Introduction
Thrombin-activatable fibrinolysis inhibitor (TAFI)
is a plasma zymogen that functions as a molecular link between
coagulation and fibrinolysis. Numerous single nucleotide
polymorphisms have been identified in carboxypeptidase basic
(CPB2), the gene encoding TAFI, and are located in the 5′-flanking
region, in the coding sequences and in the 3′-untranslated region
of the CPB2 mRNA transcript (1).
It has been suggested that CBB2 serves an important role in the
interactions among procoagulant, anticoagulant and fibrinolytic
systems (2–4). Activated TAFI (TAFIa) attenuates
fibrinolysis by removing the carboxyl-terminal lysine residues from
partially degraded fibrin that mediate positive feedback in plasmin
generation (5). Pancreatic
carboxypeptidase B (CPB) is a stable protease exhibiting high
homology with TAFI but not to TAFIa (6).
Activation of TAFI by thrombin is increased
1,250-fold in the presence of the endothelial cell membrane protein
thrombomodulin (TM) (7), which is
expressed in tumors and is a prognostic factor in human cancer
(8,9). Several studies have demonstrated that
higher TAFI levels are associated with various types of cancer
(10–13), and plasma levels of TAFI are
significantly increased in breast and lung cancer, gastric
carcinoma and multiple myeloma compared with healthy individuals,
suggesting that TAFI may serve a role in the pathogenesis of
thrombotic disorders in cancer patients (14,15).
However, the role of TAFI in tumor development remains to be fully
elucidated. In the present study, the TAFI gene was knocked down by
small interfering (si)RNA transfection to suppress the expression
of TAFI, and the effects of the TAFI signal pathway on invasion and
migration were investigated in a breast cancer cell (BCC) line.
Materials and methods
Patients and samples
The present study was approved by the ethics
committee of The Second Hospital of Shandong University (Jinan,
China) and informed consent was obtained from the participants. All
eligible specimens were collected from patients with pathologically
and clinically confirmed breast cancer who underwent surgical
resection prior to any therapy from June 2013 to December 2016. All
samples were re-evaluated by pathologists to confirm the diagnosis
and to estimate the tumor cell content. Patients were aged from 25
to 65.
Cell culture
The human BCC lines MDA-MB-231 and MCF-7 were
purchased from the American Type Cell Culture Collection (Manassas,
VA, USA). The cells were cultured in Dulbecco's modified Eagle's
medium with 10% heat-inactivated newborn calf serum (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA), 5 mmol/l
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany), 100 U/ml penicillin, and 100 U/ml
streptomycin in 5% atmospheric CO2 at 37°C.
Immunohistochemistry
Immunohistochemistry was performed to analyse TAFI
expression in breast cancer (BC) tissues and adjacent normal
tissues. In brief, human breast tissues in healthy and cancer
patients were fixed in 10% formaldehyde, and then were embedded in
paraffin. The sections were then deparaffinized with xylene,
rehydrated and then treated with 3% hydrogen peroxide to quench the
endogenous peroxidase activity. Subsequent antigen retrieval was
performed by heating in citrate buffer solution (0.01 M) using a
microwave oven. Sections were cut at 5 µm and stained with a TAFI
antibody at 4°C overnight (catalog no. sc-67300; 1:100; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) following the manufacturer's
protocol. and then sections were further incubated with
3,3-diaminobenzidine tetrahydrochloride for 5 min at room
temperature. The sections were observed and captured with a Nikon
Eclipse 90i microscope (Nikon Corporation, Tokyo, Japan).
siRNA transfection
According to the principles of siRNA design, 3×19 bp
sequences in TAFI cDNA, (GenBank accession number NM-001872.3) were
identified as target sites using a web-based online software system
(Thermo Fisher Scientific, Inc.) for computing highly effective
siRNA (https://www.thermofisher.com/order/genome-database/details/sirna/104006?CID=&ICID=uc-sirna-TAFI).
The sequences were submitted to BLAST® (National Centre
for Biotechnology Information, Bethesda, MD, USA) to ensure that
only the selected gene was targeted. The target sequences for the
TAFI gene were: siRNA-1: 5′AGU UAU AUG GCC UAU GAA CC-3′ (sense
strand) and 5′-UGGUUCAUAGGCCAUAUAACU-3′ (antisense strand),
siRNA-2: 5′-UGAUUGUUCGCAUAGAAAGAA-3′ (sense strand) and
5′-UUCUUUCUAUGCGAACAAUCA-3′ (antisense strand) and siRNA-3:
5′-UAGGUAUAAGGUUUCUGAGCC-3′ (sense strand) and
5′-GGCUCAGAAACCUUAUACCUA-3′ (antisense strand). Negative control
siRNA: 5′-GCGUAACGCGGGAAUUUACUU-3′ (sense strand) and
5′-GUAAAUUCCCGCGUUACGCUU-3′ (antisense strand). siRNA 3 achieved
the greatest knockdown of TAFI, and therefore this was selected for
further experiments. Briefly, 2 µl siRNA was diluted with 100 µl
OPTi-MEM (Invitrogen; Thermo Fisher Scientific, Inc.) and 10 µl
Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.) was
diluted with 100 µl OPTi-MEM for 30 min at room temperature.
