Introduction
Tongue cancer is one of the most globally common
malignant tumors in the oral and maxillofacial region (1,2).
However, the clinical symptoms of early tongue cancer are not
readily observed, thereby rendering a timely diagnosis difficult.
Typically, an imaging examination is performed to ensure accurate
preoperative staging and for surgical planning of the tongue mass
excision. Magnetic resonance imaging (MRI) has been widely
recognized as an ideal technique in the diagnosis of tongue cancer
due to the markedly high resolution of soft tissue that it
provides. Generally, abnormal MRI signals are flagged as lesions.
In addition, neoplastic lesions in the tongue that are located
towards the oral floor are readily detected on coronal images
(3,4). However, it is difficult to detect
potential neoplastic lesions and micrometastasis within lymph nodes
using MRI, particularly neoplasms that measure <1 cm in diameter
(5). Therefore, the sensitivity
and histological specificity of traditional MR must be improved for
the effective and early diagnosis of tongue cancer. Furthermore,
imaging modalities that provide functional and molecular imaging
would be more advantageous when compared with traditional
morphological imaging (6,7). Accordingly, advances in nano-micelle
science have led to the development of a non-invasive reagent that
provides molecular imaging with the potential for sequential and
longitudinal monitoring. In particular, super-paramagnetic iron
oxide nanoparticles (termed SPIO) have been demonstrated as a
highly effective contrast agent for MRI (8). Specifically, SPIO provide high
magnetic signal strength and relatively low cytotoxicity (9,10).
However, crude SPIO lack specificity towards a pathological site,
which potentially limits their applications. Furthermore, SPIO have
a highly hydrophobic surface, which may be efficiently coated with
plasma components, thereby leading to the rapid endocytosis of
these particles by the reticuloendothelial system (11). Therefore, surface modification of
SPIO is essential for most bio-associated applications.
To date, various nano-sized particles, including
liposomes, micelles and vesicles, have been utilized as carriers
for the delivery of SPIO to sites of interest (12,13).
Among these various modifications, micelles composed of
polyethylene glycol (PEG) have been found to block copolymers with
good biocompatibility. For example, a PEG outer shell for
nanoparticles imparts a ‘stealth’ quality to hydrophobic SPIO,
which helps to avoid potential exposure of the SPIO surface and
adsorption of blood proteins (14). In addition, poly-caprolactone (PCL)
has been widely investigated and has been applied to micelles due
to its high colloidal stability compared with small molecular
surfactants, and its enhanced solubilization (15,16).
Furthermore, PEG-PCL micelles provide a stable interface between
their inner core and the aqueous environment, and this enhances the
biocompatibility and biodegradability of these micelles to improve
their efficacy (17,18). In the present study, polymeric
micelles were designed, which incorporate a diblock copolymer of
PEG and PCL. The PEG-PCL block copolymers form bilayer micelles
that are able to encapsulate SPIO into their hydrophobic cores
(14,15).
Malignant cells, due to their high rate of cell
division, have an increased requirement for folate, as it is as an
essential component of single-carbon metabolism and nuclear
glucoside synthesis. Accordingly, the folate receptor has been
found to be overexpressed by a wide range of malignant tissues,
including ovarian, breast, oropharyngeal and liver cancer tissues
(15–17,19–21).
Furthermore, the binding efficacy of folate to the folate receptors
expressed by tumor cells is high, which renders folate an ideal
candidate for the targeted delivery of vehicles to tumor cells. To
date, folic acid (FA) has been applied as a targeting ligand in
tumor imaging diagnosis and cancer chemotherapy studies (15,19,22).
To the best of our knowledge, only a small number of
studies have described the targeted imaging of tongue cancer
(19–23). Therefore, in the present study,
folate was attached to the surface layer of SPIO-PEG-PCL copolymers
to achieve functional micelles that target tongue cells. The aim
was to produce SPIO-loaded micelles that would represent a
T2-negative contrast agent, which could be detected by
MRI to evaluate their tumor targeting efficiency noninvasively,
thereby leading to the development of a method for the early
detection of tongue cancer.
Materials and methods
Material preparation and
characterization
Monomethoxy PEG and PCL were purchased from
Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). Copolymers of
PEG-PCL and FA-PEG-PCL were synthesized via multistep reactions and
purified according to previously reported methods (23,24).
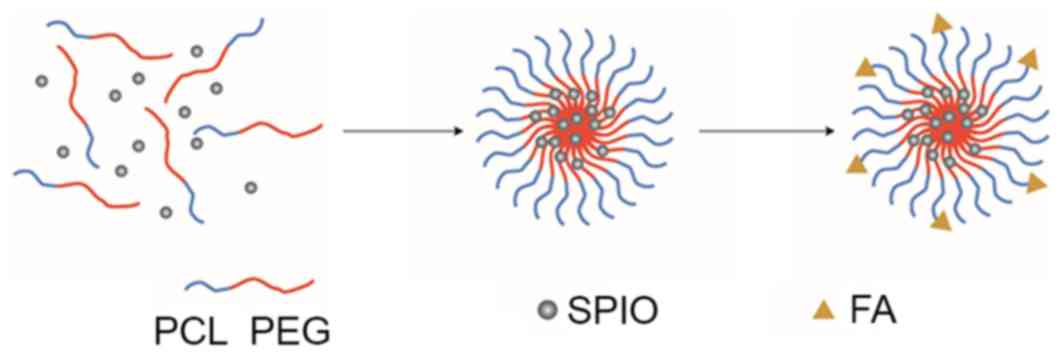
The schematic illustration of folate-targeted SPIO-PEG-PCL micelle
formation is presented in Fig. 1.
