Introduction
Breast cancer (BC) is one of the most common types
of cancer and is the leading cause of cancer mortality among
females worldwide, accounting for 25% of all cancer cases and 15%
of all cancer-associated mortality among females (1). A number of adjuvant therapies for
early BC have been developed; however, the recurrence rate remains
as high as 20–30%. Robust clinical and pathological markers
determine treatment options, including pathological evaluation of
the size of the primary tumor, number of metastatic lymph nodes,
lymphovascular invasion, as well as the expression of the estrogen
receptor (ER), progesterone receptor (PR), the Ki-67 marker of
proliferation and human epidermal growth factor receptor 2 (HER-2)
are all established markers (2).
Aside from histologic prognostic factors, a number of molecular
classifications of BC have been identified, involving the
mammaprint test or the OncotypeDX™21-Gene Recurrence Score assay,
which may be used to predict the requirement for adjuvant
chemotherapy treatment (3).
Neoadjuvant chemotherapy (NAC) describes therapeutic
intervention prior to surgery. The aim of NAC in BC is to reduce
the size of unresectable tumors in locally advanced or inflammatory
BC, thus allowing surgery to be performed (4). For operable tumors, the aim is to
downstage the tumor for improved loco-regional control and to
increase the conservative surgery rate. As NAC provides the unique
opportunity to assess response to chemotherapy within months rather
than years of follow-up, it provides the opportunity to assess the
efficacy of therapy and to change to an alternative treatment if
appropriate. NAC has recently become a popular treatment option,
which is widely used to treat patients that fulfill the criteria
for adjuvant chemotherapy following surgery, and is considered to
be a platform for testing novel therapies. Clinical studies have
made use of the in vivo response to conduct sequential
tissue biopsies and assess a range of biomarkers of resistance and
sensitivity to neoadjuvant treatment (5).
Response to NAC is an excellent indicator of disease
outcome (4). Achieving a
pathologic complete response (pCR), which is defined as the absence
of residual invasive cancer in the breast and ipsilateral axilla
lymph nodes, following NAC treatment is associated with improved
disease-free survival (DFS) in patients with luminal B/HER-2
positive tumors (6). The US Food
and Drug Administration (FDA) has recommended pCR as an end point
for the approval of novel agents for neoadjuvant treatment of
early-stage BC (7).
Angiogenesis is an essential process in the
progression of malignant tumors, as solid tumors cannot grow beyond
1–2 mm in diameter without the formation of new vessels (8). In BC, extensive neovascularization
and lymphovascular invasion have been reported to be poor
indicators of prognosis (9).
Microvascular density (MVD) has become the morphological gold
standard to assess angiogenesis in human tumors. Numerous studies
have demonstrated that the MVD of BC predicts tumor progression and
metastasis, and thus predicts prognosis (10).
Endoglin [also known as cluster of differentiation
(CD) 105], is a co-receptor of transforming growth factor-β
(TGF-β)-1 and −2, and is expressed on the endothelial cells of
peri- and intra-tumoral blood vessels and tumor stromal components
(11). Endoglin has demonstrated
to be superior to CD34 and CD31 in the evaluation of angiogenesis,
as demonstrates greater affinity for the angiogenic endothelium,
whereas CD34 and CD31 react nonspecifically with the endothelium of
healthy and pathological vessels (12).
The phosphatidylinositol 3-kinase (PI3K)-protein
kinase B (Akt)-mechanistic target of rapamycin (mTOR) signaling
pathway is an important pathway that is involved in hormone therapy
and trastuzumab resistance (13,14).
Treatment with an mTOR inhibitor may reverse resistance in advanced
BCs (15,16).
The present study examined the association between
the therapeutic effects of NAC and the expression of numerous
markers, including endoglin and mTOR, in locally advanced BC, in
order to determine whether determination of endoglin expression
prior to NAC may be used as a predictive marker of treatment
response.
Materials and methods
BC tissues
The Ethics Committee of Kaohsiung Chang Gung
Memorial Hospital (Niao-Song, Taiwan) approved this study. With
permission from the Institutional Review Board of Kaohsiung Chang
Gung Memorial Hospital (Kaohsiung, Taiwan), clinical information
and archived tissue specimens were collected from 34 patients with
BC that were diagnosed and treated in Kaohsiung Chang Gung Memorial
Hospital between 2012 and 2014. Biopsy specimens prior to NAC
treatment and tumor specimens following NAC treatment were
collected for diagnosis and the analysis of the expression of
markers. Patients provided written informed consent for the use of
their tissue samples for research purposes. In addition, all data
were analyzed anonymously. Clinical data including age at
diagnosis, clinical and pathological stage, and pathological
features, including ER, PR, Ki-67 and HER-2 expression and
responses to NAC were obtained from a combined review of clinical
and pathological records. Patients routinely underwent computed
tomography examination prior to and following NAC treatment.
Evaluation of clinical response was measured according to the
Response Evaluation Criteria In Solid Tumors guidelines (version
1.1) (17). Resected specimens
were sent for pathological examination by pathologists at Kaohsiung
Chang Gung Memorial Hospital (Kaohsiung, Taiwan).