Dilutions were mixed together and incubated at room temperature for
20 min, and 200 µl of the transfection mixture was added to each
well. Experiments were repeated three times. Then, 6 h following
transfection, the medium was replaced by the common complete medium
again. Transiently transfected cells were harvested at 24 and 48 h
following transfection for different experiments.
Semi quantitative reverse
transcription-polymerase chain reaction (RT-PCR)
Total RNA was extracted from transfected cells after
24 h using TRIzol reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocols. The purity and
concentration were determined by measuring the absorbance at 260
and 280 nm (A260/A280). Reverse transcription was performed with 1
µg of total RNA and the RevertAid First Strand cDNA Synthesis kit
(Thermo Fisher Scientific, Inc.). PCR primers used were as follows:
CPB2, forward 5′-AACTGCTTCACTGGCTACTA-3′ and reverse
5′-TATGCTTACAAAAATCCACA-3′ GAPDH forward
5′-CCACCCATGGCAAATTCCATGGCA-3′ and reverse
5′-TCTAGACGGCAGGTCAGGTCCACC-3′. PCR was carried out in a 20 µl
reaction volume containing 50 ng of genomic DNA, 10 µmol of each
primer, 200 µmmol/l of each dNTP, 4 µl5 × PCR buffer and 2 u taq
DNA polymerase. Amplification was carried out with an initial
denaturation step at 94°C for 5 min, followed by 35 cycles of
denaturation at 94°C for 40 sec, extension at 72°C for 50 sec with
a final extension at 72°C for 10 min. A densitometric scanning
instrument (model GS-800; Bio-Rad Laboratories, Inc., Hercules, CA,
USA) was used to quantify the bands and the relative amount of CPB2
and TAFI gene mRNA expression was estimated relative to the GAPDH
mRNA detected in the same sample.
Western blot analysis
Cytoplasmic proteins of different groups were
extracted using cytoplasmic extraction reagents (Beyotime Institute
of Biotechnology, Haimen, China). Protein concentration in the
supernatants was determined by bicinchoninic acid assay. A total of
20 µl protein was separated by 10% SDS-PAGE and transferred onto
polyvinylidene fluoride membranes. The membranes were blocked in 5%
skimmed milk in TBST buffer at room temperature for 1 h with gentle
agitation and then incubated overnight at 4°C with mouse anti-human
TAFI immunoglobulin (Ig)G antibody (catalog no. sc-67867; 1:200;
Santa Cruz Biotechnology, Inc.) or rabbit anti-human GAPDH IgG
antibody (catalog no. sc-25778; 1:1,000; Santa Cruz Biotechnology,
Inc.). Following washing with TBST, the membranes were incubated
with horseradish peroxidase-conjugated anti-human IgG antibody
(catalog no. ab109489; 1:1,000; Abcam). The bound antibodies were
visualized using an enhanced chemiluminescence reagent (EMD
Millipore, Billerica, MA, USA) and quantified by densitometry using
ChemiDoc XRS+ image analyzer (Bio-Rad Laboratories, Inc.). The
expression levels of TAFI and GAPDH protein were quantified by
densitometry analysis (model GS-800, Bio-Rad Laboratories, Inc.).
The signal strength of each TAFI signal was normalized against the
corresponding GAPDH control.
Cell viability assay
Viable cells were quantified by a standard
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
reduction assay. The tetrazolium salt MTT (Sigma-Aldrich; Merck
KGaA) was used to compare the cellular metabolic capacities. Each
well of a 96-well plate was seeded with 1×104 cells and
preincubated in 5% atmospheric CO2 at 37°C. Then, 48 h
following transfection, the MTT assay was performed by adding 20 µl
of MTT (5 mg/ml; Sigma-Aldrich; Merck KGaA) for 4 h. The
supernatant was removed and 150 µl DMSO added to each well. Optical
density (OD) of the solution was measured at 490 nm. Triplicate
experiments with triplicate samples were performed.
Cell migration assay
Wound healing assays were performed to quantify the
rate of cell migration. A density of 5×105 cells/well were plated
onto 24 well plates and were grown for 24 h to >90% confluence.
The medium was removed and a wound line, that is a cell-free area,
was created on the cell monolayers by manually scraping the cells
with a plastic pipette tip. Debris was removed from the culture by
washing with PBS twice and then the cells were cultured in
RPMI-1640 (Sigma-Aldrich; Merck KGaA) containing 1% FBS. Wound
sizes were verified using ImageJ software version 1.43 (National
Institutes of Health, Bethesda, MD, USA). Cell migration=0 h wound
width (1 mm)-6 h wound width. Images (original magnification, ×40)
were obtained using a Nikon Eclipse 90i microscope (Nikon
Corporation; Tokyo, Japan). These experiments were repeated three
times.