SPIO were prepared and encapsulated inside the micelle cores as
previously reported (25).
Particle sizes of the obtained nanovesicles were
determined using a Brookhaven BI-200SM dynamic light scattering
(DLS) instrument (Brookhaven Instruments Corp., Holtsville, NY,
USA). A 532-nm vertically polarized argon ion laser was used as the
light source. Measurements were made at a 90° angle at 25°C and
scattered light was collected using an autocorrelator. The particle
size of each sample was measured five times. Transmission electron
microscopy (TEM) images were obtained with a JEOL TEM-2010HR
microscope (JEOL Ltd., Tokyo, Japan) operated at 160 kV.
The loading density of the SPIO polymeric micelles
was determined using a polarized Zeeman Atomic Absorption
Spectrophotometer (Z-2000; Hitachi High Technologies, Tokyo,
Japan). Briefly, freeze-dried powders of 5-ml SPIO-loaded
nanovesicle solutions were weighed and were combined with 1 M
hydrochloric acid (HCl; 10 wt. % in water) to accelerate polymer
degradation. Complete dissolution of the SPIO was achieved after 3
days. Fe concentrations were detected at 248.3 nm, the absorption
wavelength specific for Fe, according to a previously established
calibration curve (26). SPIO
loading density was calculated as the ratio of the encapsulated
SPIO over the total vesicle weight.
Cell culture
Tca-8113 (Institute of Biochemistry and Cell
Biology; Chinese Academy of Sciences, Shanghai, China) is a human
tongue cancer cell line that overexpresses cell surface receptors
for folate. Tca-8113 cells were grown in Minimal Essential Media
(MEM; Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% heat-inactivated fetal bovine serum
(Invitrogen; Thermo Fisher Scientific, Inc.) and were maintained at
37°C in a humidified atmosphere with 5% CO2.
Biocompatibility assay
Methyl thiazolyl tetrazolium (MTT; Sigma-Aldrich;
Merck KGaA) was used to assay the cytotoxicity of folate-targeted
SPIO-PEG-PCL and folate-free SPIO-PEG-PCL micelles towards Tca-8113
cells. Briefly, Tca-8113 cells were seeded in 96-well plates
(1×105 cells/well) and the cells were incubated with
graded dilutions (1-, 2-, 4-, 8-, 16-, 32-, and 64-fold) of
nanovesicle solutions in MEM medium. The plates were incubated at
37°C in a humidified atmosphere with 5% CO2 for 3 days.
Subsequently, the folate-targeted SPIO-PEG-PCL micelles were washed
twice with phosphate-buffered saline (PBS) and incubated in fresh
medium containing 20 µl MTT solution in PBS (5 mg/ml) at 37°C.
After 4 h, the supernatant in each well was discarded and 150 ml
dimethyl sulfoxide (Sigma-Aldrich; Merck KGaA) was added to each
well to dissolve the formazen. Following gentle agitation of the
plates for 10 min, the optical density of each well at a wavelength
of 570 nm was recorded using an Infinite F200 multimode plate
reader (Tecan, Mȁnnedorf, Switzerland). Three duplicates were
measured for each experimental sample.
In vitro MRI
Tca-8113 cells (1×106 cells/well) were
incubated at 37°C with folate-targeted or folate-free SPIO-PEG-PCL
micelles at an Fe concentration of 80 µg/ml in folate-free MEM.
Following incubation periods of 0.5, 1, and 2 h in a humidified
atmosphere with 5% CO2 at 37°C, the cells were washed
with PBS three times, trypsinized, mixed with a 2% agarose solution
in PBS and scanned with a 3.0 T MRI scanner (Magnetom Avanto;
version Syngo MR B17, Siemens Healthcare Sector, Erlangen, Germany)
at room temperature. A spin echo (SE) T1-weighted
sequence [repetition time (TR), 300 msec; echo time
(TE), 17 msec], a fast spin-echo (FSE)
T2-weighted sequence (T2WI; TR, 3,000 msec;
TE, 105 msec), and T2 mapping (TR,
2,000 msec, TE, 30, 60, 90, and 120 msec, flip angle,
180°, total duration, 4 min 13 sec) were acquired with the
following parameters: Head coil; axial scanning; slice thickness,
2.0 mm; slice spacing, 0.2 mm; field-of-view, 180×180
mm2; and matrix, 256×256. MapIT software (version, Syngo
MR B17; Siemens Healthcare Sector) was applied to measure the
T2 relaxation time at the work station, with a circular
region of interest (ROI; area, 44.9 mm2). The shape and
area of the ROI were consistent. Relative T2 relaxation
time were calculated as follows (26):
T2=[(T'-T0)/T0]x100%, where T'
indicates T2 relaxation time obtained at different
points during incubation and T0 represents the
T2 relaxation time prior to incubation.
