrated with graded ethanol and sealed with neutral balsam, and
the PAS-positive material was observed in the renal glomerular and
tubular basement membranes under high-power magnification (x400)
using a BH-2 light microscope.
Western blot analysis
Proteins were extracted in ice-cold
radioimmunoprecipitation assay buffer containing a protease
inhibitor cocktail (100X; Beyotime Institute of Biotechnology), and
quantified using the BCA colorimetric method. Proteins (50 µg/lane)
were separated by 10% SDS-PAGE and transferred to polyvinylidene
difluoride membranes. Membranes were blocked with 5% skimmed milk
powder in TBST buffer [10 mM Tris (pH 7.5), 150 mM NaCl, 0.05%
Tween-20] for 2 h and incubated with anti-MMP9, anti-MMP7,
anti-TIMP1, anti-SOD, anti-AKT, anti-TGF-β1, anti-NF-κB (all 1:400)
and anti-LC3, anti-Atg3, anti-Atg5, anti-Atg7, anti-Atg12,
anti-Atg16 (all 1:1,000) antibodies for 1 h at 37°C, and then
overnight at 4°C. GAPDH served as the endogenous control. The
membranes were washed three times for 10 min each and then
incubated with horseradish peroxidase-conjugated secondary
antibodies (1:2,000) for 2 h at room temperature. The membranes
were detected using Western Blotting Luminol reagent (Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), protein bands were
visualized using the FluorChem® FC2 Imaging System (ProteinSimple;
Bio-Techne, Minneapolis, MN, USA) and the band density was
semi-quantified using AlphaView v3.2.2 software (ProteinSimple;
Bio-Techne).
Statistical analysis
Data were analyzed using SPSS v18.0 software (SPSS,
Inc., Chicago, IL, USA), and expressed as the mean ± standard
deviation of the mean. Comparisons between groups were performed
using one-way analysis of variance followed by Fisher's least
significant difference test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Changes in proteinuria
The 24 h proteinuria was significantly increased in
the STZ group (P<0.05), compared with the untreated control
group. Notably, the 24 h proteinuria level was significantly
decreased in the STZ + H2S group, following
H2S intervention (P<0.05; Table I).
 | Table I.The 24 h proteinuria in STZ-diabetic
rats. |
Table I.
The 24 h proteinuria in STZ-diabetic
rats.
| Group | 24 h proteinuria
(mg/24 h) |
|---|
| Control | 11.8±3.3 |
| STZ |
44.5±6.4a |
| STZ +
H2S |
26.6±6.1b |
| H2S | 12.6±4.0 |
Pathological morphology
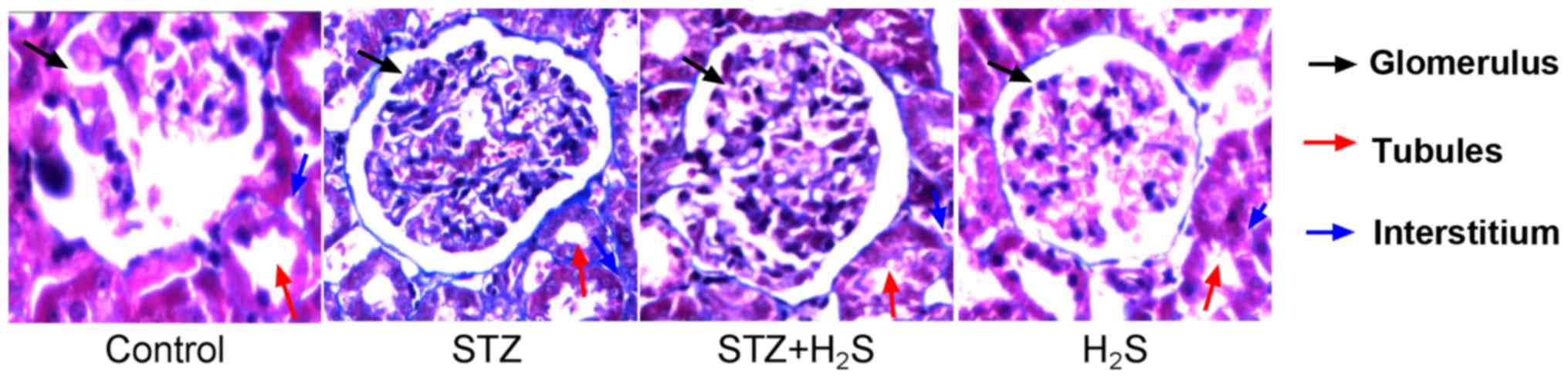
Masson's trichrome staining was performed to observe
collagen deposition; blue staining indicated the presence of
collagen in the tissues. There were no collagen fibers distinctly
observed in the renal interstitial areas of the control group,
however the glomerular and tubular basement membranes were lightly
stained. In the STZ treatment group collagen fibers were present in
the renal interstitium, combined with heavy staining of the
glomerular and tubular basement membranes. Notably, compared with
the STZ group, collagen fiber staining was markedly reduced in the
renal interstitial areas of STZ + H2S rats, and the
glomerular and tubular basement membranes were also stained lighter
in these rats. The H2S group renal interstitium did not
appear to display collagen staining, with light staining present
only on the glomerular and tubular basement membranes (Fig. 1).
