Introduction
Glioma, a well known aggressive and malignant brain
tumor, accounts for ~30% of all brain and central nervous system
tumors and 80% of malignant tumors in the brain (1). Glioma can be classified as low-grade
or high-grade tumors, depending on the 2007 World Health
Organization (WHO) grading system (2). A number of risk factors contributing
to the development of glioma have been identified, including
radiation, use of exogenous hormones, consumption of coffee and
tea, smoking status and physical conditions (3,4).
Despite improvements in the therapeutic treatment strategies for
glioma, including surgery, radiotherapy, chemotherapy, gene
therapy, immunotherapy and other novel biological therapies,
patients with this disease exhibit a median survival of 15 months
(5). The primary reasons for the
poor prognosis of gliomas is recurrence and local invasion of the
tumor into normal brain tissues (6). Therefore, it is urgent to fully
understand the molecular and cellular mechanisms of glioma and
develop more effective strategies for this malignancy.
microRNAs (miRs) are a group of endogenous,
non-coding, short (~22 nucleotides) RNAs that negatively regulate
gene expression by base pairing with the 3′-untranslated regions
(3′UTRs) of their target genes, resulting in either mRNA
degradation or suppression of translation (7). Theoretically, a single miR can
modulate a number of target genes simultaneously, while a single
gene could be regulated by multiple miRs (8). Numerous studies have demonstrated
that miRs are involved in complex regulatory networks that are
implicated in a multitude of cellular processes, including cell
proliferation, cell cycle progression, apoptosis, differentiation,
invasion, migration and metastasis (9–11).
In addition, the abnormal expression of specific miRs serves vital
roles in tumorigenesis and tumor development in human cancer, as
miRs target a number of tumor suppressors and oncogenes (12). Expression levels of certain miRs
could serve as diagnostic and prognostic indicators for patients
with cancer (13). For example,
miR-140 is downregulated in glioma and low expression is associated
with a low WHO grade and Karnofsky Performance Score (KPS) in
patients with glioma. Upregulation of miR-140 significantly
inhibited glioma cell proliferation, migration and invasion through
directly targeting disintegrin and metalloproteinase
domain-containing protein 9 (14).
Therefore, miRs have potential as novel therapeutic targets for the
diagnosis and treatment of human cancer.
miR-216b has been demonstrated to serve key roles in
the tumorigenesis and tumor development in several types of human
cancer (15–17). However, the detailed regulatory
mechanisms of miR-216b in glioma remain unknown. Therefore, the
present study aimed to investigate the expression level and
biological roles of miR-216b in glioma, as well as its underlying
molecular mechanisms.
Materials and methods
Tissue samples
A total of 48 paired human glioma tissues and
matched normal tissues were collected from patients who underwent
surgical resection at the Department of Neurosurgery, The Second
Affiliated Hospital of Nanchang University (Nanchang, China)
between June 2014 and January 2016 and their details are presented
in Table I. None of these patients
were treated with radiotherapy, chemotherapy, gene therapy,
immunotherapy or other novel biological therapies. All tissues were
flash-frozen in liquid nitrogen immediately following collection
and then stored at −80°C until RNA extraction. The present study
was approved by the Ethics Committee of the Second Affiliated
Hospital of Nanchang University. Written informed consent was also
acquired from each patient.
 | Table I.Association between miR-216b and the
clinicopathological factors of patients with glioma. |
Table I.
Association between miR-216b and the
clinicopathological factors of patients with glioma.
|
|
| miR-216b
expression |
|
|---|
|
|
|
|
|
|---|
| Clinicopathological
factors | No. of cases | Low (n) | High (n) | P-value |
|---|
| Sex |
| |
| 0.658 |
|
Male | 28 | 15 | 13 |
|
|
Female | 20 | 12 | 8 |
|
| Age, years |
|
|
| 0.715 |
|
<55 | 22 | 13 | 9 |
|
|
≥55 | 26 | 14 | 12 |
|
| KPS |
|
|
| 0.018 |
|
≥80 | 25 | 10 | 15 |
|
|
<80 | 23 | 17 | 6 |
|
| WHO grade |
|
|
| 0.022 |
|
I–II | 23 | 9 | 14 |
|
|
III | 25 | 18 | 7 |
|
Cell lines
A total of five glioma cell lines (U87, U251, U373,
LN18, A172) and primary normal human astrocytes (NHA) were
purchased from the American Type Culture Collection (Manassas, VA,
USA). The origin of the U87 cell line is unknown, but it is a
likely glioblastoma cell line (18). U373 cell line, known as a U251
derivative (19), was acquired
from the National Infrastructure of Cell line Resource (Beijing,
China). Cells were maintained in Dulbecco's modified Eagle's medium
(DMEM) with 10% fetal bovine serum (FBS), 100 U/ml penicillin and
100 mg/ml streptomycin (all from Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA). All cells were cultured in a humidified
atmosphere at 37°C with 5% CO2.
Cell transfection
A mature miR-216b mimic and an miRNA mimic negative
control (miR-NC) were obtained from Chang Jing Bio-Tech, Ltd.
(Changsha, China). The miR-216b mimic sequence was
5′-AAAUCUCUGCAGGCAAAUGUGA-3′ and the miR-NC sequence was
5′-UUCUCCGAACGUGUCACGUTT-3′. Small interfering RNA targeting
metadherin (MTDH siRNA) and its NC siRNA were designed and
synthesized by Guangzhou RiboBio Co., Ltd. (Guangzhou, China). The
MTDH siRNA sequence was 5′-GCTGTTCGAACACCTCAAA-3′ and the NC siRNA
sequence was 5′-UUCUCCGAACGUGUCACGUTT-3′. For transfection, cells
were seeded into 6-well plates at a density of 60–70% confluence.
Cells were transfected with an miR-216b mimic (100 pmol), miR-NC
(100 pmol), MTDH siRNA (100 pmol) or NC siRNA (100 pmol) using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol. At 48
h post-transfection, cells were collected and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) was
performed to evaluate the transfection efficiency.
RT-qPCR
Tissue samples and cells were subjected to RNA
isolation using the TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) following the manufacturer's protocol. The
concentration and purity of total RNA was determined using a
NanoDrop 1000 spectrophotometer (NanoDrop; Thermo Fisher
Scientific, Inc., Wilmington, DE, USA). To measure miR-216b
expression, complementary DNA (cDNA) was synthesized using a
TaqMan® MicroRNA Reverse Transcription kit and qPCR was
conducted with a TaqMan® MicroRNA Assay kit (both from
Applied Biosystems; Thermo Fisher Scientific, Inc.). The cycling
conditions were as follows: 50°C for 2 min, 95°C for 10 min; 40
cycles of denaturation at 95°C for 15 sec and annealing/extension
at 60°C for 60 sec. The primers were designed as follows: miR-216b,
5′-AAATCTCTGCAGGCAAATGTGA-3′ (forward) and 5′-GTGCAGGGTCCGAGGT-3′
(reverse); U6, 5′-GCTTCGGCAGCACATATACTAAAAT-3′ (forward) and
5′-CGCTTCACGAATTTGCGTGTCAT-3′ (reverse). To detect MTDH mRNA
expression, cDNA was synthesized using Moloney-murine leukemia
virus reverse transcription system (Promega Corporation, Madison,
WI, USA), followed by qPCR using SYBR Premix Ex Taq (Takara,
Dalian, China). The cycling conditions were as follows: 5 min at
95°C, followed by 40 cycles of 95°C for 30 sec and 65°C for 45 sec.
