Introduction
Although the incidence of pheochromocytoma is only
two cases per million of the global population (1), it leads to hypertension and
life-threatening cardiovascular complications (2). Pheochromocytoma, located in the
adrenal medulla, is a chromaffin cell neoplasm that secretes
catecholamines and various hormones, including norepinephrine,
epinephrine, dopamine, and chromogranin A. These vasoactive
hormones are responsible for the classical triad of symptoms in
pheochromocytoma: Episodic headache, sweating, and palpitations
(3). Due to the
catecholamine-secreting nature of pheochromocytomas, the diagnostic
biochemical tests for these tumors involve detection of these
hormones. However, no effective treatment exists for this tumor.
With drug therapy having no significant long-lasting benefit,
operative treatment is the only definitive cure (4,5).
Therefore, it is important to explore the mechanism underlying
pheochromocytoma pathogenesis and to investigate novel and improved
treatment methods for pheochromocytomas.
The Notch1 signaling pathway is a highly-conserved
pathway that serves important roles in cell fate specification,
differentiation, proliferation and survival (6–8).
Notch1 is a transmembrane receptor protein that is activated by
binding to the delta-like protein 1 ligand, which results in a
double proteolytic cleavage of the Notch1 protein (9). The first proteolytic cleavage is
mediated by a metalloprotease in the Notch extracellular domain,
followed by the second cleavage by the γ-secretase complex in the
transmembrane domain. Notch1 intracellular domain (NICD) then
translocates from the cytoplasm to the nucleus and binds with the
recombination signal binding protein for immunoglobulin κ J region
and other DNA binding complexes to regulate expression of genes,
including hes family bHLH transcription factor 1 (HES1), cyclin D
and hes related family bHLH transcription factor with YRPW motif 1
(10,11). Pheochromocytoma cells do not
express active Notch1, but Notch1 activators, valproic acid and
suberoyl bis-hydroxamic acid, have been reported to inhibit growth
and limit hormonal secretion by pheochromocytoma cells (12). However, the function of NICD in
pheochromocytoma cells remains unclear.
In order to investigate the role of NICD in
pheochromocytoma, a tetracycline-inducible system for NICD
overexpression in the PC12 pheochromocytoma cell line was employed.
The present study tested the hypothesis that overexpression of NICD
in PC12 cells may influence tumor cell growth.
Materials and methods
Cell culture
Rat PC12 cells were cultured in a humidified
atmosphere of 5% CO2 at 37°C in high-glucose Dulbecco's
modified Eagle's medium (DMEM; Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany), supplemented with 10% fetal calf serum
(HyClone; GE Healthcare Chicago, IL, USA), 100 U/ml penicillin and
100 mg/ml streptomycin. Cells were subcultured every 2–3 days. The
control and PC12-NICD cell lines were constructed by stable
transfection of the tet-on-EGFP and tet-on-EGFP-NICD plasmids
respectively, as described previously (13).
Cell morphology observation
PC12 cells were treated with 500 µg/ml doxycyclin
(Dox) for 36 h at room temperature and cell nuclei were stained
with DAPI at 0 and 36 h. The cells were then observed using an
inverted fluorescence microscope (IMT-2; Olympus Corporation,
Tokyo, Japan) and cell images were captured using a charge-coupled
device camera attached to the microscope.
Flow cytometry
Cells were cultured and treated with Dox for 36 h.
Cells were then trypsinized, washed twice with PBS, and incubated
with phycoerythrin (PE)-conjugated monoclonal anti-Notch1 antibody
(cat. no. 559763; 1:50; BD Biosciences, Franklin Lakes, NJ, USA),
which can also recognize NICD, for 30 min at 4°C in the dark. The
cells were washed twice with PBS, fixed with 4% formaldehyde in PBS
and centrifuged. Cells were analyzed using a FACSCanto II (BD
Biosciences). A 488 nm wavelength laser was used to excite enhanced
green fluorescent protein (EGFP) and PE and fluorescence signal was
acquired on the FL1 and FL2 spectral detection channels,
respectively. Results were analyzed with BD FACS Data-Interpolating
Vibrational Analysis software version 5.0 (BD Biosciences).