Prussian blue staining
Tca-8113 cells (5×105 cells/ml) were
incubated for 1 h at 37°C with folate-targeted and folate-free
SPIO-PEG-PCL micelles in folate-free RPMI-1640 medium (Gibco;
Thermo Fisher Scientific, Inc.) containing 80 µg/ml Fe (Gibco;
Thermo Fisher Scientific, Inc.; 61870-036). The cells were washed
three times with folate-free RPMI-1640 medium and trypsinized (10-5
mol/l). The cell suspensions were subsequently washed with PBS
three times before being fixed with 4% glutaraldehyde. After 10
min, the cells were incubated at 37°C with 2 ml Prussian blue
solution that was composed of an equal volume of 2% HCl acid
aqueous solution and 2% potassium ferrocyanide (II) trihydrate.
After 30 min, the cells were briefly stained with 0.5% neutral red
for 3 min. The resulting staining was observed under a light
microscope. The intensity of staining was defined by eye with the
following scoring system: i) 0, no staining; ii) +, weak staining;
iii) ++, moderate staining; and iv) +++, strong staining (27).
Statistical analysis
Statistical analyses were conducted with SPSS
software (version 11.0; SPSS, Inc., Chicago, IL, USA). One-way
analysis of variance and a Bonferroni post hoc test were used to
identify differences between groups, and P<0.05 was considered
to indicate a statistically significant difference.
Results
Micelle size and biocompatibility
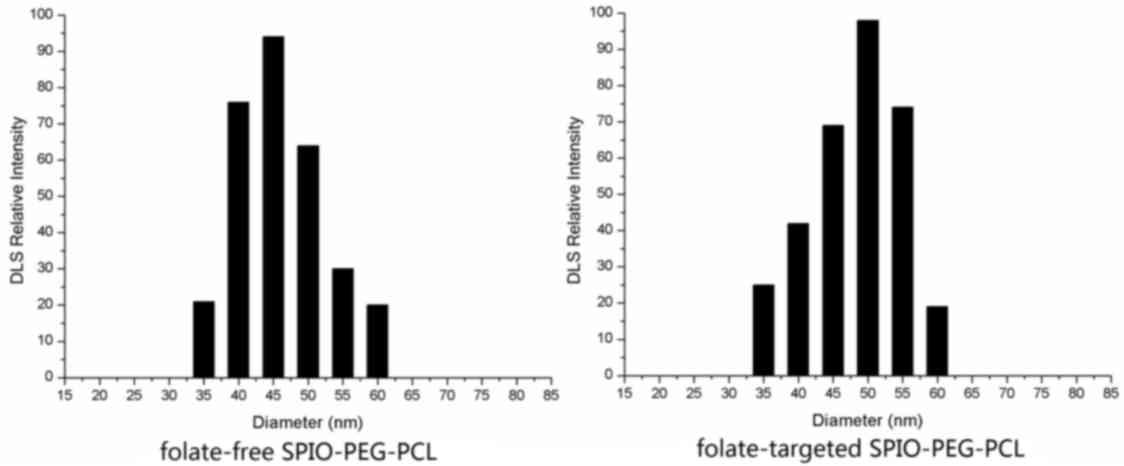
Sizes of the folate-free SPIO-PEG-PCL and
folate-targeted SPIO-PEG-PCL micelles were estimated to be 45.5 and
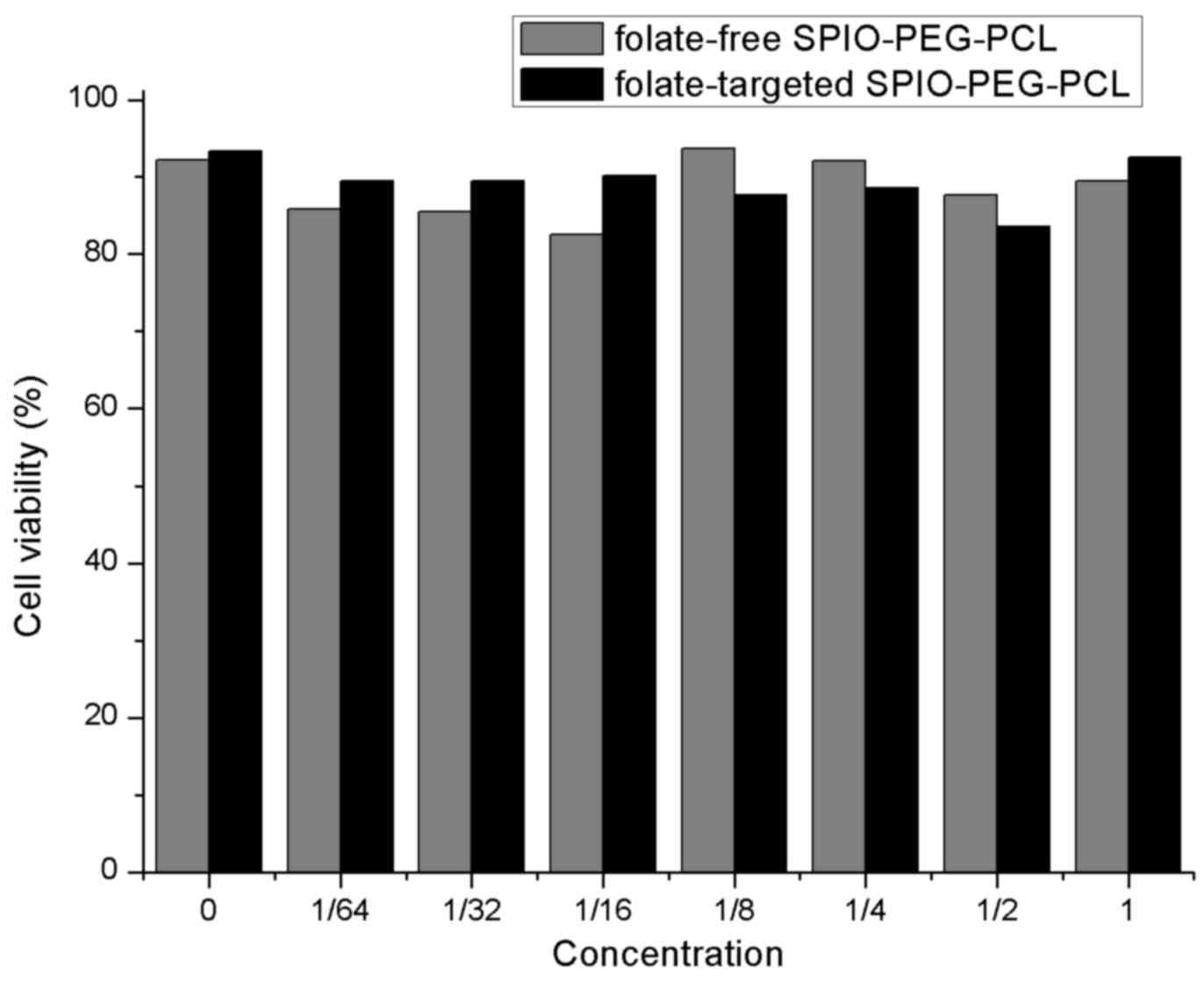
50.2 nm, respectively, according to the DLS measurement (Fig. 2). In the MTT viability assays of
the Tca-8113 cells, various SPIO concentrations were evaluated. No
statistically significant differences were identified between the