Cell invasion assay
The cell invasion ability was evaluated using a
modified Boyden chamber (BD Biosciences, San Jose, CA, USA) with a
polycarbonate filter with 8 µm pores placed between the upper and
lower chambers coated with 70 µl Matrigel (1 mg/ml) as previously
described (16). Briefly,
1×105 cells in 0.1 ml of serum-free DMEM medium were
placed in the upper chamber and the lower chamber was filled with
DMEM medium containing 15% newborn calf serum. Following incubation
for 24 h at 37°C in a 5% CO2 incubator, the cells on the
top surface of the insert were removed by wiping with a cotton
swab. Cells that migrated to the bottom surface of the insert were
fixed in 100% methanol for 2 min, stained in 0.1% Crystal Violet
for 20 min, rinsed in PBS and then subjected to microscopic
inspection (original magnification, ×100). The magnitude of cells
migration was evaluated by counting the migrated cells in 10 random
high-power microscope fields. Migration activity was expressed as a
percentage of the control group.
Statistical analysis
Data are presented as the mean ± standard deviation.
Comparisons between treatments were analyzed by one-way analysis of
variation followed by S-N-K post hoc test using SPSS software
version 18.0 (SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
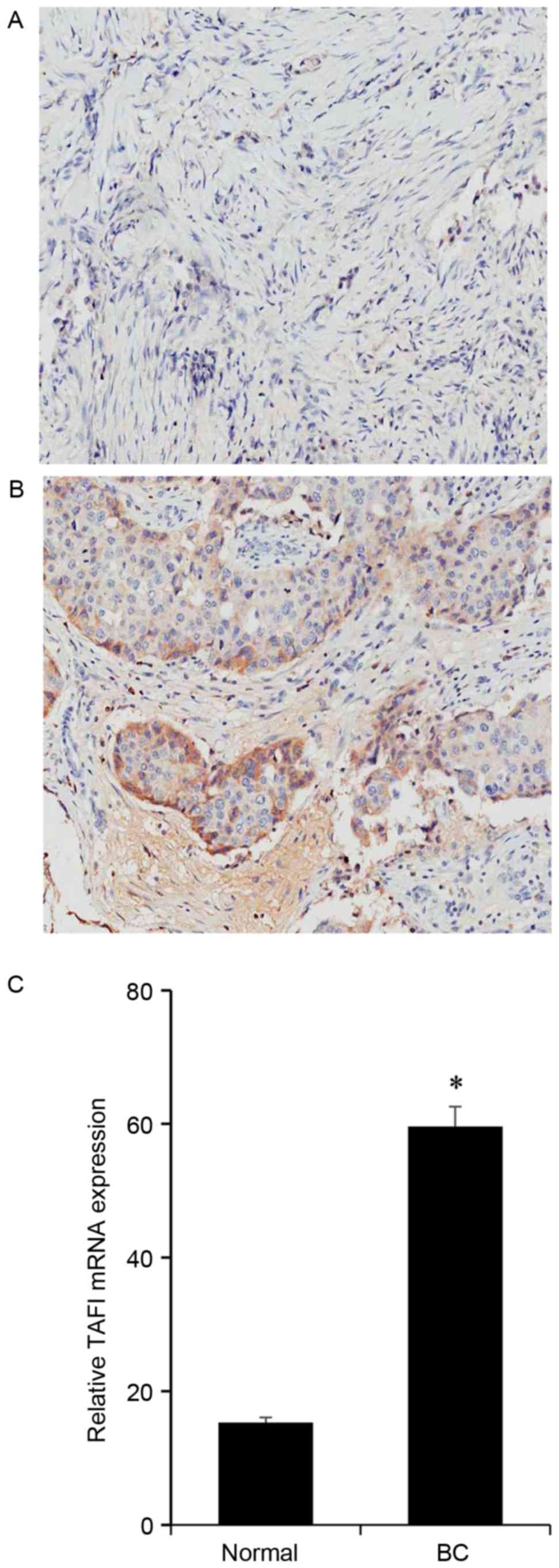
TAFI is expressed in BC tissues
BC samples, together with their adjacent normal
tissues, from 10 cases breast cancer patients were analyzed.
Immunohistochemistry demonstrated that in tumor-adjacent normal
tissues the expression of TAFI was relevantly low (Fig. 1A), while in BC tissues TAFIs were
visibly elevated (Fig. 1B). The
expression of TAFI mRNA was assessed using RT-PCR. TAFI was
expressed at higher levels in BC tissues than in matched normal
tissues (Fig. 1C).
