Introduction
Sepsis, in the context of infection, is defined as a
systemic inflammatory response syndrome (SIRS). For numerous
individuals, it is a life-threatening and profoundly damaging
condition. The consequent increase in hospitalizations and resource
utilization in providing care to patients with sepsis leads to the
increased incidence of sepsis (1).
It is one of the major causes of admissions to the intensive care
unit (ICU) and the emergency intensive care unit (EICU), and is
associated with high mortality and morbidity rates (2), in addition to multiple organ
dysfunction or injury, for example in the lungs, kidneys and bone
marrow (3–5). Sepsis is a systemic inflammatory
response, which occurs during infection (6), and its pathogenesis is a result of
immune-inflammatory and anti-inflammatory processes triggered by
the infection agent (7). As
reported frequently, the effects of sepsis on the individual
persists following the period of critical illness, and has an
effect on mortality and morbidity rates (8,9). In
addition, the inflammation present during EICU admission may cause
subsequent deleterious effects (10). Following discharge from hospital,
there is an increased risk of cardiovascular disease in patients
who survive sepsis, which suggests that an acute episode of
systemic inflammation has a long-term effect (11).
Phospholipase A2 (PLA2) is an enzyme, which is
involved in lipid metabolism, hydrolyzes phospholipids, and
liberates arachidonic acid and lysophospholipids (12). PLA2 enzymes are a diverse class of
esterases, at the sn-2 position, which preferentially cleave
glycerophospholipids to liberate a fatty acid and a
lysophospholipid (13).
Phospholipases of mammalian species are critical in transducing
cellular signals into biologically active lipid second messengers,
including lysophospholipids and arachidonic acid (12). An enzyme from human plasma was
previously isolated by investigators from the University of Utah
(14), which hydrolyzes
platelet-activating factor (PAF) and, in 1995, Tjoelker et
al (15) reported its cloning.
PAF acetyl hydrolase (PAF-AH) enzyme were later recognized as
PLA2s, one of which for oxidized lipids in plasma was independently
termed lipoprotein-associated phospholipase A2 (Lp-PLA2). In
previous years, several reviews have examined the family of PLA2s
or specific types, including secretory PLA2, cytosolic PLA2
(cPLA2), lysosomal PLA2, PAF-AH, calcium-independent PLA2 and
adipose-specific PLA2 (16–19).
In total, >30 isoforms and associated enzymes have been
identified, which are involved in inflammation and several
neoplastic conditions. Based on their localization, catalytic
mechanism, structure and evolutionary associations, the isoforms
are divided into the above six families (13). These enzymes differ in size,
function, location, substrate specificity and calcium requirements.
The classes of PLA2 inhibitors and their potential role in the
treatment of inflammatory diseases have been summarized in several
review articles (19–21).
Lp-PLA2 is a unique member of the PLA2 superfamily,
also known as PAF-AH. In humans, it circulates in an active form as
a complex with high- and low-density lipoproteins (LDLs). PLA2 has
been used as a novel biomarker in cardiac diseases, predicting the
prevalence and prognosis of chronic and acute congestive heart
failure and pulmonary hypertension (22). In Japanese cohorts, genetic
analyses have shown that loss of Lp-PLA2 function is a risk factor
for vascular and inflammatory conditions in humans harboring an
inactivating mutation at this locus (23,24).
The overexpression of Lp-PLA2 has consistently shown
anti-atherogenic and anti-inflammatory properties in certain animal
models (23,25). It is an indicator or marker of
inflammation in the vessel wall, measured in the plasma (26,27).
Primarily secreted by macrophages, Lp-PLA2 binds to the
apolipoprotein B moiety on LDL particles following release, and
remains latent until the LDL is oxidized. Following LDL
oxidization, oxidized phosphatidylcholine is released, which acts
as a requisite substrate for Lp-PLA2. Lp-PLA2 then breaks the
oxidized phosphatidylcholine into two bioactive compounds,
lysophosphatidylcholine and oxidized nonesterified free fatty acids
(27), which are considered to act
as proinflammatory factors (26).
Previous studies have shown that Lp-PLA2, which is considered to be
a candidate inflammatory factor, is often upregulated in a number
of diseases, including pancreatitis (28) and myocarditis (29), suggesting that circulating Lp-PLA2
may offer potential as a biomarker for inflammatory and infectious
diseases.
Despite the novel roles of Lp-PLA2 in the regulation
of inflammation, its functional involvement in systemic infections
remains to be elucidated. In addition, whether Lp-PLA2 has
diagnostic and prognostic value in patients with sepsis is
currently unclear. Therefore, the present study examined patients
with sepsis at an EICU and performed several measurements of serum
concentrations of Lp-PLA2 during the first days of EICU treatment.
The aim of the study was to examine the regulation and diagnostic
value of serum concentrations of Lp-PLA2 in sepsis. The
investigation focused on the expression of Lp-PLA2 and its
potential contribution in predicting prognosis in sepsis, and
whether serum levels of Lp-PLA2 can serve as a prognostic predictor
for EICU and long-term survival rates.
