Introduction
Fucoidan is a sulfated polysaccharide extracted from
brown seaweed. It has a chain structure similar to that of heparin
(1,2). It exerts various biological
activities including anticoagulation and inhibition of cell
proliferation (3–7). Fucoidan may have antitumor activity
against a variety of tumors, such as leukemia, sarcoma-180,
non-small-cell human bronchopulmonary carcinoma (NSCLC-N6), and
breast cancer (8–12). A double-blind randomized controlled
trial showed low-molecular-weight fucoidan combined with chemical
agents significantly improved the disease control rate in
metastatic colorectal cancer patients (13). However, little is known about the
mechanism underlying these biological activities. Intracellular
calcium ions are important second messengers that control a variety
of cellular responses (14).
Several newly identified T-type calcium channel blockers have been
shown to be able to inhibit the growth of human cancer cells by
blocking Ca2+ influx into the cells (15), a process that affects cell cycle
progression and cell proliferation. These blockers are potential
therapeutic agents for the tumors that depend on T-type calcium
channel to grow (16). These
studies suggest that calcium signaling may participate in
tumorigenesis and targeting their signaling could have therapeutic
values.
However, details about the effect of fucoidan on
Ca2+ signaling are largely unknown. In Sertoli cells,
fucoidan could activate the Ca2+ influx regulated by the
L-type voltage-operated Ca2+ channels by serving as an
L-selectin ligand (17). However,
the opposite effect of fucoidan on Ca2+ signaling has
also been reported. In polymorphonuclear leukocytes, fucoidan could
partly inhibit the oxLDL-induced increase in intracellular calcium
concentration [Ca2+]i by serving as a
scavenger receptor ligand (18).
Despite the fact that different cell types were used in these
studies, and that opposite effects of fucoidan on Ca2+
signaling were found, these studies commonly demonstrated that both
L-selectin receptors and scavenger receptors were sensitive to
fucoidan. Also, many tumor cells that do not express L-selectin
receptors or the scavenger receptors still exhibit Ca2+
responses (19). In HeLa cells,
for example, Ca2+ responses are induced through other
receptors (20–23). These findings indicate that a wide
variety of cell surface receptors may involve in the
Ca2+ response, and also suggest that fucoidan may
participate in the Ca2+ signaling through these cell
surface receptors.
In the present study, we investigated the effects of
fucoidan on intracellular Ca2+ responses by using a
variety of stimulants, including histamine, ATP, compound 48/80,
and acetylcholine (ACh). We found that fucoidan inhibited the
increase in [Ca2+]i induced by histamine,
ATP, compound 48/80, and acetylcholine effectively. To further
verify the effects of fucoidan on various G-protein coupled
receptors, RT-PCR was applied to identify the expression of
metabotropic histamine receptors and the purinergic P2Y receptors
after fucoidan treatment. Consistently with the effect of fucoidan
on the inhibition of [Ca2+]i, both H1R and
subtypes of P2YRs (P2YR1, P2YR2 and P2YR11) expressions were
significantly suppressed after fucoidan treatment. Taken together,
our results demonstrate that fucoidan exerts a wide spectrum of
inhibitory effects on Ca2+ responses and inhibits
various G-protein coupled receptors related to Ca2+
dynamics, suggesting that fucoidan inhibits Ca2+
signaling by directly inhibiting G-protein coupled receptors.
Materials and methods
Chemicals
Dulbecco's modified Eagle's medium (DMEM), fetal
bovine serum (FBS), penicillin, streptomycin, and 0.25%
trypsin-EGTA (ethylene glycol tetraacetic acid) were purchased from
Invitrogen (Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Fura-2 acetoxymethyl ester (fura-2/AM) was purchased from Dojindo
Laboratories (Kumamoto, Japan). Acetylcholine chloride (ACh) was
obtained from Wako Pure Chemical Industries, Ltd. (Osaka, Japan).
Fucoidan (MW=c.a. 20 kDa), adenosine-5′-triphosphate (ATP),
compound 48/80, and histamine were obtained from Sigma-Aldrich
(Merck KGaA, Darmstadt, Germany). Heparin was obtained from
Ajinomoto Co., Inc. (Tokyo, Japan).
Passage cultures of HeLa cells,
astrocytes, and HUVECs
HeLa cells and astrocytes (a gift from Dr R Susuki,
Photon Medical Research Center, Hamamatsu University School of
Medicine) were cultured at 37°C in a humidified atmosphere of 95%
air and 5% carbon dioxide (CO2) in DMEM supplemented
with 10% FBS and 100 units/ml penicillin-streptomycin. These cells
were maintained in 60-mm dishes (Corning Incorporated, Corning, NY,
USA). For studies of Ca2+ response, the cells were
subcultured in 35-mm glass-bottom dishes (Iwaki Glass, Chiba,
Japan) at approximately 0.5×105 cells per dish.
Human umbilical vein endothelial cells (HUVECs),
purchased from Health Science Research Resources Bank (HSRRB;
Osaka, Japan), were maintained in E-STIM Endothelial Cell Culture
Medium (BD Biosciences, Franklin Lakes, NJ, USA) with 10% FBS, 10
ng/ml EGF, and 200 µg/ml endothelial cell growth supplements (ECGS)
on 75-mm flasks. They were cultured in a humidified incubator
containing 5% CO2 and 95% air at 37°C. The HUVECs were
removed from the substrate with 0.25% trypsin and 0.02% EDTA (BD
Biosciences) and passaged on coverslips in 35-mm-diameter culture
dishes at a density of 1×104 cells/ml. The subcultures
were used for [Ca2+]i measurements on day 2
after passage.
Dye loading and medium
The [Ca2+]i was measured using
the fluorescent Ca2+ indicator dye fura-2. For staining,
HeLa cells, HUVECs, and astrocytes were incubated in a medium
containing fura-2/AM (2.5 µM) for 20 min at 37°C. The cells were
then rinsed twice with the medium prepared for calcium imaging. The
medium, here referred to as recording medium, contained (in mM):
NaCl, 140; KCl, 5; CaCl2, 2; MgCl2, 1.2;
glucose, 2; and HEPES (pH 7.4), 10. A calcium-free recording medium
was also used in which CaCl2 was removed,
MgCl2 was increased to 3.2 mM, and 1 mM EGTA was added
(pH 7.4).