The primers were as follows: MTDH, 5′-TGTTGAAGTGGCTGAGGG-3′
(forward) and 5′-CAGGAAATGATGCGGTTG-3′ (reverse); and GAPDH,
5′-GGTGAAGGTCGGAGTCAACG-3′ (forward) and
5′-CAAAGTTGTCATGGATGHACC-3′ (reverse). The miR-216b and MTDH mRNA
expression was normalized to those of U6 and GAPDH, respectively,
using the 2−ΔΔCq method (20).
MTT assay
Cell proliferation was evaluated using an MTT assay
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). Transfected cells
were harvested at 24 h post-transfection and then plated into
96-well plates at a density of 2,000 cells/well. Cells were
incubated in a humidified atmosphere at 37°C with 5% CO2
for 1, 2, 3 and 4 days. At specific time points, the MTT assay was
performed according to the manufacturer's protocol. A total of 10
µl MTT reagent (5 mg/ml) was added into each well and the plates
were incubated at 37°C for an additional 4 h. The culture medium
containing the MTT solution was removed and formazan crystals were
dissolved in 150 µl dimethyl sulfoxide (Sigma-Aldrich; Merck KGaA).
Cellular proliferation was determined using a microplate reader
(Bio-Rad Laboratories, Inc., Hercules, CA, USA) by measuring the
absorbance of the converted dye at 490 nm. All experiments were
performed in triplicate.
Transwell invasion assay
Transwell filters (diameter 12 mm; pore size 8-µm;
EMD Millipore, Billerica, MA, USA) coated with Matrigel®
(BD Biosciences, San Jose, CA, USA) were utilized to assess the
invasive ability of cells. A total of 48 h following transfection,
cells were collected and resuspended in FBS-free culture medium. A
total of 1×105 cells/400 µl were plated into the upper
chamber, while 600 µl culture medium containing 20% FBS was added
into the lower chamber. The plates were incubated at 37°C for 48 h.
Non-invasive cells were removed using a cotton swab. Invasive cells
were fixed with 100% methanol at room temperature for 10 min,
stained with 1% crystal violet at room temperature for 10 min,
washed at room temperature for three times and dried in air. Images
of the stained cells were captured and counted under a microscope
(magnification, ×200; IX53; Olympus Corporation, Tokyo, Japan) in
three independent fields for each Transwell filter.
The predication of miR-216b targeting
gene
The target genes of miR-216b were predicted using
PicTar (pictar.mdc-berlin. de/) and TargetScan (www.targetscan.org).
Luciferase reporter assay
For the luciferase reporter assay, the
pMIR-MTDH-3′UTR wild-type (Wt) and pMIR-MTDH-3′UTR mutant-type
(Mut) reporter vectors were synthesized by Chang Jing Bio-Tech,
Ltd. 293T cells were seeded into 24-well plates at a density of
50–60% confluence and co-transfected with an miR-216b mimic or
miR-NC, and the pMIR-MTDH-3′UTR Wt or the pMIR-MTDH-3′UTR Mut,
using Lipofectamine 2000. Following incubation for 48 h at 37°C in
5% CO2 for 48 h, the luciferase activity was detected
using the Dual-Luciferase® Reporter Assay system
(Promega Corporation). Firefly luciferase activity was normalized
to Renilla luciferase activity.
Western blotting
Transfected cells were harvested at 72 h
post-transfection and total protein was extracted using a
radioimmunoprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology, Haimen, China), which contained a protease
inhibitor. The concentration of total protein was measured using
the bicinchoninic assay kit (Pierce; Thermo Fisher Scientific,
Inc.). Equal amounts of protein (30 µg) were loaded onto 10%
SDS-PAGE gels, transferred onto polyvinylidene difluoride membranes
(EMD Millipore), blocked with 5% skimmed milk with TBS containing
0.1% Tween-20 (TBST) at room temperature for 1 h and then incubated
with the primary antibodies at 4°C overnight. The primary
antibodies used in the present study included mouse anti-human MTDH
monoclonal antibody (cat no. sc-517220; 1:1,000) and mouse
anti-human GAPDH monoclonal antibody (cat no. sc-32233 1:1,000),
rabbit anti-human polyclonal protein kinase B (AKT; cat no.
sc-8312; 1:1,000), mouse anti-human monoclonal phosphorylated
(p)-AKT (cat no. sc-514032; 1:1,000) and mouse anti-human
monoclonal phosphatase and tensin homolog (PTEN; cat no. sc-7974;
1:1,000) (all from Santa Cruz Biotechnology, Inc., Dallas, TX,
USA). The membranes were washed with TBST three times and probed
with the corresponding horseradish peroxidase-conjugated secondary
antibody (sc-2005; 1:5,000 dilution; Santa Cruz Biotechnology,
Inc.) at room temperature for 1 h. Finally, the protein bands were
visualized using Clarity Western ECL Substrate (Bio-Rad
Laboratories, Inc.) and analyzed using AlphaEase™ FC software
(version 4.0.1; ProteinSimple; Bio-Techne, Minneapolis, MN, USA).
GAPDH was used as an internal control.
Statistical analysis
Statistical analyses were performed using SPSS
(version 18.0; SPSS, Inc., Chicago, IL, USA). All results were
expressed as the mean ± standard deviation or box plots. The
Student's t-test and one-way analysis of variance (ANOVA) plus
multiple comparisons were used to analyze significant differences
between groups. The post hoc test used after ANOVA was
Student-Newman-Keuls. The correlation between miR-216b expression
and the clinicopathological factors was analyzed by the
χ2 test. Spearman's rank correlation coefficient
analysis was adopted to evaluate the association between miR-216b
and MTDH mRNA expression. P<0.05 was considered to indicate a
statistically significant difference.