Materials and methods
Study design and patient
information
The present prospective, observational study was
based in the EICU at the Affiliated Hospital of Nantong University
(Nantong, China). Between January 2008 and December 2012, patients
with sepsis admitted to the EICU were screened. Specifically,
patients with a diagnosis of sepsis according to the Surviving
Sepsis Campaign criteria for sepsis were included. Patients who met
the criteria set by the American College of Chest Physicians and
the Society of Critical Care Medicine Consensus Conference
Committee for sepsis, severe sepsis and septic shock were
classified as patients with sepsis. A total of 151 consecutive
patients (107 men and 44 women; median age 66 years, range 20–87
years) admitted to the EICU at the Affiliated Hospital of Nantong
University were enrolled in the present study (Table I). The exclusion criteria were as
follows: i) absence of informed consent; ii) patients <18 years
old; iii) patients undergoing continuous renal replacement therapy
prior to sampling; iv) patients receiving steroid therapy or
immunosuppressants; v) patients who were expected to have
short-term (<72 h) intensive care treatment due to
post-interventional observation or acute intoxication; vi) patients
with infections, including those induced by virus, chlamydia,
mycoplasma and tubercle bacillus. The medium length of admission at
the EICU was 15 days (range 1–34 days).
 | Table I.Baseline characteristics of patients
with sepsis and healthy controls. |
Table I.
Baseline characteristics of patients
with sepsis and healthy controls.
| Characteristic | Control (n=30) | Sepsis (n=39) | Severe sepsis
(n=55) | Septic shock
(n=57) |
|---|
| Age (years); mean
(range) | 61 (30–82) | 65 (49–85) | 66 (26 to 87) | 67 (20–85) |
| Gender, n (%) |
|
|
|
|
| Male | 17 (56.7) | 27 (69.2) | 39 (70.9) | 41 (71.9) |
|
Female | 13 (43.3) | 12 (30.8) | 16 (29.1) | 16 (28.1) |
| Site of infection, n
(%) |
|
|
|
|
| Lung | – | 32 (82.1) | 31 (56.3) | 33 (57.8) |
| Urinary
tract | – | – | 5 (9.1) | 3 (5.3) |
|
Abdominal | – | 7 (17.9) | 14 (25.5) | 11 (19.3) |
|
Skin | – | – | 2 (3.6) | 4 (7.0) |
|
Heart | – | – | – | 1 (1.8) |
|
Blood | – | – | 3(5.5) | 3 (5.3) |
|
Other | – | – | – | 2 (3.5) |
| Laboratory
measurements |
|
|
|
|
| White
blood cells (109/l) | – | 16.9±2.5 | 16.3±3.4 | 14.7±3.1 |
|
Platelets
(109/l) | – | 305±20 | 236±27 | 191±14 |
|
Bilirubin (µmol/l) | – | 35±5.7 | 50±7.9 | 62±13.6 |
|
Creatinine (mg/dl) | – | 1.2±0.2 | 1.4±0.3 | 2.3±0.5 |
|
C-reactive protein (mg/l) | – | 163±32 | 181±23 | 196±15 |
|
Procalcitonin (ng/ml) | – | 4.7±2.1 | 7.2±1.9 | 19.7±3.1 |
|
Interleukin 6, (pg/ml) | – | 179±69 | 1,296±723 | 21,234±12,705 |
|
Positive blood cultures, n
(%) | – | 5 (12.8) | 11 (20.0) | 29 (50.9) |
| EICU
parameters |
|
|
|
|
| EICU
(days) | – | 8±3 | 11±3 | 19±6 |
|
Ventilation (days) | – | 4±2 | 7±3 | 11±4 |
|
Catecholamine (days) | – | 0±0 | 2±2 | 6±2 |
| Renal
replacement therapy (days) | – | 0±0 | 1±0.4 | 3±1 |
| APACHE
II score | – | 19±4 | 22±3 | 27±1 |
| SOFA
score | – | 6.3±1.9 | 6.4±2.1 | 12.1±1.9 |
| All-cause
mortality, n (%) |
|
|
|
|
| EICU |
|
|
|
|
|
Succumbed to mortality | 0 (0) | 2 (5.1) | 5 (9.1) | 11 (19.3) |
|
Alive | 30 (100) | 37 (94.2) | 50 (90.9) | 46 (80.7) |
| Ovarall |
|
|
|
|
|
Succumbed to mortality | 0 (0) | 4 (10.3) | 8 (14.5) | 17 (29.8) |
|
Alive | 30 (100) | 35 (89.7) | 47 (85.5) | 40 (70.1) |
The patients' data, blood samples and clinical
information were collected prospectively. The clinical course was
observed in a follow-up period by directly contacting the patients,
the relatives or their primary care physicians. In addition, 30
healthy blood donors were analyzed as a control population, with
normal values for blood counts, liver enzymes and C-reactive
protein (CRP). All blood samples were obtained with the consent of
the patient, his or her spouse, or the appointed legal guardian.
The study protocol followed the guidelines set in the Declaration
of Helsinki and was approved by the Ethics Committee (Institutional
Review Board) at the Affiliated Hospital of Nantong University.
Definitions and determination of
relevant parameters in patients with sepsis
Serum was obtained on admission to the EICU prior to
therapeutic intervention. For the 151 patients, follow-up
measurements were available during EICU treatment. All samples were
immediately placed on ice and centrifuged for 5 min at 2000 rpm and
0°C, and serum samples were stored at −80°C. CRP, procalcitonin
(PCT) and interleukin 6 (IL-6) were measured. If a patient
succumbed to mortality within 90 days following the onset of
sepsis, this was defined as sepsis-associated mortality.
