Introduction
Breast cancer remained the leading cause of cancer
death in women although mortality is falling due to earlier
diagnosis and improved surgical techniques as well as better
chemotherapy and radiotherapy (1–3). It
is estimated that about 252,710 new cases of invasive breast cancer
will be diagnosed in women, and about 40,610 women will die from
breast cancer in 2017 in the United States (4).
About 15–20% of breast cancers are found to be
triple-negative for estrogen receptors, progesterone receptors, and
HER2, and defined as triple-negative breast cancer (TNBC) (5). TNBC has a poor prognosis and only
responds to chemotherapy and radiotherapy but not hormonal therapy
(6,7). Therefore, discovery of new molecular
targets to treat patients with TNBC has been pressing and of
significant interest (8,9).
MicroRNAs (miRNAs/miRs) are small non-coding RNAs
that play important roles in regulation of gene expression
post-transcriptionally (10).
Growing evidence has shown that some miRNAs are upregulated in
cancer and behave as oncogenic characteristics, while some miRNAs
are downregulated in cancer and act as tumor-suppressive miRNAs.
Therefore, miRNAs play critical roles in each stages of
tumorigenesis of many human cancers such as lung (11), endometrial (12), and colon cancer (13). miRNAs are promising potential
targets for cancer treatment. Previous studies have shown that
miRNAs are associated with different biological activities in
breast cancer. Huang et al showed that miR-21 improved
breast cancer cell invasion and regulated epithelial-to-mesenchymal
transition (EMT) (14).
Overexpression of miR-205 decreases cell proliferation and
increases apoptosis via regulation of HMGB3 in breast cancer.
miR-205 regulates HMGB3 and its ectopic expression significantly
inhibits cell proliferation and promotes apoptosis in breast cancer
(15). Recently, Liu et al
reported that aberrant expression of miR-374b-5p, miR-218-5p,
miR-126-3p, miR-27b-3p predicted a good prognosis in TNBC (16).
In this study, we examined the function of miR-27a
in cell proliferation, invasion and migration using TNBC cell
lines.
Materials and methods
Cell lines
MDA-MB-231 and MDA-MB-468, human TNBC cell lines
were purchased from American Type Culture Collection (ATCC,
Manassas, VA, USA). The tumor cells were grown in Dubelcco's
modified Egale's medium (DMEM) (Invitrogen, Carlsbad, CA, USA)
supplemented with 100 µg/ml streptomycin, 100 U/ml penicillin and
10% fetal bovine serum (Invitrogen), and at 37°C in a humidified
incubator with 5% CO2. When all cells reach to 70–80%
confluence, they were used in our experiments.
Cell transfection and miRNA
quantification
To transfer miR-27a mimics or anti-miR-27a inhibitor
(Invitrogen) into breast cancer cells, Lipofectamine 2000
(Invitrogen) was used. A random sequence miRNA mimic molecule was
used as a negative control (mirVana™ miRNA mimic;
Ambion, Austin, TX, USA). Then, miR-27a expression level after
transfection was examined. The total RNA was extracted from the
transfected breast cancer cells, TaqMan miRNA reverse transcription
kit (Thermo Fisher Scientific, Inc., Waltham, MA, USA) was used to
synthesize cDNA according the manufacturer's instructions. The
GAPDH, one of housekeeping genes, was used as the endogenous
reference gene.
Cell proliferation assay
To analyze the effect of miR-27a on breast cancer
cell proliferation, WST-1 assay (Roche Diagnostics, Indianapolis,
IN, USA) was performed. Briefly, the transfected breast cancer
cells were placed into 96-well plates at the density of
2×104 cells/well and cultured overnight. The cells were
continued to culture at 37°C in a humidified incubator with 5%
CO2. 20 µl of WST-1 reagent was added to each well and
incubated for at least 1 h at 37°C every 24 h. Then the absorbance
was measured at 490 nm. All experiments were performed in
triplicates.
Migration and invasion assays
To evaluate breast cancer cell migration and
invasion, the Promega migration and invasion assays were performed
according the manufacturer's instructions. Briefly, transfected
breast cancer cells were seeded on the upper Transwell chamber
either with or without Matrigel in DMEM without FBS. DMEM
containing 5% FBS was added to the lower chamber. The cells were
cultured for 18 h at 37°C. Then the non-invaded cells were removed
by cotton swabs, and the invaded cells were stained by Diff-Quik
stain. The percentage of migration and invasion was calculated and
showed as a ratio of invaded cells over cells normalized on second
day of growth curve.
Western blot analysis
The breast cancer cells were gently washed with cold
phosphate-buffered saline, and then lysed in ice-cold lysis buffer
(50 mM Tris-HCl, pH 7.5, 0.1% SDS, 150 mM NaCl, 0.5% deoxycholate,
1% NP-40, and 1X protease inhibitors), then heated at 100°C for 5
min. Protein lysates (20 µg) were loaded to the SDS-PAGE gel and
transferred to PVDF membranes (Sigma-Aldrich, St. Louis, MO, USA).
The PVDF membranes were incubated in blocking buffer for 1 h at
room temperature. Different primary antibodies [B cell lymphoma
(Bcl)-2, Bcl-2 associated X (BAX), AKT and p-AKT; Cell Signaling
Technology, Inc., Danvers, MA, USA] were added in 5% non-fat dry
milk in TBS-T buffer at 4°C overnight, followed by secondary
antibody incubation 1 h at room temperature. The immune signals
were detected with the EasySee Weatern Blot kit (TransGen Biotech
Co., Ltd., Shanghai, China).