Results
miR-216b is downregulated in glioma
tissues and cell lines
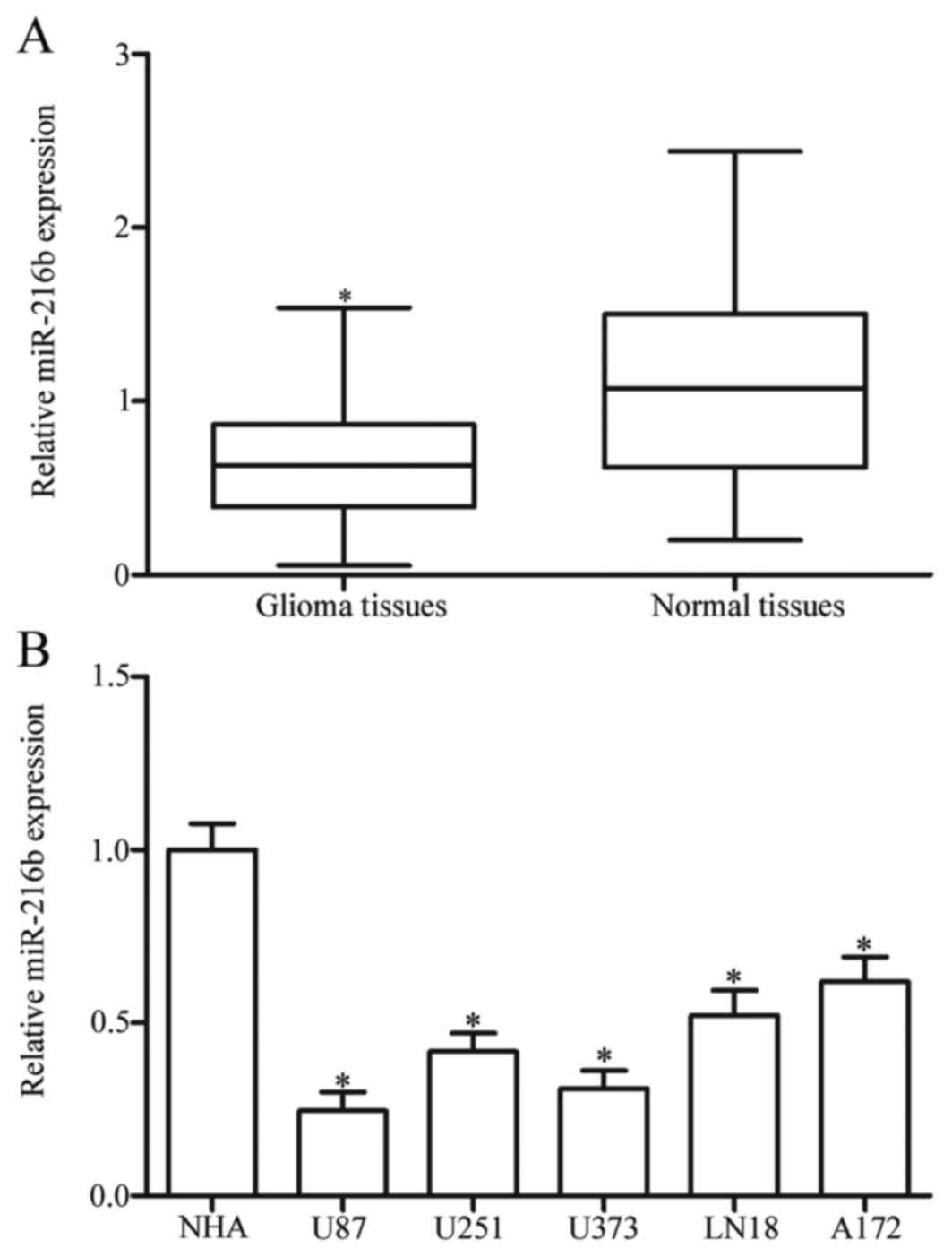
miR-216b expression was measured in glioma tissues
and matched normal tissues using RT-qPCR. The results demonstrated
that the expression level of miR-216b was significantly decreased
in the glioma tissues compared with the matched normal tissues
(P<0.05; Fig. 1A). miR-216b
expression in five glioma cell lines (U87, U251, U373, LN18 and
A172) and primary NHA was examined. As presented in Fig. 1B, all glioma cell lines exhibited
reduced expression of miR-216b in comparison with NHA (P<0.05).
These results supported the hypothesis that reduced miR-216b may be
involved in glioma formation and progression.
Low expression of miR-216b is
associated with the clinicopathological features of glioma
The association between miR-216b expression and the
clinicopathological features of patients with glioma was analyzed.
All glioma tissue samples were divided into two subgroups according
mean value (0.64); a low miR-216b group (27 cases) and a high
miR-216b group (21 cases). As demonstrated in Table I, low expression of miR-216b was
associated with a low KPS (P=0.018) and WHO grade (P=0.022) in
patients with glioma. However, no association was observed between
miR-216b expression and the sex (P=0.658) or age (P=0.715) of the
patients.
miR-216b inhibits glioma cell
proliferation and invasion
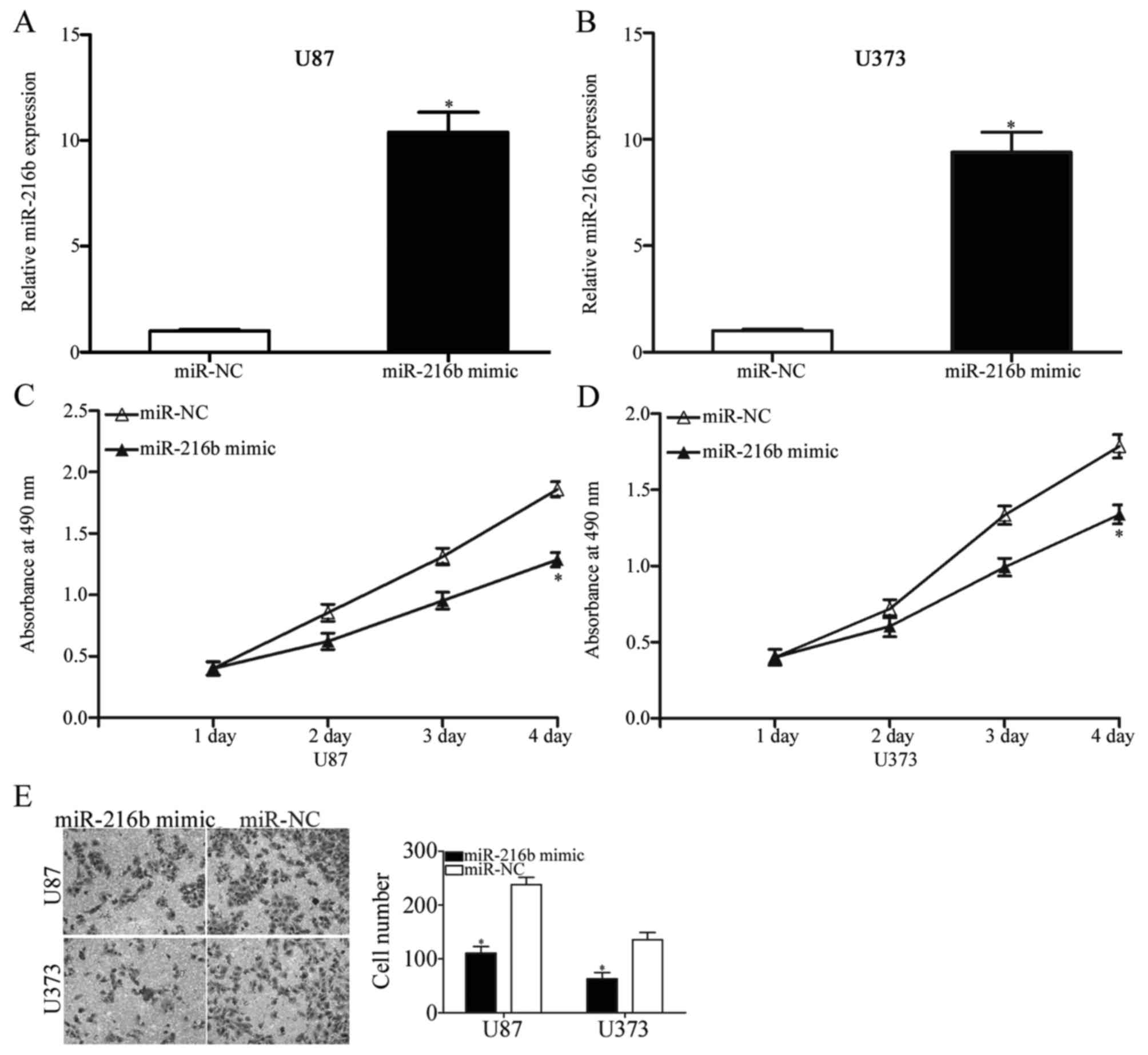
To investigate the effects of miR-216b on glioma
initiation and progression, an miR-216b mimic was introduced into
U87 and U373 cells. At 48 h post-transfection, RT-qPCR was
performed to evaluate the efficiency of overexpression and it was
demonstrated that miR-216b was upregulated in U87 and U373 cells
following transfection with an miR-216b mimic compared with cells
transfected with miR-NC (P<0.05; Fig. 2A and B). An MTT assay was conducted
to investigate the effect of miR-216b on glioma cell proliferation.