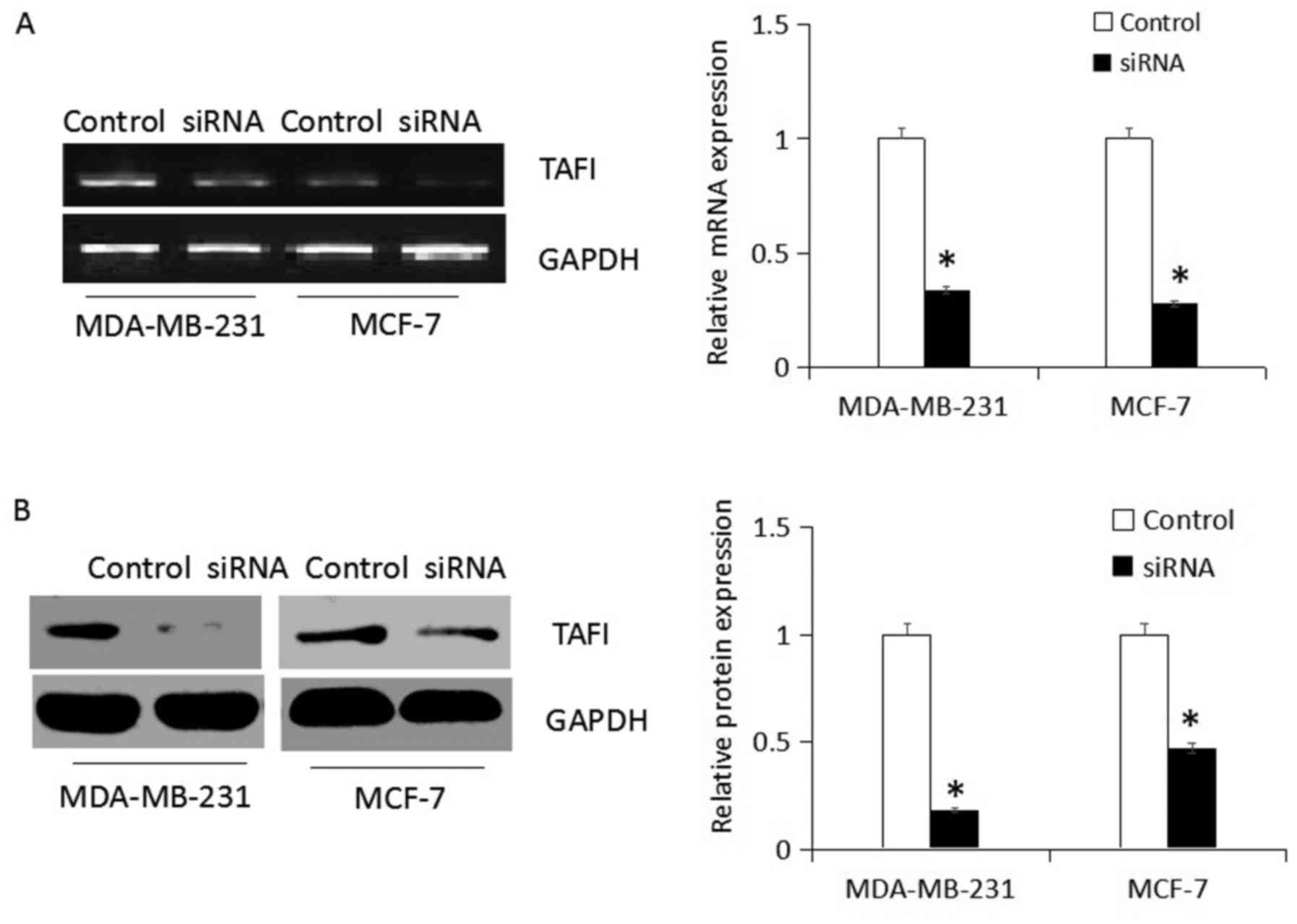
siRNA-mediates CPB2 knockdown
Specific siRNA targeting CPB2 was transfected in
MDA-MB-231 and MCF-7 cells for 48 h, then TAFI protein expression
levels were detected. In the present study, RT-PCR detection
demonstrated that siRNA blocked the expression of CPB2 gene
expression, and western blot analysis confirmed that CPB2 gene
silencing successfully suppressed expression of the corresponding
TAFI protein compared with non-transfected group (control;
P<0.05; Fig. 2A and B).