Apoptosis activity after ionizing
radiation treatment
To examine the apoptosis activity of breast cancer
cells with altered miR-27a expression, the breast cancer cells were
grown in 24-well plates at density of 2×105/well and
cultured overnight. Then the breast cancer cells were exposed to
different doses of ionizing radiation. After 24 h of culture, the
apoptosis activity was examined by measure the caspase 3/7 activity
using the Caspase-Glo3/7 assay kit (Promega Corp., Madison, WI,
USA) following the manufacturer's protocol. Briefly, Caspase-Glo
reagent was added to each well, and then the cells were incubated
with the Caspase-Glo reagent in a dark place for 8 h with gentle
shaking at room temperature. The luminescence value was measured
using 1-min lag time and 0.5 sec/well read time. All experiments
were carried out in triplicates.
Luciferase reporter assay
The pEZX-MT05 reporter vector carrying miRNA full
length binding sequence of 3′-UTR of phosphatase and tensin homolog
(PTEN) gene, BAX or control sequence (GeneCopoeia, Rockville, MD,
USA) was co-transfected to HEK-293T cells with has-miR-27a using
Lipofectamine 2000 (Invitrogen). Two days after the transfections,
the culture medium was collected and Gaussian luciferase and
alkaline phosphatase activities were measured using the secreted
pair dual luminescence kit according to the manufacturer's
instructions (GeneCopoeia). Gaussian luciferase activity was
normalized to alkaline phosphatase activity.
Xenograft assays in nude mice
Female BALB/c athymic nude mice (5-week-old)
purchased from Charles River Laboratories (Wilmington, MA, USA)
were used in xenograft assay. The mice were maintained in
accordance with the Guide for Institutional Animal Care and
guidelines for animal experiment. MDA-MB-231 breast cancer cells
(1×106) with or without miR-27a mimics were mixed with
Matrigel ECM (reconstituted basement membrane) (17) and were injected subcutaneously into
the mammary fat pads. Ten mice were included in each experimental
group. Tumor size was measured every 3 days by measuring tumor
length (L) and width (W) with calipers, and tumor volume was
calculated as: Tumor volume = πLW2/6 (18).
Statistical analysis
All of results in our experiments were shown as Mean
± standard deviation. SPSS program (version 11.0; SPSS, Inc.,
Chicago, IL, USA) was chose for statistical analyses using
Student's t-test. Differences are considered statistically
significant if P<0.05.
Results
miR-27a improved the proliferation of
TNBC cells both in vitro and in vivo
To determine the effect of miR-27a on proliferation
of TNBC cells, miR-27a was transfected to MDA-MB-231 and MDA-MB-468
TNBC cells with Lipofectamine 2000. MTT assay was performed to
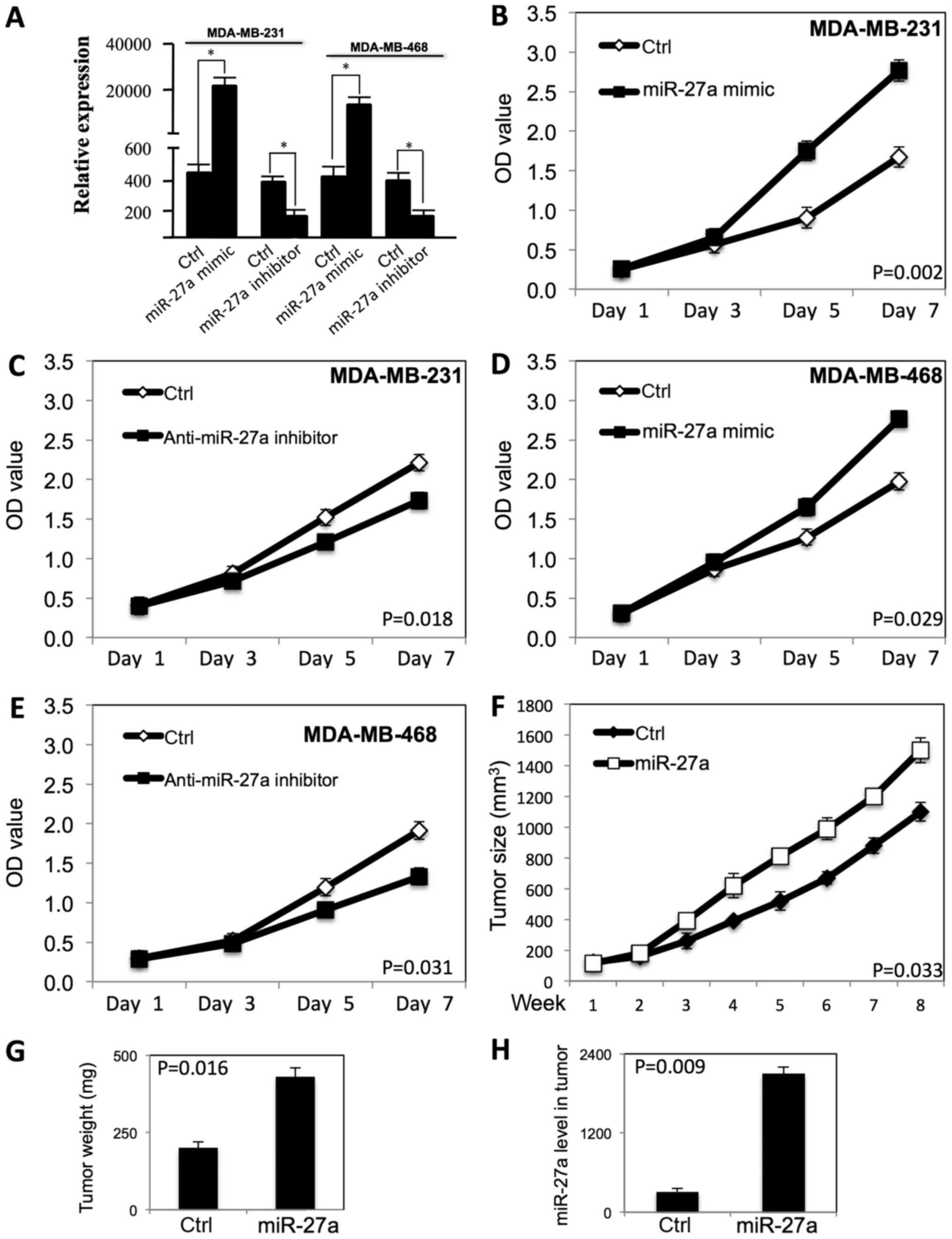
examine the growth curve (Fig. 1).
