Introduction
Human glioblastoma (GBM) is the most frequent and
aggressive type of primary brain tumor in adults, and remains an
important health problem worldwide (1). Although advances in the treatment
strategies of GBM have been made, the 5-year survival rate of GBM
patients remains poor (2). Thus,
it is important to investigate novel therapeutic strategies for GBM
treatment.
Accumulating studies have demonstrated that
microRNAs (miRNAs/miRs), a class of small and non-coding RNAs,
participate in various biological processes of cancer development
by binding to the 3′-untranslated region (3′-UTR) of mRNA (3–5). For
example, miR-300 was reported to serve as a tumor suppressor gene
and suppress GBM progression by ROCK1 (6). miR-595 was demonstrated to serve a
critical role in GBM carcinogenesis by suppression of transcription
factor SOX7 (7). Chen et al
(8) indicated that miR-22
suppresses cell proliferation, motility and invasion of GBM by
directly targeting NAD-dependent protein deacetylase sirtuin-1. To
date, the role of miR-1288 in GBM remains unclear. Gopalan et
al (9) revealed that
overexpression of miR-1288 in colon cancer cells promotes cell
proliferation and increases the percentage of G2-M phase cells. In
addition, they demonstrated that miR-1288 overexpression increases
cell proliferation and colony formation, and enhances cell
migration and cell invasion properties in oesophageal squamous cell
carcinoma by regulating forkhead box protein O1 (10). The present study observed
aberrantly increased expression of miR-1288 in GBM tissues/cells.
Next, we experimentally revealed that miR-1288 acted as a tumor
promoter by interacting with ubiquitin carboxyl-terminal hydrolase
CYLD (CYLD), and then regulating cell proliferation in GBM.
Materials and methods
Clinical specimens
Eight paired surgically-removed human glioblastoma
tissues were obtained from GBM patients, and two normal brain
tissues were obtained from individuals who passed away in traffic
accidents, and then histopathologically diagnosed at Sichuan Cancer
Hospital (Sichuan, China). The present study was approved by the
ethics committee of Sichuan Cancer Hospital. All samples were
collected and analyzed with prior written informed consent from the
patients. Tissue samples were frozen in liquid nitrogen and stored
until total RNAs or proteins were extracted.
miR-1288 expression profiles (GSE61710) in GBM
tissues were obtained from Gene Expression Omnibus (GEO; www.ncbi.nlm.nih.gov/geo/). Archived patient
samples in The Cancer Genome Atlas (TCGA) database (https://tcga-data.nci.nih.gov and https://genomecancer.ucsc.edu) were selected.
Cell culture
The LN229, LN18, U87MG, A172, D27MG and LN340 human
GBM cell lines were provided by the National Rodent Laboratory
Animal Resource (Shanghai, China) and were grown in Dulbecco's
modified Eagle's medium (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). Normal human
astrocyte (NHA) cells were obtained from Lonza Group, Ltd. (Basel,
Switzerland) and cultured in the provided astrocyte growth media
supplemented with recombinant human epidermal growth factor,
insulin, ascorbic acid, GA-1000, L-glutamine and 5% FBS. Cell lines
were cultured in a humidified incubator at 37°C in 5%
CO2.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA including miRNAs was extracted from human
tissue samples and cell lines using TRIzol reagent (Thermo Fisher
Scientific, Inc.). RNA was reverse transcribed to cDNA from RNA
using a Reverse Transcription kit (Takara Biotechnology Co., Ltd.,
Dalian, China) according to the manufacturer's protocol. qPCR was
performed with SYBR Green (Takara Biotechnology Co., Ltd.) on a ABI
7500 thermocycler. Thermocycling conditions were as follows: At
95°C for 30 sec, followed by 40 cycles of amplification at 95°C for
5 sec, at 59°C for 30 sec and at 72°C for 30 sec. The sequences of
primers were synthesized by GeneCopoeia, Inc. (Rockville, MD, USA):
miR-1288 (cat. no. HmiRQP0132), cyclin D1 (cat. no. HQP016204) and
MYC (cat. no. HQP011597). U6 and GAPDH (cat. no. HQP064347) were
used as endogenous controls for miRNA and mRNA, respectively, and
the data were analyzed according to the 2−ΔΔCq method
(11).
Plasmids, small interfering RNA and
transfection
miR-1288 mimics (miR10005942-1-5), a miR-1288
inhibitor (miR-1288-in; miR20005942-1-5), negative control
sequences and CYLD-specific small interfering (si)RNA and scramble
sequences were synthesized and purified by Guangzhou RiboBio Co.,
Ltd. (Guangzhou, China), and transfection into cells was performed
using Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol.
MTT and colony formation assays
Cell growth was measured by MTT assay. Cells were
seeded at 5×104 cells/well into a 24-well plate after
each transfection. After 1, 2, 3, 4 and 5 days of culture at 37°C,
cell viability was assessed by MTT assay. MTT solution (20 µl; 0.5
mg/ml; Sigma-Aldrich; Merck KGaA) was added to each well at 37°C.