EICU-associated mortality was defined as a patient succumbing to
mortality admission in the EICU; the overall mortality included
that within the EICU and during the observation period following
discharge from the EICU and hospital. Generally, sepsis was
diagnosed by an identifiable or suspected infection site, in
addition to evidence of SIRS manifested by at least two of the
following criteria: i) body temperature <36°C or >38°C; ii)
respiratory rate >20 breaths/min; iii) heart rate >90
beats/min; iv) white blood cell count <4,000/mm3 or
>12,000/mm3. When a patient with sepsis suffered from
dysfunction of at least one organ within 24 h h following
admission, severe sepsis was diagnosed. In the present study,
patients with sepsis, but not severe sepsis, were designated as the
sepsis group. Septic shock was defined as the beginning of
vasopressor therapy by persisting hypotension despite fluid
resuscitation and requiring vasopressor therapy, an identifiable
site of infection or evidence of a systemic inflammatory response
manifested by at least two of the following criteria: i)
temperature <36°C or >38°C; ii) respiratory rate >20
breaths/min; iii) heart rate >90 beats/min; iv) white blood cell
count <4,000/mm3 or >12,000/mm3. The
severity and the development of organ dysfunction were assessed
using the Sequential Organ Failure Assessment (SOFA) score
(30) (range 0–24) measured 24 h
into the EICU admission. According to the guidelines of the
Surviving Sepsis Campaign (31),
patients with septic shock were immediately treated with empiric
broad-spectrum antibiotherapy following admission. Several
characteristics were determined on the first day of admission,
including disease severity, underlying comorbidities, demographic
characteristics, the presence of concomitant organ dysfunction and
the Acute Physiology and Chronic Health Evaluation II (APACHE II)
score (32). The SOFA score was
also used to determine the severity of multi-organ dysfunction.
Determination of serum concentrations
of Lp-PLA2 using ELISA
The plasma concentrations of Lp-PLA2 (ng/ml) were
measured using an ELISA kit (second-generation PLAC test; diaDexus,
Inc., South San Francisco, CA, USA). All samples were analyzed in
duplicate, and the analytical coefficient of variation was
6.3%.
Patient follow-up
The patients were included in the survival model
until they succumbed to mortality, there was censoring due to loss
of follow up, or until the end of the follow-up period (90 days).
If clinically indicated, more frequent examinations were scheduled.
The mortality rates of patients with sepsis were measured as
overall survival rates. The overall survival rate was defined as
either the proportion of patients with sepsis remaining alive at a
certain time point following their sepsis episode or the occurrence
of non-sepsis-associated mortality, ensuring that the overall
survival rate did not measure excess sepsis-associated mortality.
Indication that a patient had succumbed to mortality was
ascertained by reports from family and telephone conversations, and
verified by a review of public records. The Kaplan-Meier method was
used to calculate the overall survival analysis.
Statistical analysis
All statistical analyses were performed with SPSS
19.0 statistical software (IBM SPSS, Armonk, NY, USA) as previously
described. The t-test was used for data comparison between two
groups and one-way analysis of variance followed by Tukey's post
hoc test was used for data comparisons among three groups.
Kaplan-Meier curves and log-rank test calculations were performed
to determine the effect on survival rates. The Kaplan-Meier method
was used to compute survival analyses and the log-rank test was
used to assess significant levels. All data were included for
statistical analyses. The results are expressed as the mean ±
standard error of the mean of at least three independent
experiments. Spearman's correlation tests were used to analyze the
correlations between variables. P<0.05 was considered to
indicate a statistically significant difference.
Results
Study population
The clinical characteristics of the patients with
sepsis, severe sepsis and septic shock, and healthy controls are
presented in Table I. In total,
151 patients were included in the present study: 25.8% of the
patients (n=39) suffered from sepsis, 36.5% of the patients (n=55)
suffered from severe sepsis and 37.7% of the patients (n=57)
suffered from septic shock at the start of the investigation, with
an EICU-associated mortality rate of 11.9% and an overall mortality
rate of 19.2%. The primary sources of infection were the lung (96
patients; 63.5%; pneumonia), the abdomen (32 patients; 21.2%;
peritonitis), the urinary tract (eight patients; 5.3%; UTI), the
skin (six patients; 4.0%; dermatophyte infection), the heart (one
patient; 0.7%; infectious endocarditis), blood (six patients; 4.0%;
leukemia and aplastic anemia) and others (two patients; 1.3%;
neutropenia).
Serum concentrations of Lp-PLA2 are
elevated in patients with sepsis
The serum samples from the patients were analyzed on
admission to the EICU, prior to specific therapeutic intervention.
As shown in Fig. 1, patients had
significantly higher serum concentrations of Lp-PLA2 on EICU
admission (338 ng/ml, range 206–452 ng/ml), compared with healthy
controls (123.9 ng/ml; range 12–202 ng/ml; P<0.001).