As shown in Fig. 1A, the miR-27a
expression level was significantly increased in both MDA-MB-231 and
MDA-MB-468 cells after transfection of miR-27a mimic, and the
miR-27a expression level was decreased after transfection of anti-
miR-27a inhibitor. miR-27a promoted the proliferation of MDA-MB-231
(Fig. 1B) and MDA-MB-468 cells
(Fig. 1D) (P<0.05). When the
expression level of miR-27a was decreased by the anti-miR-27a
inhibitor, the proliferation of MDA-MB-231 (Fig. 1C) and MDA-MB-468 cells (Fig. 1E) was inhibited (P<0.05).
To examine whether miR-27a regulates tumor growth
in vivo, we performed subcutaneous tumor xenograft
experiments by injection transfected MDA-MB-231 breast cancer cells
into immunodeficient nude mice. Compared to vector control group,
the tumor growth rate and tumor weight in transfected MDA-MB-231
xenografts was significantly increased (P<0.01) (Fig. 1F and G). These in vivo
results are consistent with the in vitro experiment results,
and show miR-27a improved the tumor growth of breast cancer cells
in vivo. The miR-27a expression level is high in tumor with
transfected MDA-MB-231 cells (Fig.
1H).
miR-27a enhanced migration and
invasion of TNBC cells
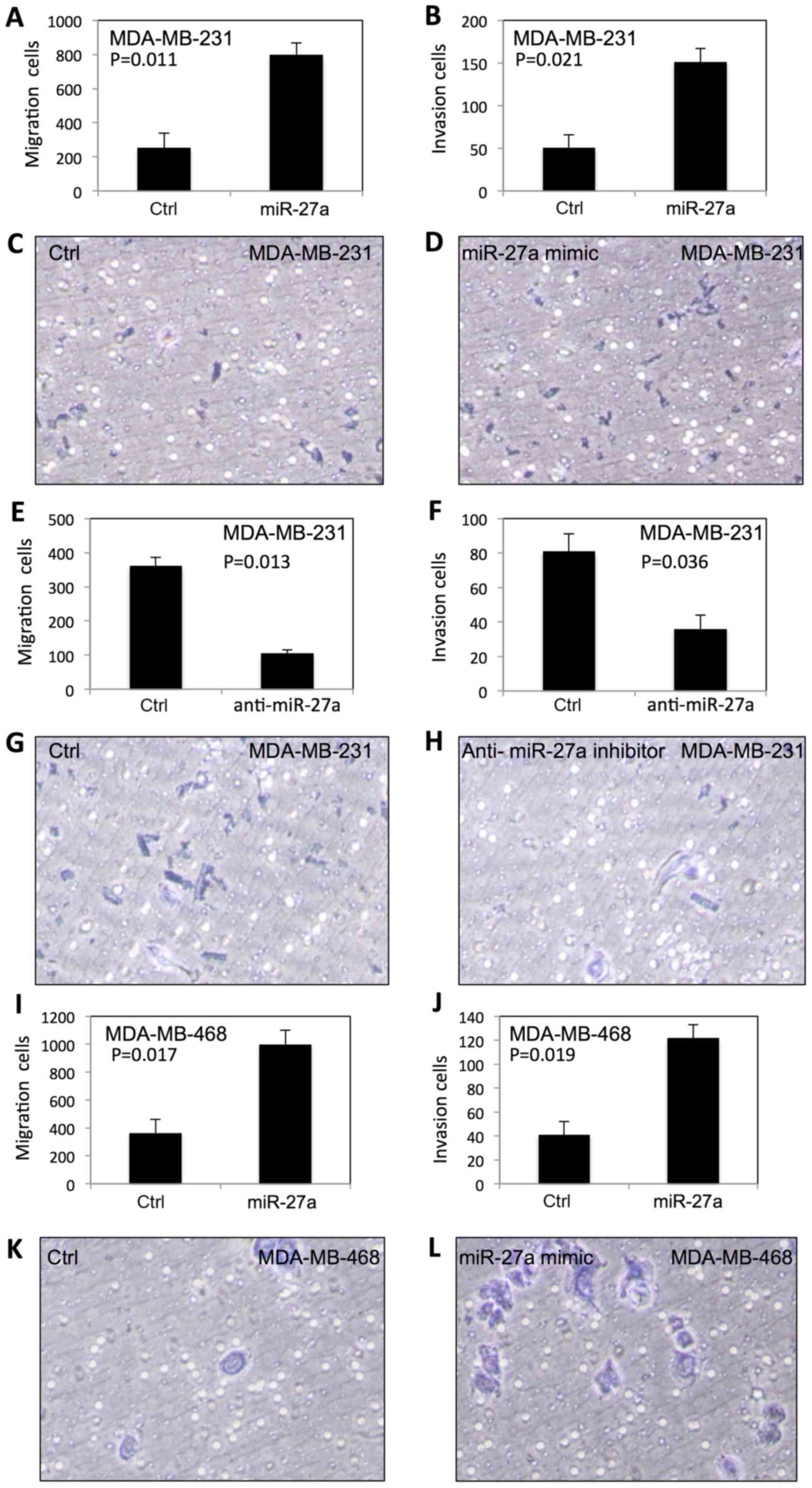
To examine the effect of miR-27a on migration and
invasion of TNBC cells, we performed the migration and Matrigel
invasion assays using BD Transwell. As shown in Fig. 2, the migration and invasion in
MDA-MB-231 (Fig. 2A-D) and
MDA-MB-468 (Fig. 2I-L) were
significantly increased after transfection of miR-27a. In contrast,
the migration and invasion of MDA-MB-231 (Fig. 2E-H) and MDA-MB-468 cells (Fig. 2M-P) were significantly decreased by
anti-miR-27a inhibitor.