Apoptotic cells detection
Apoptosis was detected by Annexin V-Phycoerythrin
(PE) and 7-Amino-Actinomycin (7-AAD) staining followed by flow
cytometric analysis. The staining was preformed using an Annexin
V-PE Apoptosis Detection kit (cat. no. 559763; BD Biosciences),
following the manufacturer's protocol. The cells were resuspended
in 400 µl 1X binding buffer at a concentration of 1×106
cells/ml, and then incubated with 5 µl Annexin V-PE and 5 µl 7-AAD
for 15 min at room temperature in the dark. Finally, the cells were
analyzed by flow cytometry.
Cell cycle assay
Cell cycle phase distribution was assessed in order
to evaluate cell proliferation in PC12 cells. Cells
(1×106) were collected and washed with ice-cold PBS,
then fixed in ice-cold 70% ethanol at −20°C for 24 h. The fixed
cells were centrifuged at 1,000 × g for 5 min, washed twice with
PBS, resuspended in PBS and incubated with 500 µl PI (cat. no.
550825; BD Biosciences) at 4°C for 30 min. Finally, the cells were
analyzed by flow cytometry.
Western blotting
Following washing with cold PBS three times, cells
were lysed in radioimmunoprecipitation assay buffer (150 mM NaCl,
10 mM Tris-HCl pH 7.4, 0.5% Triton X-100 and protease inhibitors;
Merck KGaA), homogenized on ice, and centrifuged at 12,000 × g at
4°C for 15 min. The supernatant was collected and stored at −80°C
until use. Protein concentration was determined using a
bicinchoninic acid assay kit (Pierce; Thermo Fisher Scientific,
Inc., Waltham, MA, USA). Total proteins (25 µg) were separated on
12% tris-polyacrylamide gel. The proteins were then transferred to
nitrocellulose membranes (EMD Millipore, Billerica, MA, USA). The
membranes were blocked in 5% non-fat dry milk for 1 h at 37°C,
washed in TBS with 0.05% Tween-20 (TBST) and incubated overnight
with mouse primary antibodies targeting microtubule associated
protein 1 light chain 3 B (LC3B), Beclin1, autophagy-related (ATG)5