Calcium imaging
Fluorescence imaging for
[Ca2+]i was performed 24 h after subculture
for HeLa cells and astrocytes, and 48 h after subculture for
HUVECs. The cells in each culture dish loaded with fura-2 AM were
placed on the stage of an inverted microscope (IX 70; Olympus,
Tokyo, Japan). They were superfused continuously using silicone
tubing warmed to 37°C and connected to a reservoir syringe held at
a height of 70 cm. The cells were illuminated at wavelengths of 340
and 380 nm, alternating every 3 sec. Time-lapse images of the
fluorescence emitted were obtained at 510 nm with a 40× objective
lens (UApo 40x/340, NA 0.9; Olympus). An intensified charge-coupled
device (CCD) camera (C4742-95; Hamamatsu Photonics K.K., Hamamatsu,
Japan) was used to capture the images. Approximately 10–20 cells
were observed in a microscopic field, and the changes in
[Ca2+]i in individual cells were determined
using the fluorescence ratio of 340/380 nm and an image-analysis
software (Aquacosmos, Hamamatsu Photonics). The changes in
[Ca2+]i, here referred to as Ca2+
responses, were quantified by calculating the areas under the
response curve recorded in each cell image, which are expressed as
the integrated amplitude of Ca2+ responses.
Reverse transcription-polymerase chain
reaction (RT-PCR)
In order to examine the effects of fucoidan on
expressions of specific G-protein coupled receptors, RT-PCR was
used to identify the mRNA expressions of H1R and P2YRs. HeLa cells
were serum-starved for 24 h and treated with 0.5 and 1.0 mg/ml
fucoidan respectively, for 3 h. The cells were harvested and
extracted with RNA. Total RNA pellet was resolved in 10 µl of
diethylpyrocarbonate-treated water, and 1 µg of each RNA sample was
used for the RT reaction. RNA samples were reverse-transcribed to
cDNA by RT EasyTM II kit (First-strand cDNA for Real-Time PCR)
(Foregene Co., Ltd., Chengdu, China). RT-qPCR was conducted by Real
Time PCR EasyTM-SYBR-Green I kit (Foregene Co., Ltd.). TaqMan
primers and the probe for H1R were listed in Table I. Amplicon size and reaction
specificity were confirmed by agarose gel electrophoresis.
Identities of the PCR products were verified by sequencing using a
genetic analysis system. Semi-quantitive RT-PCR was used to test
the P2YRs expressions. cDNA was amplified by PCR using 2X Taq PCR
StarMix (GenStar Biosolutions Co., Ltd., Beijing, China) according
to the manufacturer's instructions. The conditions consisted of an
initial denaturation step at 95°C for 2 min, followed by 40 cycles
of 30 sec denaturation step at 95°C, 30 sec annealing step at
53.5°C to 56.5°C, and 1 min extension step at 72°C. A final
extension step of 5 min at 72°C was also performed. PCR products
were separated by electrophoresis on a 1% agarose gel. All PCR
results were derived with cycle number producing a signal in the
linear portion of the amplification curve. The primers were
designed as presented in Table
II. The GAPDH gene was used as the internal standard, and data
were expressed as the ratio of H1R mRNA to GAPDH mRNA or P2YRs mRNA
to GADPH mRNA, respectively.
 | Table I.Primers for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primers for reverse
transcription-quantitative polymerase chain reaction.
| Primer | Sequence
(5′-3′) |
|---|
| H1R | F:
CAGAGGATCAGATGTTAGGTGATAGC |
|
| R:
AGCGGAGCCTCTTCCAAGTAA |
| TaqMan probe |
FAM-CTTCTCTCGAACGGACTCAGATACCACC-TAMRA |
| GAPDH | F:
CGGATTTGGTCGTATTGGG |
|
| R:
CGGATTTGGTCGTATTGGG |
 | Table II.Primers for semi-quantitative
polymerase chain reaction. |
Table II.
Primers for semi-quantitative
polymerase chain reaction.
| Primer | Sequence
(5′-3′) |
|---|
| P2YR1 | F:
GACTTCTTGTACGTGCTGACTCT |
|
| R:
GACCTCTTGTCACCTGATACGTG |
| P2YR2 | F:
CTCTACTTTGTCACCACCAG |
|
| R:
TTCTGCTCCTACAGCCGAAT |
| P2YR11 | F:
GAGGCCTGCATCAAGTGTCTG |
|
| R:
ACGTTGAGCACCCGCATGATG |
| GAPDH | F:
ATTCCATGGCACCGTCAAGGCT |
|
| R:
TCAGGTCCACCACTGACACGTT |
Statistical analysis
For the quantification of Ca2+ responses,
10–20 cells were analyzed in each observation. All observations
were repeated using 3–5 culture dishes. The integrated amplitude of
the Ca2+ response was calculated using an extended
baseline for subtraction. Statistical significance was determined
by one-way ANOVA followed by Student's t-test, and differences were
considered significant at P<0.05.
Results
Effects of fucoidan on Ca2+
responses induced by histamine in HeLa cells
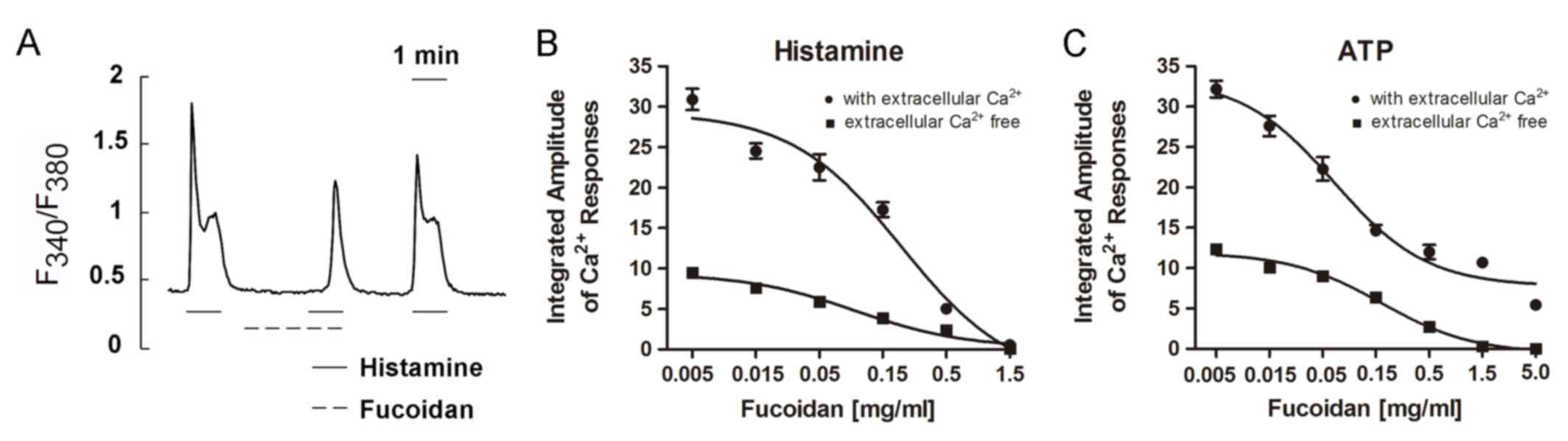
Histamine (2.5 µM) was applied three times to induce
Ca2+ responses. The first challenge induced a rapid rise
in [Ca2+]i followed by a sustained plateau in
HeLa cells in the presence of extracellular Ca2+
(Fig. 1A). When the cells were
superfused with the recording medium containing fucoidan (0.5
mg/ml), the second histamine challenge (in the same medium) was
followed, and only a small response was induced, i.e., a
small peak with a small or no plateau preceded by a long delay time
(Fig. 1A). The third challenge,
which took place after the removal of fucoidan, induced a quite
large response (Fig. 1A),
suggesting that the suppressive effect of fucoidan was reversible.