Association of serum concentrations of
Lp-PLA2 with disease severity in patients with sepsis
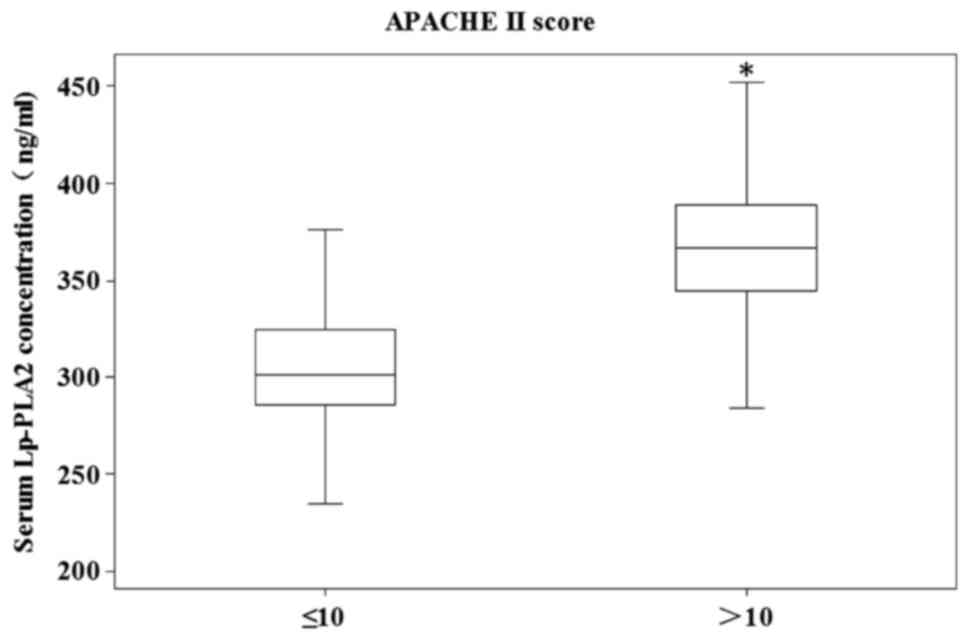
High serum levels of Lp-PLA2 were associated with
the severity of disease; patients with high APACHE II scores
(>10) exhibited a further increase in Lp-PLA2 levels (median
360.8 ng/ml; range 264–452 ng/ml), compared with patients with low
APACHE II scores (≤10; median 305.8 ng/ml; range 206–390 ng/ml;
P=0.005), as shown in Fig. 2.
These data indicated that elevated levels of Lp-PLA2 were primarily
associated with disease severity in patients with sepsis.
All patients with sepsis were divided into three
groups: Sepsis (median 297 ng/ml; range 206–388 ng/ml), severe
sepsis (median 336.6 ng/ml; range 247–410 ng/ml) and septic shock
(median 366.3 ng/ml; range 258–452 ng/ml). The serum concentration
of Lp-PLA2 in the septic shock group was significantly higher,
compared with that in the severe sepsis group (P<0.05; Fig. 3), and the serum concentration of
Lp-PLA2 in the severe sepsis group was significantly higher,
compared with that in the sepsis group (P<0.001; Fig. 3).
Correlation between serum levels of
Lp-PLA2 in sepsis and other laboratory markers
To determine which factors may have promoted the
elevated concentrations of Lp-PLA2 in patients with sepsis,
correlation analyses were performed with extensive sets of
laboratory parameters, using Spearman's rank correlation test. As
shown in Table II, the serum
concentrations of Lp-PLA2 were correlated with markers of systemic
inflammation in patients with sepsis, including CRP (P=0.006), PCT
(P<0.0001) and IL-6 (P=0.001). Compared with CRP and IL-6, PCT
was more relevant to serum concentrations of Lp-PLA2 (r=0.55), and
higher than CRP (r=0.37) and IL-6 (r=0.39). Consequently, an
association was identified between serum concentrations of Lp-PLA2
on EICU admission with established clinical scores, including
APACHE II (r=0.27; P=0.004) and SOFA (r=0.23; P=0.007) scores
(Table II).
 | Table II.Correlations between serum
concentrations of Lp-PLA2 and other laboratory markers in patients
with sepsis. |
Table II.
Correlations between serum
concentrations of Lp-PLA2 and other laboratory markers in patients
with sepsis.
|
| Lp-PLA2, vs.
marker/score on admission |
|---|
|
|
|
|---|
| Marker/score | r | P-value |
|---|
| C-reactive
protein | 0.37 | 0.006 |
| Procalcitonin | 0.55 | <0.0001 |
| Interleukin 6 | 0.39 | 0.001 |
| APACHE II | 0.27 | 0.004 |
| SOFA | 0.23 | 0.007 |
Correlation of serum levels of Lp-PLA2
with EICU and overall mortality rates
Based on the associations between serum
concentrations of Lp-PLA2, inflammatory markers and prognostic
clinical scores, the present study hypothesized that Lp-PLA2
measurements can predict the risk of mortality in patients with
sepsis. Therefore, the concentrations of Lp-PLA2 on admission in
patients that succumbed to mortality during EICU treatment were
compared with those of survivors at overall follow-up. In the
cohort of patients with sepsis, 11.9% (18 cases) succumbed to
mortality in the EICU, whereas the overall mortality rate increased
to 19.2% (29 cases) during the follow-up period. During the time
spent in EICU, those who succumbed to mortality exhibited a further
increase in levels of Lp-PLA2 (median 391.6 ng/ml; range 305–452
ng/ml), compared with the survivors (median 330.2 ng/ml; range
206–401 ng/ml; P=0.002) as shown in Fig. 4. During the overall follow-up, the
non-survivors had higher levels of Lp-PLA2 (median 376.5 ng/ml;
range 293–452 ng/ml), compared with the survivors (median 328
ng/ml; range 206–401 ng/ml; P=0.03; Fig. 5), which showed that a high level of
Lp-PLA2 was a prognostic predictor for mortality rates.