and ATG7 (cat. nos. 3868, 3495, 12994 and 8558; 1:1,000; Cell
Signaling Technology, Inc., Danvers, MA, USA;) at 4°C. Mouse
anti-β-actin (cat. no. sc-47778, 1:1,000; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA.) was used as an internal control. The
membranes were washed thrice in TBST and incubated for 1 h with
secondary antibodies conjugated to horseradish peroxidase (HRP)
(cat. no. SC-2004; 1:2,000; Santa Cruz Biotechnology, Inc.) at
37°C, washed thrice in TBST, and treated with Immun-Star HRP
peroxide buffer and Luminol/Enhancer (Beijing Zhongshan Golden
Bridge Biotechnology Co., Ltd., Beijing, China) for
chemiluminescent detection of protein bands. The computer
gray-scale value was analyzed using Quantity One software version
4.6.2 (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
Data were analyzed using SPSS 16.0 software (SPSS,
Inc., Chicago, IL, USA) and were presented as the mean ± standard
deviation. Significance analysis was performed using Student's
t-test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Dox treatment induces NICD expression
in PC12 cells
A tetracycline (tet)-inducible system was used to
drive overexpression of NICD in PC12 cells. PC12 cells were
transfected with either a tet-on-EGFP plasmid (control) or a
NICD-expressing tet-on-EGFP-NICD plasmid (NICD), and protein
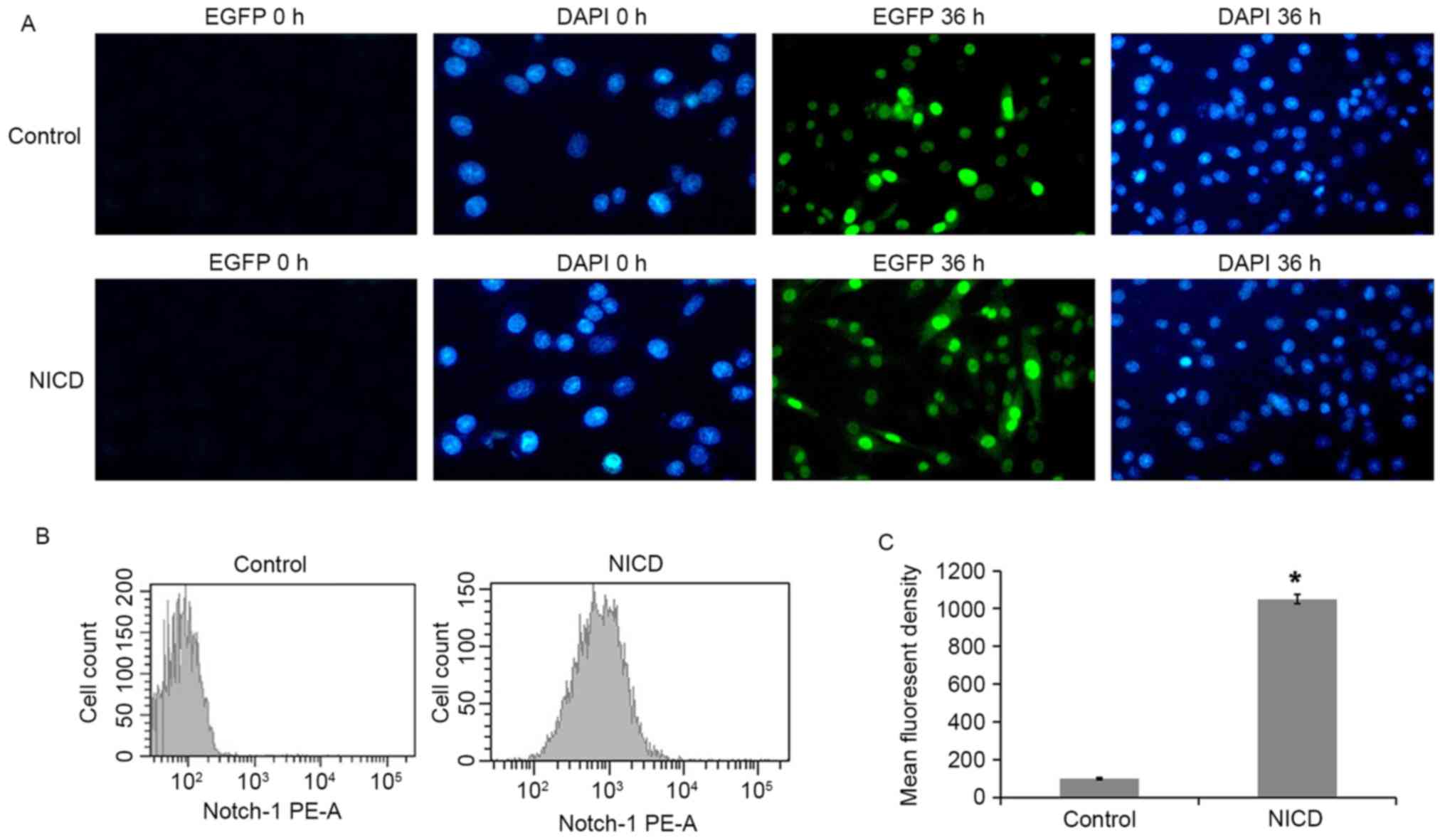
expression was then induced with Dox. EGFP fluorescence was
assessed in the Dox-induced PC12 cells at 36 h and it was observed
that 90.4% of the total cells were EGFP-positive in the NICD group
(Fig. 1A). Next, NICD expression
was examined by staining with a PE-conjugated specific antibody and
flow cytometry analysis. PE fluorescence was significantly enhanced
following induction with Dox for 36 h (Fig. 1B and C). These results establish
that NICD was overexpressed in the PC12-NICD cells at 36 h
post-induction with Dox.