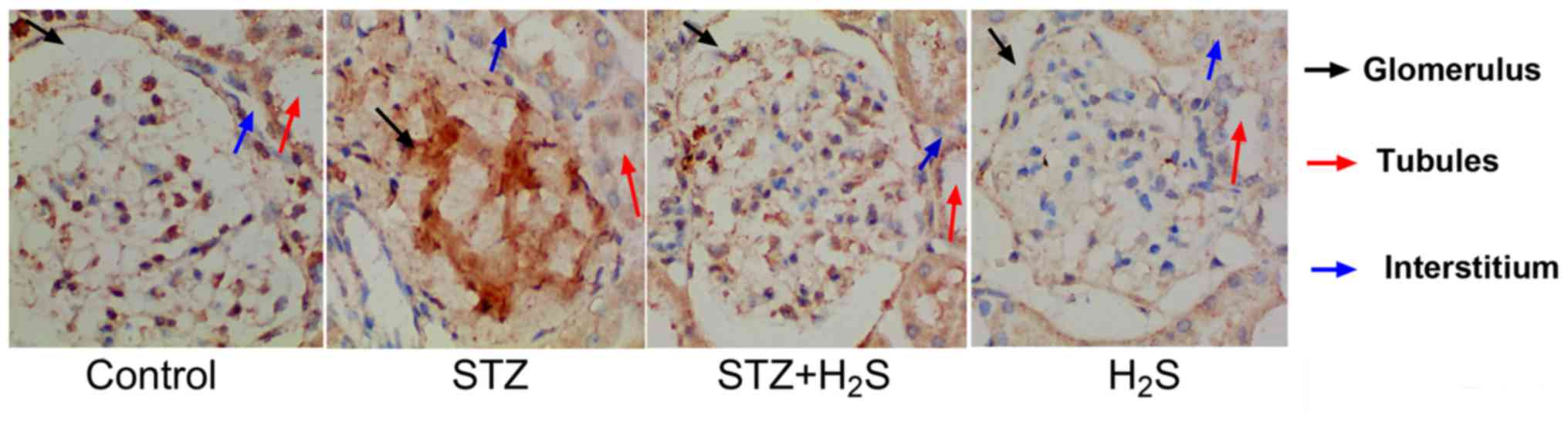
Immunohistochemistry
Immunohistochemical analysis was performed to
observe collagen IV deposition and brown staining indicated the
presence of collagen IV in the tissues. In the control group,
collagen IV was lightly observed in the glomerulus, tubules and
interstitial areas. In the STZ group, collagen IV positivity was
observed in the glomerulus, tubules and interstitial areas.
However, in the STZ + H2S group, collagen IV positive
staining was decreased in these areas, compared with the STZ group.
In the H2S group, collagen IV was not observed in the
renal tubules, the glomerulus or the renal interstitium (Fig. 2).
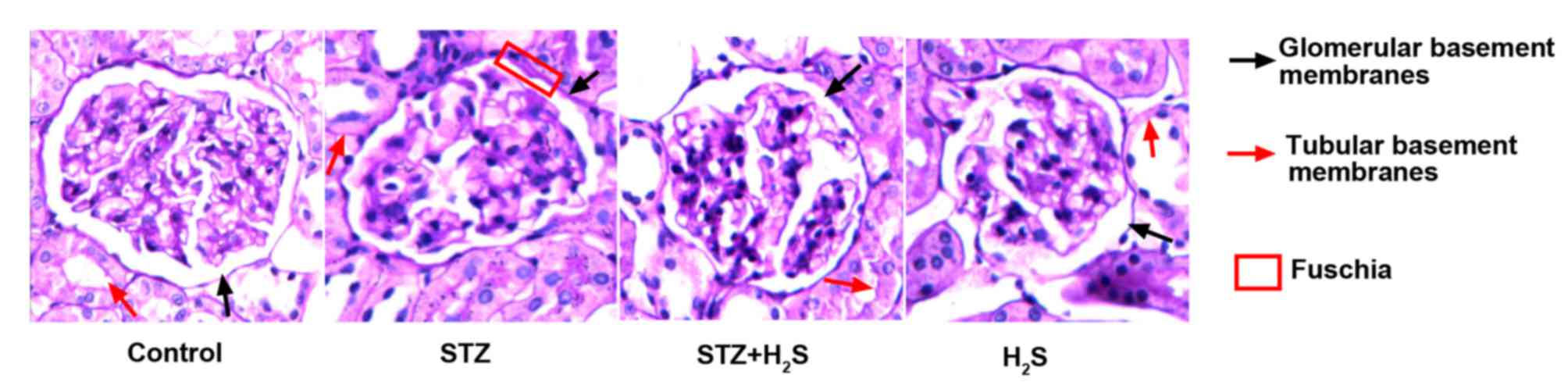
PAS staining
Fuchsia staining represented PAS positive material.
PAS staining was lowest in the renal glomerular and tubular
basement membranes of the control group. Marked PAS staining of the
renal glomerular and tubular basement membranes was observed in the
STZ group. Compared with the STZ group, the presence of PAS
positive material in renal glomerular and tubular basement
membranes was markedly improved in the STZ + H2S group.
PAS positive staining of the renal glomerular and tubular basement
membranes was low in the H2S group (Fig. 3).