Serum concentrations of Lp-PLA2 are
associated with survival rates of patients with sepsis
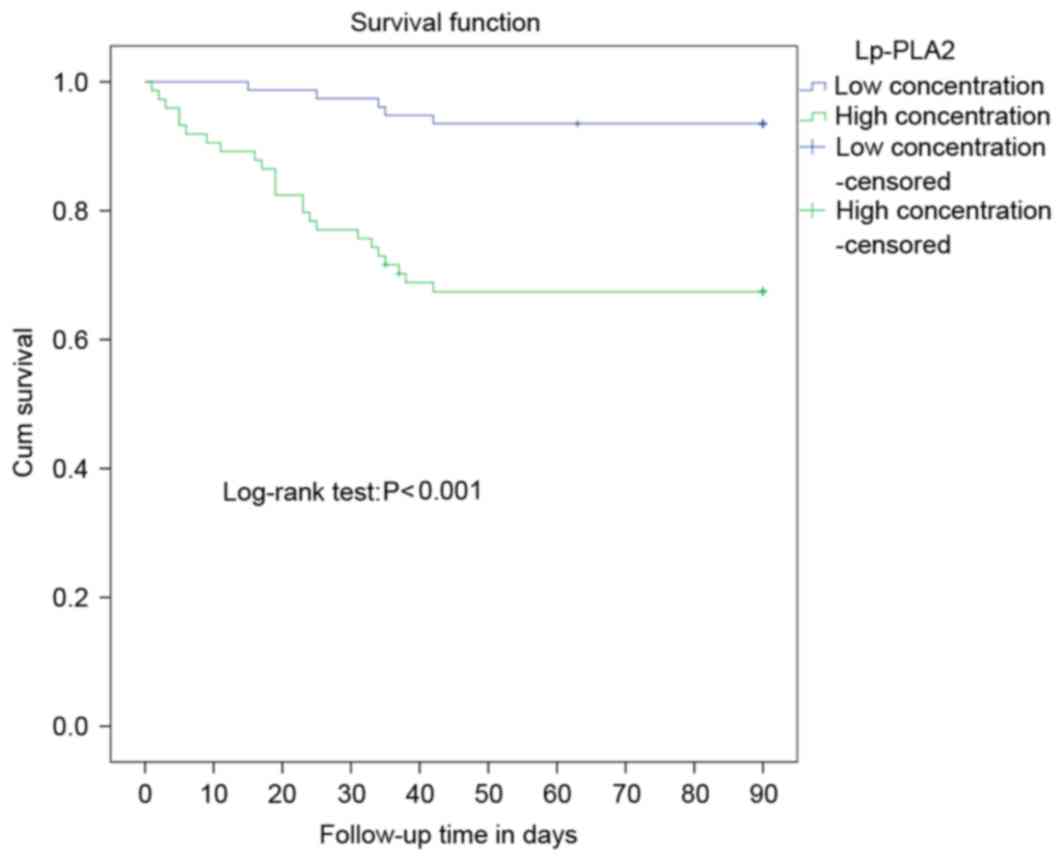
To further substantiate the results in terms of the
potential prognostic value of Lp-PLA2 measurements, Kaplan-Meier
survival curves analysis was performed. The prognostic role of
Lp-PLA2 on the overall survival rate of patients with sepsis was
investigated by comparing the 90-day-survival rate of patients with
high or low serum concentrations of Lp-PLA2 in sepsis using
Kaplan-Meier survival curves and the log-rank test. Using the
quartile limits of serum expression of Lp-PLA2 to divide patient
the population into low and high levels allowed the interquartile
range to be set as a cut-off, and a significant correlation between
the serum expression of Lp-PLA2 and survival rates was established.
The median serum expression of Lp-PLA2 was 346 ng/ml, dividing the
samples into two groups: Low concentration (≤346 ng/ml) and high
concentration (>346 ng/ml). There were 74 cases in the high
concentration group, 24 of which succumbed to mortality and two
cases were lost to follow-up, and the 90-day-overall survival rate
was 64.8%. In the low concentration group, there were 77 cases,
five of which succumbed to mortality and one was lost during
follow-up. The 90-day-overall survival rate for the negative group
was 92.2%. The overall survival rate of the low concentration group
was significantly higher, compared with that of the high
concentration group (P<0.001; Fig.
6).
Thus, the data obtained indicated that measuring the
levels of Lp-PLA2 in a medical EICU environment may be valuable for
evaluating the short-term and long-term prognoses of a patient with
sepsis.
Discussion
The present study focused on the expression of serum
Lp-PLA2 in sepsis. The concentrations of Lp-PLA2 were examined on
admission to the EICU, prior to specific therapeutic interventions,
in a well-characterized cohort of patients with sepsis. The primary
finding of the present study was that Lp-PLA2 provided as a marker
of inflammation and severity of illness, which correlated with
long-term prognosis (up to 90 days) following the episode of
sepsis. From the serum measurements, there was a significant
difference in the concentration of Lp-PLA2 between the patients
with sepsis and the healthy controls. Subsequently, the association
between serum concentrations of Lp-PLA2 and the severity of disease
was determined. As expected, serum levels of Lp-PLA2 on admission
to EICU were significantly elevated in patients with sepsis with
high initial APACHE II scores, compared with those with low APACHE
II scores. Therefore, the serum concentrations of Lp-PLA2 were
positively correlated with the severity of disease. The present
study then analyzed the associations between serum concentrations
of Lp-PLA2, inflammatory markers and prognostic clinical scores,
which showed that serum concentrations of Lp-PLA2 were associated
with inflammatory markers and prognostic clinical scores.