folate-free SPIO-PEG-PCL and folate-targeted SPIO-PEG-PCL groups
(F=0.698; P=0.676), thereby demonstrating that increased
concentrations of either type of SPIO-PEG-PCL micelle did not
increase cytotoxicity (Fig.
3).
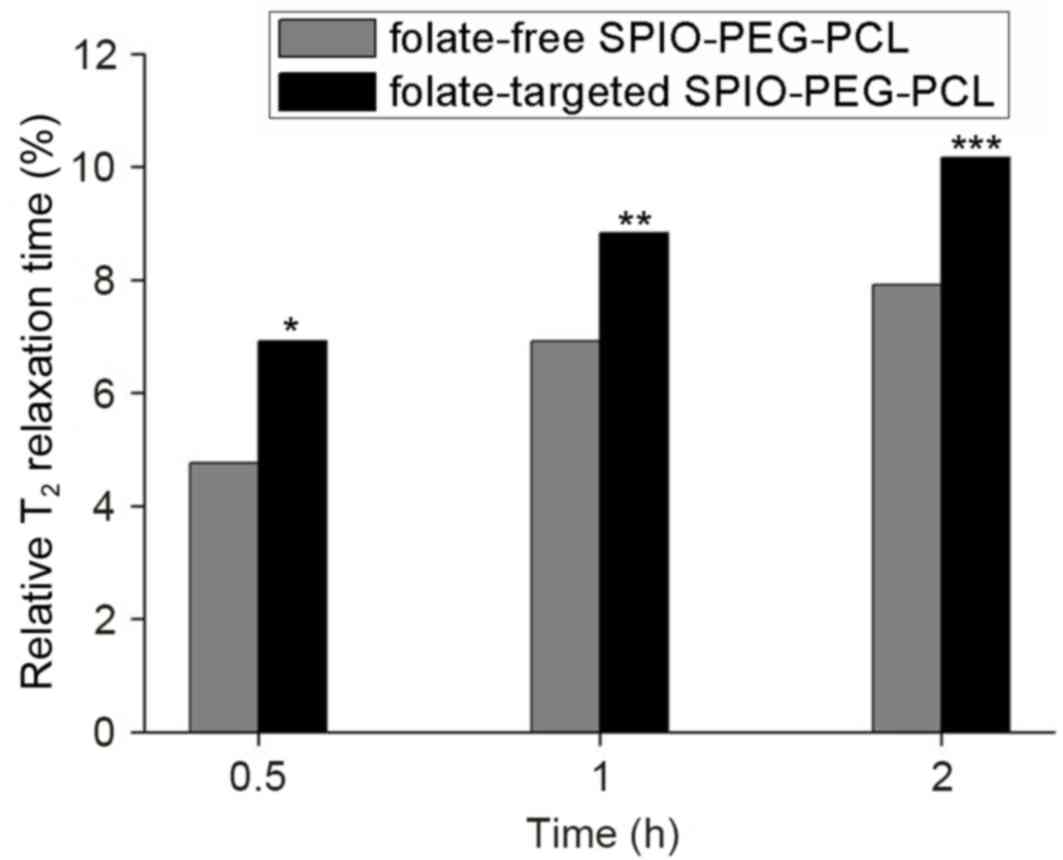
In vitro MRI
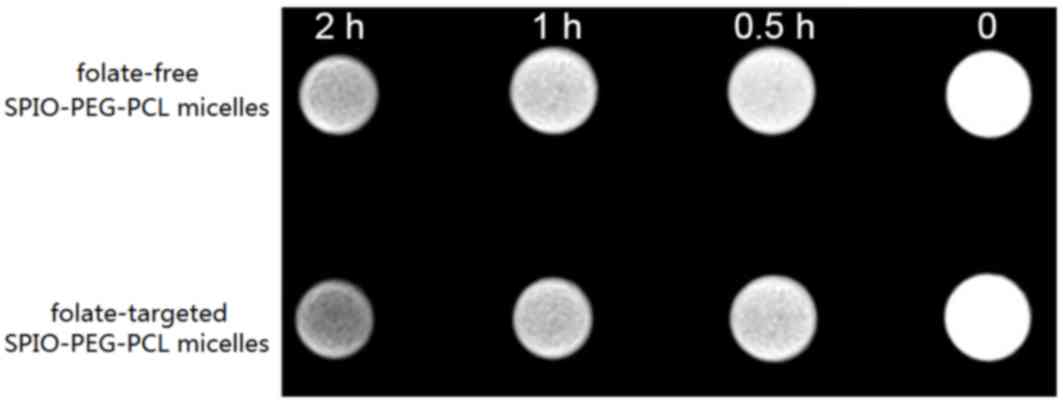
To validate the tumor targeting ability of the
nanoparticles generated, MRI signal intensity was measured for each
set of cells that was incubated with folate-targeted SPIO-PEG-PCL
and folate-free SPIO-PEG-PCL micelles, respectively. The
T2WI signal intensity of the two sets of cells decreased
marginally after 0.5 h (Fig. 4),
and a time-dependent decrease in signal intensity was observed for
the two groups. However, compared with the folate-targeted
SPIO-PEG-PCL micelles, the decrease in signal intensity for the
folate-free SPIO-PEG-PCL micelles was less marked (Fig. 4). Relative T2 relaxation
time was calculated and, under the same conditions, the MRI signals
of the cells incubated with the folate-targeted SPIO-PEG-PCL
micelles exhibited a significantly greater weakening in signal
compared with the folate-free SPIO-PEG-PCL micelles (P=0.002;
Fig. 5).
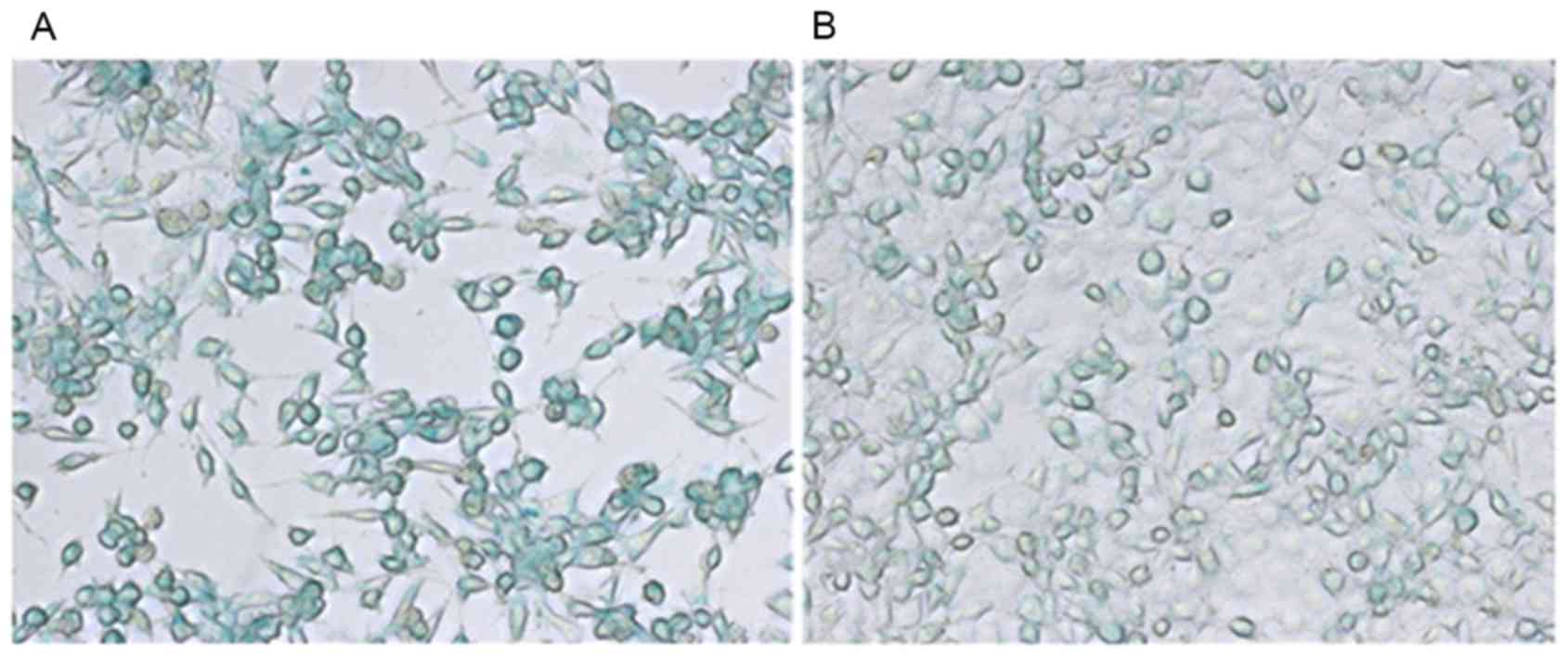
Prussian blue staining
Entrapment of the SPIO in the nanomicelles allowed
direct visualization of their uptake by Tca-8113 cells. As shown in
Fig. 6, Prussian blue staining
experiments demonstrated that the cells incubated with the
folate-targeted SPIO-PEG-PCL micelles exhibited strong staining
(+++), which was markedly stronger staining than the cells that
were incubated with the folate-free SPIO-PEG-PCL micelles (moderate
staining, ++).
Discussion
Advances in imaging technology and their application
to cellular biology have brought molecular imaging to the forefront