The recovery of the responses was observed within 2 min after
removal of fucoidan.
A dose-response curve for the suppression of
histamine-induced responses was obtained by using different
concentrations of fucoidan (Fig.
1B). In the presence of extracellular Ca2+ (filled
circles), the Ca2+ responses were gradually suppressed
by fucoidan in a dose-dependent manner. The response was completely
suppressed at a concentration of 1.5 mg/ml. Similarly, in the
absence of extracellular Ca2+ (EGTA 1 mM present)
(filled squares), fucoidan suppressed Ca2+ responses
gradually as its concentration increased. In both the presence and
absence of extracellular Ca2+, 1.5 mg/ml of fucoidan was
sufficient to completely abolish histamine-induced Ca2+
responses.
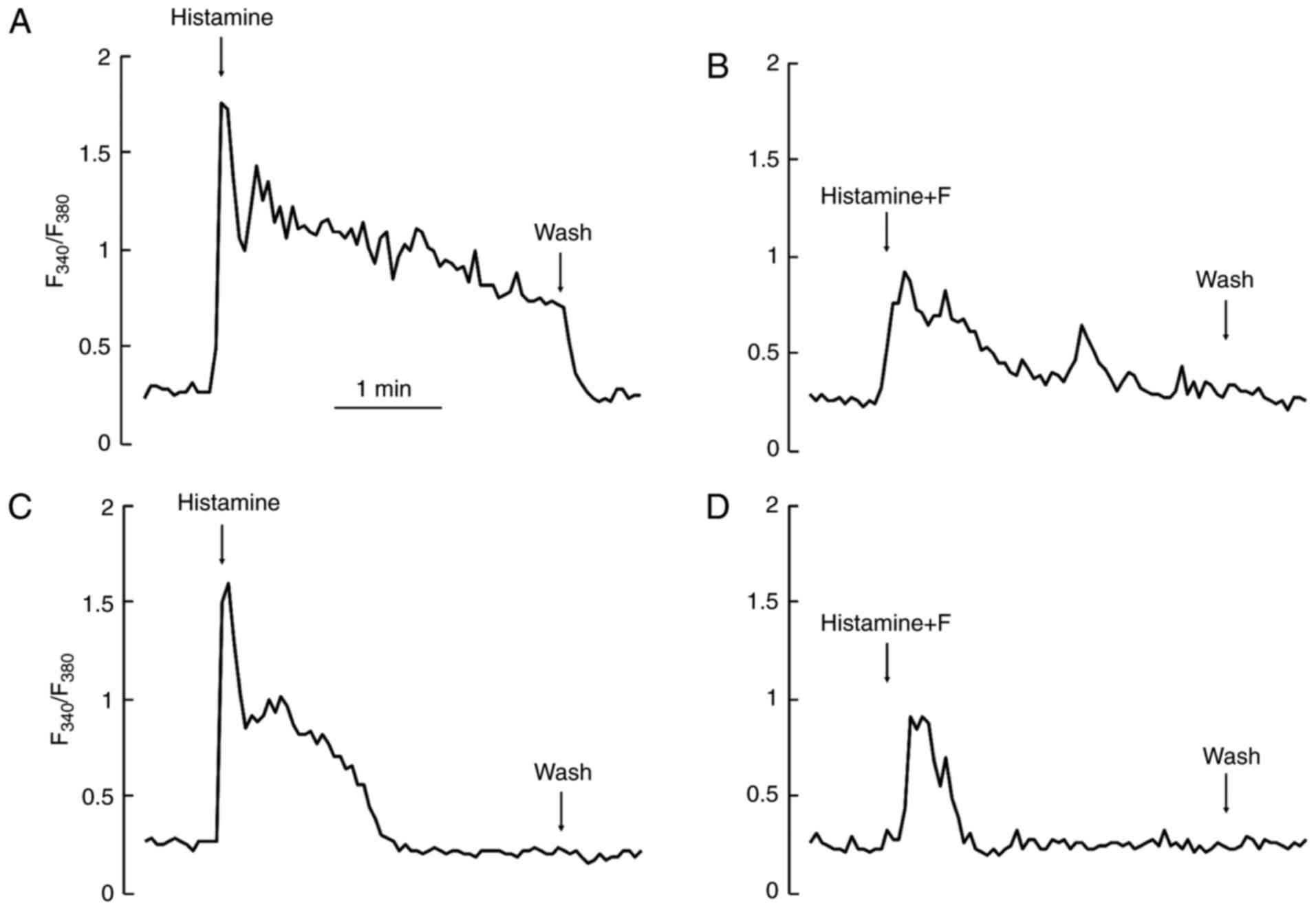
In the presence of extracellular Ca2+,
challenges with histamine induced Ca2+ responses of a
similar amplitude each time. However, in the absence of
extracellular Ca2+, stimulation with an agonist, such as
histamine, depleted intracellular Ca2+ pool, and thus
the second or the third challenge in the same cell always induced a
very small or no response at all. Therefore, we compared these
responses statistically in the absence or presence of extracellular
Ca2+ by integrating the amplitudes of the first
responses induced by the first 3-min challenge only. For the
control, we used 3-min applications of agonists after recording the
baseline for 3 min. For the test, fucoidan was added to the
recording medium during the same baseline period (3 min). Then,
without removal of fucoidan, the subsequent doses of agonists were
further added to the medium (Fig. 2A
and C). In both presence and absence of extracellular
Ca2+, fucoidan at a concentration of 0.5 mg/ml
suppressed the Ca2+ responses induced by histamine very
effectively (Fig. 2B and D) as
compared to the control.