Overexpression of NICD increases PC12
cell apoptosis
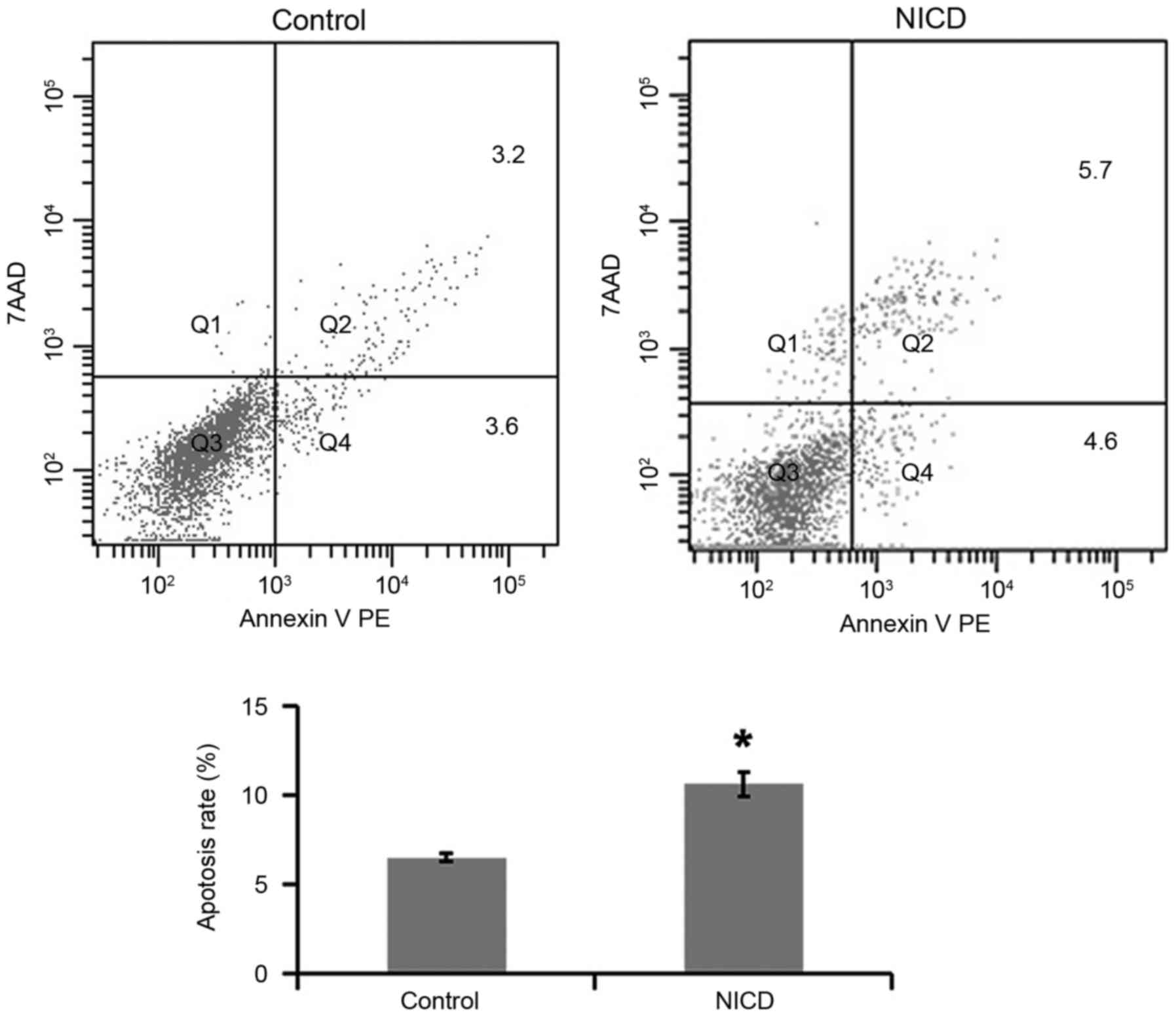
To investigate the effect of NICD overexpression on
apoptosis, PC12 cells were induced with Dox for 36 h and then
double-stained with Annexin V and PI prior to flow cytometry
analysis. PC12 cells in which NICD was overexpressed exhibited an
increased apoptosis rate compared with control cells (Fig. 2).