The medium was removed from each well and the formazan was
solubilized in 150 µl dimethyl sulfoxide (Sigma-Aldrich; Merck
KGaA). The absorbance of each well at 490 nm was measured using a
Multiskan GO microplate spectrophotometer (Thermo Fisher
Scientific, Inc.).
For the colony formation assay, a density of 1,000
indicated U87MG cells/well upon different treatments were seeded
into a 6-well cell culture plate and incubated for 14 days at room
temperature. Colonies were fixed with 4% paraformaldehyde for 5 min
and stained at room temperature with 1.0% crystal violet for 30
sec. The number of colonies was counted under a microscope (Motic
AE30 inverted fluorescence microscope; Microscope Systems Limited,
Glasgow, UK) at magnification, ×100.
Anchorage-independent growth
assay
Cells (1,000) were suspended in 2 ml complete medium
plus 0.3% agar (Sigma-Aldrich; Merck KGaA) and then plated on top
of a bottom layer containing 0.5% complete medium agar mixture.
After 14 days, viable colonies that were larger than 0.1 mm in
diameter were counted by microscopy (Motic AE30 inverted
fluorescence microscope; Microscope Systems Limited).
Bromodeoxyuridine (BrdU) staining
U87MG cells after transfection were incubated with
10 µM BrdU for 1 h and for an extra hour after the transfer to
fresh medium, fixed with 4% paraformaldehyde for 30 min at room
temperature and then stained at 4°C overnight with BrdU antibodies
(1:500; cat no. 61273; Upstate Biotechnology, Inc., Lake Placid,
NY, USA) according to the manufacturer's protocol. After incubation
at 37°C for 1 h with horseradish peroxidase (HRP)-conjugated
secondary antibodies (1:5,000; Abcam, Cambridge, UK), images were
acquired using a laser scanning microscope (Axioskop 2 plus; Zeiss
GmbH, Jena, Germany). All experiments were performed at least three
times.
MiRNA target prediction and luciferase
assays
Potential target genes of miR-1288 were predicted
using TargetScan online software version 3.1 (http://www.targetscan.org). Cells were co-transfected
with the wild-type CYLD 3´UTR (GeneCopoeia, Inc.) or the
control-luciferase plasmid and miR-1288 or miR-1288-mutant (mut).
Each group was also co-transfected with 5 ng control pRL-TK
Renilla plasmid (Promega Corporation, Madison, WI, USA)
using Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific,
Inc.). Luciferase and Renilla activities were assessed 48 h
after transfection using a Dual Luciferase Reporter Assay kit
(Promega Corporation) according to the manufacturer's protocol.
Western blot analysis
Protein lysates were prepared using
radioimmunoprecipitation assay lysis buffer (Cell Signaling
Technology, Inc., Danvers, MA, USA). The protein concentration was
determined using a bicinchoninic acid protein assay. Equal amounts
(40 µg) of proteins were separated by 10% SDS-PAGE and
electrotransferred onto polyvinylidene difluoride membranes (EMD
Millipore, Billerica, MA, USA). Membranes were subsequently blocked
in TBS containing 0.5% Tween-20 with 5% milk for 2 h at room
temperature and incubated at 4°C with the following primary
antibodies overnight: Anti-CYLD (cat no. 8462; 1:1,000),
anti-cyclin D1 (cat no. 2978; 1:1,000) and anti-c-MYC (cat no.
5605; 1:1,000; all Cell Signaling Technology, Inc.). An
anti-α-tubulin monoclonal antibody (cat no. T6199; 1:5,000;
Sigma-Aldrich; Merck KGaA) served as a loading control. The blots
were then incubated at 37°C for 2 h with a HRP-conjugated
anti-rabbit immunoglobulin secondary antibody (cat no. P0023D;
1:5,000; Beyotime Institute of Biotechnology, Haimen, China). The
signals were visualized using enhanced chemiluminescence following
the manufacturer's protocol.