miR-27a altered the radiation-induced
inhibitory effect on proliferation of TNBC cells
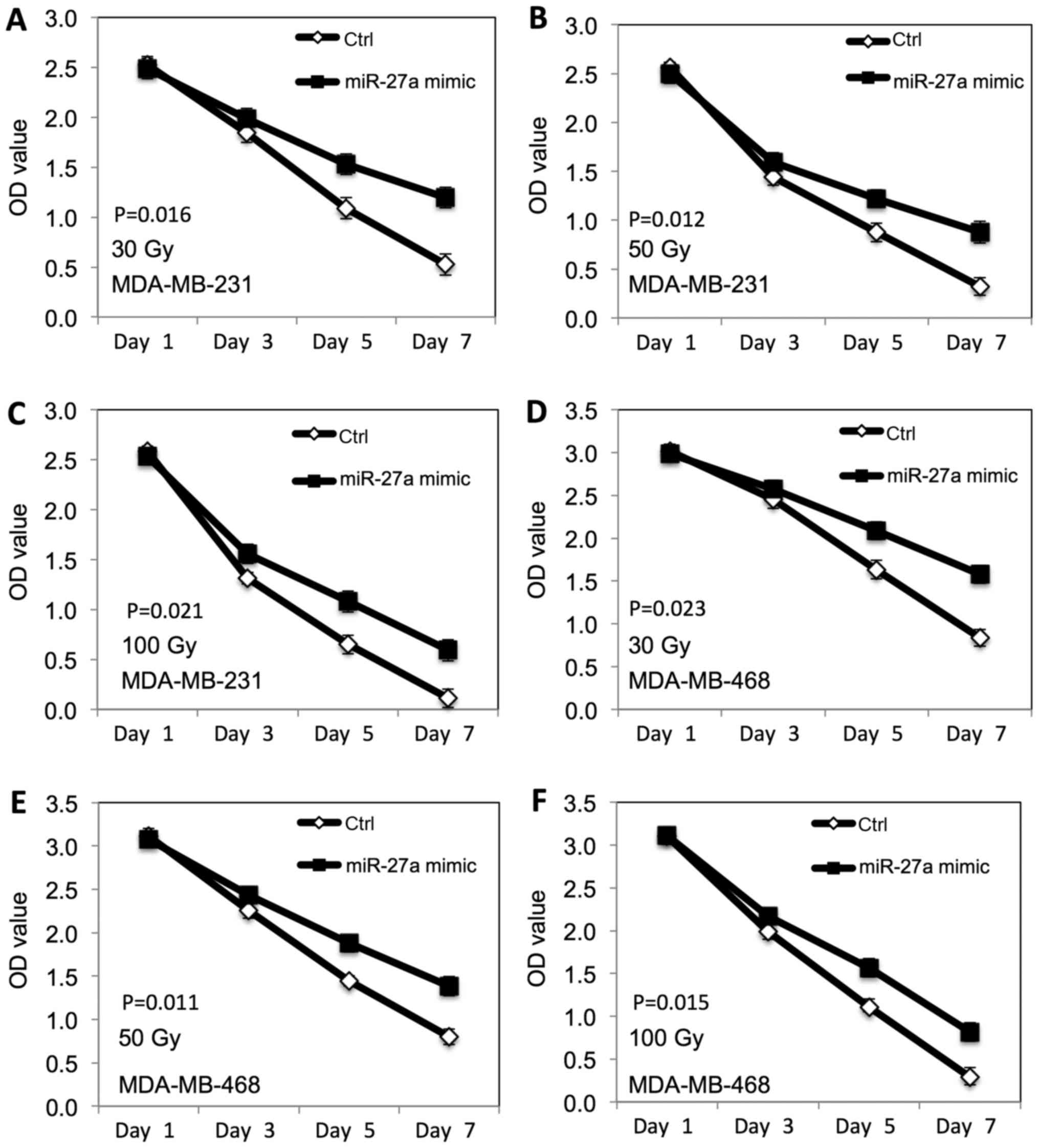
We further examined the radiation-induced inhibitory
effect of miR-27a on the proliferation of TNBC cells. The
MDA-MB-231 and MDA-MB-468 breast cancer cells transfected with
miR-27a were exposed to different doses of ionizing radiation (30,
50 and 100 Gy). MTT assay was performed to evaluate the
proliferation. As shown in Fig. 3,
the radiation-induced inhibitory effect on proliferation of both
MDA-MB-231 (Fig. 3A-C) and
MDA-MB-468 (Fig. 3D-F) cells was
decreased at dose dependent manner after miR-27a overexpression,
compared to the control cells.