Tissue microarray (TMA)
Tumor specimens (following treatment) from archived
specimens were collected for TMA blocks. TMA blocks were
constructed using the TMA Grand Master system (3DHISTECH Ltd.,
Budapest, Hungary). Target regions for the array (areas with BC)
were identified by marking the areas on hematoxylin and
eosin-stained sections from each paraffin-embedded sample. A total
of 3 tissue cores with a diameter of 3 mm were transferred from
each donor block to the recipient TMA block. Liver or skeletal
muscle tissues were placed in the first lane core of the three
upper right cores of the TMA block to ensure correct
orientation.
Immunohistochemical (IHC)
staining
IHC staining procedures were followed as previously
described (18). Biopsy specimens
and TMA blocks constructed from formalin-fixed paraffin-embedded
human BC tissue were sectioned at 3-µm thickness and dried
overnight at 37°C. Slides were deparaffinized in xylene and
rehydrated through a graded alcohol series to water. For antigen
retrieval, the slides were incubated with an anti-endoglin primary
antibody (cat. no. NCL-CD105; 1:50; Novocastra™; Leica Biosystems,
Ltd., Milton Keynes, UK) for 3 h at room temperature and anti-mTOR
primary antibody (cat. no. ab2732; 1:100; Abcam, Cambridge, MA,
USA) 1:100 for 2 h at room temperature. Following a wash step with
PBS, the UltraVision™ Quanto Detection system HRP (cat. no.
TL-125-QHL; 1:10; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
for 10 min at room temperature was added. The slides were then
analyzed using the Dako Liquid DAB+ Substrate Chromogen system
(cat. no. K3468; Dako; Agilent Technologies GmbH, Waldbronn,
Germany), followed by counterstaining with hematoxylin 1:1 at room
temperature for 1 min and mounting onto coverslips using
Entellan® New Mounting Medium (cat. no. 107961; Merck
Millipore, Darmstadt, Germany). Incubation of slides without the
primary antibody was used as a negative control. Slides were
scanned using the Pannoramic SCAN scanner (3DHISTECH Ltd.) for
analysis. The staining intensity of these markers was determined by
two independent pathologists, and classified as low or high.
Evaluation of MVD
MVD was evaluated as described previously (18). Endoglin positive single cells or
clusters of cells clearly in the vessel lumen were considered to be
an individual vessel. Areas of inflammation, fibrosis or necrosis,
and vessels with a muscle wall were excluded. The sections were
scanned (magnification, ×100) by two observers simultaneously to
select the hotspots (regions with the highest endoglin staining) of
the three tissue array spots for every patient. The number of
microvessels in each hotspot were counted (magnification level,
×200) and their density was expressed as the mean number/high-power
field. Mean values of endoglin staining were calculated for each
individual tumor and used for further analysis.
Statistical analysis
The association between IHC findings and clinical
features, including alterations of tumor stage, pathological stage
and expression markers were analyzed by Spearman's rank correlation
coefficient. SPSS software (version, 10.0; SPSS Inc., Chicago, IL,
USA) was used for all calculations. P<0.05 was considered to
indicate a statistically significant association.
Results
Tumor responses to NAC
A total of 34 paired specimens were available for
staining and analysis from 34 patients. The mean age of the
patients was 55 years. All tumors were invasive ductal carcinoma.
All patients received NAC including anthracycline and taxane,
either administered sequentially or in combination. For breast
tumors with HER-2 overexpression, trastuzumab was added into the
treatment regimen. The overall response rate of primary tumors was
67.6% (Table I); only 5 (14.7%)
patients exhibited disease progression during NAC treatment.
 | Table I.Clinical information of patients. |
Table I.
Clinical information of patients.