As demonstrated in Fig. 2C and D,
ectopic expression of miR-216b suppressed U87 and U373 cells
proliferation (P<0.05). A Transwell invasion assay was used to
evaluate the roles of miR-216b on glioma cell metastasis. The
results indicated that expression of miR-216b decreased the
invasive ability of U87 and U373 cells (Fig. 2E, P<0.05). These results
suggested that miR-216b exerts a tumor suppressor role in
glioma.
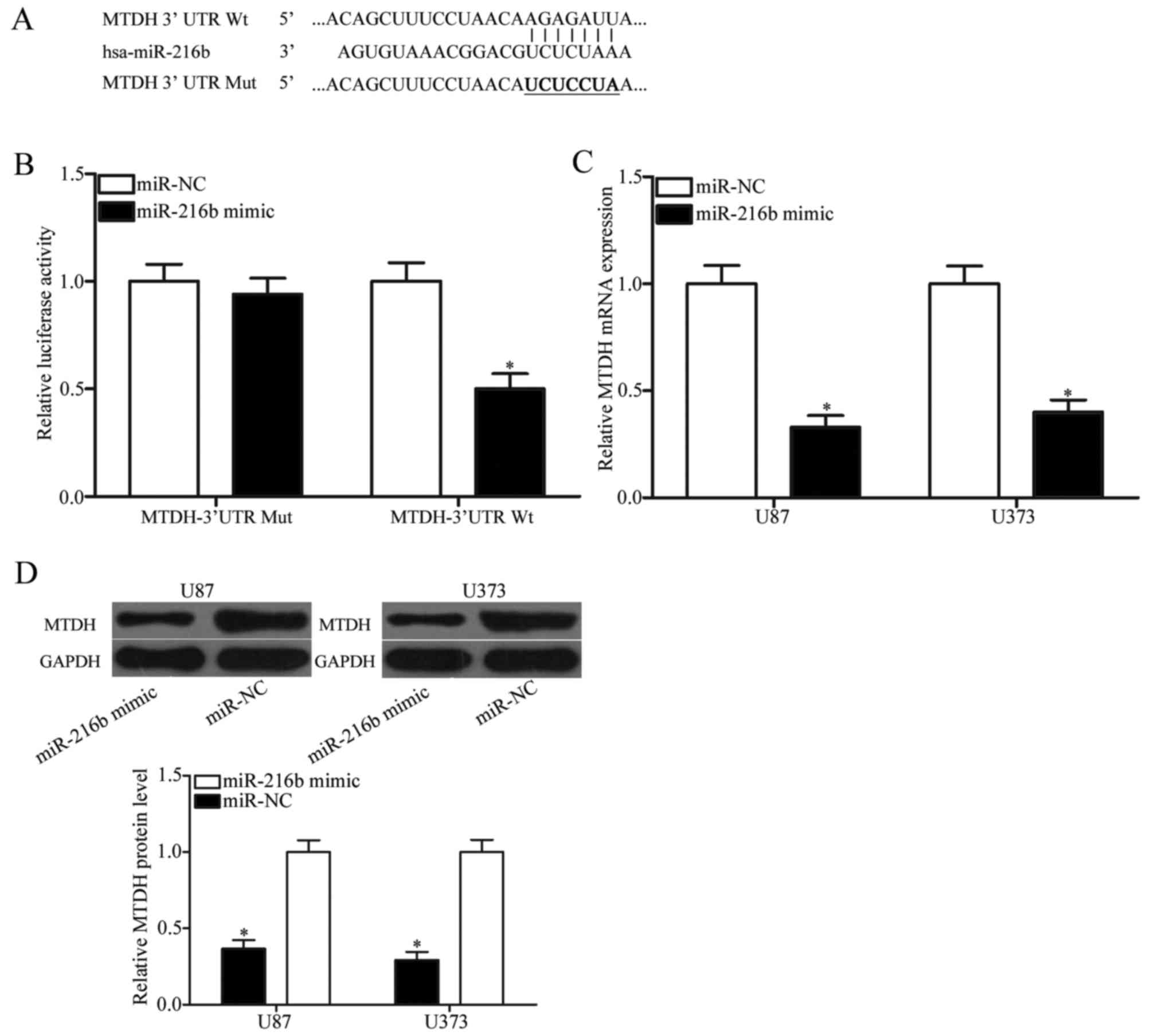
MTDH is a direct target of miR-216b in
glioma
Based on bioinformatic analysis using PicTar and
TargetScan, a potential list of target genes was predicated. Among
these genes, MTDH was of interest (Fig. 3A). MTDH is upregulated in glioma
(21) and involved in glioma
occurrence, and development (22)
indicating that MTDH may be a direct target of miR-216b in glioma.
To confirm whether MTDH was a direct target of miR-216b, a
luciferase reporter assay was performed. 293T cells were
transfected with luciferase reporter vectors, together with an
miR-216b mimic or miR-NC. The results demonstrated that the
luciferase activity was reduced by the co-transfection with an
miR-216b mimic and pMIR-MTDH-3′UTR Wt (P<0.05; Fig. 3B); however, co-transfection of an
miR-216b mimic and pMIR-MTDH-3′UTR Mut did not affect the
luciferase activity in 293T cells. To further determine the
regulatory effects of miR-216b on endogenous MTDH expression,
RT-qPCR and western blotting was performed in U87 and U373 cells
following transfection with an miR-216b mimic or miR-NC. As
demonstrated in Fig. 3C and D,
upregulation of miR-216b suppressed MTDH mRNA and protein
expression in U87 and U373 cells (all P<0.05). These results
demonstrated that MTDH is a direct target gene of miR-216b in
glioma.