Overexpression of NICD inhibits PC12
cell proliferation
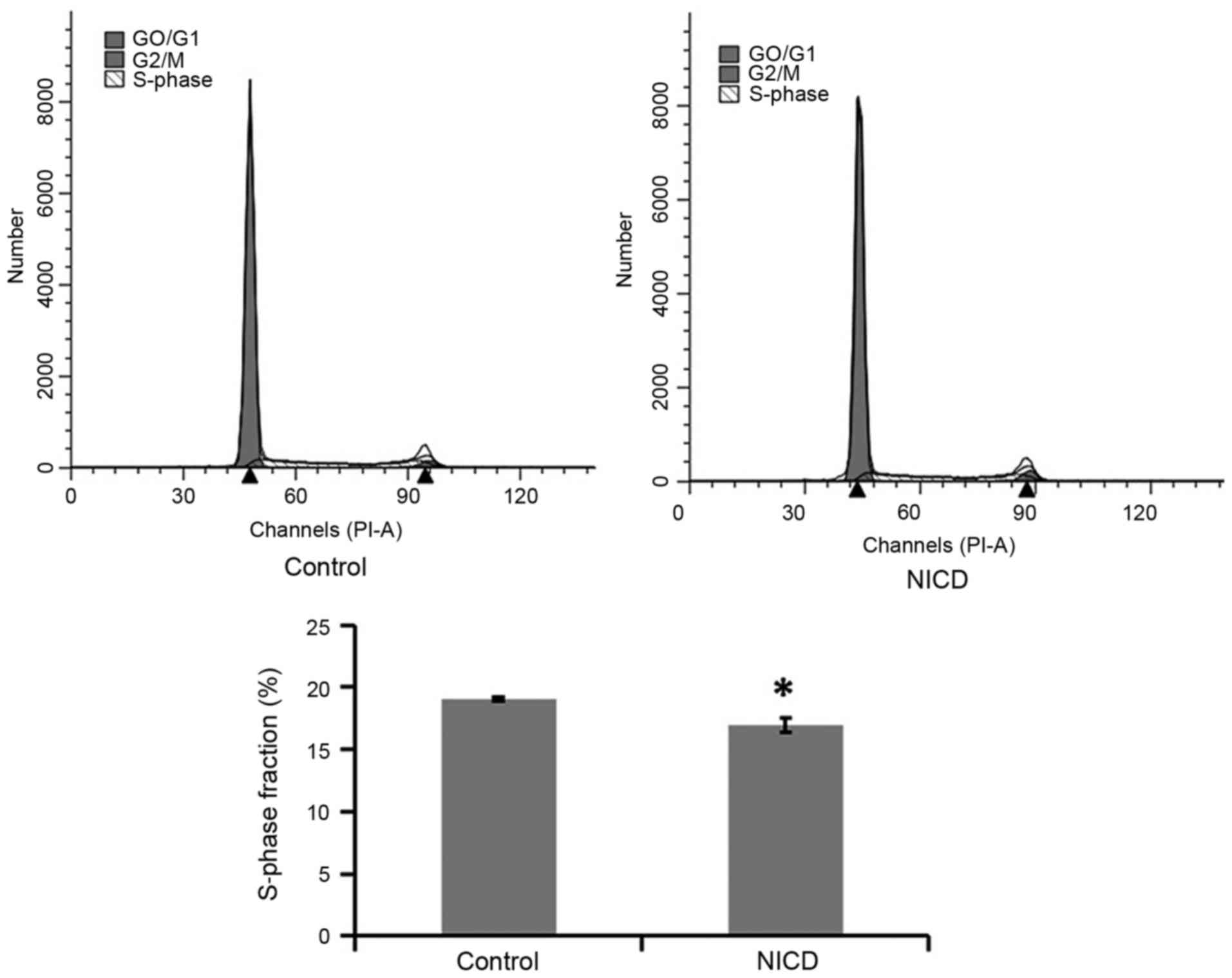
To investigate the effect of NICD overexpression on
PC12 cell proliferation, cell cycle analysis was performed. PC12
cells were induced with Dox for 36 h, stained with PI and then
analyzed by flow cytometry for cell cycle phase distribution. The
results indicated that the % of cells in the S-phase of the cell
cycle was suppressed in the NICD group compared with the control
group, which suggested that NICD overexpression significantly
inhibited the growth of PC12 cells via regulating S-phase cell
cycle arrest (Fig. 3).