Therefore, the present study compared concentrations of Lp-PLA2 on
admission in patients who succumbed to mortality during EICU
treatment and in survivors at overall follow-up. The results showed
that Lp-PLA2 measurements predicted the mortality rates in patients
with sepsis. In addition, the results of Kaplan-Meier survival
curves and the log-rank test showed that the overall survival rate
of the low concentration group was significantly higher compared
with that of the high concentration group. In these patients, serum
concentrations of Lp-PLA2 were found to have a close association
with the severity of disease, mortality rates and prognosis.
Sepsis is one of the most common contributors to
mortality rates worldwide. Unfortunately, the prognosis of sepsis
has improved only gradually despite advances in intensive care
medicine. Evidence that PLA2 and inflammation are tightly coupled
has accumulated in previous years (23). In previous reports, all six types
of PLA2 have been discussed in relation to subgroups, terms of
groups, mechanism of action, structure and interaction with
membranes, role in disease, the various forms, biological
activities and development in selective inhibitors (15–21).
The secreted enzymes may occur in various intracellular vesicles.
The PAF-AH and certain cPLA2 enzymes are
Ca2+−independent (33).
As reported in a previous study, Lp-PLA2, which is upregulated by
oxidized phospholipids in oxidized LDL, acts on oxidized
phospholipids to promote it to produce the two pro-inflammatory
mediators, lysophosphatidylcholines and oxidized non-esterified
fatty acids (34). In a previous
study of acute pancreatitis, important pathophysiological roles of
PLA2 have been shown; the group II A secretory phospholipase A2
(PLA2-II) is considered to be important in cell injury and
inflammation (35). PLA2-II
appears to be the major enzyme in acute pancreatitis responsible
for the systemic inflammatory process (36). A previous study also showed that
the expression of PLA2-II was increased in the pancreas, induced by
4% sodium taurocholate injected into the pancreatic duct, whereas
knockdown of the PLA2-II gene mediated by small interfering (si)RNA
relieved the severity of pancreatitis (35). This suggests that PLA2-II is
important in inflammation. PLA2 also correlates with the appearance
of SIRS in severe acute pancreatitis (37). In addition to these findings, the
results of the present study, showed that Lp-PLA2 was associated
with the severity, and the mortality and survival rates of sepsis.
Of note, PCT exhibited higher correlation with the serum
concentrations of Lp-PLA2, compared with CRP and IL-6, however the
underlying mechanism remains to be elucidated. It was hypothesized
that this may be caused by calcium ions, as mentioned above, as
certain forms of PLA2 have been recognized as cytosolic
Ca2+−independent. Procalcitonin, a precursor of
calcitonin manufactured in the thyroid, is significantly
upregulated in several types of bacterial infection (38,39).
Future investigations aim to focus on the underlying mechanism of
this. Future investigations also aim to focus on the role of the
downregulated expression of Lp-PLA2, including siRNA-mediated gene
knockdown.
Although the present study obtained valuable results
regarding Lp-PLA2 in sepsis, there were several limitations. The
study did not record all causes of mortality, which makes it
difficult to adequately establish a link between inflammation and
the cause of mortality. In addition, no data were collected on
terminal disease status, which may have an effect on results. In
future investigations of Lp-PLA2, the potential prognostic value of
Lp-PLA2 measurements requires substantiation using multivariate Cox
regression analysis, with the markers of infection/inflammation,
includingCRP, white blood cell count, renal (creatinine) and
hepatic (bilirubin, INR) function, to determine whether Lp-PLA2 is
an independent significant prognostic parameter to predict ICU
survival rates.
In conclusion, the data obtained in the present
study suggested a possible role for Lp-PLA2 as a prognostic marker
in sepsis, and provided background evidence for larger trials to
evaluate the clinical and pathophysiologic role of Lp-PLA2 in
sepsis, compared with other markers of inflammation and hypoxia.
Persistently elevated serum concentrations of Lp-PLA2 were
associated with an unfavorable outcome in patients with sepsis. In
addition to a possible pathogenic role of Lp-PLA2 in sepsis, the
present study indicated the potential value for Lp-PLA2 as a
prognostic biomarker in patients with sepsis during the early
course of EICU treatment.
Acknowledgements
The present study was supported by the Natural
Science Foundation of Nantong (grant no. MS32015032) and the
National Natural Science Foundation of China (grant no.
81402226).
References
|
1
|
Iskander KN, Osuchowski MF,
Stearns-Kurosawa DJ, Kurosawa S, Stepien D, Valentine C and Remick
DG: Sepsis: Multiple abnormalities, heterogeneous responses, and
evolving understanding. Physiol Rev. 93:1247–1288. 2013. View Article : Google Scholar :
|
|
2
|
Edman-Wallér J, Ljungström L, Jacobsson G,
Andersson R and Werner M: Systemic symptoms predict presence or
development of severe sepsis and septic shock. Infect Dis (Lond).
48:209–214. 2016. View Article : Google Scholar
|
|
3
|
Mayeux PR and MacMillan-Crow LA:
Pharmacological targets in the renal peritubular microenvironment:
Implications for therapy for sepsis-induced acute kidney injury.