| Clinical
information | No. of patients |
|---|
| Mean age at time of
diagnosis (years) | 55 (33–72) |
| Clinical stage prior
to NAC |
|
| Stage
II | 13 |
| Stage
III | 18 |
| Stage
IV | 3 |
| Subtype |
|
| Luminal
A | 9 |
| Luminal
B | 9 |
| HER-2
enriched | 8 |
| Triple
negative | 8 |
| NAC regimen |
|
| EC
×4 | 8 |
| ET
×6 | 18 |
| ETH
×6 | 8 |
| Response to
NAC |
|
|
Progressive disease | 5 |
| Stable
disease | 6 |
| Partial
response | 18 |
|
Complete response | 5 |
High endoglin expression correlates
with improved response to NAC
The expression of endoglin was assessed by
immunohistochemistry to evaluate the MVD of tumors. A mean value of
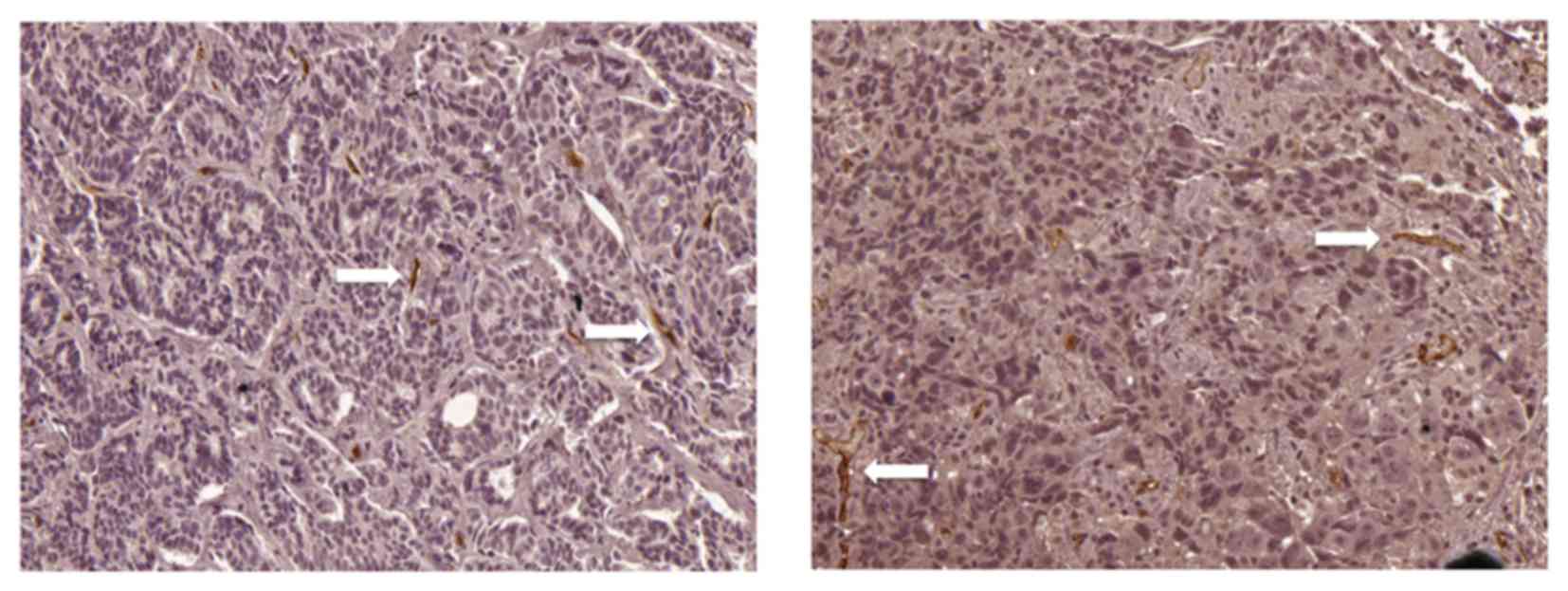
14 was selected as the cut-off point for MVD; a value of ≤14 was
considered as low expression (Fig.
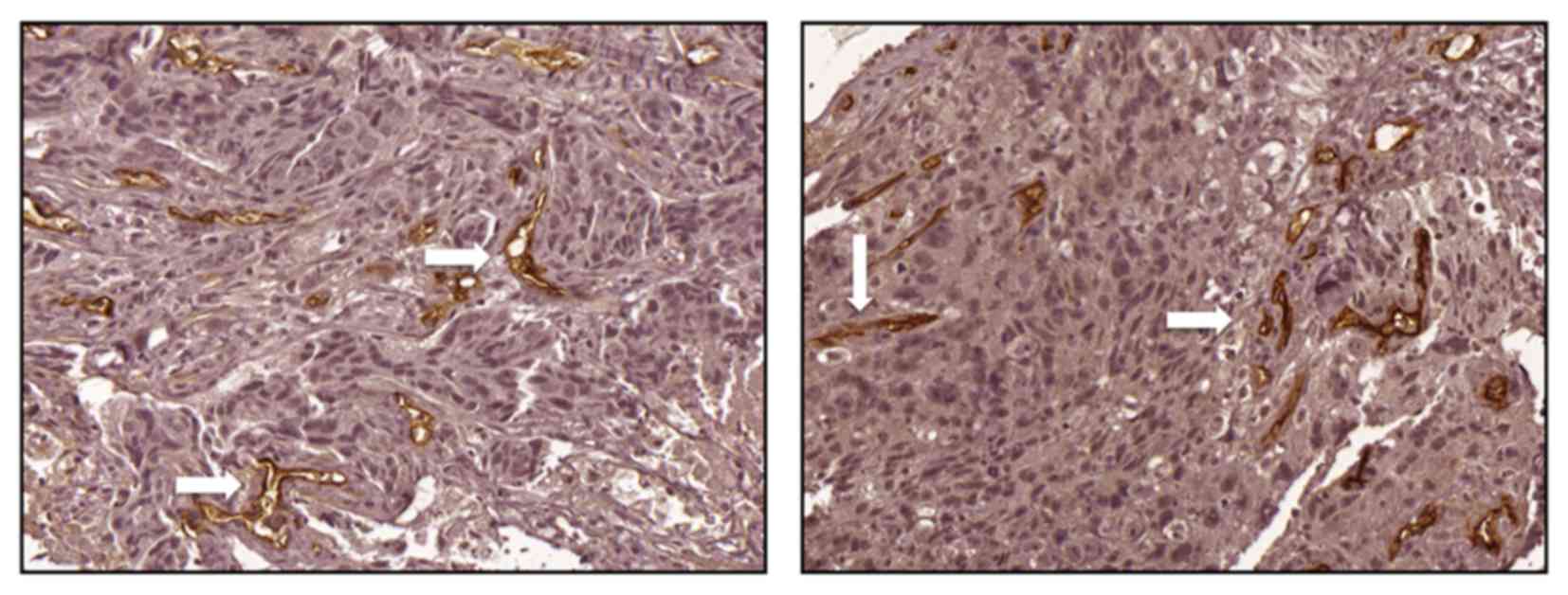
1) and a value of>14 was considered as high expression
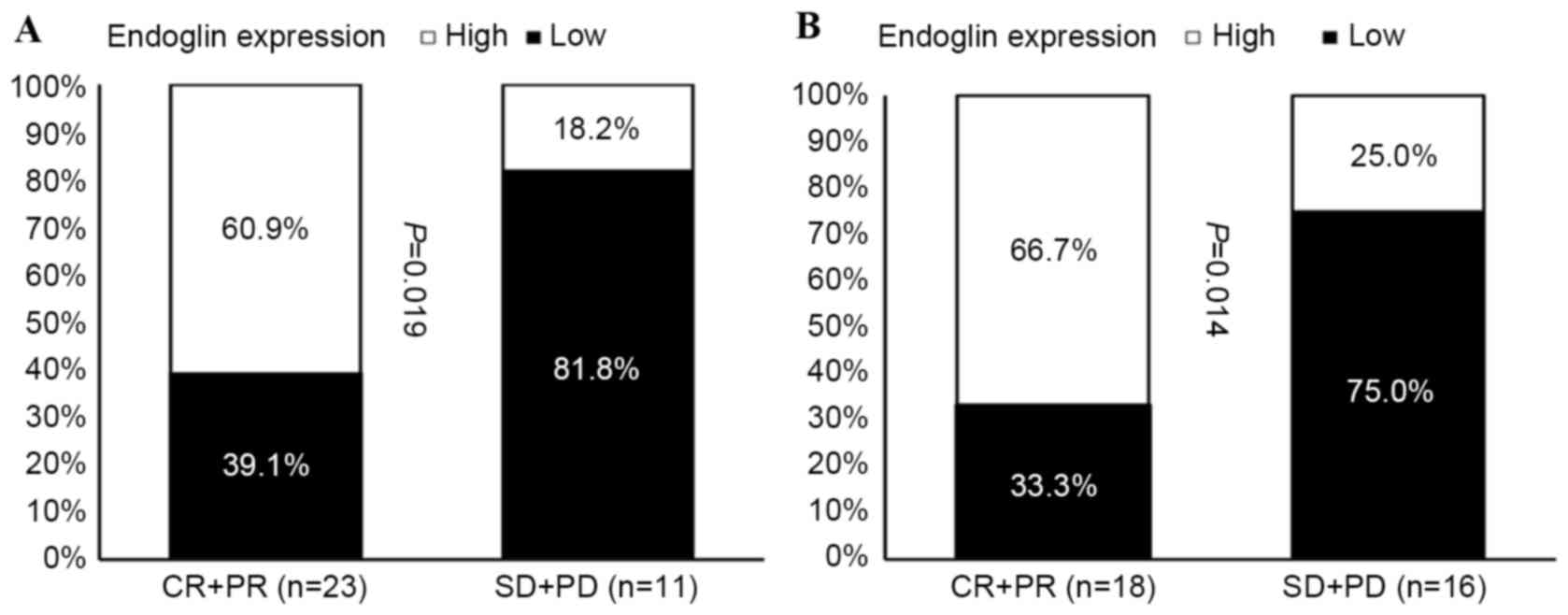
(Fig. 2). A high MVD score in the
tumor biopsy samples obtained prior to NAC was significantly
associated with an improved patient response rate of primary tumors
(Fig. 3A), and of primary tumors
with regional lymph node involvement (Fig. 3B).