NICD overexpression influences
autophagy-related protein expression
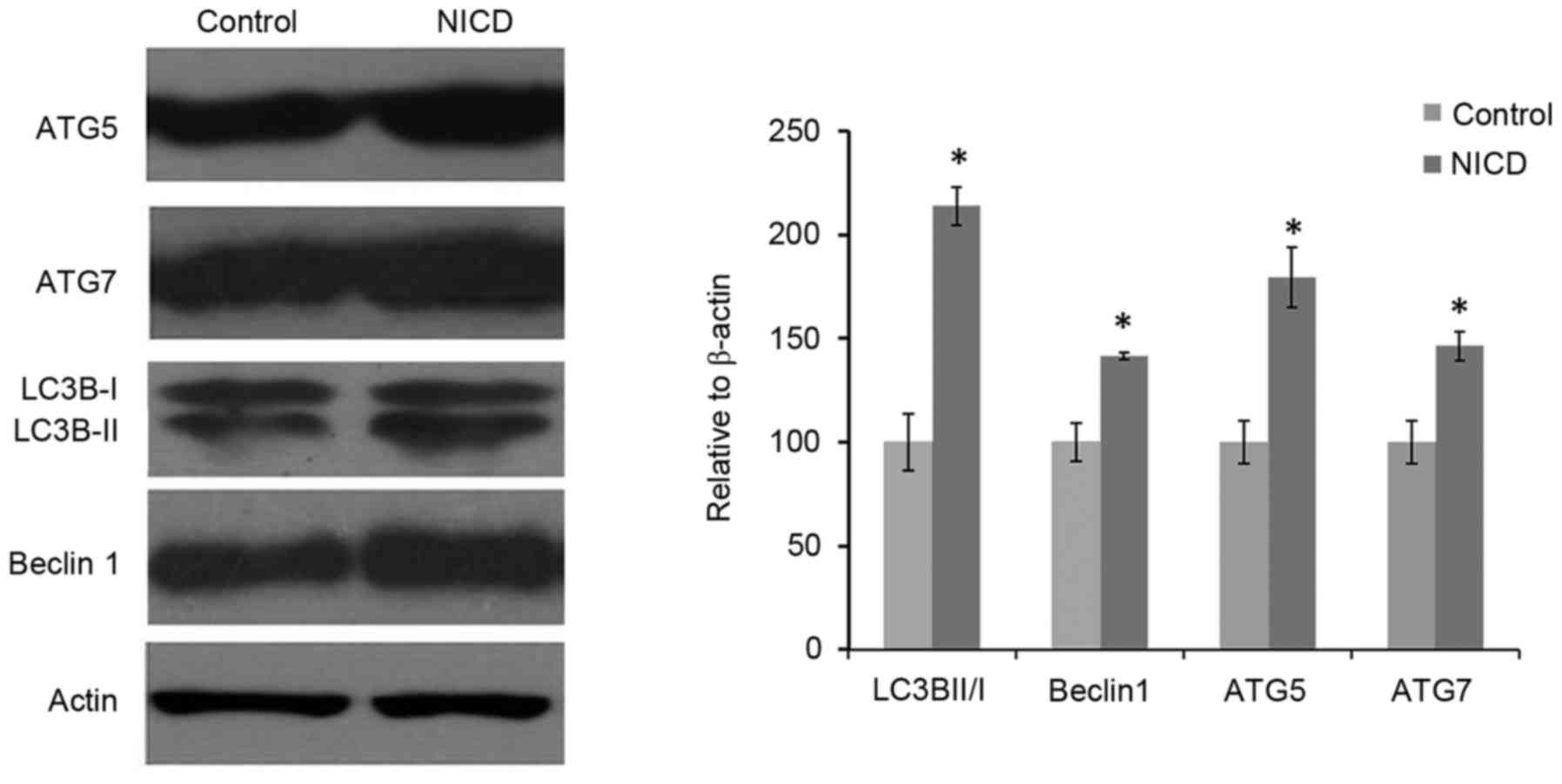
To evaluate the effect of NICD on autophagy, the
protein expression levels of autophagy-related proteins ATG5, ATG7,
LC3B and Beclin1 were analyzed by western blotting in cells that
were induced with Dox for 36 h. The results demonstrated that
Dox-induced NICD expression significantly increased the level of
LC3II/I ratio, and the expression levels of ATG5, ATG7 and Beclin1
compared with the group (Fig. 4).
These results suggested that NICD may be involved in regulating
PC12 cell growth in part through autophagy-dependent pathways.