(28). Simultaneously, the
identification of molecular targets has been applied to the
development and design of various molecular imaging probes for
solid tumors (29). As a
diagnostic technique, MRI is currently one of the most powerful,
non-invasive imaging modalities that is used in clinical medicine
today (30), and SPIO represent
one of the most effective MRI contrast agents. In the present
study, polymeric micelles were generated for the delivery of SPIO,
and these SPIO were attached to folate to achieve a
targeted-micelle system. The folate receptor is overexpressed by a
wide range of malignant cells, including tongue cancer cells.
Therefore, the aim of the present study was to combine folate with
SPIO-PEG-PCL copolymers to provide tumor-specific imaging following
folate receptor-mediated endocytosis of these micelles. In the MRI
experiments conducted, this folate-targeted micelle system
effectively weakened the MRI signal of Tca-8113 cells. Prussian
blue staining further confirmed the endocytosis of the targeted
micelles by Tca-8113 cells. The folate-targeted SPIO-PEG-PCL
demonstrated potential as a platform for diagnostic imaging
applications.
It has been shown that tumor vasculature exhibits
poor architecture due to the absence of a pericyte lining and a
poor lymphatic drainage system (31). Correspondingly, this leaky
vasculature leads to a stronger differential interstitial pressure
at the center of tumors when compared with the tumor periphery, and
this is referred to as the enhanced permeability and retention
(EPR) effect (31,32). For particles ranging in size from
10–100 nm, the EPR effect is consistent with the preferential
accumulation of particles in a tumor and extended blood circulation
times (33,34). In the present study, DLS