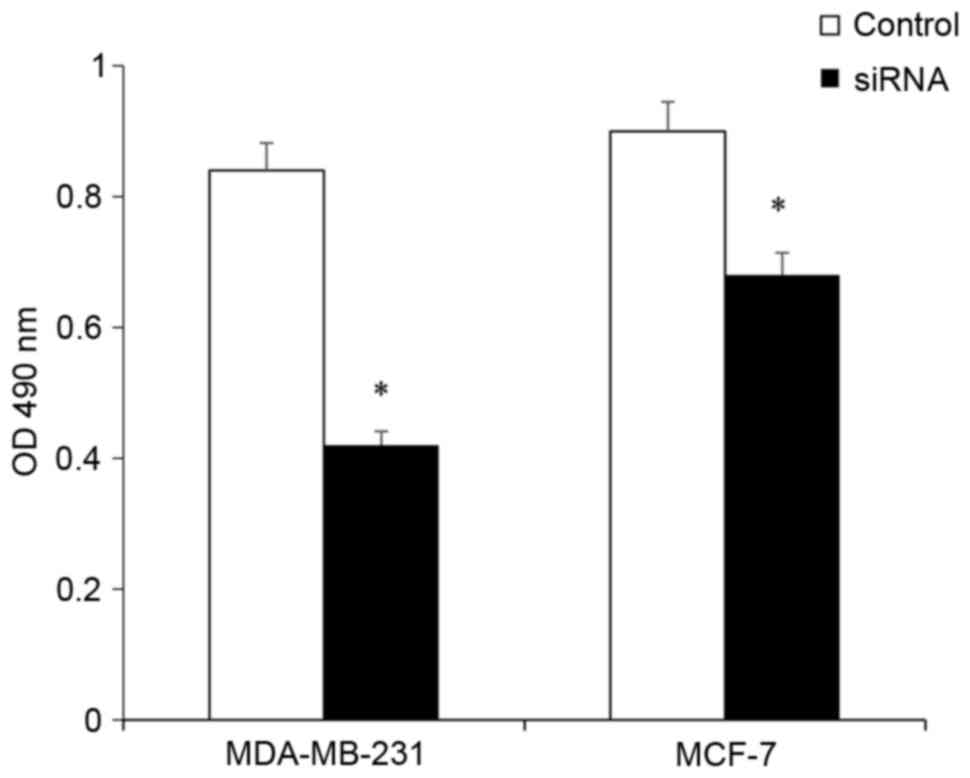
TAFI depletion reduces BCC
viability
The effect of TAFI depletion on cell viability was
evaluated using the MTT assay. Following treatment with siRNA for
48 h, the cell viability exhibited a significant decrease in
MDA-MB-231 and MCF-7 cells (P<0.05; Fig. 3). This indicated that TAFI has an
effect on the regulation of BCC viability.
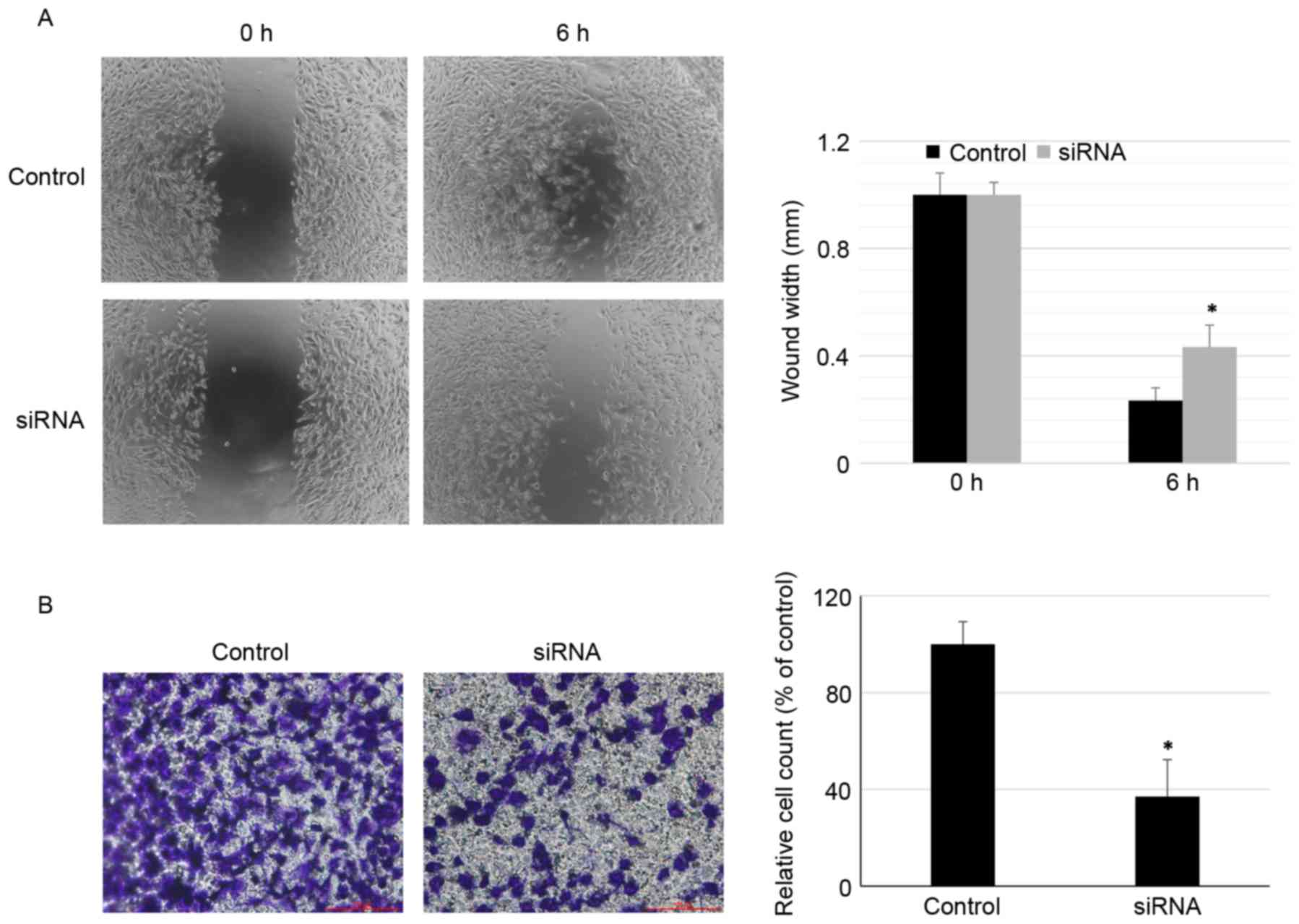
Effects of RNA interference TAFI on
cell migration and invasion
Next, the role of TAFI in migration and invasion of
BCCs was determined using wound scratch and Transwell chamber
assays, respectively. As demonstrated in Fig. 4A and B, transfection with TAFI
siRNA significantly inhibited migration and invasion in MDA-MB-231
cells compared with non-transfected group (control; P<0.05). The
present study revealed similar effects of TAFI inhibition on cell
migration and invasion in the less metastatic MCF-7 cells compared
with the more metastatic MDA-MB-231 cells (data not shown).