Effects of fucoidan on Ca2+
responses induced by ATP in HeLa cells
Application of 5 µM ATP to HeLa cells rapidly
induced oscillating Ca2+ responses, as visualized by
fura-2 imaging in the presence and absence of extracellular
Ca2+. The amplitudes of such responses were as large as
those induced by histamine. We measured Ca2+ responses
by varying the concentration of fucoidan in the presence and
absence of extracellular Ca2+. In the absence of calcium
ions (EGTA, 1 mM) (filled squares, Fig. 1C), Ca2+ responses were
gradually suppressed as fucoidan concentration increased from 0.005
to 5.0 mg/ml. Responses were almost completely suppressed at a
fucoidan concentration of 5.0 mg/ml. The suppression of ATP-induced
Ca2+ responses by fucoidan appeared to be
dose-dependent. Although the dose-dependent suppression curve
observed in the presence of external Ca2+ (filled
circles) was roughly parallel to that observed in the absence of
extracellular Ca2+ (plus EGTA), the curve obtained with
2 mM extracellular Ca2+ did not reach zero. Even at a
fucoidan concentration of 5.0 mg/ml, responses could not be
suppressed completely in the presence of extracellular
Ca2+.
Effects of fucoidan on Ca2+
responses induced by compound 48/80 in HeLa cells
Compound 48/80 is an oligomeric mixture of the
condensation products of p-methoxyphenethylamine and formaldehyde.
It activates G proteins by a mechanism analogous to that of
mastoparan, which interacts directly with G proteins to mimic the
role of the intracellular loop of G-protein-coupled receptors
(24,25). We measured the Ca2+
responses induced by compound 48/80 in the absence of extracellular
Ca2+. Compound 48/80 induced rapid Ca2+
responses in HeLa cells (Fig. 3A),
and it was suppressed significantly by fucoidan at a concentration
of 0.5 mg/ml (Fig. 3A). The
responses induced by compound 48/80 were very sensitive to
fucoidan.
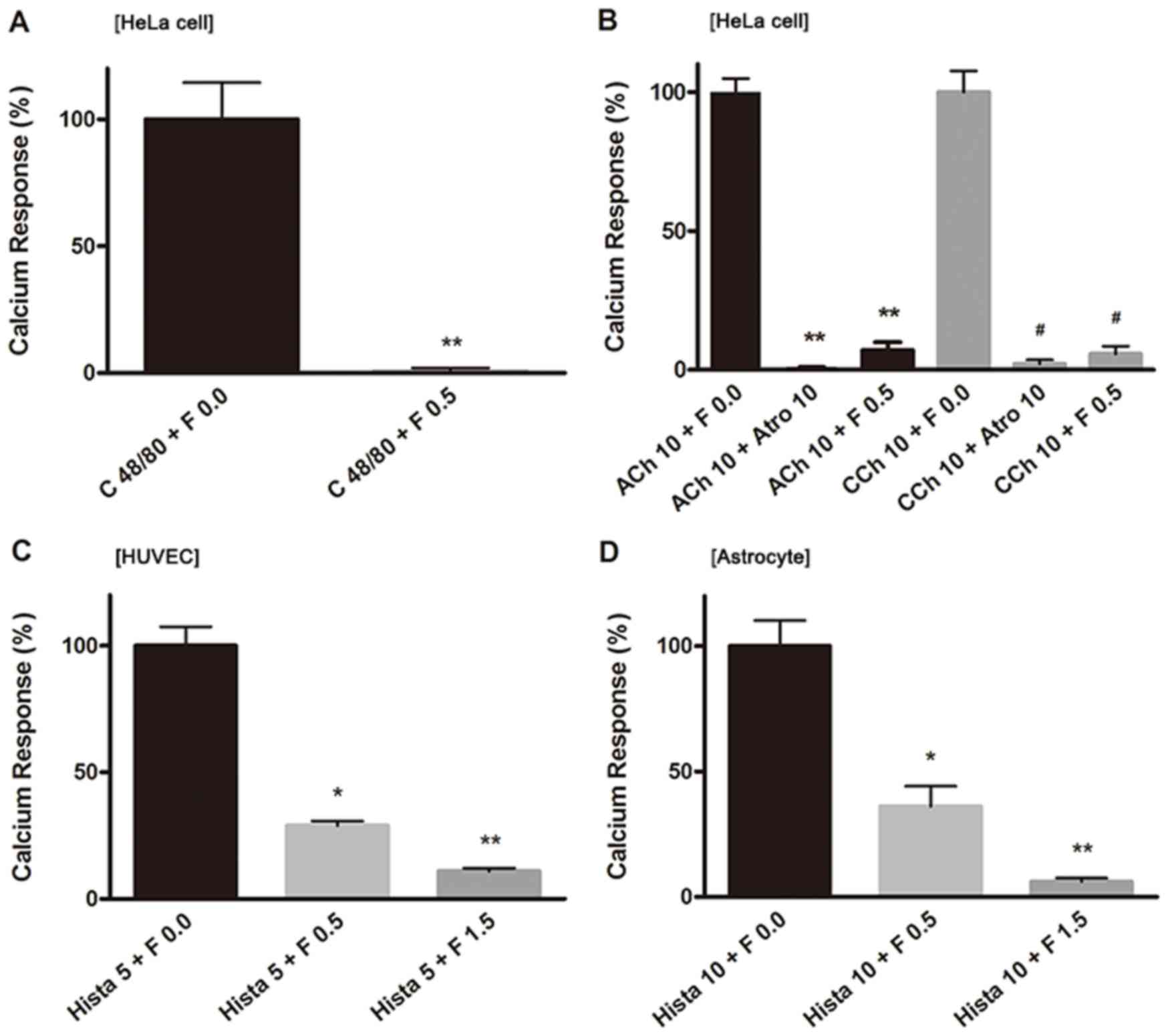 | Figure 3.Ca2+ responses induced by
various chemicals in different cells. The percentage of
Ca2+ responses were analyzed in the following treatment
groups: (A) Compound 48/80 (100 µM) and fucoidan (0.5 mg/ml) in
HeLa cells; (B) 10 µM ACh or 10 µM CCh, atropine (10 µM) and
fucoidan (0.5 mg/ml) in HeLa cells; (C) histamine (5 µM) and
fucoidan (0.5 and 1.5 mg/ml) in HUVECs; and (D) histamine (10 µM)
and fucoidan (0.5 and 1.5 mg/ml) in astrocytes. In each
measurement, 10–20 cells were selected arbitrarily for analysis.
The ordinate is expressed in reference to the control value. All
data points indicate an average of 3–5 independent measurements
(dishes), and data are presented as the mean ± standard error of
the mean. #P<0.01, *P<0.05 and **P<0.01 vs. the
corresponding 0 mg/ml fucoidan group. C 48/80, compound 48/80; F,
fucoidan; ACh, acetylcholine; Atro, atropine; CCh, carbachol; Hist,
histamine; HUVEC, human umbilical vein endothelial cells. |
Effects of fucoidan on Ca2+
responses induced by cholinergic agonists in HeLa cells
Acetylcholine (ACh) has been shown to act through
two major types of receptors, nicotinic and muscarinic receptors.