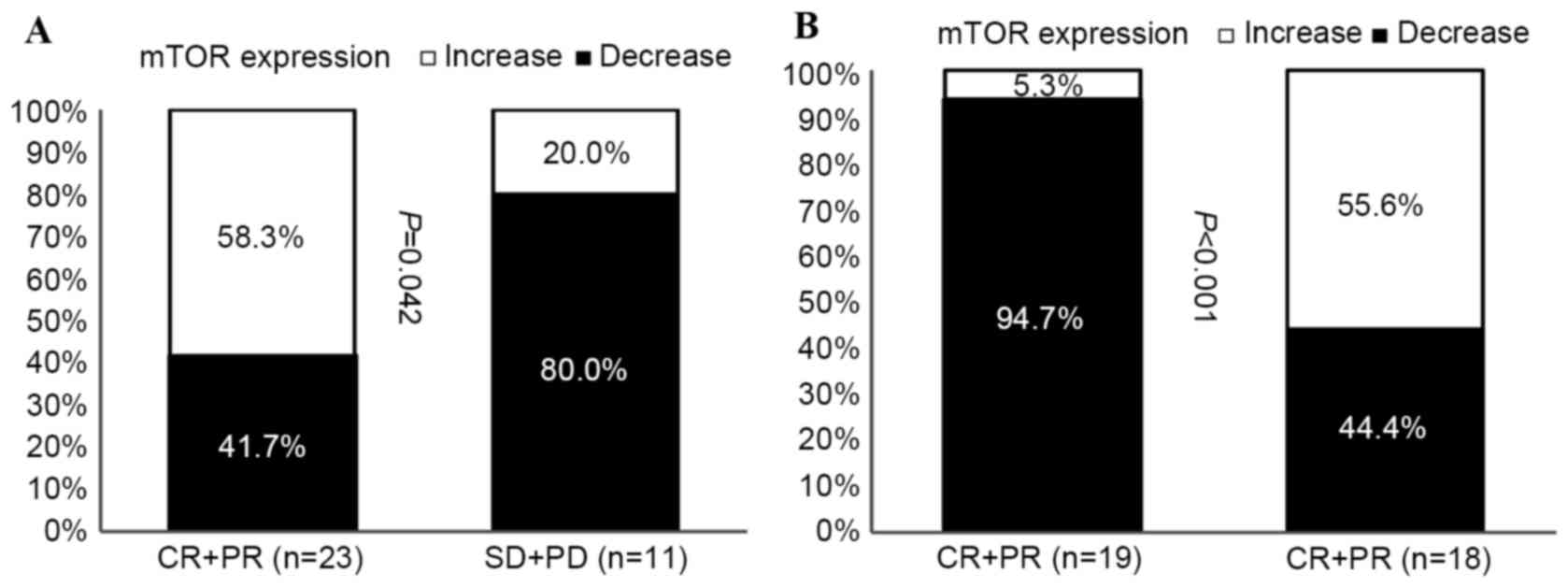
Increased mTOR expression is linked to
improved NAC response
The expression of mTOR in tumors removed prior to
NAC and in residual tumors following NAC were compared in primary
tumors (Fig. 4A), and in primary
tumors with regional lymph node involvement (Fig. 4B). The expression of mTOR was
increased in tumors with improved responses to NAC treatment, while
decreased mTOR expression was observed in tumors that progressed
following NAC treatment (Fig.
4A).
Discussion
BC is currently one of the most common types of
cancer among females in Taiwan (19). Although the first step to determine
treatment for BC depends on the Tumor Node Metastasis stage
(20), there are a variety of
biomarkers that have been reported for prognostic and predictive
purposes. The most common biomarkers used are hormone receptors,
HER-2 expression status, and the Ki-67 index, which classifies BC
types into luminal A, luminal B, HER-2-enriched and basal
triple-negative breast cancers (21). These classifications are associated
with different prognoses and treatment options. Identification of
novel markers has led to a greater understanding of the importance
of existing biomarkers, and a more definitive insight into tumor
biology.
NAC is the current standard of treatment for
patients with locally advanced BC, and is frequently used for
patients with operable BC, with the aim of downstaging the tumor
and improving the success rate of breast-conserving surgery
(22). Response to NAC may predict
a reduction in the micrometastatic burden, and allow for the
individualization of systemic treatments. Traditionally, patients
are administered with adjuvant chemotherapy following resection of
primary tumors. However, determining the effectiveness of adjuvant
chemotherapy is difficult, as there is no evaluable lesion
following surgery. With NAC treatment, responses to drug activity
are rapidly available, and valuable information may be collected
from proof-of-concept studies that involve a relatively small
number of patients.
It is the general consensus that the absence of
residual invasive cancer in the breast and lymph nodes is the
preferred definition of pCR that provides the best indicator of
clinical outcome (23).
For breast tumors overexpressing HER-2,
HER-2-targeted agents have been reported to improve the pCR rate
and DFS. A novel anti-HER-2 antibody, known as pertuzumab, obtained
FDA approval for the treatment of HER-2-positive BC due to the
significant improvement in pCR when combined with trastuzumab as
part of the NAC regimen (24).
Patients with triple-negative breast cancer (TNBC) demonstrate a
worse prognosis once pCR is not achieved; however, they display an
increased probability of obtaining pCR when compared with non-TNBC
patients. Once TNBC patients attain pCR following NAC treatment,
they demonstrate an excellent survival rate (25).