Statistical analysis
All data are expressed as the mean ± standard
deviation. The data were analyzed using Student's t-test for
pair-wise comparisons or one-way analysis of variance followed by a
post hoc Tukey test for multiple comparisons. Statistical analysis
was performed using SPSS 19.0 software (IBM Corp., Armonk, NY,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
miR-1288 expression is upregulated in
GBM
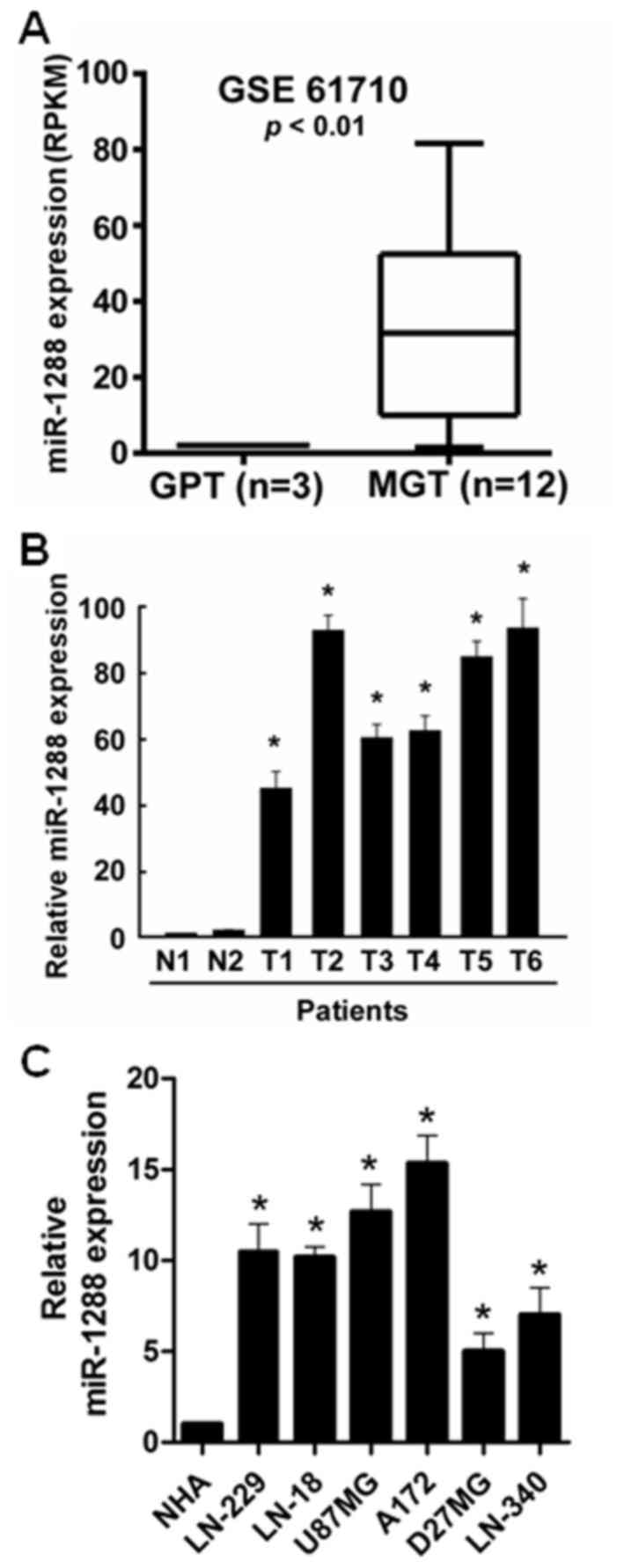
To reveal the role of miR-1288 in GBM, the
expression data downloaded from the Gene Expression Omnibus (GEO,
accession number GSE61710) was analyzed. The results of the GEO
analysis demonstrated that miR-1288 levels were significantly
increased in malignant glioma tissues compared with glioma
peritumoral tissues (Fig. 1A). To
further confirm this observation, the expression of miR-1288 in
human glioblastoma tissues and normal brain tissues was measured by
RT-qPCR. miR-1288 expression was significantly upregulated in GBM
tissues compared with normal brain tissues (Fig. 1B). The expression of miR-1288 in
LN229, LN443, LN18, U87MG, A172, D27MG and LN340 GBM cells was