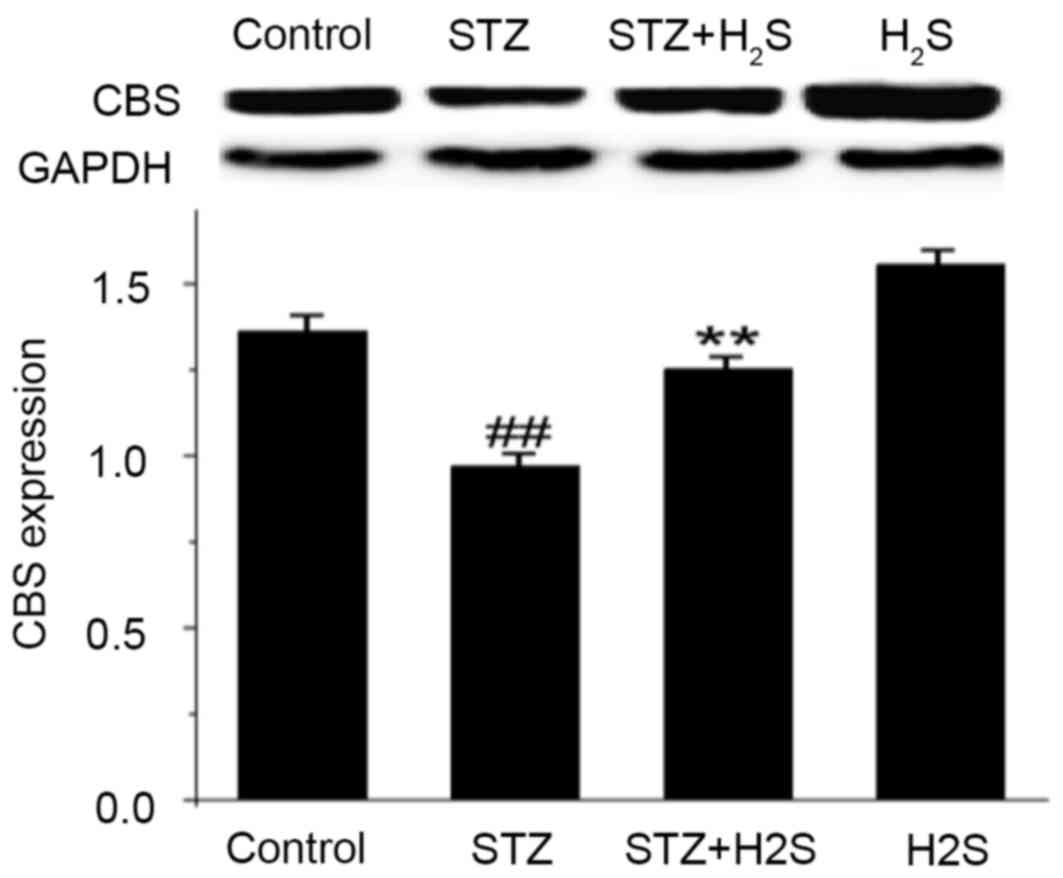
Effects of STZ and H2S on
CBS protein expression
CBS is an enzyme that can endogenously produce
H2S in kidney tissue. The present study observed that
CBS expression was significantly decreased in the STZ group
(P<0.05). Compared with the STZ group, however, the expression
levels of CBS protein were significantly increased in the STZ +
H2S group (P<0.05; Fig.
4). These results indicated that renal fibrosis was elevated
following STZ treatment, and was markedly improved with
H2S treatment; H2S may therefore improve
renal tissue fibrosis.
Effects of STZ and H2S on
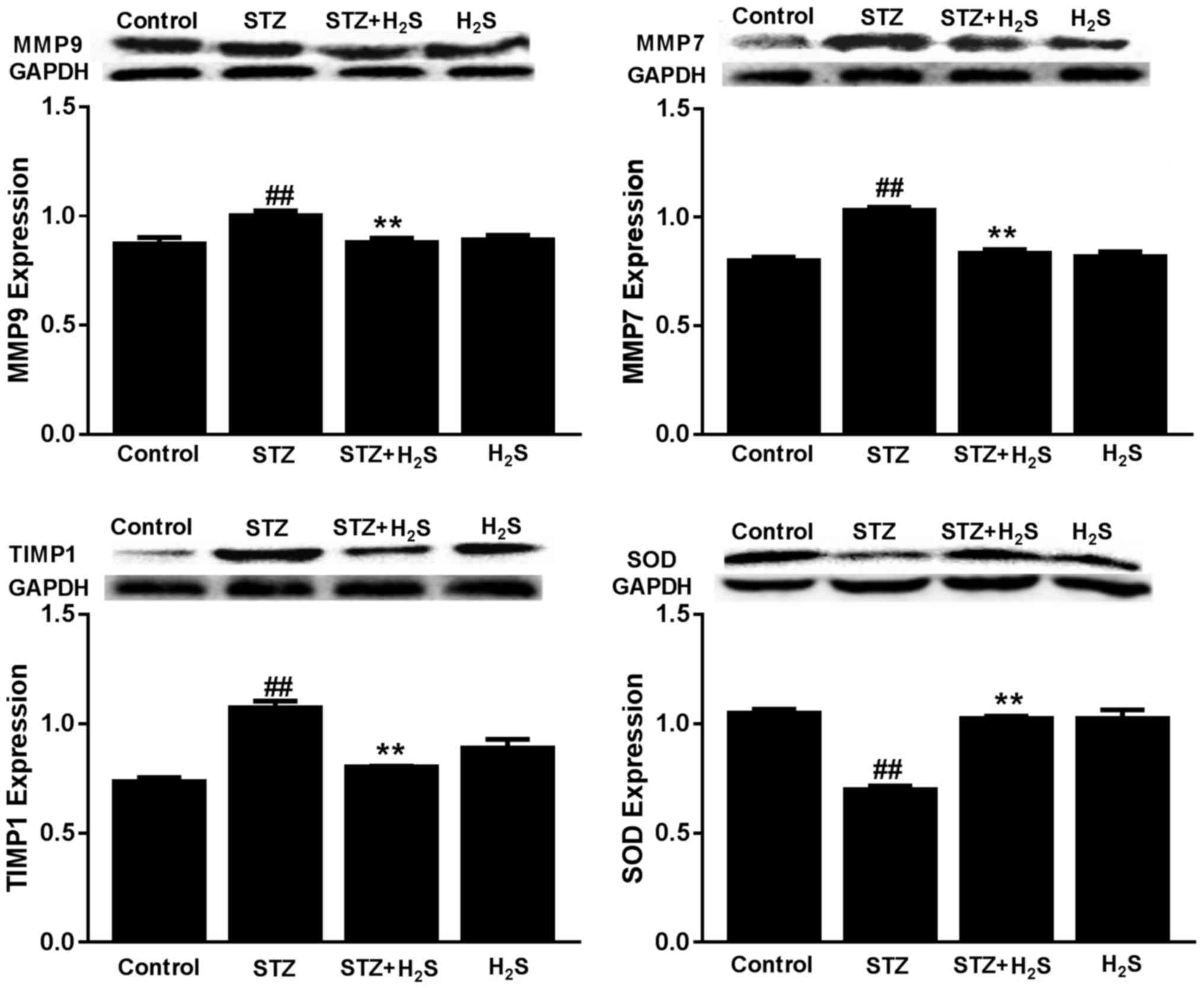
MMP9, MMP7, TIMP1 and SOD expression
The expression of MMP9, MMP7 and TIMP1 protein were
significantly increased in the STZ group compared with the control