Angiogenesis is important for cancer cells to
proliferate, grow and metastasize. Inhibiting angiogenesis
therefore leads to inhibition of these characteristics, which is
may be detrimental to tumor growth and survival (8). Currently, there are numerous agents
that target the neovascularization pathway; however, a predictive
marker of response remains to be detected (26). Although numerous types of
malignancies are hypervascular tumors, it is uncertain to what
extent the angiogenesis signaling pathway is involved, as
anti-angiogenetic agents only moderately prolong the overall
survival rate of these cancers (27–29).
The plasma levels of vascular growth factor-A (VEGFA) prior to
treatment have been evaluated retrospectively in a previous study
(30). Until recently, elevated
levels of VEGFA have been demonstrated to be an indicator of poor
prognosis; however, it is unable to predict response to
anti-angiogenic therapies, including bevacizumab (31). MVD has become the pathological gold
standard for assessing angiogenesis in solid tumors. Studies have
demonstrated that the angiogenic potential of BC, as assessed by
MVD, correlates with the potential of tumor progression and
metastasis, and therefore may be used to predict clinical outcome
(8,32).
Traditionally, pan endothelial markers, including
CD31, CD34 and Von Willebrand factor are used to assess MVD
histologically (33,34). However, endoglin is reportedly a
more effective marker than CD34 and CD31 in the evaluation of
neovascularization of tumors, as it demonstrates a greater affinity
for endothelial cells in tumor tissues, whereas CD34 and CD31 react
nonspecifically with pathological and healthy vessels (12).
A previously study demonstrated that MVD, as
determined by endoglin staining, was correlated positively with
HER-2 expression, and negatively with hormone receptor expression.
The importance of MVD on overall survival is greater for early
stage BCs (18). The present study
revealed that MVD, as evaluated by endoglin staining, correlated
with tumor response to NAC treatment. Therefore, endoglin may be a
suitable predictor for patient response following NAC
treatment.
Endoglin is an accessory receptor for TGF and is
upregulated during hypoxia via induction of hypoxia-inducible
factor 1α. Therefore, its expression is elevated in the actively
proliferating endothelium (35,36).
A clear positive correlation has been reported between several
markers of cell proliferation, including cyclin-A and Ki-67, and
endoglin expression levels. Therefore, endoglin has been suggested
as an appropriate marker for tumor-associated neovascularization
(36). Additionally, endoglin has
been demonstrated to be a potential marker for tumor diagnosis and
prognosis in a previous study (36).
As high endoglin expression correlates with the
rapid proliferation of tumor cells, and as chemotherapy effective
for inhibiting the growth of rapidly proliferating tumor cells
(37), this may explain why high
endoglin expression correlated with an improved response rate of
primary tumors to NAC treatment in the present study. However,
whether this response translates to improved DFS or overall
survival remains to be elucidated.
The present study additionally demonstrated that
mTOR expression levels were elevated in tumors responsive to NAC.
Endocrine therapy inhibits the growth-promoting effects of estrogen
via ERs, and may therefore be considered as the cornerstone of
direct target therapy. Approximately 70–75% of BCs express ERs,
indicating a high level of dependence on estrogen for tumor growth
(38). Although endocrine therapy
continues to be an effective treatment for ER-positive (ER+) BC,
many patients with advanced ER + BC develop de novo or
acquired resistance and require more intensive and toxic
treatments, such as chemotherapy. Novel approaches which augment
the benefit of existing endocrine therapies, by prolonging time to
disease progression, preventing or overcoming resistance, and
delaying the use of chemotherapy are required. The PI3K/Akt/mTOR
signaling pathway is a key intracellular signaling system that
induces cellular growth and survival. A previous study demonstrated
that the hyper activation of this signaling pathway is implicated
in the tumorigenesis of ER+ BC and resistance to endocrine therapy.
Furthermore, a previous study reported that inhibition of the
PI3K/AKT/mTOR signaling pathway may augment the benefit of
endocrine therapy in ER+ BC (39).
AspCR is considered to be the best response for cancer cells that
survive following NAC, elevated mTOR expression may serve as an
alternative signaling pathway for residual tumors. Further
follow-up is required to assess the outcome of these patients.
The limitations of the present study included the
use of insufficient samples for further analysis. This was due to
the fact that patients and doctors in Taiwan remain hesitant to
accept NAC, even though NAC is used in routine clinical practice in
numerous Western countries. Furthermore, a number of patients
achieved pCR; therefore, there were no residual tumors for the
assessment of mTOR expression levels. Additionally, there was a
limited duration of follow-up; therefore, it could not be confirmed
whether response to NAC or the expression of endoglin prior to NAC
correlated with improved DFS or overall survival.
In conclusion, the present study demonstrated that
the expression of endoglin in BC tissue samples prior to NAC may be
a useful predictor of treatment response. Long-term follow-up of
clinical outcome is required to explain the elevation of mTOR
expression levels following NAC treatment in responsive but non-pCR
tumors.