Nicotinic receptors are ligand-gated ion channels (nAChR, also
known as ionotropic cholinergic receptors), while muscarinic
receptors belong to the G-protein coupled receptor superfamily of
seven transmembrane domain proteins (mAChR, also known as
metabotropic cholinergic receptors). Our fura-2 Ca2+
imaging showed that nAChR and mAChR agonists ACh (10 µM) and
carbachol (CCh, 10 µM) increased the [Ca2+]i
in HeLa cells. Both of these Ca2+ transients were
completely blocked by addition of atropine (10 µM) (Fig. 3B), a muscarinic antagonist. When
fucoidan was applied at 0.5 mg/ml, the ACh- or CCh-induced
Ca2+ responses were suppressed to an extent similar to
that of inhibition by atropine (10 µM) (Fig. 3B). This suppressive effect was
reversed immediately after fucoidan was removed from the
medium.
Effects of fucoidan on Ca2+
responses induced by histamine in HUVECs and astrocytes
In order to examine the cell specificity of the
effects of fucoidan on Ca2+ responses, we verified the
sensitivity of Ca2+ responses induced by an agonist
common to different cell types, specifically HUVECs and astrocytes.
Histamine induced Ca2+ response in HUVECs and astrocytes
similar to those induced in HeLa cells. In HUVECs, the
Ca2+ responses induced by histamine (5 µM) were
suppressed to approximately 30% the magnitude of the first
uninhibited application by the addition of fucoidan at 0.5 mg/ml
and to approximately 10% by 1.5 mg/ml (Fig. 3C). In astrocytes, Ca2+
responses induced by histamine (10 µM) were reduced to
approximately 36 and 6% the magnitude of the first uninhibited
application when the cells were given fucoidan at 0.5 and 1.5
mg/ml, respectively (Fig. 3D),
suggesting that histamine receptors expressed both in HUVECs and
astrocytes were sensitive to fucoidan in a manner similar to that
of HeLa cells.
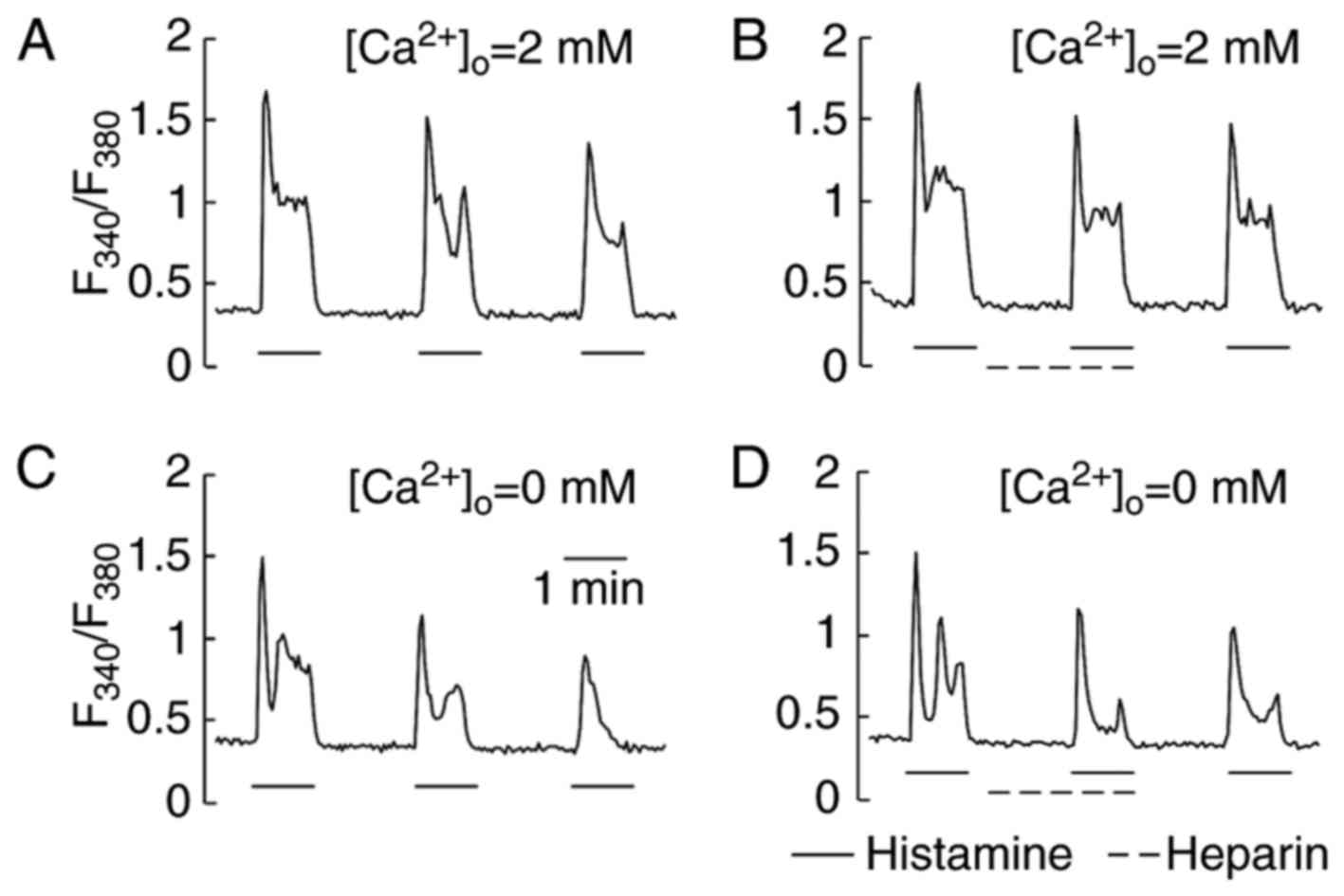
Effect of heparin on Ca2+
responses induced by histamine in HeLa cells
Heparin is also a sulfated polysaccharide with a
structure similar to that of fucoidan. It has been shown that
heparin via micropipette-injection into cells inhibited
Ca2+ responses by inhibiting the inositol
1,4,5-trisphosphate (InsP3) receptor (26,27).
In our study, heparin was applied extracellularly. Fig. 4 shows a representative single cell
trace of [Ca2+]i during a heparin test. In a
series of trials, HeLa cells were stimulated first with histamine
(2.5 µM) and then superfused with a recording medium containing 40
unit/ml of heparin. Without removal of heparin, the same
concentration of histamine (2.5 µM) was re-administered to evaluate
the effects of heparin on the Ca2+ response. By
comparing the peak Ca2+ response induced by histamine in
the presence of extracellular Ca2+ (Fig. 4A and B) with that in the absence
(Fig. 4C and D), heparin was found
to neither facilitate nor suppress the generation of
Ca2+ responses. The removal of heparin did not increase
the peak of Ca2+ responses induced by the next histamine
stimulation. The Ca2+ responses induced by ATP (5 µM)
were also insensitive to heparin (data not shown).