miR-27a decreased radiation-induced
apoptosis in TNBC cells
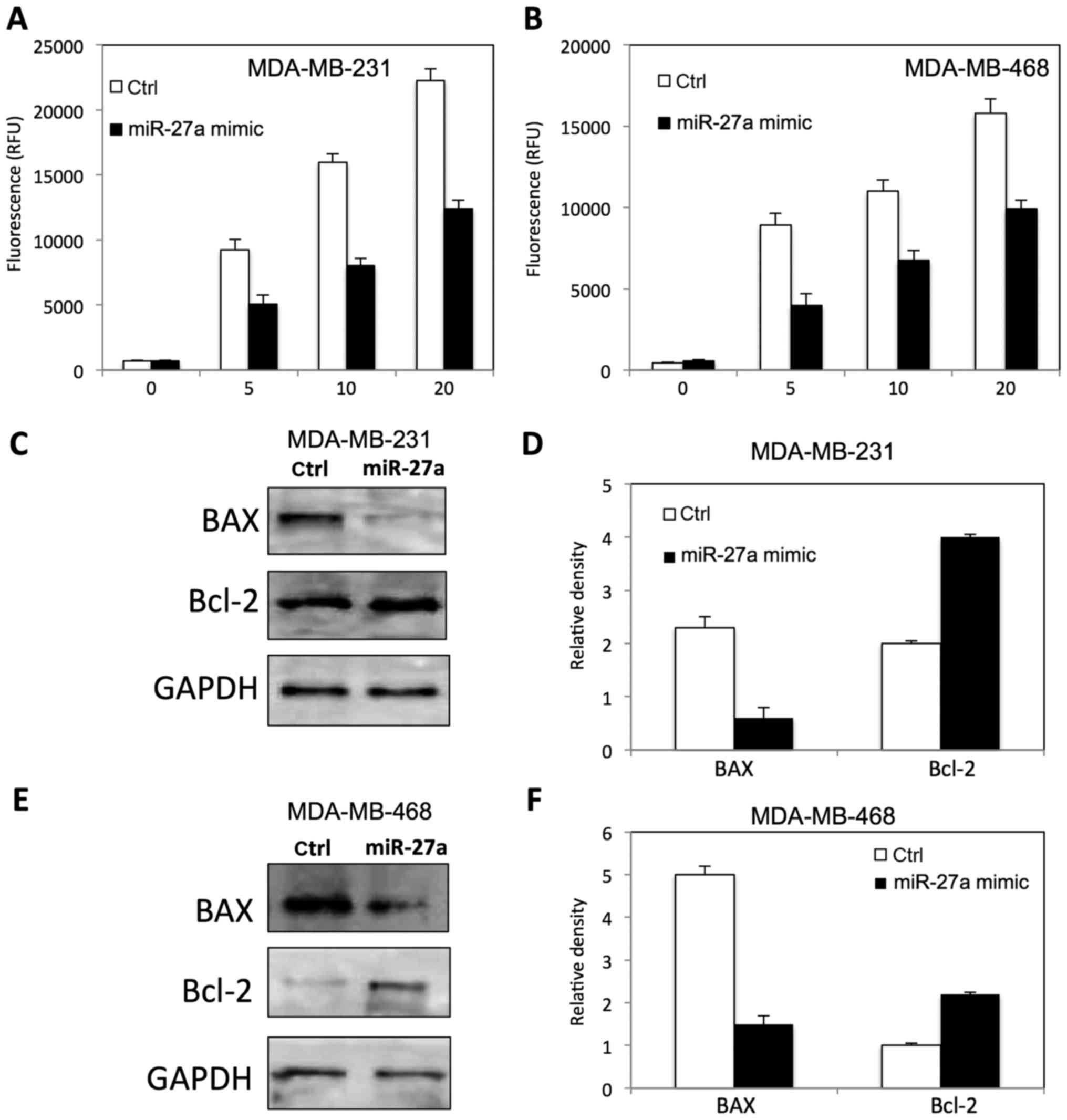
To determine the role of miR-27a on
radiation-induced apoptosis in TNBC cells by measuring caspase 3/7
activity, and apoptotic related protein. As shown in Fig. 4, miR-27a inhibited the caspase 3/7
activity in both MDA-MB-231 (Fig.
4A) and MDA-MB-468 (Fig. 4B)
cells at dose dependent manner. The expression of BAX and Bcl-2 was
altered in MDA-MB-231 (Fig. 4C and
D) and MDA-MB-468 (Fig. 4E and
F) after miR-27a overexpression, compared to the control
cells.