measurement demonstrated that the mean diameter for the
folate-targeted PEG-PCL-SPIO micelle was ~50.2 nm. This diameter is
suitable for efficient targeting in MRI applications, it is highly
desirable for achieving a longer blood circulation time, and it
facilitates the uptake of SPIO by cancer cells (35).
In the present study, MTT assays were performed to
evaluate the possible cytotoxicity of the generated micelles and no
obvious decrease in cell viability was observed despite the
increasing concentrations of the polymers in these assays.
Furthermore, when the micelles were incubated in the 1-fold
dilution, the level of cytotoxicity detected was considered to be
acceptable. Thus, copolymers of the folate-targeted SPIO-PEG-PCL
exhibited minimal cytotoxicity towards the Tca-8113 cells
examined.
In the MRI conducted in the present study,
incubation of the Tca-8113 cells with the folate-targeted
SPIO-PEG-PCL micelles resulted in a significant decrease in signal
intensity and relative T2 relaxation time. Furthermore,
these same decreases were not observed for the Tca-8113 cells that
were incubated with the folate-free SPIO-PEG-PCL micelles.
Consistent with these results, stronger Prussian blue staining was
observed for the folate-targeted SPIO-PEG-PCL micelles versus the
folate-free SPIO-PEG-PCL micelles, thereby indicating a higher
intracellular Fe concentration in the former. Thus, cellular uptake
of the micelles appeared to be dependent on the presence of the
folate ligand. These results are consistent with those of Yang
et al (23) and Hong et
al (29), who demonstrated
that hydrophobic SPIO nanocrystals that were loaded into
folate-targeted SPIO-PEG-PCL micelles provided targeted delivery of
an anticancer drug to hepatocellular carcinoma.