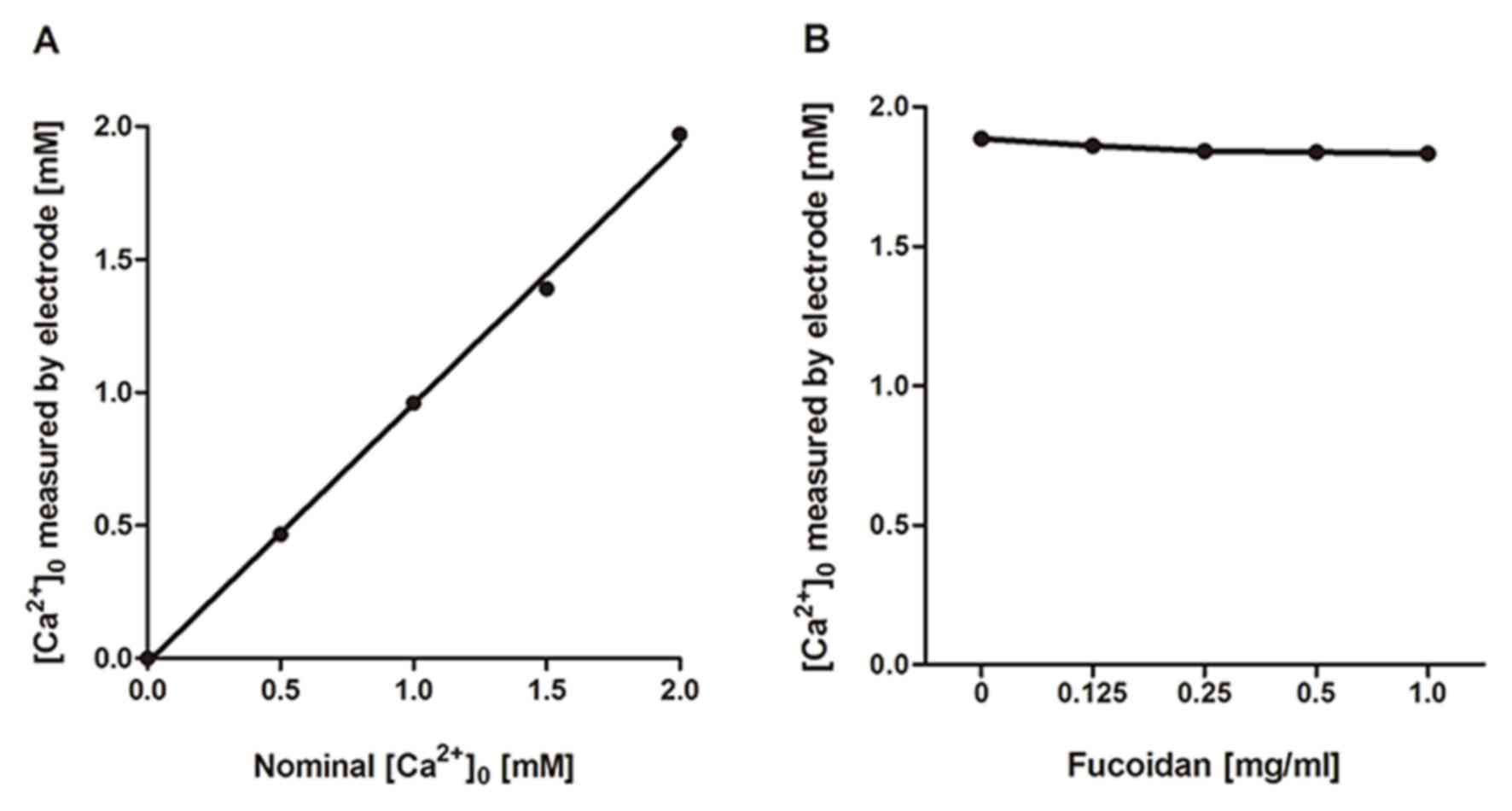
Effects of fucoidan on calcium ions in
the medium
As described above, fucoidan showed inhibitory
effects on Ca2+ responses linked to a wide range of
receptors. We suspected that the inhibition of Ca2+
responses by fucoidan would be attributable to one of its physical
properties related to interaction with calcium ions. Fucoidan is a
sulfated polysaccharide and has many negatively charged sulfate
groups. These may chelate positively charged Ca2+ in the
extracellular medium. Assuming that this is the case, the shortage
of external Ca2+ would lead to a reduction or complete
loss of Ca2+ response. Our data, obtained using a
Ca2+−sensitive electrode (Fig. 5), show that fucoidan has only a
very small effect on the effective concentration of calcium ions in
solution. This excluded the possibility that the suppression of
Ca2+ response by fucoidan was due to its effect on
Ca2+ chelating. The biological effects of fucoidan can
then be solely attributed to its interactions with membrane
proteins.
Effects of fucoidan on expressions of
H1R and P2YRs in HeLa cells
To identify the histamine receptors and P2YRs
expressed in HeLa cells and the effect of fucoidan on expressions
of H1R and P2YRs, RT-PCR of H1R, P2YR1, P2YR2, and P2YR11 were
performed. H1R expression in HeLa cells treated by 0.5 or 1.0 mg/ml
fucoidan for 3 h was significantly lower than that of untreated
cells (Fig. 6A). Consistently, the
expressions of P2YR1 and P2YR11 in HeLa cells induced by either 0.5
or 1.0 mg/ml for 3 h were decreased significantly compared with
untreated cells. And the expression of P2YR2 was also inhibited by
1.0 mg/ml fucoidan treatment for 3 h compared with that of the
untreated cells (Fig. 6B, C and
D). These results suggested that fucoidan suppressed the
histamine-induced Ca2+ response by interacting with the
H1 type metabotropic histamine receptors, and inhibited ATP-evoked
[Ca2+]i by inhibiting the purinergic
G-protein-coupled P2Y receptors.