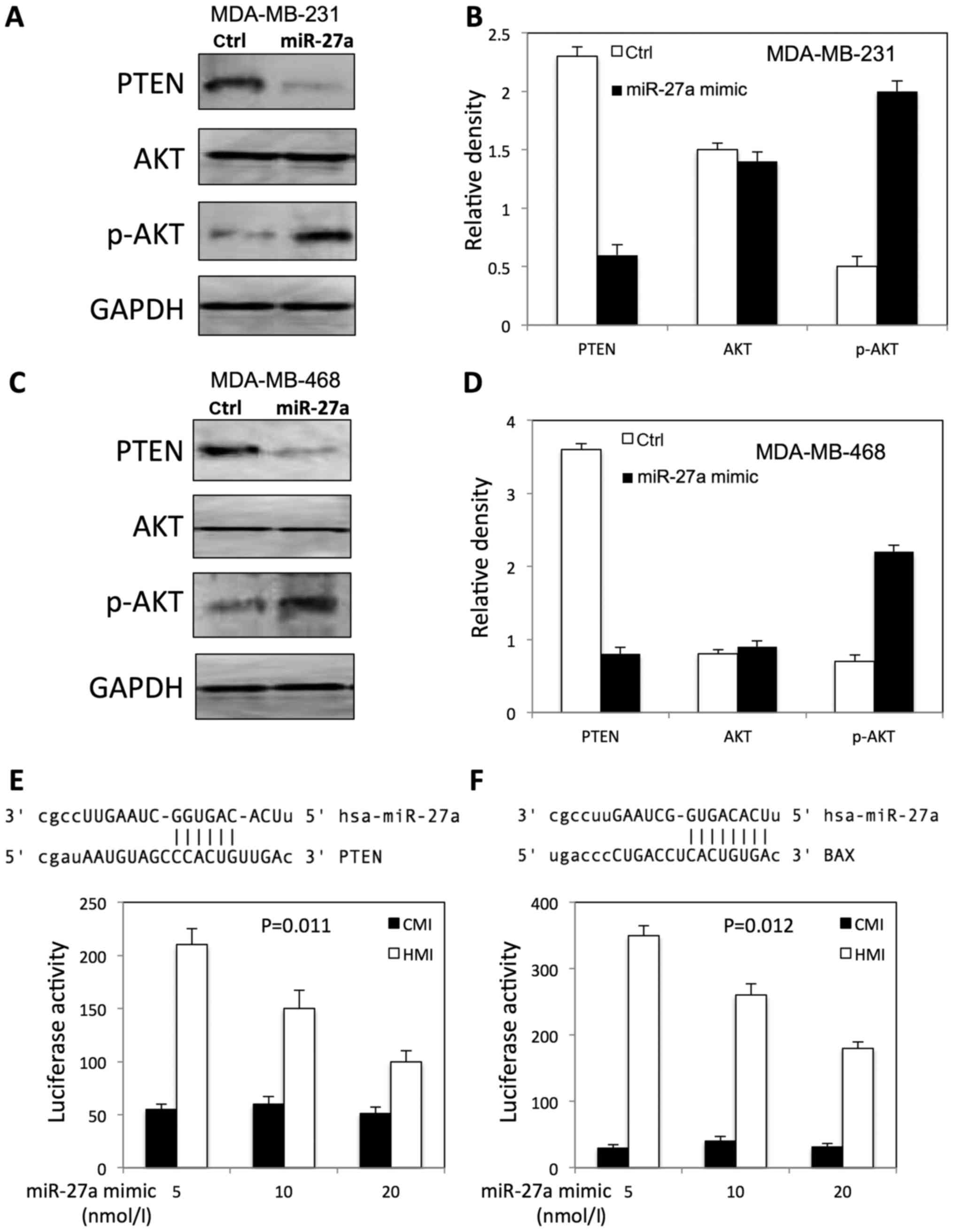
miR-27a activated AKT in TNBC
cells
We further examined the expression of p-AKT, total
AKT, and PTEN in both MDA-MB-231 and MDA-MB-468 cells after miR-27a
overexpression. We found that miR-27a increased the expression of
p-AKT, and decreased the PTEN expression level in both MDA-MB-231
(Fig. 5A and B) and MDA-MB-468
(Fig. 5C and D) cells.
To investigate whether miR-27a is able to regulate
PTEN and BAX expression directly, we searched the miR-27a potential
binding sites within the PTEN 3′-UTR and BAX 3′-UTR by miRNA target
gene prediction software (TargetScanHuman). The search showed that
miR-27a has a potential binding site within the PTEN 3′-UTR and BAX
3′-UTR (Fig. 5E and F).
To evaluate the effect of miR-27a on PTEN and BAX
expression, we performed luciferase assay with a reporter (HMI)
containing 3′-UTR of PTEN and BAX gene. The luciferase activity of
the construct (HMI) significantly increased in a dosage-dependent
manner by co-transfection of miR-27a mimic in a dosage-dependent
manner (Fig. 5E and F). However,
miR-27a transfection had no effect on luciferase activity in
control plasmid (CMI) lacking the PTEN 3′-UTR fusion (Fig. 5E and F).