group (P<0.05), whereas SOD protein was significantly decreased
in this group (P<0.05). However, the expression of MMP9, MMP7
and TIMP1 protein were significantly decreased and SOD protein was
significantly increased in the STZ + H2S group, compared
with the STZ group (P<0.05). There was no difference in MMP9,
MMP7, TIMP1 and SOD protein expression between the control and
H2S-only groups (Fig.
5).
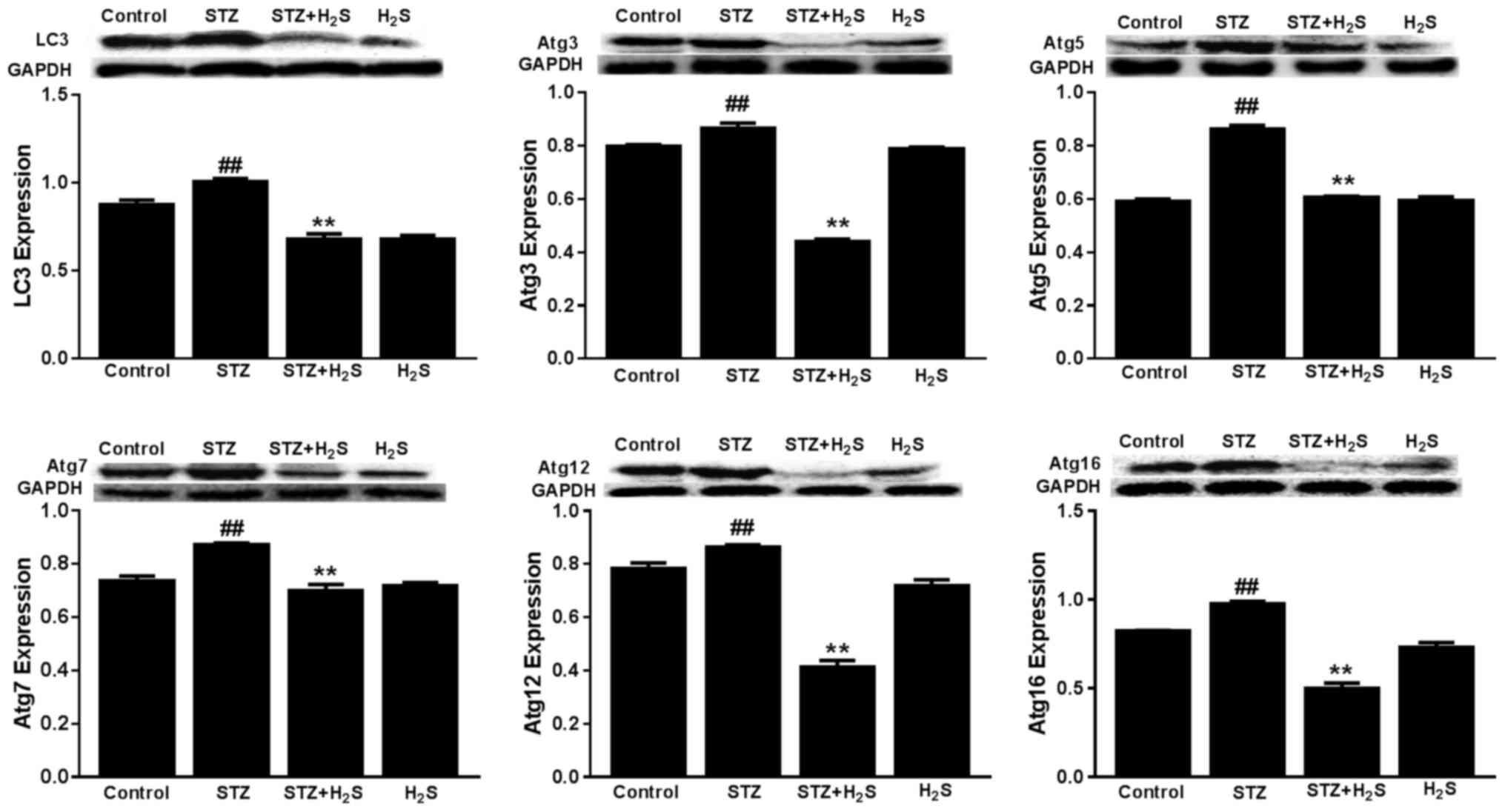
Effects of STZ and H2S on
LC3, Atg3, Atg5, Atg7, Atg12 and Atg16 expression
The expression of LC3, Atg3, Atg5, Atg7, Atg12 and
Atg16 was significantly increased in the STZ group, compared with
the control group (P<0.05). However, the expression levels of
these proteins were significantly decreased in the STZ +
H2S group, compared with the STZ group (P<0.05).