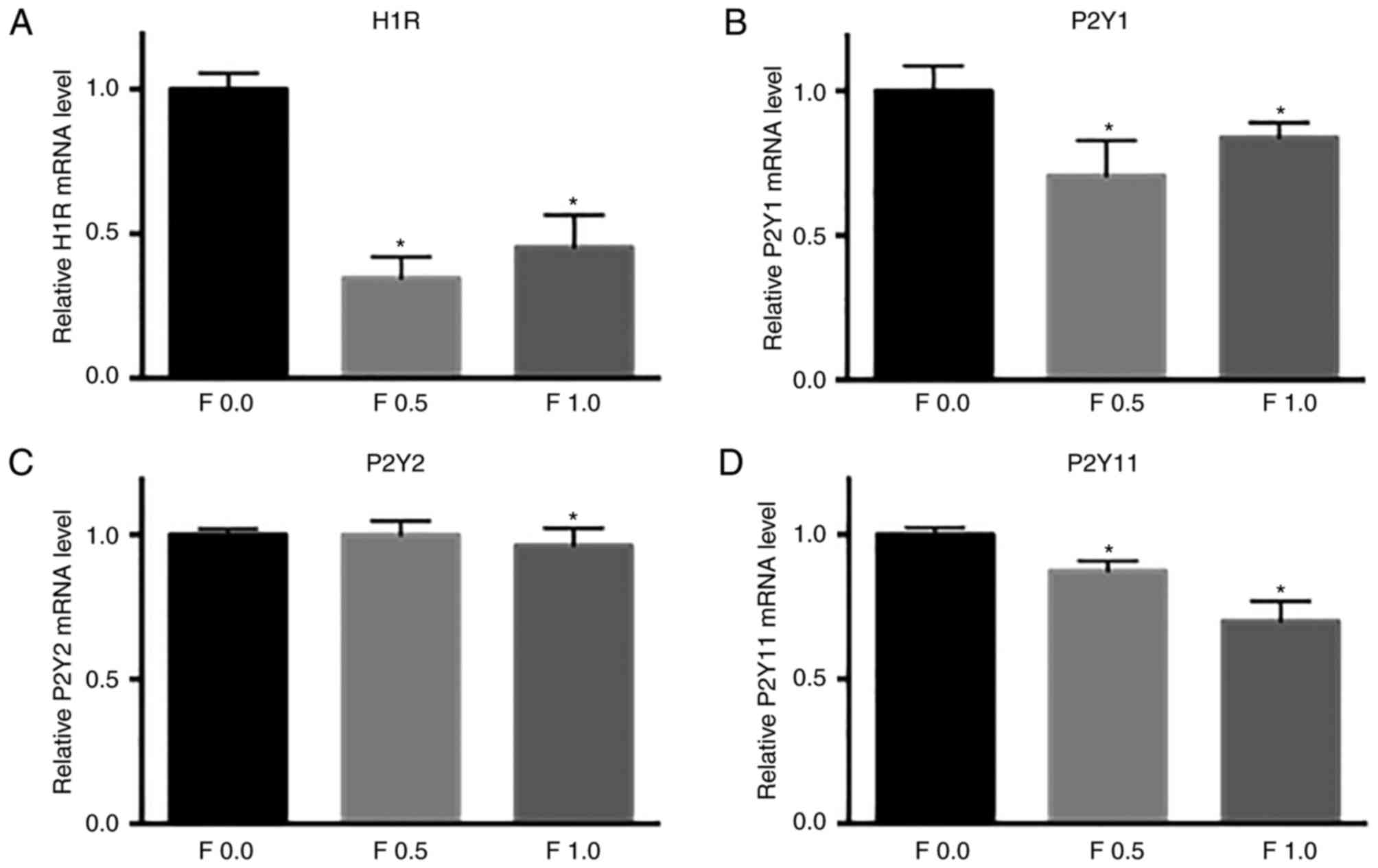 | Figure 6.Effects of fucoidan on the
expressions of H1R and the P2YRs in HeLa cells. GAPDH was used as
the internal standard. (A) The ratio of H1R mRNA to GAPDH mRNA in
the untreated cells, or cells treated with either 0.5 or 1.0 mg/ml
fucoidan, as indicated. The relative mRNA expressions of (B) P2YR1,
(C) P2YR2 and (D) P2YR11, in the untreated cells, or cells treated
with either 0.5 mg/ml or 1.0 mg/ml fucoidan, as indicated, were
also analyzed. *P<0.05 vs. 0 mg/ml fucoidan (control). H1R,
histamine receptor 1; P2YR, purinergic receptor P2Y, G-protein
coupled; F, fucoidan. |
Discussion
In this report, we described the effects of fucoidan
on intracellular Ca2+ responses induced by the ligands
for various receptors to explore the potentials of direct
interactions of fucoidan with these receptors. We showed that
irrespective of cell types, fucoidan had an inhibitory effect on
increases in [Ca2+]i induced by ligands for
different receptors in a dose-dependent manner. Our results
demonstrated a dose-dependent pattern of fucoidan suppression on
histamine- and ATP-induced Ca2+ responses. Furthermore,
we showed that such inhibitory effects of fucoidan were not
affected by the external calcium ions. It has been found that
fucoidan interacts with a wide variety of receptors, and more
importantly, fucoidan has a large number of sulfate residues, it is
unlikely that such molecules could diffuse into the cells. In
addition, RT-PCR assay identified that fucoidan inhibits mRNA
expressions of various G-protein-coupled receptors H1R and P2YRs.
Taken together, our results strongly suggest that fucoidan
inhibition of Ca2+ responses may be due to a direct
inhibition of different receptors in different cell types.
Histamine receptors are a class of G-protein-coupled
receptors (GPCR) with histamine as their endogenous ligand
(28). HeLa cells display well
characterized Ca2+ responses upon histamine stimulation.
Ca2+ responses should be attributed to activation of
metabotropic histamine receptors. Our results showed that the
integrated amplitude of Ca2+ responses was approximately
three times larger in the presence of extracellular Ca2+
than in its absence (Fig. 1B).
Previous studies have shown that histamine evokes an increase in
[Ca2+]i, of which the initial transient
increase is independent of extracellular Ca2+. This is
followed by a sustained increase that can be abolished by the
removal of extracellular Ca2+ (29,30).
Previous studies have also demonstrated that all histamine-induced
Ca2+ responses are completely inhibited by the H1
receptor antagonist pyrilamine but not by cimetidine, an inhibitor
of H2 receptors. It has been suggested that the
histamine H1-receptor mediates these responses in HeLa cells
(31,32).
Histamine liberation from mast cells can be induced
by compound 48/80 (33). This
stimulates the metabotropic receptor family. This receptor is also
expressed on the plasma membranes of HeLa cells and is very
sensitive to fucoidan (Fig. 3A).
Furthermore, mRNA of histamine was significantly inhibited by
fucoidan suggesting that fucoidan probably suppressed the
histamine-induced Ca2+ response (Fig. 1B) by interacting with the H1 type
of metabotropic histamine receptors (Fig. 6A).
Purinergic receptors include ligand-gated P2X
receptors and G-protein-coupled P2Y receptors. ATP is a stimulant
of both P2X receptors and P2Y receptors (34,35).