further detected, and the result indicated that compared with NHA
cells, miR-1288 expression was significantly increased in GBM cells
(Fig. 1C). miR-1288 expression in
U87MG cells was less abundant than in A172 cells, but was more
abundant compared with the other GBM cell lines. Therefore, the
U87MG cell line may the most appropriate model for studying the
expression of miR-1288 in relation to GBM.
miR-1288 promotes, while miR-1288-in
inhibits GBM cell proliferation
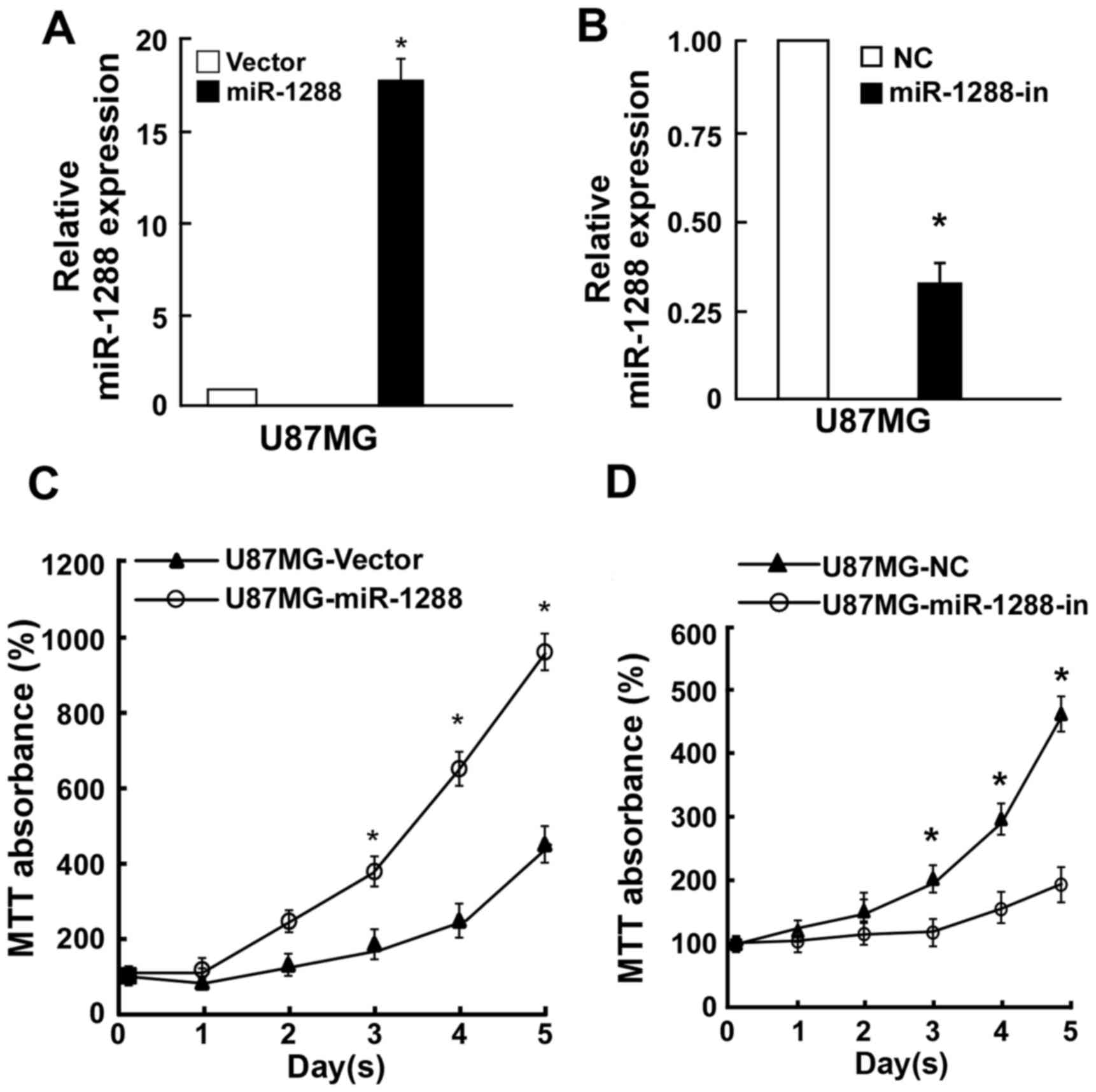
To investigate whether GBM cell proliferation was
regulated by miR-1288, U87MG cells were cotransfected with miR-1288
or miR-1288-in or the respective controls. RT-qPCR analysis was
used to verify relative miR-1288 expression (Fig. 2A and B). MTT assay revealed that
ectopic overexpression of miR-1288 markedly enhanced cell
proliferation of U87MG cells, while suppression of cell
proliferation by miR-1288-in (Fig. 2B
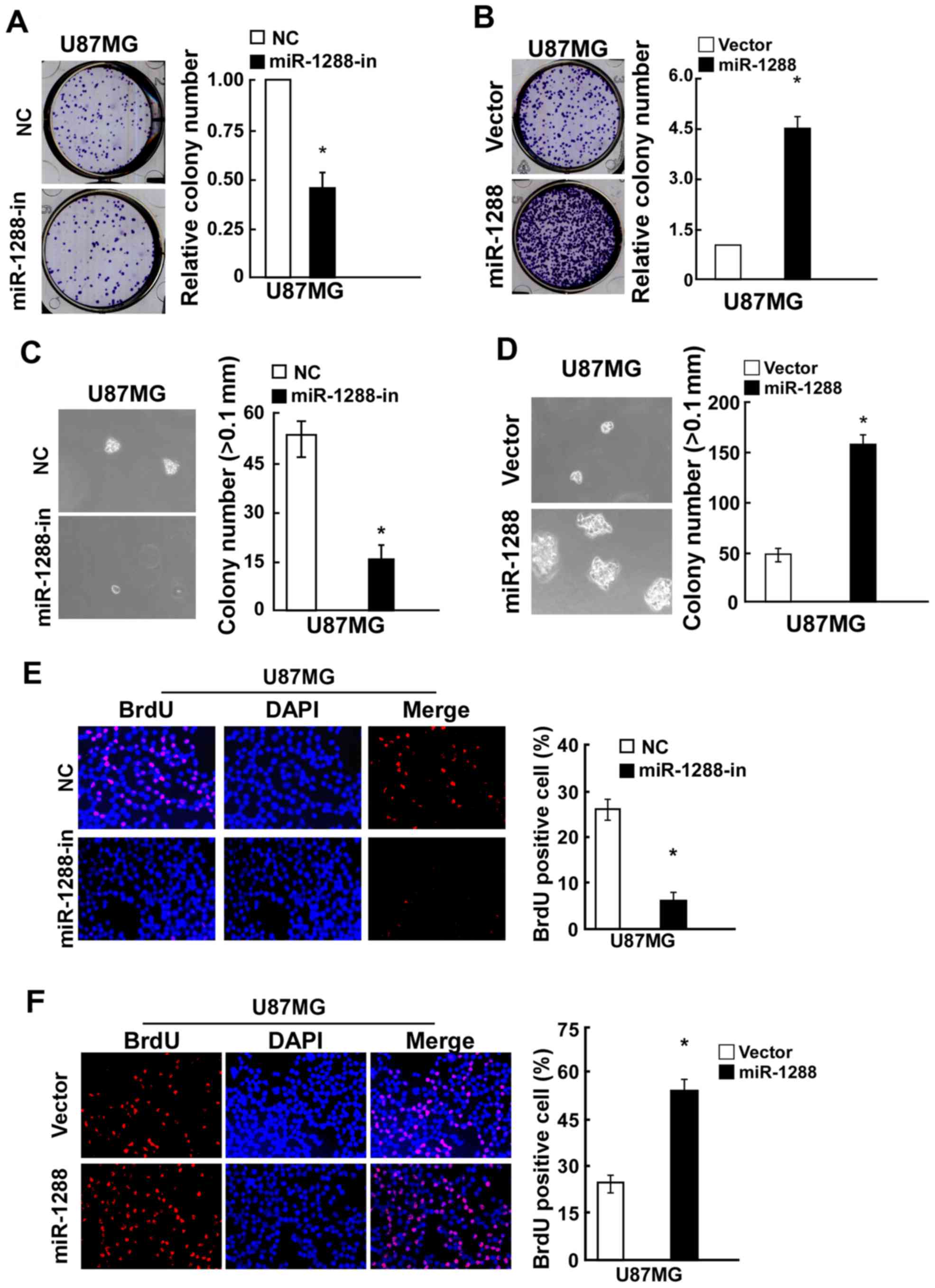
and C). Similarly, a colony formation assay demonstrated that
compared with the relative negative control, miR-1288 significantly
promoted while miR-1288-in markedly suppressed colony formation of
U87MG cells (Fig. 3A and B).