Discussion
Notch signaling served an important role in cell
fate specification, differentiation, proliferation, and survival
(14). Previous studies have
demonstrated that Notch signaling is significant in neurogenesis
(15). Increased expression of
active Notch1 protein inhibits cell growth and hormone secretion in
carcinoid norepinephrine tumors and medullary thyroid cancer cells
(16,17), and Notch1 activator/histone
deacetylase inhibitor compounds lead to a decrease in proliferation
in PC12 cells (12). In another
study, treatment with the Notch1 signaling pathway inhibitor
N-[N-(3,5-difluorophenacetyl)-L-alanyl]-S-phenylg lycinet-butyl
ester and with the amyloid-β peptide 25–35, resulted in prolonged
survival and decreased expression of caspase 3, 8 and 9 in PC12
cells (18). However, the function
of NICD in pheochromocytoma remains unknown.
In order to explore the role of NICD in
pheochromocytoma, a tet-inducible system for NICD expression in the
PC12 cell line was used. Through transfection and drug selection,
PC12 cells expressing tet-inducible NICD (PC12-NICD cells) were
obtained, in which NICD expression is under a tight regulation by
Dox (13). At 36 h post-Dox
induction, NICD protein expression levels were significantly
enhanced in the PC12-NICD cells compared with the control cells.
The results demonstrated that NICD overexpression suppressed cell
proliferation and increased the rate of apoptosis in PC12
cells.
Autophagy is an intracellular degradation system
that delivers proteins and organelles to the lysosome and provides
cells with nutrients by recycling the degradation products
(19–21). Mutants of the Notch gene,
glp-1, lead to inhibition of germline proliferation and to
increase in autophagy levels in the nematode Caenorhabditis
elegans (22,23). Another study on T-cell leukemia
reported that activation of the Notch target gene HES1 regulates
the expression of phosphatase and tensin homolog (24). Inhibition of Notch signaling
increases autophagy activity. Autophagy has been reported to occur
downstream of the Notch pathway receptor activation during
Drosophila melanogaster zygote development, and a decrease
in autophagy resulted in precocious activation of Notch signaling
in ovarian follicle cells (25).
These studies suggest a link between Notch signaling and autophagy,
that remains to be elucidated. In the present study, expression
levels of autophagy-related proteins LC3B, ATG5, ATG7 and Beclin 1
were significantly increased in PC12 cells following overexpression
of NICD, which implied that NICD, a fragment of the Notch1 protein,
is sufficient to induce autophagy in PC12 cells. Whether
NICD-mediated autophagy contributes to suppressed cell
proliferation and increased apoptosis needs to be further explored
in the future. Recently, Wu et al (26) reported that developmental retention
of early-stage cells and the differentiation of stem cells is
delayed in the Atg16L1 mutation mouse model, which suggests that
autophagy regulates Notch degradation and modulates stem cell
development and neurogenesis.
In summary, the present study indicated that
overexpression of NICD suppressed cell proliferation, promoted cell
apoptosis, and activated increased autophagy-realted protein
expression in PC12 cells. The present results suggest that NICD may
be a promising target towards developing novel and effective
treatment strategies against pheochromocytoma.
Acknowledgements
The present study was funded by the National Natural
Science Foundation of China (grant nos. 31100790 and 81171250).
References
|
1
|
Fung MM, Viveros OH and O'Connor DT:
Diseases of the adrenal medulla. Acta Physiol (Oxf). 192:325–335.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Eisenhofer G: Screening for
pheochromocytomas and paragangliomas. Curr Hypertens Rep.
14:130–137. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lenders JW, Duh QY, Eisenhofer G,
Gimenez-Roqueplo AP, Grebe SK, Murad MH, Naruse M, Pacak K and
Young WF Jr: Endocrine Society: Pheochromocytoma and paraganglioma:
An endocrine society clinical practice guideline. J Clin Endocrinol
Metab. 99:1915–1942. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Adler JT, Mack E and Chen H: Isolated
adrenal mass in patients with a history of cancer: Remember
pheochromocytoma. Ann Surg Oncol. 14:2358–2362. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Adler JT, Meyer-Rochow GY, Chen H, Benn
DE, Robinson BG, Sippel RS and Sidhu SB: Pheochromocytoma: Current
approaches and future directions. Oncologist. 13:779–793. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Qi S, Zhao X, Li M, Zhang X, Lu Z, Yang C,
Zhang C, Zhang H and Zhang N: Aberrant expression of
Notch1/numb/snail signaling, an epithelial mesenchymal transition
related pathway, in adenomyosis. Reprod Biol Endocrinol. 13:962015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kangsamaksin T, Tattersall IW and
Kitajewski J: Notch functions in developmental and tumour
angiogenesis by diverse mechanisms. Biochem Soc Trans.