Discussion
Recent studies have shown that miR-27a plays
important roles in tumorgenesis in many organs, including kidney
(19), breast (20), stomach (21) and cervix (22). For instance, Pan et al found
that miR-27a improved proliferation, migration and invasion of
human osteosarcoma cells by regulating MAP2K4 expression (23). The overexpression of miR-27a is
associated with metastasis in gastric cancer cells by regulating
EMT (24). It has been
demonstrated that Wnt/β-catenin signaling pathway is associated
with the proliferation and migration of breast cancer. Kong et
al found that miR-27a promoted proliferation of breast cancer
cells by targeting SFRP1 via Wnt/β-catenin signaling pathway both
in vitro and in vivo (25). In addition, Drayton et al
reported that miR-27a contributed to cisplatin resistance by
targeting cysteine/glutamate exchanger SLC7A11 (26). Recently study has shown that
miR-27a might act as a prognostic marker for breast cancer
(27). Furthermore, miR-27a may
coordinate with other miRNAs in breast cancer cells (28). In our study, we found that miR-27a
improved the proliferation of TNBC cells. Moreover, we showed that
overexpression of miR-27a enhanced migration and invasion in both
MDA-MB-231 and MDA-MB-468 cells. However, downregulation of miR-27a
decreased the migration and invasion in both MDA-MB-231 and
MDA-MB-468 cells. It has been known that TNBC is more likely to
recur and has poor prognosis (29). Metastasis is one of important cause
for the recurrence of TNBC. Therefore, our study indicated that
miR-27a play important roles in TNBC progression.
Radiotherapy is one of highly effective adjuvant
treatments in patients with breast cancer after surgery (30). TNBC is characterized as rapid
growth and local recurrence. It has been found that radiotherapy
can decrease the locoregional recurrence in patients with T1-2N0
disease after modified radical mastectomy (31). In the present study, we found that
miR-27a enhanced the survival of TNBC cells after irradiation.
Meanwhile, miR-27a inhibited the radiation-induced apoptosis of
TNBC cells.
PTEN, the second most frequently mutated tumor
suppressor gene in human cancer, plays critical roles in
proliferation, apoptosis and cell cycle in tumor cells. PTEN
involves tumor development by targeting several signaling pathways,
such as MAPK pathway, FAK pathway and PI3K/AKT pathway. Recent
studies have demonstrated that PI3K/AKT pathway is the key pathway
by which PTEN displays antioncogenic effects (32). The PI3K/AKT pathway regulates
multiple biological processes and mediates the downstream responses
including cell proliferation, apoptosis and metabolism. It has been
found that PI3K/AKT is activated in TNBC due to PTEN loss (33). Our results showed that PTEN
expression level was decreased, and p-AKT expression level was
increased in TNBC cells after overexpression miR-27a. In addition,
luciferase assay showed that PTEN and BAX are downregulated by
miR-27a by binding to 3′-UTR. These results indicated miR-27a
regulates proliferation of TNBC cells by targeting PI3K/AKT
signaling pathway. Further studies are needed to demonstrate the
molecular mechanism.
miR-27a regulated tumorigenesis and malignant
progression of TNBC. The present study indicates miR-27a might be
used as a potential biomarker to predict the radiotherapy response
and prognosis in TNBC.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Webb PM, Cummings MC, Bain CJ and Furnival
CM: Changes in survival after breast cancer: Improvements in
diagnosis or treatment? Breast. 13:7–14. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Di Leo A, Curigliano G, Diéras V, Malorni
L, Sotiriou C, Swanton C, Thompson A, Tutt A and Piccart M: New
approaches for improving outcomes in breast cancer in Europe.
Breast. 24:321–330. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
American Cancer Society: Breast Cancer.
http://www.cancer.org/cancer/breastcancer/detailedguide/breast-cancer-key-statistics
|
|
5
|
Boyle P: Triple-negative breast cancer:
Epidemiological considerations and recommendations. Ann Oncol. 23
Suppl 6:vi7–vi12. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Negi P, Kingsley PA, Jain K, Sachdeva J,
Srivastava H, Marcus S and Pannu A: Survival of triple negative
versus triple positive breast cancers: Comparison and contrast.
Asian Pac J Cancer Prev. 17:3911–3916. 2016.PubMed/NCBI
|
|
7
|
Braicu C, Chiorean R, Irimie A, Chira S,
Tomuleasa C, Neagoe E, Paradiso A, Achimas-Cadariu P, Lazar V and
Berindan-Neagoe I: Novel insight into triple-negative breast
cancers, the emerging role of angiogenesis, and antiangiogenic
therapy. Expert Rev Mol Med. 18:e182016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Linklater ES, Tovar EA, Essenburg CJ,
Turner L, Madaj Z, Winn ME, Melnik MK, Korkaya H, Maroun CR,
Christensen JG, et al: Targeting MET and EGFR crosstalk signaling
in triple-negative breast cancers. Oncotarget. 7:69903–69915.
2016.PubMed/NCBI
|
|
9
|
Gray MJ, Gong J, Hatch MM, Nguyen V,
Hughes CC, Hutchins JT and Freimark BD:
Phosphatidylserine-targeting antibodies augment the
anti-tumorigenic activity of anti-PD-1 therapy by enhancing immune
activation and downregulating pro-oncogenic factors induced by
T-cell checkpoint inhibition in murine triple-negative breast
cancers. Breast Cancer Res. 18:502016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Krol J, Loedige I and Filipowicz W: The
widespread regulation of microRNA biogenesis, function and decay.