There was no difference in LC3, Atg3, Atg5, Atg7, Atg12 or Atg16
protein expression between the control group and the
H2S-only group (Fig.
6).
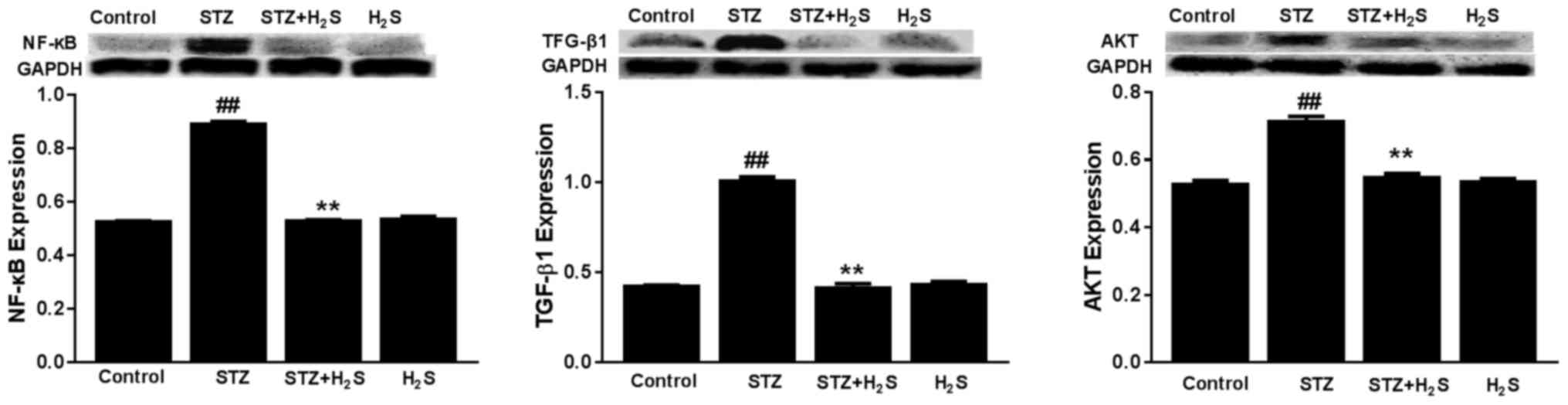
Effects of STZ and H2S on
TGF-β1, NF-κB and AKT expression
The expression levels of TGF-β1, NF-κB and AKT were
significantly increased in the STZ group compared with the control
group (P<0.05). The expression levels of TGF-β1, NF-κB and AKT
were significantly decreased in the STZ + H2S group,
compared with the STZ group (P<0.05). There was no difference in
TGF-β1, NF-κB or AKT expression between the control group and the
H2S group (Fig. 7).
Discussion
The results of the present study demonstrated a
significant increase in urinary protein content in diabetic rats.
Immunohistochemical examination discovered prominent renal tissue
fibrosis and significant upregulation of renal tissue collagen IV
expression amongst STZ-induced diabetic rats. The results of
Masson's staining indicated that collagen fibers were present in
the renal interstitium in the STZ group and the presence of
PAS-positive material in renal glomerular and tubular basement
membranes was markedly increased in the STZ group compared with the
control group. These results also indicated that there was
dysregulation of MMPs and TIMP expression in the renal tissue of
diabetic rats. Collectively, these results suggested the presence
of renal tissue fibrosis in diabetic rats. Furthermore, the present
study observed an elevation of activated autophagy biomarkers,
including LC3, Atg3, Atg5, Atg7, Atg12 and Atg16. These biomarkers
were upregulated in the renal tissue of diabetic rats, suggesting
that autophagic activation may be associated with renal tissue
fibrosis in these rats. Previous research has indicated that renal
fibrosis is accompanied by an upregulation of autophagy (6), whereas a different study suggested
that a downregulation of autophagy occurs in diabetic nephropathy
(8). Such a difference may be
associated with disease duration and stage of diabetic nephropathy;
the majority of the autophagic downregulation is observed in early
stages of diabetes and is associated with diabetic kidney
hypertrophy, whereas enhanced autophagy is often observed in the
late stages of diabetes and is associated with diabetic kidney
fibrosis (8). Evidence of cellular
autophagy inhibition was observed in the proximal and distal
tubules of early STZ-induced diabetic rats by electron microscopy
analysis, three days following STZ administration (8). Whereas some studies have demonstrated
the presence of autophagy in renal tissue fibrosis (9,10).