Acknowledgements
The authors would like to thank Mr. Hsiao JK, MS
(Kaohsiung, Taiwan), for his technical support, the Tissue Bank and
Biobank Laboratory (grant no. CLRPG8B00330), Chang Gung Medical
Foundation (grant no. CMRPG890112) and the Kaohsiung Chang Gung
Memorial Hospital for providing the materials.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dai X, Xiang L, Li T and Bai Z: Cancer
hallmarks, biomarkers and breast cancer molecular subtypes. J
Cancer. 7:1281–1294. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cianfrocca M and Gradishar W: New
molecular classifications of breast cancer. CA Cancer J Clin.
59:303–313. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gralow JR, Burstein HJ, Wood W, Hortobagyi
GN, Gianni L, von Minckwitz G, Buzdar AU, Smith IE, Symmans WF,
Singh B and Winer EP: Preoperative therapy in invasive breast
cancer: Pathologic assessment and systemic therapy issues in
operable disease. J Clin Oncol. 26:814–819. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
von Minckwitz G, Untch M, Blohmer JU,
Costa SD, Eidtmann H, Fasching PA, Gerber B, Eiermann W, Hilfrich
J, Huober J, et al: Definition and impact of pathologic complete
response on prognosis after neoadjuvant chemotherapy in various
intrinsic subtypes. J Clin Oncol. 30:1796–1804. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cortazar P, Zhang L, Untch M, Mehta K,
Costantino JP, Wolmark N, Bonnefoi H, Cameron D, Gianni L,
Valagussa P, et al: Pathological complete response and long-term
clinical benefit in breast cancer: The CTNeoBC pooled analysis.
Lancet. 384:164–172. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Prowell TM and Pazdur R: Pathological
complete response and accelerated drug approval in early breast
cancer. N Engl J Med. 366:2438–2441. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Folkman J, Watson K, Ingber D and Hanahan
D: Induction of angiogenesis during the transition from hyperplasia
to neoplasia. Nature. 339:58–61. 1989. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gasparini G: Clinical significance of the
determination of angiogenesis in human breast cancer: Update of the
biological background and overview of the Vicenza studies. Eur J
Cancer. 32A:1–2493. 1996.
|
|
10
|
Uzzan B, Nicolas P, Cucherat M and Perret
GY: Microvessel density as a prognostic factor in women with breast
cancer: A systematic review of the literature and meta-analysis.
Cancer Res. 64:2941–2955. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li C, Hampson IN, Hampson L, Kumar P,
Bernabeu C and Kumar S: CD105 antagonizes the inhibitory signaling
of transforming growth factor beta1 on human vascular endothelial
cells. FASEB J. 14:55–64. 2000.PubMed/NCBI
|
|
12
|
Tanaka F, Otake Y, Yanagihara K, Kawano Y,
Miyahara R, Li M, Yamada T, Hanaoka N, Inui K and Wada H:
Evaluation of angiogenesis in non-small cell lung cancer:
Comparison between anti-CD34 antibody and anti-CD105 antibody. Clin
Cancer Res. 7:3410–3415. 2001.PubMed/NCBI
|
|
13
|
Schiff R, Massarweh SA, Shou J, Bharwani
L, Mohsin SK and Osborne CK: Cross-talk between estrogen receptor
and growth factor pathways as a molecular target for overcoming
endocrine resistance. Clin Cancer Res. 10 Suppl:331S–336S. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Berns K, Horlings HM, Hennessy BT,
Madiredjo M, Hijmans EM, Beelen K, Linn SC, Gonzalez-Angulo AM,
Stemke-Hale K, Hauptmann M, et al: A functional genetic approach
identifies the PI3K pathway as a major determinant of trastuzumab
resistance in breast cancer. Cancer Cell. 12:395–402. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Baselga J, Campone M, Piccart M, Burris HA
III, Rugo HS, Sahmoud T, Noguchi S, Gnant M, Pritchard KI, Lebrun
F, et al: Everolimus in postmenopausal hormone-receptor-positive
advanced breast cancer. N Engl J Med. 366:520–529. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Andre F, O'Regan R, Ozguroglu M, Toi M, Xu
B, Jerusalem G, Masuda N, Wilks S, Arena F, Isaacs C, et al:
Everolimus for women with trastuzumab-resistant, HER2-positive,
advanced breast cancer (BOLERO-3): A randomised, double-blind,
placebo-controlled phase 3 trial. Lancet Oncol. 15:580–591. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rau KM, Huang CC, Chiu TJ, Chen YY, Lu CC,
Liu CT, Pei SN and Wei YC: Neovascularization evaluated by CD105
correlates well with prognostic factors in breast cancers. Exp Ther
Med. 4:231–236. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Health Promotion Administration, Ministry
of Health and Welfare, Taiwan, . 2013, Taiwan Cancer Registry
annual report. Taiwan, Taipei: 2016, http://www.hpa.gov.tw/File/Attach/5191/File_6166.pdfJune
14–2017
|
|
20
|
American Joint Committee on Cancer, .
https://cancerstaging.org/references-tools/deskreferences/Pages/default.aspxSeptember
9–2017
|
|
21
|
Sorlie T, Perou CM, Tibshirani R, Aas T,
Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey
SS, et al: Gene expression patterns of breast carcinomas
distinguish tumor subclasses with clinical implications. Proc Natl
Acad Sci USA. 98:10869–10874. 2001; View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Berruti A, Brizzi MP, Generali D, Ardine
M, Dogliotti L, Bruzzi P and Bottini A: Presurgical systemic
treatment of nonmetastatic breast cancer: Facts and open questions.