Together these results demonstrated that knockdown of TAFI markedly
inhibited BCC migration (at 6 h) and invasion (at 24 h).
Discussion
BC is one of the most frequent malignancies
worldwide. There is an upward trend in incidence; the prognosis of
patients with advanced BC is closely associated with metastasis
with few effective treatments (17). BC initiation and progression is a
complicated process which is associated with the loss of the normal
regulatory pathways between cell proliferation, differentiation and
apoptosis (18,19). In recent years, the incidence of BC
in China has been increasing, according to the national cancer
centre statistics (20), BC is the
predominant female malignant tumor (21). Tumor metastasis is the basic
characteristic of malignant tumors, and also the main difficulty in
curing them. Previous studies have demonstrated that an abnormal
increase in circulating coagulation protein conducive to
thrombophilia is associated with different types of cancer
(12,13). Coagulation-associated abnormality
in cancer patients is an increased plasma fibrinogen level.
Thrombotic and hemorrhagic complications are among the common
causes of mortality in cancer patients. Impairment of coagulation
may be an important early symptom of tumor progression (22). Studies have identified that cancer
patients with tumor growth, angiogenesis and end-organ damage, may
also experience coagulation activation (15,23).
TAFI represents the important molecular link between
the coagulation and fibrinolytic pathways (24). TAFI activity is generated in the
process of coagulation through binding to thrombin, thrombin-TM
complex and orplasmin, which in turn cleave TAFI protein at Arg114
(residue Arg92 following removal of the signal peptide) into
N-terminal activation peptide and catalytic domains, leading to
exposure of the active site cleft of activated TAFIa (25–27).
The plasma concentration of TAFI is controlled by genetic and
non-genetic factors. It is suggested that a variety of diseases are
positively associated with plasma TAFI levels (28). Reports showed that (12,13)
databases indicate a high expression of TAFI levels in breast,
ovarian and hepatic cancer cell lines. Plasma levels of TAFI are
also increased in a number of types of cancers, including BC
(10,29). In addition, higher levels of TAFI
have been associated with more advanced stages of cancer (30). The fibrinolytic system, more
appropriately referred to as the plasminogen activator system,
controls not only the intravascular fibrin deposition but is also
involved in a wide variety of physiologic and pathologic processes.
These components are involved in tumor growth, invasion and
metastasis in cancer cells (31).
The pathogenesis of the hypercoagulable state of the patients is
extremely complex. However, the enhanced coagulation of tumor cells
serves an important function in it. Malignant tumors are involved
in the hemostasis system by several pathways. Studies have
demonstrated that the cytokines which tumor cells excrete
contribute to the adhesiveness of the epithelium, thus aiding
metastasis (32,33). Anticoagulant drugs like
acetaminophen (34) may reduce the
migration of tumor cells by inhibiting coagulation. Cancer cell
invasion and metastasis require the degradation of the
extracellular matrix during local invasion, angiogenesis,
intravasation and extravasation. Invasive tumor cells express a
plasminogen activation system composed of a urokinase- and/or
tissue-type plasminogen activator, plasminogen and protease
receptors on their membrane surface. There is a negative
correlation between the TM expression level and cell malignancy;
patients with low TM expression in BC tissues have a significantly
lower rate of disease-free survival compared with patients with
high TM expression (35). However,
soluble type TM reduced invasion and metastasis of cancer cells,
indicating that TM is not only an anticoagulant protein but also a
regulator of tumor invasion and metastasis, (36,37).
However, the mechanisms are not completely understood and the
importance of TAFI in homeostasis and fibrinolysis remains to be
elucidated.
The present study inhibited TAFI expression using
siRNA in BCC lines and the results demonstrated that decreasing
TAFI expression may reduce cell growth, migration and invasion in
two types of BCC cells. Although the mechanism of TAFI synthesis in
the BCC cells remains to be elucidated, the present study may offer
a novel therapeutic approach for the treatment of BC
metastasis.
Acknowledgements
We are grateful to Central Research Laboratory, the
Second Hospital of Shandong University for technical assistance and
the generous support. This study was supported by a grant from the
Youth Foundation of the Second Hospital of Shandong University
(grant no. Y2014010044) and the Natural Science Foundation of
Shandong Province (grant no. ZR2014HM023)..