Therefore, it is speculated that the level of autophagy may be
upregulated when significant renal tissue fibrosis occurs in DN.
This phenomenon was also observed in another experimental report
(11). Such time-dependent
alterations also conform to the characteristics of autophagy: Early
mild damage leads to protective autophagy, whereas sustained
serious damage may result in traumatic autophagy. However, whether
protective autophagy or traumatic autophagy occurred in the present
study remains unclear.
Excessive oxidative stress and autophagy has
previously been demonstrated in DN. Reactive oxygen species (ROS)
are mediators of autophagic activation, furthermore, ROS-induced
activation of autophagy may result in alternative outcomes that
promote either cell death or survival (12). The activation of autophagy is
frequently associated with ROS and oxidative stress. The
downregulation of SOD expression in the STZ-induced DN tissues
observed in the present study suggests an associated increase in
oxidative stress, and this may be associated with the activation of
autophagy.
The regulatory mechanism of autophagy is closely
associated with the AKT signaling pathway (13). Inhibition of AKT may activate
autophagy, however the opposite result has also been reported
(14). Furthermore, suppression of
the phosphatidylinositol-3-kinase/AKT pathway may attenuate renal
fibrosis; previous findings indicated that phospho-AKT expression
was upregulated in rat kidneys with chronic allograft nephropathy,
and these rats presented with severe interstitial fibrosis and
tubular atrophy (15,16). A previous study indicated that AKT
may have a bidirectional regulation role in autophagy and produce
time-dependent effects, however this is also likely to be
associated with the feedback regulation of AKT by Atg5 (17). In the present study, the level of
autophagy was significantly increased in the renal tissue of
diabetic rats, however the expression of AKT was also markedly
increased. These results suggest that AKT upregulation may be
associated with feedback regulation by Atg5, and other regulatory
pathways of autophagy may be involved in the development of renal
fibrosis associated with DN.
Similar to AKT, TGF-β1 and NF-κB also have critical
roles in the pathogenesis of DN (18,19),
and are common signaling pathways involved in autophagic
regulation. TGF-β has a central role in the pathogenesis of tissue
fibrosis, and overexpression of this protein in renal tubular
epithelial cells resulted in widespread peritubular fibrosis and
induction of autophagy (20).
Furthermore, cell culture studies indicated that TGF-β may activate
autophagy in tubular cells (21).
Therefore, it seems that TGF-β may regulate autophagy, which in
turn may regulate several critical aspects of kidney fibrosis, such
as tubulointerstitial fibrosis and diabetic nephropathy. Regarding
NF-κB, Haar et al (22)
reported that acute high-fat feeding mediates cardioprotection
against ischemia-reperfusion injury, and this was associated with a
NF-κB-dependent increase in autophagy. The results of the present
study indicate that the mechanism of renal interstitial fibrosis in
diabetic rats may be associated with the activation of TGF-β and
the NF-κB pathways, with a subsequent increase in the level of
autophagy.
Previous evidence has demonstrated that
H2S has acritical role in the pathogenesis of DN
(23,24). H2S is an endogenous
signaling gas, which possesses potent anti-inflammatory,
anti-oxidant and other regulatory functions (7). Together with nitric oxide and carbon
monoxide, H2S has important regulatory roles in
different physiological and pathological situations. Accumulating
evidence has indicated that H2S may have an antifibrotic
effect in the development of fibrosis in the heart, liver and
kidneys (25–27). One study demonstrated a decrease in
H2S-producing enzymes and subsequently a decrease in the
endogenous H2S level in plasma and tissues (28), whereas administration of exogenous
H2S was able to inhibit the development of fibrosis. The
production of H2S from L-cysteine is catalyzed primarily
by two enzymes, cystathionine γ-lyase and CBS (29). The results of the present study
demonstrated that CBS expression was significantly decreased in the
STZ group. Compared with the control group, and the expression
level of CBS protein was significantly increased in the STZ +
H2S group compared with the STZ group. In a rat model of
unilateral ureteral obstruction, H2S inhibited renal
fibrosis by attenuating excessive collagen production and ECM
protein expression (30). The
results of the present study indicated that H2S may
attenuate mesangial matrix deposition and renal tissue fibrosis,
and may inhibit excessive autophagy in the diabetic rat kidney. The
protective mechanism of H2S against diabetic nephropathy
may be associated with the downregulation of autophagy.
The present study aimed to investigate whether
exogenous H2S was able to protect against the
development of diabetic nephropathy. The results indicated that
H2S was able to improve proteinuria, to reduce the
presence of PAS-positive zones in the renal glomerular and tubular
basement membranes, and to reduce renal tissue fibrosis in
STZ-induced diabetic rats. Furthermore, administration of
H2S increased the expression of SOD and decreased the
production of collagen IV and expression of AKT, TGF-β1 and NF-κB.