Nat Rev Genet. 11:597–610. 2010.PubMed/NCBI
|
|
11
|
Xue J, Yang J, Luo M, Cho WC and Liu X:
MicroRNA-targeted therapeutics for lung cancer treatment. Expert
Opin Drug Discov. 12:141–157. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Canlorbe G, Wang Z, Laas E, Bendifallah S,
Castela M, Lefevre M, Chabbert-Buffet N, Daraï E, Aractingi S,
Méhats C and Ballester M: Identification of microRNA expression
profile related to lymph node status in women with early-stage
grade 1–2 endometrial cancer. Mod Pathol. 29:391–401. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mullany LE, Herrick JS, Wolff RK, Buas MF
and Slattery ML: Impact of polymorphisms in microRNA biogenesis
genes on colon cancer risk and microRNA expression levels: A
population-based, case-control study. BMC Med Genomics. 9:212016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang TH, Wu F, Loeb GB, Hsu R,
Heidersbach A, Brincat A, Horiuchi D, Lebbink RJ, Mo YY, Goga A and
McManus MT: Up-regulation of miR-21 by HER2/neu signaling promotes
cell invasion. J Biol Chem. 284:18515–18524. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu J, Mao Q, Liu Y, Hao X, Zhang S and
Zhang J: Analysis of miR-205 and miR-155 expression in the blood of
breast cancer patients. Chin J Cancer Res. 25:46–54.
2013.PubMed/NCBI
|
|
16
|
Liu Y, Cai Q, Bao PP, Su Y, Cai H, Wu J,
Ye F, Guo X, Zheng W, Zheng Y and Shu XO: Tumor tissue microRNA
expression in association with triple-negative breast cancer
outcomes. Breast Cancer Res Treat. 152:183–191. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Peng Y, Chen F, Melamed J, Chiriboga L,
Wei J, Kong X, McLeod M, Li Y, Li CX, Feng A, et al: Distinct
nuclear and cytoplasmic functions of androgen receptor cofactor p44
and association with androgen-independent prostate cancer. Proc
Natl Acad Sci USA. 105:5236–5241. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Minn AJ, Gupta GP, Siegel PM, Bos PD, Shu
W, Giri DD, Viale A, Olshen AB, Gerald WL and Massagué J: Genes
that mediate breast cancer metastasis to lung. Nature. 436:518–524.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gottardo F, Liu CG, Ferracin M, Calin GA,
Fassan M, Bassi P, Sevignani C, Byrne D, Negrini M, Pagano F, et
al: Micro-RNA profiling in kidney and bladder cancers. Urol Oncol.
25:387–392. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mertens-Talcott SU, Chintharlapalli S, Li
X and Safe S: The oncogenic microRNA-27a targets genes that
regulate specificity protein transcription factors and the G2-M
checkpoint in MDA-MB-231 breast cancer cells. Cancer Res.
67:11001–11011. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu T, Tang H, Lang Y, Liu M and Li X:
MicroRNA-27a functions as an oncogene in gastric adenocarcinoma by
targeting prohibitin. Cancer Lett. 273:233–242. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang X, Tang S, Le SY, Lu R, Rader JS,
Meyers C and Zheng ZM: Aberrant expression of oncogenic and
tumor-suppressive microRNAs in cervical cancer is required for
cancer cell growth. PLoS One. 3:e25572008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pan W, Wang H, Jianwei R and Ye Z:
MicroRNA-27a promotes proliferation, migration and invasion by
targeting MAP2K4 in human osteosarcoma cells. Cell Physiol Biochem.
33:402–412. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang Z, Liu S, Shi R and Zhao G: miR-27
promotes human gastric cancer cell metastasis by inducing
epithelial-to-mesenchymal transition. Cancer Genet. 204:486–491.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kong LY, Xue M, Zhang QC and Su CF: In
vivo and in vitro effects of microRNA-27a on proliferation,
migration and invasion of breast cancer cells through targeting of
SFRP1 gene via Wnt/β-catenin signaling pathway. Oncotarget.
8:15507–15519. 2017.PubMed/NCBI
|
|
26
|
Drayton RM, Dudziec E, Peter S, Bertz S,
Hartmann A, Bryant HE and Catto JW: Reduced expression of miRNA-27a
modulates cisplatin resistance in bladder cancer by targeting the
cystine/glutamate exchanger SLC7A11. Clin Cancer Res. 20:1990–2000.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tang W, Zhu J, Su S, Wu W, Liu Q, Su F and
Yu F: MiR-27 as a prognostic marker for breast cancer progression
and patient survival. PLoS One. 7:e517022012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Guttilla IK and White BA: Coordinate
regulation of FOXO1 by miR-27a, miR-96, and miR-182 in breast
cancer cells. J Biol Chem. 284:23204–23216. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dent R, Hanna WM, Trudeau M, Rawlinson E,
Sun P and Narod SA: Pattern of metastatic spread in triple-negative
breast cancer. Breast Cancer Res Treat. 115:423–428. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
PDQ Pediatric Treatment Editorial Board:
Childhood craniopharyngioma treatment (PDQ®): Health
professional versionIn: PDQ Cancer Information Summaries. National
Cancer Institute (US); Bethesda, MD: 2002
|
|
31
|
Abdulkarim BS, Cuartero J, Hanson J,
Deschênes J, Lesniak D and Sabri S: Increased risk of locoregional
recurrence for women with T1-2N0 triple-negative breast cancer
treated with modified radical mastectomy without adjuvant radiation
therapy compared with breast-conserving therapy. J Clin Oncol.
29:2852–2858. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cully M, You H, Levine AJ and Mak TW:
Beyond PTEN mutations: The PI3K pathway as an integrator of
multiple inputs during tumorigenesis. Nat Rev Cancer. 6:184–192.
2006. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
López-Knowles E, O'Toole SA, McNeil CM,
Millar EK, Qiu MR, Crea P, Daly RJ, Musgrove EA and Sutherland RL:
PI3K pathway activation in breast cancer is associated with the
basal-like phenotype and cancer-specific mortality. Int J Cancer.
126:1121–1131. 2010. View Article : Google Scholar : PubMed/NCBI
|