42:1563–1568. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Capaccione KM and Pine SR: The Notch
signaling pathway as a mediator of tumor survival. Carcinogenesis.
34:1420–1430. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Penton AL, Leonard LD and Spinner NB:
Notch signaling in human development and disease. Semin Cell Dev
Biol. 23:450–457. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guruharsha KG, Kankel MW and
Artavanis-Tsakonas S: The Notch signalling system: Recent insights
into the complexity of a conserved pathway. Nat Rev Genet.
13:654–666. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hori K, Sen A and Artavanis-Tsakonas S:
Notch signaling at a glance. J Cell Sci. 126:2135–2140. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Adler JT, Hottinger DG, Kunnimalaiyaan M
and Chen H: Histone deacetylase inhibitors upregulate Notch-1 and
inhibit growth in pheochromocytoma cells. Surgery. 144:956–962.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu YM, Duan P, Huang CT, Li B, Han XF, Xu
Y, Yan WH and Xing Y: Construction of inducible lentiviral vector
containing human Notch1 and EGFP gene and its expression in PC12
cells. Zhongguo Ying Yong Sheng Li Xue Za Zhi. 29:232–237. 2013.(In
Chinese). PubMed/NCBI
|
|
14
|
Louvi A and Artavanis-Tsakonas S: Notch
and disease: A growing field. Semin Cell Dev Biol. 23:473–480.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kong L, Hu Y, Yao Y, Jiao Y, Li S and Yang
J: The coumarin derivative osthole stimulates adult neural stem
cells, promotes neurogenesis in the hippocampus, and ameliorates
cognitive impairment in APP/PS1 transgenic mice. Biol Pharm Bull.
38:1290–1301. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kunnimalaiyaan M, Vaccaro AM, Ndiaye MA
and Chen H: Overexpression of the NOTCH1 intracellular domain
inhibits cell proliferation and alters the neuroendocrine phenotype
of medullary thyroid cancer cells. J Biol Chem. 281:39819–39830.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nakakura EK, Sriuranpong VR,
Kunnimalaiyaan M, Hsiao EC, Schuebel KE, Borges MW, Jin N, Collins
BJ, Nelkin BD, Chen H and Ball DW: Regulation of neuroendocrine
differentiation in gastrointestinal carcinoid tumor cells by notch
signaling. J Clin Endocrinol Metab. 90:4350–4356. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liang H, Zhang Y, Shi X, Wei T and Lou J:
Role of Notch-1 signaling pathway in PC12 cell apoptosis induced by
amyloid beta-peptide (25–35). Neural Regen Res. 9:1297–1302. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Levine B and Klionsky DJ: Development by
self-digestion: Molecular mechanisms and biological functions of
autophagy. Dev Cell. 6:463–477. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mizushima N, Levine B, Cuervo AM and
Klionsky DJ: Autophagy fights disease through cellular
self-digestion. Nature. 451:1069–1075. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Thumm M and Kadowaki T: The loss of
Drosophila APG4/AUT2 function modifies the phenotypes of cut
and Notch signaling pathway mutants. Mol Genet Genomics.
266:657–663. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lapierre LR, Gelino S, Melendez A and
Hansen M: Autophagy and lipid metabolism coordinately modulate life
span in germline-less C. elegans. Curr Biol. 21:1507–1514.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang MC, O'Rourke EJ and Ruvkun G: Fat
metabolism links germline stem cells and longevity in C.
elegans. Science. 322:957–960. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Palomero T, Sulis ML, Cortina M, Real PJ,
Barnes K, Ciofani M, Caparros E, Buteau J, Brown K, Perkins SL, et
al: Mutational loss of PTEN induces resistance to NOTCH1 inhibition
in T-cell leukemia. Nat Med. 13:1203–1210. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Barth JM, Hafen E and Köhler K: The lack
of autophagy triggers precocious activation of Notch signaling
during Drosophila oogenesis. BMC Dev Biol. 12:352012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wu X, Fleming A, Ricketts T, Pavel M,
Virgin H, Menzies FM and Rubinsztein DC: Autophagy regulates Notch
degradation and modulates stem cell development and neurogenesis.
Nat Commun. 7:105332016. View Article : Google Scholar : PubMed/NCBI
|