Therefore, these folate-targeted SPIO-PEG-PCL
micelles demonstrated high sensitivity for MRI. This indicates that
this type of micelle has the potential capacity to be an ideal MRI
contrast agent for tongue tumors. Furthermore, the results of the
Prussian blue staining in the present study revealed that
folate-targeted micelles revealed a superior targeting effect on
tongue cancer cells than the folate-free micelles. Thus,
folate-targeted micelles have a greater potential as a drug
delivery system to treat tongue tumors than folate-free
micelles.
However, there were two main limitations associated
with the present study. The examination of folate targeting was
only performed in vitro and was not investigated in
vivo. In addition, the delivery efficiency of the micelles was
not evaluated using an anti-cancer drug. Thus, future studies will
include monitoring of the transfer efficiency of SPIO and
anti-cancer drugs to tongue cancers in vivo in order to
validate the present findings and to confirm the potential for this
method to provide early detection of tongue cancer.
In conclusion, compared with folate-free
SPIO-PEG-PCL micelles, folate-targeted SPIO-PEG-PCL micelles
exhibited superior targeting for Tca-8113 cells and achieved an
ideal imaging effect in vitro MRI. This novel targeted
micelle may be used as an effective contrast agent for the early
diagnosis of tongue cancer.
Acknowledgements
The authors received grants from the Natural Science
Foundation of Guangdong Province (grant no. 2014A030310484) and the
S&T Programs of Guangdong Province (grant no.
2012B031800086).
References
|
1
|
Hartl DM, Dauchy S, Escande C, Bretagne E,
Janot F and Kolb F: Quality of life after free-flap tongue
reconstruction. J Laryngol Otol. 123:550–554. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Patel SC, Carpenter WR, Tyree S, Couch ME,
Weissler M, Hackman T, Hayes DN, Shores C and Chera BS: Increasing
incidence of oral tongue squamous cell carcinoma in young white
women, age 18 to 44 years. J Clin Oncol. 29:1488–1494. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu Z, Wang H and Li Q: Tongue tumor
detection inmedical hyperspectral images. Sensors (Basel).
12:162–174. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ong CK and Chong VF: Imaging of tongue
carcinoma. Cancer Imaging. 6:186–193. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen W, Zhang C, Zhang S, Liang L, Zhang
B, Liu C, Zhang Z and Liang C: Application value of MRI combined
with positron emission tomography (PET)/CT in diagnosis and
preoperative staging of tongue squamous cell carcinoma. J Med
Imaging Radiat Oncol. 59:170–178. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim BY, Rutka JT and Chan WC:
Nanomedicine. N Engl J Med. 363:2434–2443. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Baetke SC, Lammers T and Kiessling F:
Applications of nanoparticles for diagnosis and therapy of cancer.
Br J Radiol. 88:201502072015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rosen JE, Chan L, Shieh DB and Gu FX: Iron
oxide nanoparticles for targeted cancer imaging and diagnostics.
Nanomedicine. 8:275–290. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Raji MA, Amara M, Amoabediny G, Tajik P,
Barin A, Magierowski S and Ghafar-Zadeh E: Cytotoxicity of
synthesized iron oxide nanoparticles: Toward novel biomarkers of
colon cancer. Conf Proc IEEE Eng Med Biol Soc. 2014:6179–6182.
2014; PubMed/NCBI
|
|
10
|
Landmark KJ, Dimaggio S, Ward J, Kelly C,
Vogt S, Hong S, Kotlyar A, Myc A, Thomas TP, Penner-Hahn JE, et al:
Synthesis, characterization, and in vitro testing of
superparamagneticiron oxide nanoparticles targeted using folic
acid-conjugated dendrimers. ACS Nano. 2:773–783. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hahn MA, Singh AK, Sharma P, Brown SC and
Moudgil BM: Nanoparticles as contrast agents for in-vivo
bioimaging: Current status and future perspectives. Anal Bioanal
Chem. 399:3–27. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rosen JE, Chan L, Shieh DB and Gu FX: Iron
oxide nanoparticles for targeted cancer imaging and diagnostics.
Nanomedicine. 8:275–290. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Barreto JA, O'Malley W, Kubeil M, Graham
B, Stephan H and Spiccia L: Nanomaterials: Applications in cancer
imaging and therapy. Adv Mater. 23:H18–H40. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hong G, Yuan R, Liang B, Shen J, Yang X
and Shuai X: Folate-functionalized polymeric micelle as hepatic
carcinoma-targeted, MRI-ultrasensitive delivery system of antitumor
drugs. Biomed Microdevices. 10:693–700. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nasongkla N, Bey E, Ren J, Ai H, Khemtong
C, Guthi JS, Chin SF, Sherry AD, Boothman DA and Gao J:
Multifunctional polymeric micelles as cancer-targeted,
MRI-ultrasensitive drug delivery systems. Nano Lett. 6:2427–2430.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li R, Li X, Xie L, Ding D, Hu Y, Qian X,
Yu L, Ding Y, Jiang X and Liu B: Preparation and evaluation of
PEG-PCL nanoparticles for local tetradrine delivery. Int J Pharm.