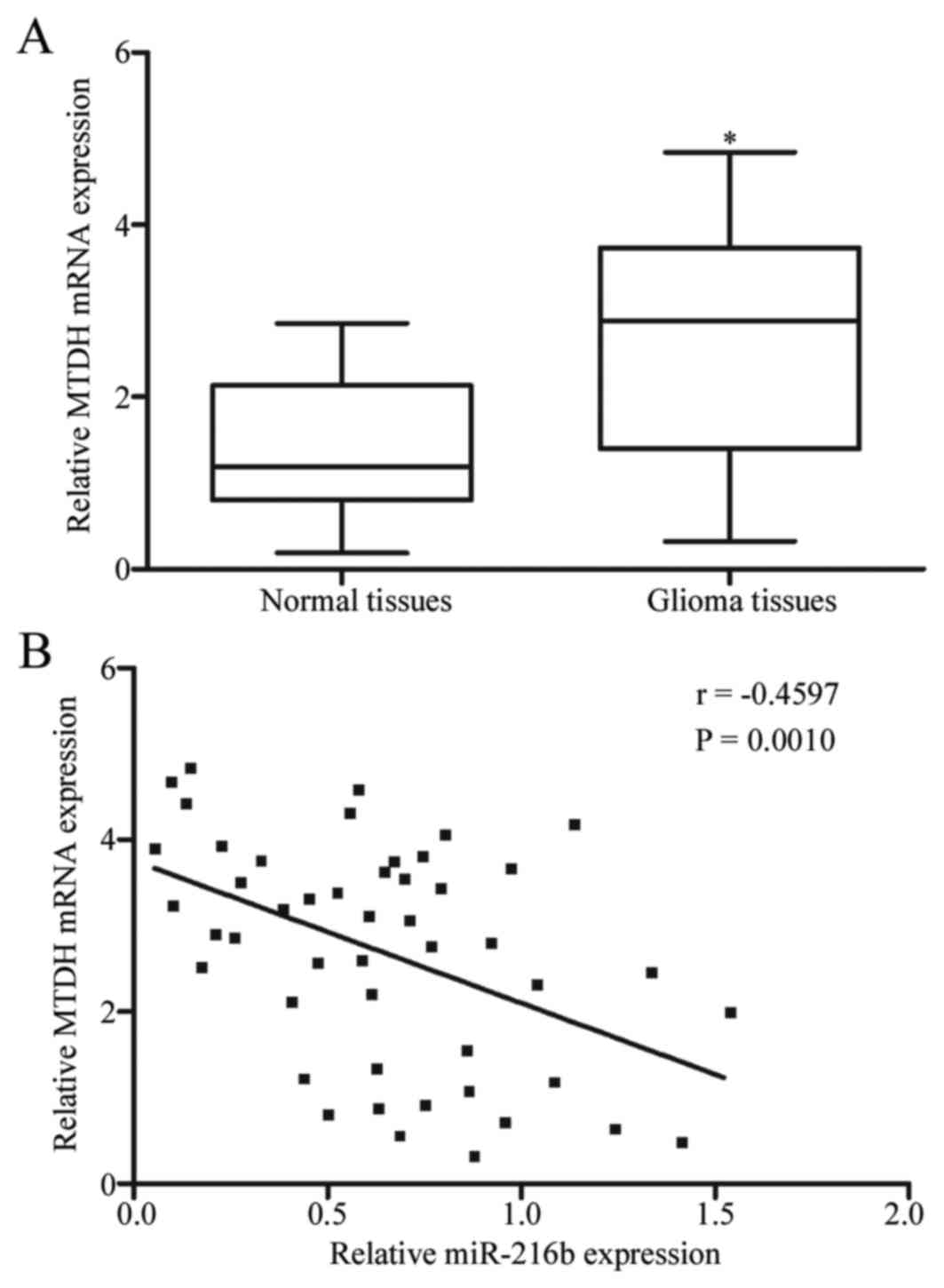
miR-216b was negatively correlated
with MTDH expression in glioma tissues
As miR-216b was lowly expressed in glioma tissues
and miR-216b inhibited glioma cell proliferation and invasion by
negative regulation of MTDH, it was hypothesized that the
expression level of miR-216b may be inversely correlated with
miR-216b expression in glioma tissues. MTDH expression was examined
in glioma tissues and matched normal tissues using RT-qPCR. As
demonstrated in Fig. 4A, elevated
expression of MTDH was observed in glioma tissues compared with the
matched normal tissues (P<0.05). Furthermore, Spearman's rank
correlation coefficient analysis indicated an inverse correlation
between miR-216b and MTDH mRNA expression in glioma tissues
(P=0.001; r=-0.4597; Fig. 4B).
These results confirmed that MTDH is a direct target of miR-216b in
glioma.
miR-216b exerts its tumor suppressive
roles by downregulating MTDH expression
As MTDH was identified as a direct target of
miR-216b, the roles of MTDH in cell proliferation and invasion in
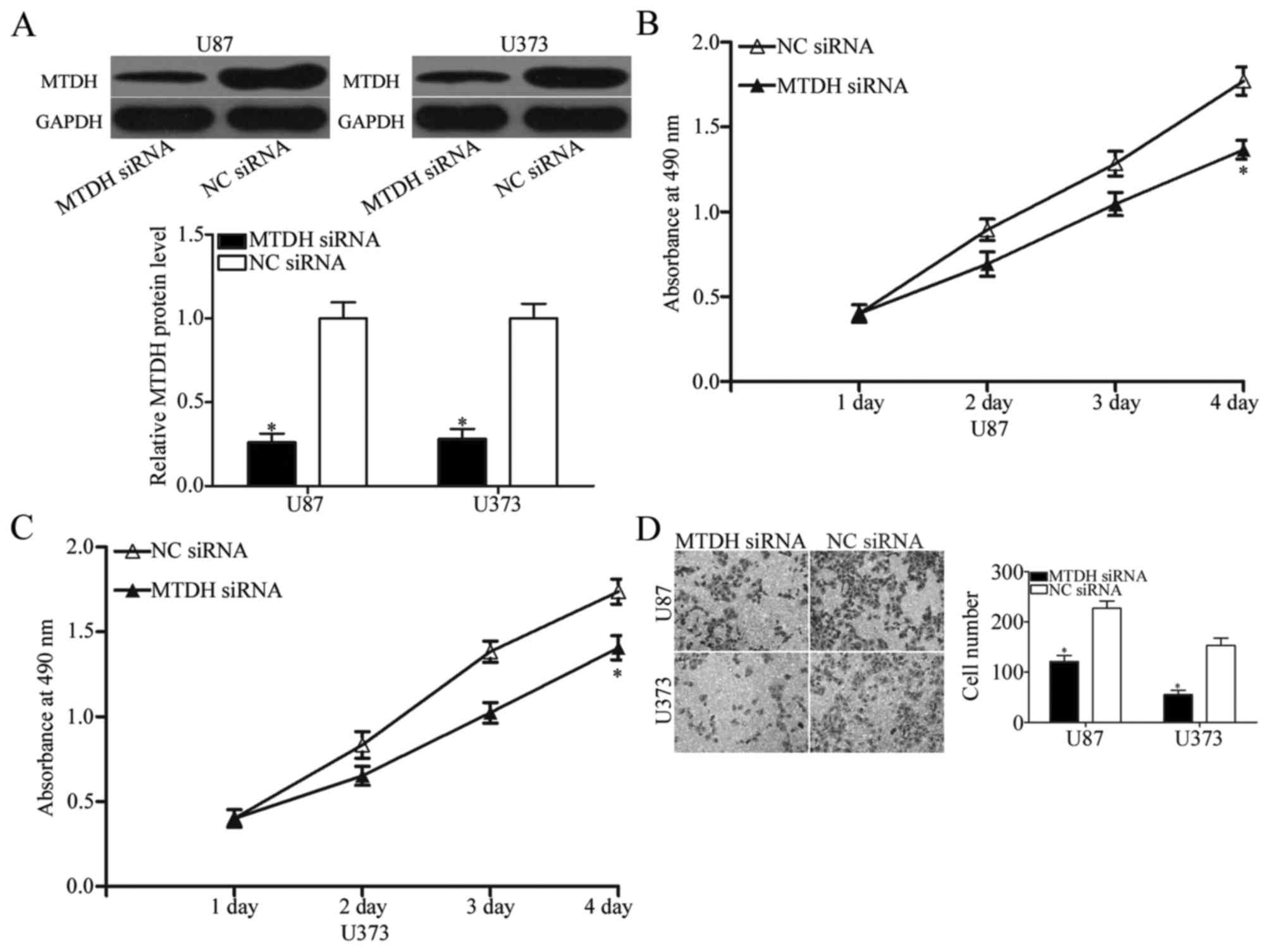
glioma were investigated. MTDH expression was silenced using MTDH
siRNA and confirmed by western blotting (P<0.05; Fig. 5A). MTT and Transwell invasion
assays were used to assess the effects of MTDH-knockdown on cell
proliferation and invasion in glioma. The results demonstrated that
the inhibition of MTDH expression by transfection with MTDH siRNA
decreased U87 and U373 cell proliferation (P<0.05; Fig. 5B and C) and invasion (P<0.05;
Fig. 5D). These results suggest
that miR-216b inhibited glioma cell proliferation and invasion,
partially through downregulation of MTDH expression.