Pharmacol Ther. 134:139–155. 2012. View Article : Google Scholar :
|
|
4
|
Gill SE, Taneja R, Rohan M, Wang L and
Mehta S: Pulmonary microvascular albumin leak is associated with
endothelial cell death in murine sepsis-induced lung injury in
vivo. PLoS One. 9:e885012014. View Article : Google Scholar :
|
|
5
|
Crouser E, Exline M, Knoell D and Wewers
MD: Sepsis: Links between pathogen sensing and organ damage. Curr
Pharm Des. 14:1840–1852. 2008. View Article : Google Scholar :
|
|
6
|
de Pablo R, Monserrat J, Prieto A and
Alvarez-Mon M: Role of circulating lymphocytes in patients with
sepsis. Biomed Res Int. 2014:6710872014. View Article : Google Scholar :
|
|
7
|
Hotchkiss RS and Karl IE: The
pathophysiology and treatment of sepsis. N Engl J Med. 348:138–150.
2003. View Article : Google Scholar
|
|
8
|
Sakr Y, Vincent JL, Ruokonen E,
Pizzamiglio M, Installe E, Reinhart K and Moreno R: Sepsis
Occurrence in Acutely Ill Patients Investigators: Sepsis and organ
system failure are major determinants of post-intensive care unit
mortality. J Crit Care. 23:475–483. 2008. View Article : Google Scholar
|
|
9
|
Yende S, Waterer GW, Tolley EA, Newman AB,
Bauer DC, Taaffe DR, Jensen R, Crapo R, Rubin S, Nevitt M, et al:
Inflammatory markers are associated with ventilatory limitation and
muscle dysfunction in obstructive lung disease in well functioning
elderly subjects. Thorax. 61:10–16. 2006. View Article : Google Scholar
|
|
10
|
Najafi A, Mojtahedzadeh M, Ahmadi KH,
Abdollahi M, Mousavi M, Chelkeba L, Najmeddin F and Ahmadi A: The
immunological benefit of higher dose N-acetyl cysteine following
mechanical ventilation in critically ill patients. Daru. 22:572014.
View Article : Google Scholar :
|
|
11
|
Yende S, D'Angelo G, Mayr F, Kellum JA,
Weissfeld L, Kaynar AM, Young T, Irani K and Angus DC: GenIMS
Investigators: Elevated hemostasis markers after pneumonia
increases one-year risk of all-cause and cardiovascular deaths.
PLoS One. 6:e228472011. View Article : Google Scholar :
|
|
12
|
Moon SH, Jenkins CM, Liu X, Guan S,
Mancuso DJ and Gross RW: Activation of mitochondrial
calcium-independent phospholipase A2γ (iPLA2γ) by divalent cations
mediating arachidonate release and production of downstream
eicosanoids. J Biol Chem. 287:14880–14895. 2012. View Article : Google Scholar :
|
|
13
|
Dennis EA, Cao J, Hsu YH, Magrioti V and
Kokotos G: Phospholipase A2 enzymes: Physical structure, biological
function, disease implication, chemical inhibition, and therapeutic
intervention. Chem Rev. 111:6130–6185. 2011. View Article : Google Scholar :
|
|
14
|
Stafforini DM, Elstad MR, McIntyre TM,
Zimmerman GA and Prescott SM: Human macrophages secret
platelet-activating factor acetylhydrolase. J Biol Chem.
265:9682–9687. 1990.
|
|
15
|
Tjoelker LW, Wilder C, Eberhardt C,
Stafforini DM, Dietsch G, Schimpf B, Hooper S, Le Trong H, Cousens
LS, Zimmerman GA, et al: Anti-inflammatory properties of a
platelet-activating factor acetylhydrolase. Nature. 374:549–553.
1995. View
Article : Google Scholar
|
|
16
|
Kudo I and Murakami M: Phospholipase A2
enzymes. Prostaglandins Other Lipid Mediat. 68–69:3–58. 2002.
View Article : Google Scholar
|
|
17
|
Menschikowski M, Hagelgans A and Siegert
G: Secretory phospholipase A2 of group IIA: Is it an offensive or a
defensive player during atherosclerosis and other inflammatory
diseases? Prostaglandins Other Lipid Mediat. 79:1–33. 2006.
View Article : Google Scholar
|
|
18
|
Nevalainen TJ, Graham GG and Scott KF:
Antibacterial actions of secreted phospholipases A2. Review.
Biochim Biophys Acta. 1781:1–9. 2008. View Article : Google Scholar
|
|
19
|
Karabina SA, Gora S, Atout R and Ninio E:
Extracellular phospholipases in atherosclerosis. Biochimie.
92:594–600. 2010. View Article : Google Scholar
|
|
20
|
Rosenson RS: Phospholipase A2 inhibition
and atherosclerotic vascular disease: Prospects for targeting
secretory and lipoprotein-associated phospholipase A2 enzymes. Curr
Opin Lipidol. 21:473–480. 2010. View Article : Google Scholar
|
|
21
|
Suckling K: Phospholipase A2s: Developing
drug targets for atherosclerosis. Atherosclerosis. 212:357–366.
2010. View Article : Google Scholar
|
|
22
|
Passacquale G, Di Giosia P and Ferro A:
The role of inflammatory biomarkers in developing targeted
cardiovascular therapies: Lessons from the cardiovascular
inflammation reduction trials. Cardiovasc Res. 109:9–23. 2016.