Additionally, the anchorage-independent growth ability of U87MG
cells transfected with miR-1288 was significantly increased, and
miR-1288-in treatment caused a decrease in the
anchorage-independent growth ability of U87MG cells (Fig. 3C and D). BrdU assay results
demonstrated that BrdU-positive cells were significantly increased
in the U87MG cell line after transfection with miR-1288, while
miR-1288-in exhibited the opposite effect (Figs. 2E and 3E). These results demonstrated that
miR-1288 increases GBM cell tumorigenicity in vitro.
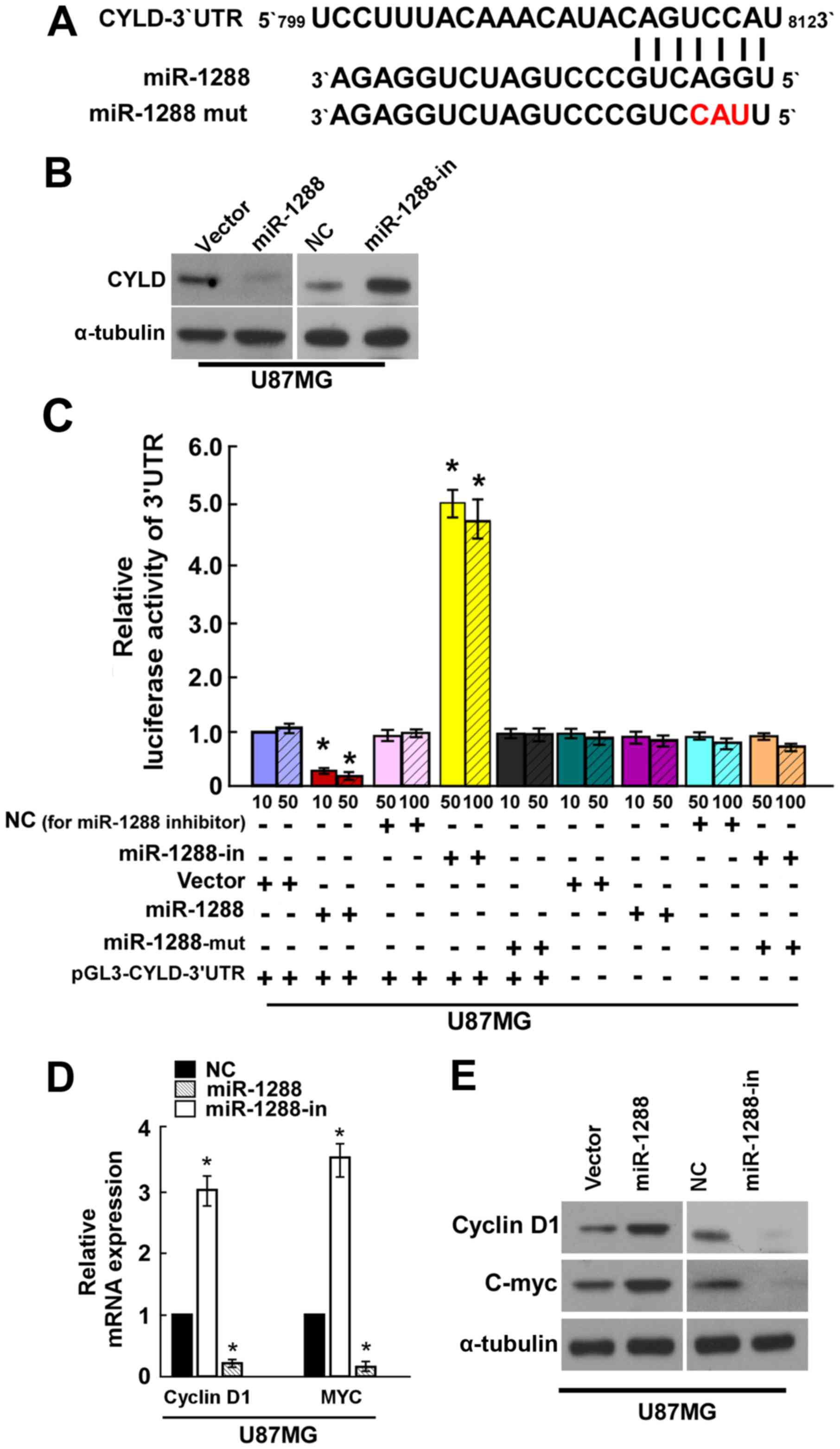
miR-1288 directly targets CYLD by
binding to its 3′-UTR
Bioinformatics methods (http://www.targetscan.org) identified CYLD as a
potential target of miR-1288 (Fig.