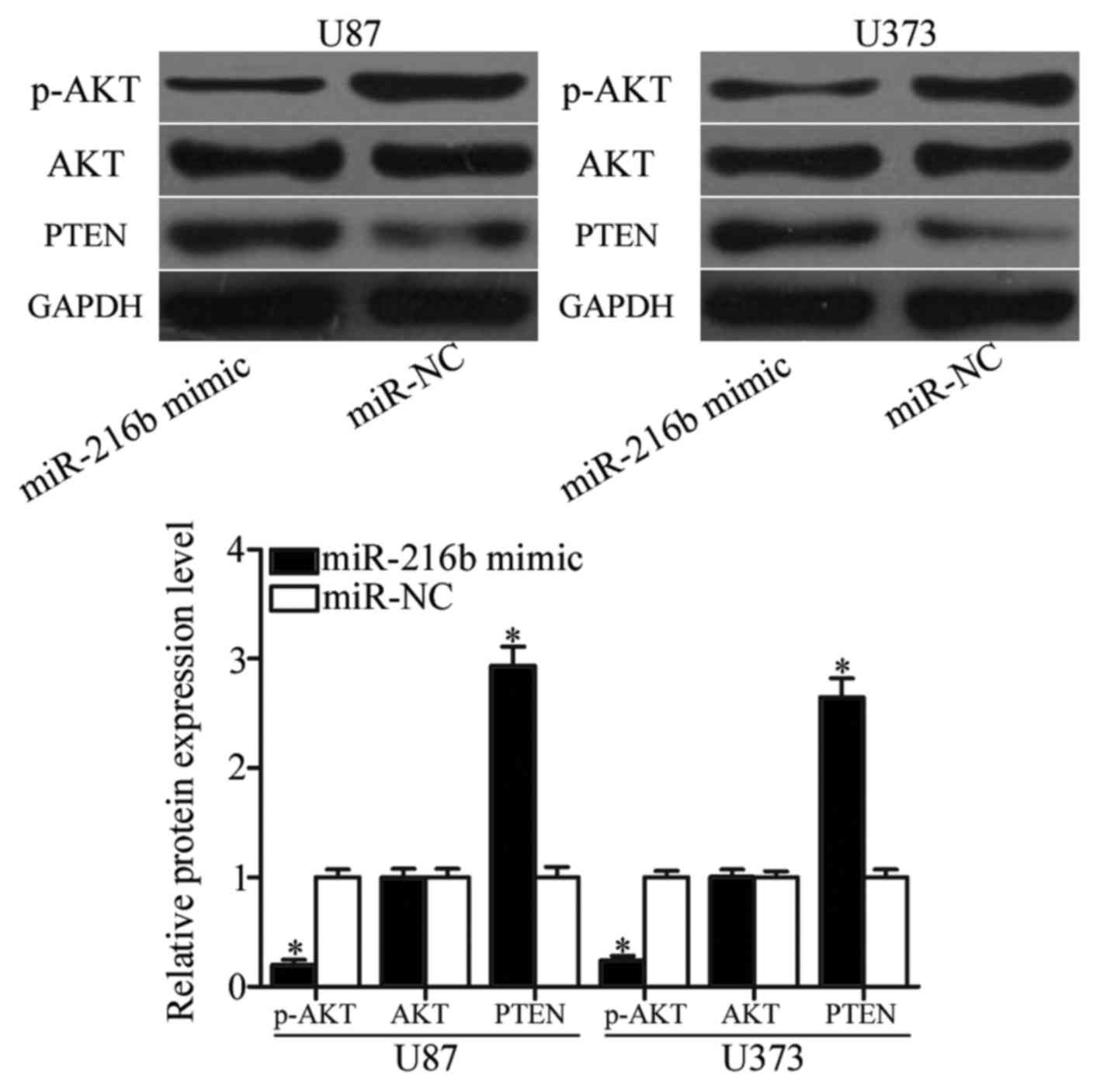
miR-216b inhibits the PTEN/AKT
signaling pathway
Previous studies demonstrated that MTDH could
negatively regulate PTEN expression via blocking its transcription
(23,24). Therefore, PTEN, AKT and p-AKT
expression was measured in U87 and U373 cells following
transfection with an miR-216b mimic or miR-NC. As demonstrated in
Fig. 6, upregulation of miR-216b
enhanced PTEN expression and reduced p-AKT expression, whereas
miR-216b did not affect AKT expression in U87 and U373 cells
(P<0.05). These results indicated that miR-216b is involved in
PTEN/AKT signaling pathway through regulation of MTDH.
Discussion
The heterogeneity, aggressive nature and angiogenic
behavior of glioma results in high morbidity, high recurrence rate,
high mortality and low cure rate, and therefore results in a poor
prognosis and short survival time (6,25,26).
A number of studies have demonstrated that miRs serve important
roles in the formation and progression of glioma as oncogenes or
tumor suppressor genes through negative regulation of their targets
(27–29). miRs are considered to be potential
targets for diagnosis, therapy and prognosis in glioma.
Previous studies have demonstrated that miR-216b is
abnormally expressed in several types of human cancer. For
instance, in nasopharyngeal carcinoma, miR-216b was downregulated
in tumor tissues and cell lines. Reduced miR-216b expression was
strongly correlated with advanced clinical stage and lymph node
metastasis (15). Liu et al
(16) reported that miR-216b was
lower in hepatocellular carcinoma tissues and was associated with
tumor volume, hepatitis B virus (HBV) infection, HBV DNA level and
vascular invasion in patients with this disease. Egeli et al
(30) demonstrated that miR-216b
expression was low in pancreatic ductal adenocarcinoma. Expression
level of miR-216b was correlated with aggressive tumor
characteristics and shortened disease-free survival of patients
with pancreatic ductal adenocarcinoma. Aberrant downregulation of
miR-216b was also observed in breast (31,32)
and gastric cancer (33). However,
the expression pattern of miR-216b in glioma remains unclear.
RT-qPCR was used to examine miR-216b expression in glioma tissues
and cell lines. Results demonstrated that expression level of
miR-216b was reduced in glioma tissues and cell lines. Low miR-216b
expression was correlated with the KPS and WHO grade of gliomas.