References
|
1
|
Boffa MB, Maret D, Hamill JD, Bastajian N,
Crainich P, Jenny NS, Tang Z, Macy EM, Tracy RP, Franco RF, et al:
Effect of single nucleotide polymorphisms on expression of the gene
encoding thrombin-activatable fibrinolysis inhibitor: A functional
analysis. Blood. 111:183–189. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bajzar L: Thrombin activatable
fibrinolysis inhibitor and an antifibrinolytic pathway.
Arterioscler Thromb Vasc Biol. 20:2511–2518. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Redlitz A, Tan AK, Eaton DL and Plow EF:
Plasma carboxypeptidases as regulators of theplasminogen system. J
Clin Invest. 96:2534–2538. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang W, Boffa MB, Bajzar L, Walker JB and
Nesheim ME: A study of the mechanism of inhibition of fibrinolysis
by activated thrombin-activable fibrinolysis inhibitor. J Biol
Chem. 273:27176–27181. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Boffa MB, Hamill JD, Bastajian N, Dillon
R, Nesheim ME and Koschinsky ML: A role for CCAAT/enhancer-binding
protein in hepatic expression of thrombin-activable fibrinolysis
inhibitor. J Biol Chem. 277:25329–25336. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pereira PJ Barbosa, Segura-Martin S, Oliva
B, Ferrer-Orta C, Avilés FX, Coll M, Gomis-Rüth FX and Vendrell J:
Human procarboxypeptidase B: Three-dimensional structure and
implications for thrombin-activatable fibrinolysis inhibitor
(TAFI). J Mol Biol. 321:537–547. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hosaka Y, Takahashi Y and Ishii H:
Thrombomodulin in human plasma contributes to inhibit fibrinolysis
through acceleration of thrombin-dependent activation of plasma
procarboxypeptidase B. Thromb Haemost. 79:371–377. 1998.PubMed/NCBI
|
|
8
|
Tamura A, Hebisawa A, Hayashi K, Sagara Y,
Fukushima K, Kurashima A, Yotsumoto H, Mori M and Komatsu H:
Prognostic significance of thrombomodulin expression and vascular
invasion in stage I squamous cell carcinoma of the lung. Lung
Cancer. 34:375–382. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Reijerkerk A, Meijers JC, Havik SR, Bouma
BN, Voest EE and Gebbink MF: Tumor growth and metastasis are not
affected in thrombin-activatable fibrinolysis inhibitor-deficient
mice. J Thromb Haemost. 2:769–779. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fawzy MS, Mohammed EA, Ahmed AS and
Fakhr-Eldeen A: Thrombin-activatable fibrinolysis inhibitor
Thr325Ile polymorphism and plasma level in breast cancer: A pilot
study. Meta Gene. 4:73–84. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fawzy MS and Toraih EA: Data supporting
the structural and functional characterization of
Thrombin-Activatable Fibrinolysis Inhibitor in breast cancer. Data
Brief. 5:981–989. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yeung TL, Leung CS, Wong KK, Samimi G,
Thompson MS, Liu J, Zaid TM, Ghosh S, Birrer MJ and Mok SC: TGF-β
modulates ovarian cancer invasion by upregulating CAF-derived
versican in the tumor microenvironment. Cancer Res. 73:5016–5028.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Miller TW, Balko JM, Fox EM, Ghazoui Z,
Dunbier A, Anderson H, Dowsett M, Jiang A, Smith RA, Maira SM, et
al: ERα-dependent E2F transcription can mediate resistance to
estrogen deprivation in human breast cancer. Cancer Discov.
1:338–351. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hataji O, Taguchi O, Gabazza EC, Yuda H,
D'Alessandro-Gabazza CN, Fujimoto H, Nishii Y, Hayashi T, Suzuki K
and Adachi Y: Increased circulating levels of thrombin-activatable
fibrinolysis inhibitor in lung cancer patients. Am J Hematol.
76:214–219. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fidan E, Kavgaci H, Orem A, Yilmaz M,
Yildiz B, Fidan S, Akcan B, Ozdemir F and Aydin F: Thrombin
activatable fibrinolysis inhibitor and
thrombin-antithrombin-III-complex levels in patients with gastric
cancer. Tumour Biol. 33:1519–1525. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huang Z, Miao X, Luan Y, Zhu L, Kong F, Lu
Q, Pernow J, Nilsson G and Li N: PAR1-stimulated platelet releasate
promotes angiogenic activities of endothelial progenitor cells more
potently than PAR4-stimulated platelet releasate. J Thromb Haemost.
13:465–476. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cui J, Hollmén M, Li L, Chen Y, Proulx ST,
Reker D, Schneider G and Detmar M: New use of an old drug:
Inhibition of breast cancer stem cells by benztropine mesylate.
Oncotarget. 8:1007–1022. 2017.PubMed/NCBI
|
|
18
|
Chung SS and Vadgama JV: Curcumin and
epigallocatechin gallate inhibit the cancer stem cell phenotype via
down-regulation of STAT3-NFκB signaling. Anticancer Res. 35:39–46.