TGF-β1 is an essential regulator of extracellular matrix synthesis
and cell proliferation, and is considered to be a marker of renal
fibrogenesis, whereas collagen IV and collagen II are major
extracellular matrix components (31). NF-κB activation has been documented
to be associated with renal inflammation and renal fibrogenesis
(32). In neonatal rat
cardiomyocytes exposed to hypoxia/reoxygenation, H2S was
able to significantly reverse the reduced cell viability and
augmented cell injury, via the inhibition of autophagy (25). Jung et al (33) revealed that treatment with
H2 Sattenuated unilateral ureteral obstruction-induced
oxidative stress and kidney fibrosis, and activated TGF-β1 and
NF-κB. The antifibrotic mechanisms of H2S may involve
anti-oxidative stress and autophagy-suppressing roles, together
with blockade of TGF-β1 and NF-κB signaling. In the present study,
H2S was observed to alleviate renal fibrogenesis and
autophagy activation in diabetic kidney tissue, and this possibly
occurred via regulation of SOD, AKT, TGF-β1 and NF-κB
signaling.
The results of the present study demonstrated that
H2S may improve renal tissue fibrosis of STZ-induced
diabetic rats, and may reverse the dysregulation of MMPs/TIMPs and
the activation of autophagy. Furthermore, H2S
intervention may inhibit oxidative stress and activation of the
AKT, TGF-β1 and NF-κB pathways, indicating that these signaling
pathways may be involved in the pathogenesis of renal tissue
fibrosis of diabetic rats, and may be associated with the
regulation of autophagy.
In conclusion, the present study demonstrated that
autophagic suppression may represent a protective mechanism of
H2S in the development of DN, by attenuating the
imbalance of MMPs/TIMPs and the excessive deposition of collagen.
H2S may alleviate renal tissue fibrosis and activation
of autophagy in STZ-induced diabetes, and this may occur via
anti-oxidative stress mechanisms, together with blockade of AKT,
TGF-β1 and NF-κB signaling. Further potential mechanisms of
autophagic suppression by H2S remain poorly defined, and
should be explored in the future.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant no. 81202830).
Glossary
Abbreviations
Abbreviations:
|
DN
|
diabetic nephropathy
|
|
H2S
|
hydrogen sulfide
|
|
NaHS
|
sodium hydrosulfide
|
|
ECM
|
extracellular matrix
|
|
MMP
|
matrix metalloproteinases
|
|
TIMP
|
tissue inhibitor of
metalloproteinases
|
|
CBS
|
cystathionine β synthase
|
|
SOD
|
superoxide dismutase
|
|
AKT
|
AKT serine/threonine kinase
|
|
TGF-β1
|
transforming growth factor-β1
|
|
NF-κB
|
nuclear factor-κB
|
References
|
1
|
Bentata Y, Haddiya I, Latrech H, Serraj K
and Abouqal R: Progression of diabetic nephropathy, risk of
end-stage renal disease and mortality in patients with type-1
diabetes. Saudi J Kidney Dis Transpl. 24:392–402. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu Y: Cellular and molecular mechanisms
of renal fibrosis. Nat Rev Nephrol. 7:684–696. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ding Y and Choi ME: Autophagy in diabetic
nephropathy. J Endocrinol. 224:R15–R30. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Choi AM, Ryter SW and Levine B:
Autophagyin human health and disease. N Engl J Med. 368:651–662.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Velentzas PD, Velentzas AD, Mpakou VE,
Antonelou MH, Margaritis LH, Papassideri IS and Stravopodis DJ:
Detrimental effects of proteasome inhibition activity in Drosophila
melanogaster: Implication of ER stress, autophagy, and apoptosis.
Cell Biol Toxicol. 29:13–37. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim WY, Nam SA, Song HC, Ko JS, Park SH,
Kim HL, Choi EJ, Kim YS, Kim J and Kim YK: The role of autophagy in
unilateral ureteral obstruction rat model. Nephrology (Carlton).
17:148–159. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Otunctemur A, Ozbek E, Dursun M, Sahin S,
Besiroglu H, Ozsoy OD, Cekmen M, Somay A and Ozbay N: Protective
effect of hydrogen sulfide on gentamicin-induced renal injury. Ren
Fail. 36:925–931. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Han K, Zhou H and Pfeifer U: Inhibition
and restimulation by insulin of cellular autophagy in distal
tubular cells of the kidney in early diabetic rats. Kidney Blood
Press Res. 20:258–263. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li L, Zepeda-Orozco D, Black R and Lin F:
Autophagy is a component of epithelial cell fate in obstructive
uropathy. Am J Pathol. 176:1767–1778. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ding Y and Choi ME: Regulation of
autophagy by TGF-β: Emerging role in kidney fibrosis. Semin
Nephrol. 34:62–71. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Forbes MS, Thornhill BA, Minor JJ, Gordon
KA, Galarreta CI and Chevalier RL: Fight-or-flight: Murine
unilateral ureteral obstruction causes extensive proximal tubular
degeneration, collecting duct dilatation, and minimal fibrosis. Am
J Physiol Renal Physiol. 303:F120–F129. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee HB, Yu MR, Yang Y, Jiang Z and Ha H:
Reactive oxygen species-regulated signaling pathways in diabetic
nephropathy. J Am Soc Nephro. 14 8 Suppl 3:S241–S245. 2003.