4A). To elucidate whether CYLD is indeed directly targeted by
miR-1288, the present study investigated whether miR-1288
recognizes the 3′UTR of CYLD mRNA using western blot analysis and
dual-luciferase reporter assay. Western blot analysis demonstrated
that CYLD was decreased in miR-1288-transfected U87MG cells, while
miR-1288-in clearly increased CYLD protein expression (Fig. 4B). A CYLD 3′-UTR wild-type vector
was co-transfected into U87MG cells with miR-1288, miR-1288-in or
miR-NC, and then measured by luciferase activity. The results
revealed that transfection of miR-1288 markedly suppressed the
luciferase activity of CYLD 3′-UTR wild-type in U87MG cells,
transfection of miR-1288-in significantly increased the luciferase
activity of CYLD 3′-UTR wild -type in U87MG cells, and miR-1288-mut
demonstrated no effect on the luciferase activity of CYLD 3′-UTR
wild-type in U87MG cells (Fig.
4C). Taken together, these results suggested that CYLD is a
target of miR-1288.
To further determine the mechanism by which the
miR-1288-CYLD axis regulates cell proliferation of GBM, the
expression of cell proliferation-associated genes were measured.
Decreased cyclin D1 and MYC expression were detected after
treatment with the miR-1288 by RT-qPCR and western blot analysis
(Fig. 4D and E, respectively).
Collectively, these results indicated that miR-1288 functionally
modulates the cellular proliferation regulators cyclin D1 and MYC;
thus, may mediate GBM cell proliferation.
Silencing of CYLD expression reversed
the cell proliferation promotion by miR-1288-in in GBM
The present study hypothesized that the phenotypes
associated with miR-1288-in expression would be reversed by the
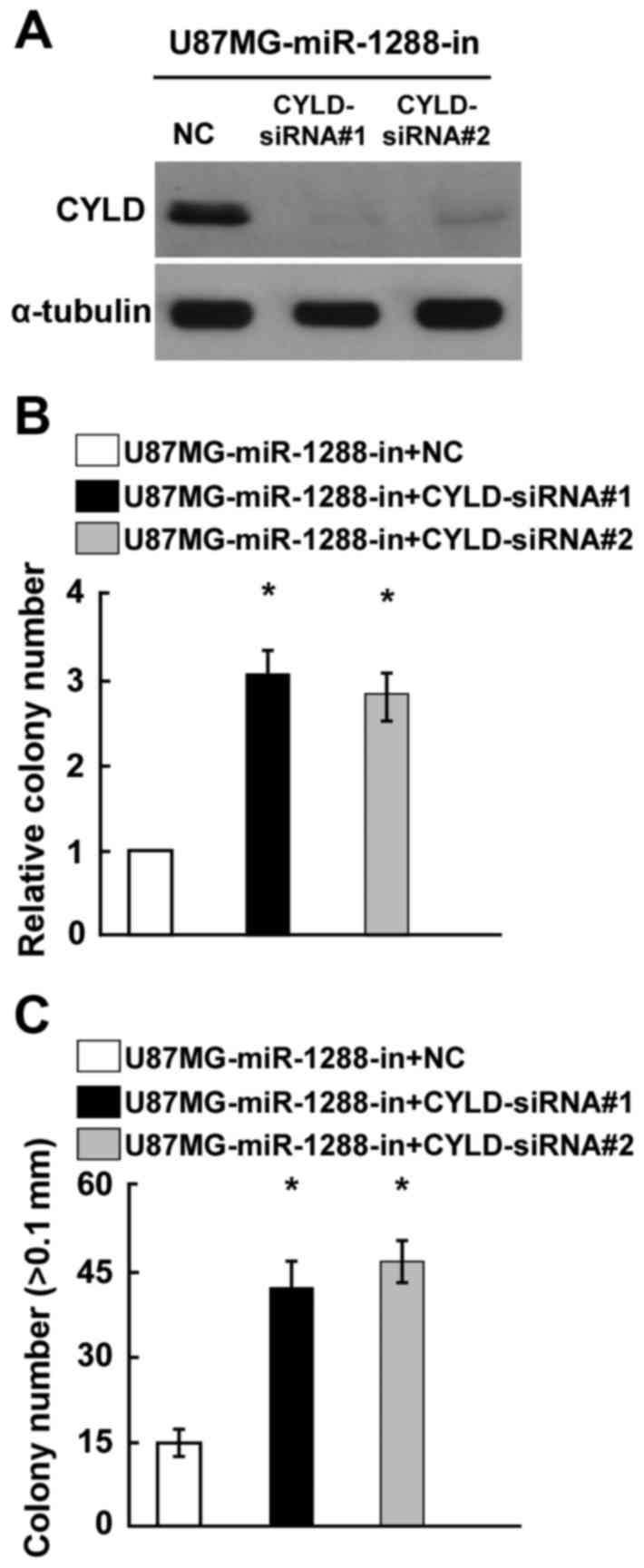
silencing of CYLD expression. CYLD expression was confirmed by
western blotting (Fig. 5A). Colony
formation and anchorage-independent growth assays demonstrated that
knockout of CYLD expression significantly reversed the
miR-1288-in-induced promotion of GBM cell proliferation (Fig. 5B and C). Taken together, these
results indicated that CYLD serves an essential role in cell
proliferation of GBM, potentially acting as a mediator of
miR-1288.