2015.PubMed/NCBI
|
|
19
|
Ramachandran C and You W: Differential
sensitivity of human mammary epithelial and breast carcinoma cell
lines to curcumin. Breast Cancer Res Treat. 54:269–278. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hu X, Jiang L, Li Q and Gu Y: Quantitative
assessment of background parenchymal enhancement in breast magnetic
resonance images predicts the risk of breast cancer. Oncotarget.
8:10620–10627. 2017.PubMed/NCBI
|
|
21
|
Zhang F, Zhang Y, Deng Z, Xu P, Zhang X,
Jin T and Liu Q: Genetic variants in the acylphosphatase 2 gene and
the risk of breast cancer in a Han Chinese population. Oncotarget.
7:86704–86712. 2016.PubMed/NCBI
|
|
22
|
Kvolik S, Jukic M, Matijevic M, Marjanovic
K and Glavas-Obrovac L: An overview of coagulation disorders in
cancer patients. Surg Oncol. 19:e33–e46. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
De Cicco M: The prothrombotic state in
cancer: Pathogenic mechanisms. Crit Rev Oncol Hematol. 50:187–196.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mosnier LO and Bouma BN: Regulation of
fibrinolysis by thrombin activatable fibrinolysis inhibitor, an
unstable carboxypeptidase B that unites the pathways of coagulation
and fibrinolysis. Arterioscler Thromb Vasc Biol. 26:2445–2453.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Antovic JP and Blombäck M:
Thrombin-activatable fibrinolysis inhibitor antigen and TAFI
activity in patients with APC resistance caused by factor V Leiden
mutation. Thromb Res. 106:59–62. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Miah MF and Boffa MB: Functional analysis
of mutant variants of thrombin-activatable fibrinolysis inhibitor
resistant to activation by thrombin or plasmin. J Thromb Haemost.
7:665–672. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nesheim M: Thrombin and fibrinolysis.
Chest. 124:(Suppl 3). 33S–39S. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Boffa MB and Koschinsky ML: Curiouser and
curiouser: Recent advances in measurement of thrombin-activatable
fibrinolysis inhibitor (TAFI) and in understanding its molecular
genetics, gene regulation, and biological roles. Clin Biochem.
40:431–442. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chengwei X, Xiaoli M, Yuan Z, Li P,
Shengjiang W, Chao Y and Yunshan W: Plasma thrombin-activatable
fibrinolysis inhibitor levels and its Thr325Ile polymorphism in
breast cancer. Blood Coagul Fibrinolysis. 24:698–703. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Balcik OS, Albayrak M, Uyar ME, Dagdas S,
Yokus O, Ceran F, Cipil H, Kosar A and Ozet G: Serum thrombin
activatable fibrinolysis inhibitor levels in patients with newly
diagnosed multiple myeloma. Blood Coagul Fibrinolysis. 22:260–263.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Higuchi T, Nakamura T, Kakutani H and
Ishii H: Thrombomodulin suppresses invasiveness of HT1080 tumor
cells by reducing plasminogen activation on the cell surface
through activation of thrombin-activatable fibrinolysis inhibitor.
Biol Pharm Bull. 32:179–185. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Luo J, Lee S Ok, Liang L, Huang CK, Li L,
Wen S and Chang C: Infiltrating bone marrow mesenchymal stem cells
increase prostate cancer stem cell population and metastatic
ability via secreting cytokines to suppress androgen receptor
signaling. Oncogene. 33:2768–2778. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Eichbaum C, Meyer AS, Wang N, Bischofs E,
Steinborn A, Bruckner T, Brodt P, Sohn C and Eichbaum MH: Breast
cancer cell-derived cytokines, macrophages and cell adhesion:
Implications for metastasis. Anticancer Res. 31:3219–3227.
2011.PubMed/NCBI
|
|
34
|
Hasanein P and Sharifi M: Effects of
rosmarinic acid on acetaminophen-induced hepatotoxicity in male
Wistar rats. Pharm Biol. 55:1809–1816. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bazzi ZA, Lanoue D, El-Youssef M,
Romagnuolo R, Tubman J, Cavallo-Medved D, Porter LA and Boffa MB:
Activated thrombin-activatable fibrinolysis inhibitor (TAFIa)
attenuates breast cancer cell metastatic behaviors through
inhibition of plasminogen activation and extracellular proteolysis.
BMC Cancer. 16:3282016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
McMahon B and Kwaan HC: The plasminogen
activator system and cancer. Pathophysiol Haemost Thromb.
36:184–194. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Vairaktaris E, Yapijakis C, Nkenke E,
Vassiliou S, Vylliotis A, Nixon AM, Derka S, Ragos V, Spyridonidou
S, Tsigris C, et al: The 1040C/T polymorphism influencing thermal
stability and activity of thrombin activatable fibrinolysis
inhibitor is associated with risk for oral cancer. Am J Hematol.
82:1010–1012. 2007. View Article : Google Scholar : PubMed/NCBI
|