Oncologist. 13:1137–1148. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
von Minckwitz G, Untch M, Blohmer JU,
Costa SD, Eidtmann H, Fasching PA, Gerber B, Eiermann W, Hilfrich
J, Huober J, et al: Definition and impact of pathologic complete
response on prognosis after neoadjuvant chemotherapy in various
intrinsic breast cancer subtypes. J Clin Oncol. 30:1796–1804. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gianni L, Pienkowski T, Im YH, Roman L,
Tseng LM, Liu MC, Lluch A, Staroslawska E, de la Haba-Rodriguez J,
Im SA, et al: Efficacy and safety of neoadjuvant pertuzumab and
trastuzumab in women with locally advanced, inflammatory, or early
HER2-positive breast cancer (NeoSphere): A randomised multicentre,
open-label, phase 2 trial. Lancet Oncol. 13:25–32. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liedtke C, Mazouni C, Hess KR, André F,
Tordai A, Mejia JA, Symmans WF, Gonzalez-Angulo AM, Hennessy B,
Green M, et al: Response to neoadjuvant therapy and long-term
survival in patients with triple-negative breast cancer. J Clin
Oncol. 26:1275–1281. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schneider BP, Shen F and Miller KD:
Pharmacogenetic biomarkers for the prediction of response to
antiangiogenic treatment. Lancet Oncol. 13:e427–e436. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pang R and Poon RT: Angiogenesis and
antiangiogenic therapy in hepatocellular carcinoma. Cancer Lett.
242:151–167. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Webber K, Cooper A, Kleiven H, Yip D and
Goldstein D: Management of metastatic renal cell carcinoma in the
era of targeted therapies. Intern Med J. 41:594–605. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Llovet JM, Ricci S, Mazzaferro V, Hilgard
P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A,
et al: Sorafenib in advanced hepatocellular carcinoma. N Engl J
Med. 359:378–390. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fakhrejahani E and Toi M: Antiangiogenesis
therapy for breast cancer: An update and perspectives from clinical
trials. Jpn J Clin Oncol. 44:197–207. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Burstein HJ, Chen YH, Parker LM, Savoie J,
Younger J, Kuter I, Ryan PD, Garber JE, Chen H, Campos SM, et al:
VEGF as a marker for outcome among advanced breast cancer patients
receiving anti-VEGF therapy with bevacizumab and vinorelbine
chemotherapy. Clin Cancer Res. 14:7871–7877. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kraby MR, Krüger K, Opdahl S, Vatten LJ,
Akslen LA and Boflin AM: Microvascular proliferation in luminal A
and basal-like breast cancer subtypes. J Clin Pathol. 68:891–897.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fox SB, Gasparini G and Harris AL:
Angiogenesis: Pathological, prognostic, and growth-factor pathways
and their link to trial design and anticancer drugs. Lancet Oncol.
2:278–289. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mineo TC, Ambrogi V, Baldi A, Rabitti C,
Bollero P, Vincenzi B and Tonini G: Prognostic impact of VEGF,
CD31, CD34, and CD105 expression and tumour vessel invasion after
radical surgery for IB-IIA non-small cell lung cancer. J Clin
Pathol. 57:591–537. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Miller DW, Graulich W, Karges B, Stahl S,
Ernst M, Ramaswamy A, Sedlacek HH, Müller R and Adamkiewicz J:
Elevated expression of endoglin, a component of the
TGF-beta-receptor complex, correlates with proliferation of tumor
endothelial cells. Int J Cancer. 81:568–572. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Nassiri F, Cusimano MD, Scheithauer BW,
Rotondo F, Fazio A, Yousef GM, Syro LV, Kovacs K and Lloyd RV:
Endoglin (CD105): A review of its role in angiogenesis and tumor
diagnosis, progression and therapy. Anticancer Res. 31:2283–2290.
2011.PubMed/NCBI
|
|
37
|
Klijn JG, Berns EM and Foekens JA:
Prognostic factors and response to therapy in breast cancer. Cancer
Surv. 18:165–198. 1993.PubMed/NCBI
|
|
38
|
Systemic treatment of early breast cancer
by hormonal, cytotoxic, or immune therapy. 133 randomised trials
involving 31,000 recurrences and 24,000 deaths among 75,000 women.
Early Breast Cancer Trialists' Collaborative Group. Lancet.
339:71–85. 1992.PubMed/NCBI
|
|
39
|
Ciruelos Gil EM: Targeting the
PI3K/AKT/mTOR pathway in estrogen receptor-positive breast cancer.
Cancer Treat Rev. 40:862–871. 2014. View Article : Google Scholar : PubMed/NCBI
|