These results suggested that miR-216b appeared to be involved in
carcinogenesis and cancer progression.
miR-216b has been investigated in the development of
a number of human cancers. In nasopharyngeal carcinoma, restoration
of expression of miR-216b produced a suppressive effect on cell
proliferation, invasion in vitro, as well as tumor growth
in vivo (15,17). In breast cancer, the introduction
of miR-216b inhibited cell proliferation, migration, invasion and
induced apoptosis (31,32). In hepatocellular carcinoma,
miR-216b overexpression suppressed cell growth and metastasis and
enhanced cell cycle arrest, and apoptosis (16,34).
In gastric cancer, upregulation of miR-216b attenuated cell
proliferation and cell cycle progression (33). In the present study, it was
demonstrated that ectopic expression of miR-216b repressed glioma
cell proliferation and invasion in vitro. These results
suggested that miR-216b was characterized as an important tumor
suppressor and may therefore serve as a potential therapeutic
target for the treatment of these types of cancer.
miRs are known to regulate target mRNAs through
directly binding to the 3′UTR of target gene, (9). Several oncogenes have been identified
as direct targets of miR-216b, including protein kinase C α
(17), GTPase KRAS (15) in nasopharyngeal carcinoma,
insulin-like growth factor 2 mRNA-binding protein 2
(16), forkhead box protein M1
(34) in hepatocellular carcinoma
and histone deacetylase 8 (33) in
gastric cancer, syntenin-1 (35)
in breast cancer, and fibroblast growth factor receptor 1 (30) in pancreatic cancer. A previous
study also indicated that expression level of miR-216b could be
regulated by C/EBP-homologous protein 10/growth arrest and DNA
damage-inducible protein 153 (36). In the present study it was
confirmed that MTDH was a novel, direct and functional target of
miR-216b in glioma. Bioinformatic analysis was performed to
predicate the candidate targets of miR-216b. MTDH was selected for
further investigation. A luciferase reporter assay revealed that
miR-216b was able to directly target the 3′UTR of MTDH. In
addition, MTDH mRNA and protein expression could be negatively
regulated by miR-216b in glioma cells. Furthermore, the negative
association between miR-216b and MTDH mRNA expression in glioma
tissues further confirmed that MTDH is a direct target of miR-216b.
MTDH knockdown mimicked the effects of miR-216b overexpression on
glioma cell proliferation and invasion.
MTDH, also known as astrocyte elevated gene-1, was
first identified in human fetal astrocytes in 2002 (37). MTDH is located at chromosome 8q22
and encodes a 582-amino acid protein, which is expressed in all
organs and distributed throughout the cytoplasm, membrane, nucleus
and endoplasmic reticulum of the cell (38). Previous studies reported that MTDH
was frequently over-expressed in breast (39), gastric (40), cervical (41) and bladder cancer (42). In glioma, MTDH was significantly
upregulated at the mRNA and protein level. Additionally, the MTDH
expression level was associated with the histological grade of
patients with glioma (43).
Experiments have demonstrated that MTDH acts as an oncogene and is
associated with tumorigenesis and tumor development in glioma
(22,44,45).
Therefore, targeting MTDH in glioma may prolong survival time and
improve the outcome of patients afflicted with this aggressive and
invariably fatal disease. The present study suggested that miR-216b
targeted MTDH to inhibit cell proliferation and invasion of glioma.
Therefore, miR-216b may be useful as a therapeutic target for the
suppression of the rapid growth and metastasis of gliomas.
In conclusion, the results of the present study
indicated that miR-216b repressed cell proliferation and invasion
through inhibiting the expression of MTDH, and highlight the
therapeutic potential of miR-216b in glioma. Future work is
required to investigate the upstream regulatory mechanism of
miR-216b in glioma and investigate whether the potential of
miR-216b may be fully realised in patients with this
malignancy.
References
|
1
|
Goodenberger ML and Jenkins RB: Genetics
of adult glioma. Cancer Genet. 205:613–621. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vigneswaran K, Neill S and Hadjipanayis
CG: Beyond the World Health Organization grading of infiltrating
gliomas: Advances in the molecular genetics of glioma
classification. Ann Transl Med. 3:952015.PubMed/NCBI
|
|
3
|
Malerba S, Galeone C, Pelucchi C, Turati
F, Hashibe M, La Vecchia C and Tavani A: A meta-analysis of coffee
and tea consumption and the risk of glioma in adults. Cancer Causes
Control. 24:267–276. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ohgaki H: Epidemiology of brain tumors.
Methods Mol Biol. 472:323–342. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Thorne AH, Meisen WH, Russell L, Yoo JY,
Bolyard CM, Lathia JD, Rich J, Puduvalli VK, Mao H, Yu J, et al:
Role of cysteine-rich 61 protein (CCN1) in macrophage-mediated
oncolytic herpes simplex virus clearance. Mol Ther. 22:1678–1687.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Giese A, Bjerkvig R, Berens ME and
Westphal M: Cost of migration: Invasion of malignant gliomas and
implications for treatment. J Clin Oncol. 21:1624–1636. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Orang A Valinezhad, Safaralizadeh R and
Kazemzadeh-Bavili M: Mechanisms of miRNA-mediated gene regulation
from common downregulation to mRNA-specific upregulation. Int J
Genomics. 2014:9706072014.PubMed/NCBI
|
|
8
|
Luo W and Sehgal A: Regulation of
circadian behavioral output via a MicroRNA-JAK/STAT circuit. Cell.
148:765–779. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hwang HW and Mendell JT: MicroRNAs in cell
proliferation, cell death and tumorigenesis. Br J Cancer. 96
Suppl:R40–R44. 2007.PubMed/NCBI
|
|
10
|
Kloosterman WP and Plasterk RH: The
diverse functions of microRNAs in animal development and disease.
Dev Cell. 11:441–450. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zimmerman AL and Wu S: MicroRNAs, cancer
and cancer stem cells. Cancer Lett. 300:10–19. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen R, Liu H, Cheng Q, Jiang B, Peng R,
Zou Q, Yang W, Yang X, Wu X and Chen Z: MicroRNA-93 promotes the
malignant phenotypes of human glioma cells and induces their
chemoresistance to temozolomide. Biol Open. 5:669–677. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pencheva N and Tavazoie SF: Control of
metastatic progression by microRNA regulatory networks. Nat Cell
Biol. 15:546–554. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu X, Wang S, Yuan A, Yuan X and Liu B:
MicroRNA-140 represses glioma growth and metastasis by directly
targeting ADAM9. Oncol Rep. 36:2329–2338. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Deng M, Tang H, Zhou Y, Zhou M, Xiong W,
Zheng Y, Ye Q, Zeng X, Liao Q, Guo X, et al: miR-216b suppresses
tumor growth and invasion by targeting KRAS in nasopharyngeal
carcinoma. J Cell Sci. 124:2997–3005. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu FY, Zhou SJ, Deng YL, Zhang ZY, Zhang
EL, Wu ZB, Huang ZY and Chen XP: miR-216b is involved in
pathogenesis and progression of hepatocellular carcinoma through
HBx-miR-216b-IGF2BP2 signaling pathway. Cell Death Dis.
6:e16702015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Deng M, Liu JF, Gu YX, Zheng GP and He ZM:
miR-216b suppresses cell proliferation and invasion by targeting
PKCα in nasopharyngeal carcinoma cells. Zhonghua Zhong Liu Za Zhi.