View Article : Google Scholar
|
|
13
|
Cuyàs E, Corominas-Faja B, Joven J and
Menendez JA: Cell cycle regulation by the nutrient-sensing
mammalian target of rapamycin (mTOR) pathway. Methods Mol Biol.
1170:113–144. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Heras-Sandoval D, Pérez-Rojas JM,
Hernández-Damián J and Pedraza-Chaverri J: The role of
PI3K/AKT/mTOR pathway in the modulation of autophagy and the
clearance of protein aggregates in neurodegeneration. Cell Signal.
26:2694–2701. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang C, Cao Y, Zhang Y, Li L, Xu M, Long
Y, Rong R and Zhu T: Cyclic helix B peptide inhibits ischemia
reperfusion-induced renal fibrosis via the PI3K/Akt/FoxO3a pathway.
J Transl Med. 13:3552015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhou LN, Wang N, Dong Y, Zhang Y, Zou H,
Li Q, Shi Y, Chen L, Zhou W, Han C and Wang Y: Increased Expression
of p-Akt correlates with Chronic Allograft Nephropathy in Rat
Kidney Model. Cell Biochem Biophys. 71:1685–1693. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hu N, Kong LS, Chen H, Li WD, Qian AM,
Wang XY, Du XL, Li CL, Yu XB and Li XQ: Autophagy protein 5
enhances the function of rat EPCs and promotes EPCs homing and
thrombus recanalization via activating AKT. Thromb Res.
136:642–651. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sharma K and Ziyadeh FN: Hyperglycemia and
diabetic kidney disease. The case for transforming growth
factor-beta as a key mediator. Diabetes. 44:1139–1146. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fornoni A, Ijaz A, Tejada T and Lenz O:
Role of inflammation in diabetic nephropathy. Curr Diabetes Rev.
4:10–17. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Koesters R, Kaissling B, LeHir M, Picard
N, Theilig F, Gebhardt R, Glick AB, Hähnel B, Hosser H, Gröne HJ
and Kriz W: Tubular overexpression of transforming growth
factor-beta1 induces autophagy and fibrosis but not mesenchymal
transition of renal epithelial cells. Am J Pathol. 177:632–643.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ding Y, Kim JK, Kim SI, Na HJ, Jun SY, Lee
SJ and Choi ME: TGF-{beta}1 protects against mesangial cell
apoptosis via induction of autophagy. J Biol Chem. 285:37909–37919.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Haar L, Ren X, Liu Y, Koch SE, Goines J,
Tranter M, Engevik MA, Nieman M, Rubinstein J and Jones WK: Acute
consumption of a high-fat diet prior to ischemia-reperfusion
results in cardioprotection through NF-κB-dependent regulation of
autophagic pathways. Am J Physiol Heart Circ Physiol.
307:H1705–H1713. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhou X, Feng Y, Zhan Z and Chen J:
Hydrogen sulfide alleviates diabetic nephropathy in a
streptozotocin-induced diabetic rat model. J Biol Chem.
289:28827–28834. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Aminzadeh MA and Vaziri ND: Downregulation
of the renal and hepatic hydrogen sulfide (H2S)-producing enzymes
and capacity in chronic kidney disease. Nephrol Dial Transplant.
27:498–504. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jiang H, Xiao J, Kang B, Zhu X, Xin N and
Wang Z: PI3K/SGK1/GSK3β signaling pathway is involved in inhibition
of autophagy in neonatal rat cardiomyocytes exposed to
hypoxia/reoxygenation by hydrogen sulfide. Exp Cell Res.
345:134–140. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhao DA, Liu J, Huang Q and Han ZM: Change
in plasma H2S level and therapeutic effect of H2S supplementation
in tubulointerstitial fibrosis among rats with unilateral ureteral
obstruction. Zhongguo Dang Dai Er Ke Za Zhi. 15:903–908.
2013.PubMed/NCBI
|
|
27
|
Cheng P, Wang F, Chen K, Shen M, Dai W, Xu
L, Zhang Y, Wang C, Li J, Yang J, et al: Hydrogen sulfide
ameliorates ischemia/reperfusion-induced hepatitis by inhibiting
apoptosis and autophagy pathways. Mediators Inflamm.
2014:9352512014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kimura H: Physiological role of hydrogen
sulfide and polysulfide in the central nervous system. Neurochem
Int. 63:492–497. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Szabó C: Hydrogen sulphide and its
therapeutic potential. Nat Rev Drug Discov. 6:917–935. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kundu S, Pushpakumar SB, Tyagi A, Coley D
and Sen U: Hydrogen sulfide deficiency and diabetic renal
remodeling: Role of matrix metalloproteinase-9. Am J Physiol
Endocrinol Metab. 304:E1365–E1378. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Santibañez JF, Quintanilla M and Bernabeu
C: TGF-β/TGF-β receptor system and its role in physiological and
pathological conditions. Clin Sci (Lond). 121:233–251. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yoon JJ, Lee YJ, Lee SM, Kang DG and Lee
HS: Oryeongsan suppressed high glucose-induced mesangial fibrosis.
BMC Complement Altern Med. 15:302015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jung KJ, Jang HS, Kim JI, Han SJ, Park JW
and Park KM: Involvement of hydrogen sulfide and homocysteine
transsulfuration pathway in the progression of kidney fibrosis
after ureteral obstruction. Biochim Biophys Acta. 1832:1989–1997.
2013. View Article : Google Scholar : PubMed/NCBI
|