Discussion
The results of the present study revealed the
following novel findings: i) miR-1288 was upregulated in GBM; ii)
in vitro experiments confirmed that miR-1288 overexpression
promoted cell proliferation of GBM; iii) miR-1288 targeted CYLD in
GBM cells and cells negatively expressing CYLD.
Previous studies have demonstrated that miRNAs serve
essential roles in tumor development and progression of various
types of cancer by targeting genes associated with cell
proliferation, apoptosis, invasion, migration and angiogenesis
(3,12–16).
miR-146b-5p serves as a tumor suppressor and predicts the prognosis
of human gliomas (17). miR-182-5p
was reported to promote tumorigenesis of glioma by inducing STAT3
activation (18). Guo et al
(19) indicated that miR-141 and
miR-200c inhibit glioma cell growth and migration by suppressing
zinc finger E-box-binding homeobox 1 expression. In particular, the
function of miR-1288 and its regulated targets in GBM remains
unknown. The present study demonstrated that expression of miR-1288
was significantly upregulated in GBM tissues and cells.
miR-1288 has previously been reported to be
associated with pathological staging and serve an essential role in
the progression of colorectal cancer (9). Gopalan et al (20) demonstrated that miR-1288 expression
was upregulated in oesophageal squamous cell carcinoma tissues, and
overexpression of miR-1288 promoted cell proliferation, migration
and invasion of oesophageal squamous cell carcinoma. Similarly, the
present study indicated that overexpression of miR-1288 could
promote cell proliferation of GBM in vitro.
CYLD, a mutated gene in familial cylindromatosis, is
known to serve as tumor suppressor gene in multiple types of cancer
by regulating various signaling pathways, including Wnt/β-catenin,
nuclear factor-κB and transforming growth factor-β (21–25).
In the present study, CYLD was demonstrated to serve as a
functional target of miR-1288, using a bioinformatics prediction.
Western blotting and a dual-luciferase reporter assay were used to
verify that miR-1288 targets CYLD by interacting with the 3′UTR of
CYLD to reduce CYLD expression. Further functional experiments
revealed that the suppression of CYLD reversed the cell
proliferation promotion by miR-1288-in in GBM.
In conclusion, the present study demonstrated that
miR-1288 expression was increased in GBM. miR-1288 was identified
as a tumor promoter in GBM by inhibiting of CYLD, suggesting that
the miR-1288/CYLD axis may represent a potential therapeutic target
for the treatment of GBM.
Acknowledgements
The present study was supported by the Department of
Radiation Oncology, Sichuan Cancer Hospital (Sichuan, China) and
the Research project of Sichuan Provincial Health and Family
Planning Commission (grant no. 16PJ511).
References
|
1
|
Van Meir EG, Hadjipanayis CG, Norden AD,
Shu HK, Wen PY and Olson JJ: Exciting new advances in
neuro-oncology: The avenue to a cure for malignant glioma. CA
Cancer J Clin. 60:166–193. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Franceschi E, Depenni R, Paccapelo A,
Ermani M, Faedi M, Sturiale C, Michiara M, Servadei F, Pavesi G,
Urbini B, et al: Which elderly newly diagnosed glioblastoma
patients can benefit from radiotherapy and temozolomide? A PERNO
prospective study. J Neurooncol. 128:157–162. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang W, Chen J, Dai J, Zhang B, Wang F and
Sun Y: MicroRNA-16-1 inhibits tumor cell proliferation and induces
apoptosis in A549 non-small cell lung carcinoma cells. Oncol Res.
24:345–351. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhou W, Bi X, Gao G and Sun L: miRNA-133b
and miRNA-135a induce apoptosis via the JAK2/STAT3 signaling
pathway in human renal carcinoma cells. Biomed Pharmacother.
84:722–729. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu F, Zhang S, Zhao Z, Mao X, Huang J, Wu
Z, Zheng L and Wang Q: MicroRNA-27b up-regulated by human
papillomavirus 16 E7 promotes proliferation and suppresses
apoptosis by targeting polo-like kinase2 in cervical cancer.