35:645–650. 2013.(In Chinese). PubMed/NCBI
|
|
18
|
Allen M, Bjerke M, Edlund H, Nelander S
and Westermark B: Origin of the U87MG glioma cell line: Good news
and bad news. Sci Transl Med. 8:354re32016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Torsvik A, Stieber D, Enger PØ,
Golebiewska A, Molven A, Svendsen A, Westermark B, Niclou SP, Olsen
TK, Enger M Chekenya and Bjerkvig R: U-251 revisited: Genetic drift
and phenotypic consequences of long-term cultures of glioblastoma
cells. Cancer Med. 3:812–824. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kang DC, Su ZZ, Sarkar D, Emdad L, Volsky
DJ and Fisher PB: Cloning and characterization of HIV-1-inducible
astrocyte elevated gene-1, AEG-1. Gene. 353:8–15. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Emdad L, Sarkar D, Lee SG, Su ZZ, Yoo BK,
Dash R, Yacoub A, Fuller CE, Shah K, Dent P, et al: Astrocyte
elevated gene-1: A novel target for human glioma therapy. Mol
Cancer Ther. 9:79–88. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Du C, Yi X, Liu W, Han T, Liu Z, Ding Z,
Zheng Z, Piao Y, Yuan J, Han Y, et al: MTDH mediates trastuzumab
resistance in HER2 positive breast cancer by decreasing PTEN
expression through an NFκB-dependent pathway. BMC Cancer.
14:8692014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xu C, Kong X, Wang H, Zhang N, Kong X,
Ding X, Li X and Yang Q: MTDH mediates estrogen-independent growth
and tamoxifen resistance by down-regulating PTEN in MCF-7 breast
cancer cells. Cell Physiol Biochem. 33:1557–1567. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Paw I, Carpenter RC, Watabe K, Debinski W
and Lo HW: Mechanisms regulating glioma invasion. Cancer Lett.
362:1–7. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shi Q, Bao S, Song L, Wu Q, Bigner DD,
Hjelmeland AB and Rich JN: Targeting SPARC expression decreases
glioma cellular survival and invasion associated with reduced
activities of FAK and ILK kinases. Oncogene. 26:4084–4094. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Peng G, Liao Y and Shen C: miRNA-429
inhibits astrocytoma proliferation and invasion by targeting BMI1.
Pathol Oncol Res. 23:369–376. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Karsy M, Arslan E and Moy F: Current
progress on understanding microRNAs in glioblastoma multiforme.
Genes Cancer. 3:3–15. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Peng T, Zhang S, Li W, Fu S, Luan Y and
Zuo L: MicroRNA-141 inhibits glioma cells growth and metastasis by
targeting TGF-β2. Am J Transl Res. 8:3513–3521. 2016.PubMed/NCBI
|
|
30
|
Egeli U, Tezcan G, Cecener G, Tunca B,
Sevinc E Demirdogen, Kaya E, Ak S, Dundar HZ, Sarkut P, Ugras N, et
al: miR-216b Targets FGFR1 and Confers Sensitivity to radiotherapy
in pancreatic ductal adenocarcinoma patients without EGFR or KRAS
Mutation. Pancreas. 45:1294–1302. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jana S, Sengupta S, Biswas S, Chatterjee
A, Roy H and Bhattacharyya A: miR-216b suppresses breast cancer
growth and metastasis by targeting SDCBP. Biochem Biophys Res
Commun. 482:126–133. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zheng L, Zhang X, Yang F, Zhu J, Zhou P,
Yu F, Hou L, Xiao L, He Q and Wang B: Regulation of the P2X7R by
microRNA-216b in human breast cancer. Biochem Biophys Res Commun.
452:197–204. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang Y, Xu P, Yao J, Yang R, Shi Z, Zhu X,
Feng X and Gao S: MicroRNA-216b is down-regulated in human gastric
adenocarcinoma and inhibits proliferation and cell cycle
progression by targeting oncogene HDAC8. Target Oncol. 11:197–207.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zheng WW, Zhou J, Zhang CH and Liu XS:
MicroRNA-216b is downregulated in hepatocellular carcinoma and
inhibits HepG2 cell growth by targeting Forkhead box protein M1.
Eur Rev Med Pharmacol Sci. 20:2541–2550. 2016.PubMed/NCBI
|
|
35
|
Jana S, Sengupta S, Biswas S, Chatterjee
A, Roy H and Bhattacharyya A: miR-216b suppresses breast cancer
growth and metastasis by targeting SDCBP. Biochem Biophys Res
Commun. 482:126–133. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xu Z, Bu Y, Chitnis N, Koumenis C, Fuchs
SY and Diehl JA: miR-216b regulation of c-Jun mediates
GADD153/CHOP-dependent apoptosis. Nat Commun. 7:114222016.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Su ZZ, Kang DC, Chen Y, Pekarskaya O, Chao
W, Volsky DJ and Fisher PB: Identification and cloning of human
astrocyte genes displaying elevated expression after infection with
HIV-1 or exposure to HIV-1 envelope glycoprotein by rapid
subtraction hybridization, RaSH. Oncogene. 21:3592–3602. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lee SG, Kang DC, DeSalle R, Sarkar D and
Fisher PB: AEG-1/MTDH/LYRIC, the beginning: Initial cloning,
structure, expression profile, and regulation of expression. Adv
Cancer Res. 120:1–38. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li J, Zhang N, Song LB, Liao WT, Jiang LL,
Gong LY, Wu J, Yuan J, Zhang HZ, Zeng MS and Li M: Astrocyte
elevated gene-1 is a novel prognostic marker for breast cancer
progression and overall patient survival. Clin Cancer Res.
14:3319–3326. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Dong L, Qin S, Li Y, Zhao L, Dong S, Wang
Y, Zhang C and Han S: High expression of astrocyte elevated gene-1
is associated with clinical staging, metastasis, and unfavorable
prognosis in gastric carcinoma. Tumour Biol. 36:2169–2178. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yu JQ, Zhou Q, Zhu H, Zheng FY and Chen
ZW: Overexpression of astrocyte elevated gene-1 (AEG-1) in cervical
cancer and its correlation with angiogenesis. Asian Pac J Cancer
Prev. 16:2277–2281. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yang G, Zhang L, Lin S, Li L, Liu M, Chen
H, Cao M, Liu D, Huang YR and Bo J: AEG-1 is associated with tumor
progression in nonmuscle-invasive bladder cancer. Med Oncol.
31:9862014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
He Z, He M, Wang C, Xu B, Tong L, He J,
Sun B, Wei L and Chu M: Prognostic significance of astrocyte
elevated gene-1 in human astrocytomas. Int J Clin Exp Pathol.
7:5038–5044. 2014.PubMed/NCBI
|
|
44
|
Yang Y, Wu J, Guan H, Cai J, Fang L, Li J
and Li M: MiR-136 promotes apoptosis of glioma cells by targeting
AEG-1 and Bcl-2. FEBS Lett. 586:3608–3612. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Liu L, Wu J, Ying Z, Chen B, Han A, Liang
Y, Song L, Yuan J, Li J and Li M: Astrocyte elevated gene-1
upregulates matrix metalloproteinase-9 and induces human glioma
invasion. Cancer Res. 70:3750–3759. 2010. View Article : Google Scholar : PubMed/NCBI
|