View Article : Google Scholar
|
|
23
|
Rosenson RS and Stafforini DM: Modulation
of oxidative stress, inflammation, and atherosclerosis by
lipoprotein-associated phospholipase A2. J Lipid Res. 53:1767–1782.
2012. View Article : Google Scholar :
|
|
24
|
Bachelerie F, Ben-Baruch A, Burkhardt AM,
Combadiere C, Farber JM, Graham GJ, Horuk R, Sparre-Ulrich AH,
Locati M, Luster AD, et al: International union of basic and
clinical pharmacology. [corrected]. LXXXIX. Update on the extended
family of chemokine receptors and introducing a new nomenclature
for atypical chemokine receptors. Pharmacol Rev. 66:1–79. 2013.
View Article : Google Scholar
|
|
25
|
Kones R: Molecular sources of residual
cardiovascular risk, clinical signals, and innovative solutions:
Relationship with subclinical disease, undertreatment, and poor
adherence: Implications of new evidence upon optimizing
cardiovascular patient outcomes. Vasc Health Risk Manag. 9:617–670.
2013. View Article : Google Scholar :
|
|
26
|
Zalewski A, Macphee C and Nelson JJ:
Lipoprotein-associated phospholipase A2: A potential therapeutic
target for atherosclerosis. Curr Drug Targets Cardiovasc Haematol
Disord. 5:527–532. 2005. View Article : Google Scholar
|
|
27
|
Macphee CH, Nelson J and Zalewski A: Role
of lipoprotein-associated phospholipase A2 in atherosclerosis and
its potential as a therapeutic target. Curr Opin Pharmacol.
6:154–161. 2006. View Article : Google Scholar
|
|
28
|
Bedirli A, Gokahmetoglu S, Sakrak O,
Soyuer I, Ince O and Sozuer E: Beneficial effects of recombinant
platelet-activating factor acetylhydrolase and BN 52021 on
bacterial translocation in cerulein-induced pancreatitis. Eur Surg
Res. 36:136–141. 2004. View Article : Google Scholar
|
|
29
|
Onyimba JA, Coronado MJ, Garton AE, Kim
JB, Bucek A, Bedja D, Gabrielson KL, Guilarte TR and Fairweather D:
The innate immune response to coxsackievirus B3 predicts
progression to cardiovascular disease and heart failure in male
mice. Biol Sex Differ. 2:22011. View Article : Google Scholar :
|
|
30
|
Hutchings L, Watkinson P, Young JD and
Willett K: Defining multiple organ failure after major trauma: A
comparison of the Denver, Sequential Organ Failure Assessment, and
Marshall scoring systems. J Trauma Acute Care Surg. 82:534–541.
2017. View Article : Google Scholar :
|
|
31
|
Shrestha GS, Kwizera A, Lundeg G, Baelani
JI, Azevedo LCP, Pattnaik R, Haniffa R, Gavrilovic S, Mai NTH,
Kissoon N, et al: International Surviving Sepsis Campaign
guidelines 2016: The perspective from low-income and middle-income
countries. Lancet Infect Dis. 17:893–895. 2017. View Article : Google Scholar
|
|
32
|
Angstwurm MW, Dempfle CE and Spannagl M:
New disseminated intravascular coagulation score: A useful tool to
predict mortality in comparison with Acute Physiology and Chronic
Health Evaluation II and Logistic Organ Dysfunction scores. Crit
Care Med. 314–320; quiz 328. 2006. View Article : Google Scholar
|
|
33
|
Kojima M, Aiboshi J, Shibata M, Kobayashi
T and Otomo Y: Novel role of group VIB Ca2+-independent
phospholipase A2γ in leukocyte-endothelial cell interactions: An
intravital microscopic study in rat mesentery. J Trauma Acute Care
Surg. 79:782–789. 2015. View Article : Google Scholar
|
|
34
|
Wang WY, Li J, Yang D, Xu W, Zha RP and
Wang YP: OxLDL stimulates lipoprotein-associated phospholipase A2
expression in THP-1 monocytes via PI3K and p38 MAPK pathways.
Cardiovasc Res. 85:845–852. 2010. View Article : Google Scholar
|
|
35
|
Zhang KJ, Zhang DL, Jiao XL and Dong C:
Effect of phospholipase A2 silencing on acute experimental
pancreatitis. Eur Rev Med Pharmacol Sci. 17:3279–3284. 2013.
|
|
36
|
Isenmann R, Rau B and Beger HG: Early
severe acute pancreatitis: Characteristics of a new subgroup.
Pancreas. 22:274–278. 2001. View Article : Google Scholar
|
|
37
|
Lausevic Z, Lausevic M,
Trbojevic-Stankovic J, Krstic S and Stojimirovic B: Predicting
multiple organ failure in patients with severe trauma. Can J Surg.
51:97–102. 2008.
|
|
38
|
Schuetz P, Amin DN and Greenwald JL: Role
of procalcitonin in managing adult patients with respiratory tract
infections. Chest. 141:1063–1073. 2012. View Article : Google Scholar
|
|
39
|
Becker KL, Snider R and Nylen ES:
Procalcitonin assay in systemic inflammation, infection, and
sepsis: Clinical utility and limitations. Crit Care Med.
36:941–952. 2008. View Article : Google Scholar
|