Oncotarget. 7:19666–19679. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhou F, Li Y, Hao Z, Liu X, Chen L, Cao Y,
Liang Z, Yuan F, Liu J, Wang J, et al: MicroRNA-300 inhibited
glioblastoma progression through ROCK1. Oncotarget. 7:36529–36538.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hao Y, Zhang S, Sun S, Zhu J and Xiao Y:
MiR-595 targeting regulation of SOX7 expression promoted cell
proliferation of human glioblastoma. Biomed Pharmacother.
80:121–126. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen H, Lu Q, Fei X, Shen L, Jiang D and
Dai D: miR-22 inhibits the proliferation, motility, and invasion of
human glioblastoma cells by directly targeting SIRT1. Tumour Biol.
37:6761–6768. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gopalan V, Pillai S, Ebrahimi F,
Salajegheh A, Lam TC, Le TK, Langsford N, Ho YH, Smith RA and Lam
AK: Regulation of microRNA-1288 in colorectal cancer: Altered
expression and its clinicopathological significance. Mol Carcinog.
53 Suppl 1:E36–E44. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gopalan V, Islam F, Pillai S, Tang JC,
Tong DK, Law S, Chan KW and Lam AK: Overexpression of microRNA-1288
in oesophageal squamous cell carcinoma. Exp Cell Res. 348:146–154.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zheng F, Zhang J, Luo S, Yi J, Wang P,
Zheng Q and Wen Y: miR-143 is associated with proliferation and
apoptosis involving ERK5 in HeLa cells. Oncol Lett. 12:3021–3027.
2016.PubMed/NCBI
|
|
13
|
Shen L, Liu L, Ge L, Xie L, Liu S, Sang L,
Zhan T and Li H: miR-448 downregulates MPPED2 to promote cancer
proliferation and inhibit apoptosis in oral squamous cell
carcinoma. Exp Ther Med. 12:2747–2752. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tao WY, Wang CY, Sun YH, Su YH, Pang D and
Zhang GQ: MicroRNA-34c suppresses breast cancer migration and
invasion by targeting GIT1. J Cancer. 7:1653–1662. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Song L, Yang J, Duan P, Xu J, Luo X, Luo
F, Zhang Z, Hou T, Liu B and Zhou Q: MicroRNA-24 inhibits
osteosarcoma cell proliferation both in vitro and in vivo by
targeting LPAATβ. Arch Biochem Biophys. 535:128–135. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pan Y, Robertson G, Pedersen L, Lim E,
Hernandez-Herrera A, Rowat AC, Patil SL, Chan CK, Wen Y, Zhang X,
et al: miR-509-3p is clinically significant and strongly attenuates
cellular migration and multi-cellular spheroids in ovarian cancer.
Oncotarget. 7:25930–25948. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu J, Xu J, Li H, Sun C, Yu L, Li Y, Shi
C, Zhou X, Bian X, Ping Y, et al: miR-146b-5p functions as a tumor
suppressor by targeting TRAF6 and predicts the prognosis of human
gliomas. Oncotarget. 6:29129–29142. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xue J, Zhou A, Wu Y, Morris SA, Lin K,
Amin S, Verhaak R, Fuller G, Xie K, Heimberger AB and Huang S:
miR-182-5p induced by STAT3 activation promotes glioma
tumorigenesis. Cancer Res. 76:4293–4304. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Guo E, Wang Z and Wang S: MiR-200c and
miR-141 inhibit ZEB1 synergistically and suppress glioma cell
growth and migration. Eur Rev Med Pharmacol Sci. 20:3385–3391.
2016.PubMed/NCBI
|
|
20
|
Gopalan V, Islam F, Pillai S, Tang JC,
Tong DK, Law S, Chan KW and Lam AK: Overexpression of microRNA-1288
in oesophageal squamous cell carcinoma. Exp Cell Res. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bignell GR, Warren W, Seal S, Takahashi M,
Rapley E, Barfoot R, Green H, Brown C, Biggs PJ, Lakhani SR, et al:
Identification of the familial cylindromatosis tumour-suppressor
gene. Nat Genet. 25:160–165. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Massoumi R: CYLD: A deubiquitination
enzyme with multiple roles in cancer. Future Oncol. 7:285–297.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hayashi M, Jono H, Shinriki S, Nakamura T,
Guo J, Sueta A, Tomiguchi M, Fujiwara S, Yamamoto-Ibusuki M,
Murakami K, et al: Clinical significance of CYLD downregulation in
breast cancer. Breast Cancer Res Treat. 143:447–457. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang L, Ding Y, Yuan Z, Liu J, Sun J, Lei
F, Wu S, Li S and Zhang D: MicroRNA-500 sustains nuclear factor-κB
activation and induces gastric cancer cell proliferation and
resistance to apoptosis. Oncotarget. 6:2483–2495. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ge WL, Xu JF and Hu J: Regulation of oral
squamous cell carcinoma proliferation through crosstalk between
SMAD7 and CYLD. Cell Physiol Biochem. 38:1209–1217. 2016.
View Article : Google Scholar : PubMed/NCBI
|