379:158–166. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pridgen EM, Langer R and Farokhzad OC:
Biodegradable, polymeric nanoparticle delivery systems for cancer
therapy. Nanomedicine (Lond). 2:669–680. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gong C, Shi S, Dong P, Kan B, Gou M, Wang
X, Li X, Luo F, Zhao X, Wei Y, et al: Synthesis and
characterization of PEG-PCL-PEG thermosensitive hydrogel. Int J
Pharm. 365:89–99. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hong G, Yuan R, Liang B, Shen J, Yang X
and Shuai X: Folate-functionalized polymeric micelle as hepatic
carcinoma-targeted, MRI-ultrasensitive delivery system of antitumor
drugs. Biomed Microdevices. 10:693–700. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xie M, Zhang H, Xu Y, Liu T, Chen S, Wang
J and Zhang T: Expression of folate receptors in nasopharyngeal and
laryngeal carcinoma and folate receptor-mediated endocytosis by
molecular targeted nanomedicine. Int J Nanomedicine. 8:2443–2451.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lu Y and Low PS: Immunotherapy of folate
receptor-expressing tumors: Review of recent advances and future
prospects. J Control Release. 91:17–29. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim YK, Minai-Tehrani A, Lee JH, Cho CS,
Cho MH and Jiang HL: Therapeutic efficiency of folated
poly(ethylene glycol)-chitosan-graft-polyethylenimine-Pdcd4
complexes in H-ras12V mice with liver cancer. Int J Nanomedicine.
8:1489–1498. 2013.PubMed/NCBI
|
|
23
|
Yang X, Deng W, Fu L, Blanco E, Gao J,
Quan D and Shuai X: Folate-functionalized polymeric micelles for
tumor targeted delivery of a potent multidrug-resistance modulator
FG020326. J Biomed Mater Res A. 86:48–60. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shuai X, Ai H, Nasongkla N, Kim S and Gao
J: Micellar carriers based on block copolymers of
poly(epsilon-caprolactone) and poly(ethylene glycol) for
doxorubicin delivery. J Control Release. 98:415–426. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sun S, Zeng H, Robinson DB, Raoux S, Rice
PM, Wang SX and Li G: Monodisperse MFe2O4 (M=Fe, Co, Mn)
nanoparticles. J Am Chem Soc. 126:273–279. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Feng ST, Li J, Luo Y, Yin T, Cai H, Wang
Y, Dong Z, Shuai X and Li ZP: pH-Sensitive nanomicelles for
controlled and efficient drug delivery to human colorectal
carcinoma LoVo cells. PloS One. 9:e1007322014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Prom-u-thai C, Dell B, Thomson G and
Rerkasem B: Easy and rapid detection of iron in rice grain.
ScienceAsia. 29:203–207. 2003. View Article : Google Scholar
|
|
28
|
Margolis DJ, Hoffman JM, Herfkens RJ,
Jeffrey RB, Quon A and Gambhir SS: Molecular imaging techniques in
body imaging. Radiology. 245:333–356. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hong GB, Zhou JX and Yuan RX:
Folate-targeted polymeric micelles loaded with ultrasmall
superparamagnetic iron oxide: Combined small size and high MRI
sensitivity. Int J Nanomedicine. 7:2863–2872. 2012.PubMed/NCBI
|
|
30
|
Bautista MC, Bomati-Miguel O, Zhao X,
Morales MP, Gonzalez-Carreno T, de Alejo Perez R, Ruiz-Cabello J
and Veintemillas-Verdaguer S: Comparative study of ferrofluids
based on dextran-coated iron oxide and metal nanoparticles for
contrast agents in magnetic resonance imaging. Nanotechnology.
15:154–159. 2004. View Article : Google Scholar
|
|
31
|
Maeda H, Nakamura H and Fang J: The EPR
effect for macromolecular drug delivery to solid tumors:
Improvement of tumor uptake, lowering of systemic toxicity, and
distinct tumor imaging in vivo. Adv Drug Deliv Rev. 65:71–79. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Greish K: Enhanced permeability and
retention of macromolecular drugs in solid tumors: A royal gate for
targeted anticancer nanomedicines. J Drug Target. 15:457–464. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Byrne JD, Betancourt T and Brannon-Peppas
L: Active targeting schemes for nanoparticle systems in cancer
therapeutics. Adv Drug Deliv Rev. 60:1615–1626. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ranganathan R, Madanmohan S, Kesavan A,
Baskar G, Krishnamoorthy YR, Santosham R, Ponraju D, Rayala SK and
Venkatraman G: Nanomedicine: Towards development of
patient-friendly drug-delivery systems for oncological
applications. Int J Nanomedicine. 7:1043–1060. 2012.PubMed/NCBI
|
|
35
|
Lee JH, Huh YM, Jun YW, Seo JW, Jang JT,
Song HT, Kim S, Cho EJ, Yoon HG, Suh JS, et al: Artificially
engineered magnetic nanoparticles for ultra-sensitive molecular
imaging. Nat Med. 13:95–99. 2007. View
Article : Google Scholar : PubMed/NCBI
|