In the presence of extracellular Ca2+, Ca2+
responses resulted from both P2X and P2Y receptors. Fucoidan showed
dose-dependence in inhibiting Ca2+ responses induced by
ATP (5 µM) in the presence of extracellular Ca2+
([Ca2+]o=2 mM) (Fig. 1C). However, this inhibition is
partial, suggesting that fucoidan must affect either the P2X or P2Y
purinergic receptors but not both. In the absence of extracellular
Ca2+ (1 mM EGTA added), P2X receptors lost their
functions, and only P2Y receptors contributed to the increase in
[Ca2+]i. Fucoidan at 5.0 mg/ml completely
inhibited the increase in [Ca2+]i in the
absence of extracellular Ca2+
([Ca2+]o=0 mM) (Fig. 1C). Fig. 1C shows that fucoidan suppresses
ATP-induced responses by inhibiting the metabotropic P2Y but not
ionotropic P2X receptors. To further identify whether fucoidan
impacts on the metabotropic P2Y receptors or not, RT-PCR was used
to verify the effect of fucoidan on the expressions of different
subtypes of P2YRs. Consistently with the previous results, both
treatments of 0.5 and 1.0 mg/ml fucoidan for 3 h inhibit the
expression of P2YR1 and P2YR11 significantly, and 1.0 mg/ml
fucoidan treatment for 3 h suppresses the expression of P2YR2,
which suggests that fucoidan suppresses the P2YRs expression
directly, by which inhibits the ATP-evoked Ca2+ response
(Fig. 6B-D).
Two main classes of ACh receptors are nAChRs and
mAChRs: the former are stimulated by nicotine and ACh, and the
latter are stimulated by muscarine and ACh. ACh-induced
Ca2+ responses can be completely blocked by atropine in
HeLa cells. Atropine is a competitive antagonist only against the
mAChR, suggesting that no nAChR was expressed in the HeLa cells.
Fucoidan inhibited the ACh- or CCh-induced Ca2+
responses to an extent similar to that of atropine. This
demonstrated that fucoidan can block Ca2+ responses
induced by mAChRs in HeLa cells.
We examined the inhibitory effects of fucoidan on
Ca2+ responses both in the presence and absence of
extracellular Ca2+. In the absence of extracellular
Ca2+ (chelated by EGTA), the Ca2+ channel
does not contribute to Ca2+ response. The
Ca2+ response in HeLa cells could not be induced by Bay
K8644, an L-type Ca2+ channel activator, suggesting that
the major Ca2+ channel (L-type) is not expressed in HeLa
cells (data not shown). This is similar to the results reported by
Gavazzo et al (36).
We conclude that, in the case of HeLa cells, HUVECs,
and astrocytes, Ca2+ responses induced by histamine,
ATP, compound 48/80, and ACh can be abolished by fucoidan through
an inhibition of G-protein-coupled receptors. Specifically, the
downstream of signal transduction is inhibited at the individual
sites of the membrane receptors.
In summary, a broad range of membrane receptors,
including metabotropic receptors, are strongly sensitive to
fucoidan (Table III). The
following points indicate that fucoidan interacts with the cell
membrane by a direct extracellular approach only: i) Fucoidan is a
large molecule (approximately 20 kDa). ii) Fucoidan is a negatively
charged molecule with many sulfate residues, making diffusion
through the cell membrane difficult. iii) The effects of fucoidan
appear immediately after application and disappear soon after
removal. iv) Fucoidan suppresses endocytosis dramatically, so it
could not enter the cell in this way (37). It is quite reasonable to assume
that receptors can be internalized when they are occupied by their
own ligand (the cell membrane itself is endocytosed). This is
visualized by other researchers in histamine receptors and others.
So, probably, there is a feedback mechanism in the cell when the
density of receptor proteins in intracellular vesicles or in Golgi
apparatus become high, expression of mRNA is reduced.
 | Table III.Spectrum of effects induced by
fucoidan. |
Table III.
Spectrum of effects induced by
fucoidan.
|
| HeLa cells |
|
|
|---|
|
|
|
|
|
|---|
| Membrane
receptor | Hist | ATP | ACh | 48/80 | BK | HUVECs Hist | Astrocytes ATP |
|---|
| GPCR | + | + | + | + | / | + | + |
| Ion channel | / | − | / | / | / | / | / |
It is known that heparin is an inhibitor of
InsP3 receptors. Because fucoidan has a structure
similar to that of heparin, a function similar to that of heparin
(inhibition of intracellular InsP3 receptor pathway) can
be expected to underlie its inhibitory effect. Heparin was tested
by direct injection into the cytoplasm to inhibit the release of
Ca2+ from the endoplasmic reticulum (26,27).
In the present study, we applied heparin extracellularly to HeLa
cells without any membrane treatment. Heparin did not show any
inhibitory effect on the Ca2+ responses induced by
histamine either in the presence or absence of external
Ca2+ (Fig. 4).
Therefore, the fucoidan effect is very difficult to be explained by
assuming that the molecule binds to the InsP3 receptors
located on the endoplasmic reticulum. Fucoidan exerts its
inhibitory effects on Ca2+ responses very fast after its
application, and these inhibitory effects are reversed within 3 min
of its removal from the medium. These findings also support the
idea that fucoidan inhibits receptor proteins to suppress
Ca2+ responses.
The present work demonstrates that fucoidan has a
wide spectrum of effects, most of them somehow connected to inhibit
a Ca2+ response induced by diverse types of agonists and
that these effects occur in a dose-dependent manner. Inhibition was
found to be associated with the inhibitory effects on the
activities of G-protein-coupled receptors irrespective of cell
types. HeLa cells, HUVECs, and astrocytes showed the similar
results. The clinical use of fucoidan must be considered with a
great care because it has immediate, strong effects on receptor
activity, endocytosis and delayed effects on cell proliferation.
The degradation of fucoidan into smaller units can be presumed to
reduce these effects (although we did not examine this). When
administered orally (and degraded by digestive acids), fucoidan
might be less effective. While clinical use by injection or local
spray inside the body may yield significant results, but which can
be quite unpredictable because of the large number of fucoidan
targets.
Acknowledgements
The present study was mainly supported by the grant
‘Medical Photonics’ the 21st century Center of Excellence (COE: No.
F13) from the Ministry of Education, Culture, Sports, Science and
Technology, Japan and Collaborative Innovation Center of Henan
University of Chinese Medicine, China.
References
|
1
|
Berteau O and Mulloy B: Sulfated fucans,
fresh perspectives: Structures, functions and biological properties
of sulfated fucans and an overview of enzymes active toward this
class of polysaccharide. Glycobiology. 13:29R–40R. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mourão PA and Pereira MS: Searching for
alternatives to heparin: Sulfated fucans from marine invertebrates.
Trends Cardiovasc Med. 9:225–232. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kuznetsova TA, Besednova NN, Mamaev AN,
Momot AP, Shevchenko NM and Zvyagintseva TN: Anticoagulant activity
of fucoidan from brown algae fucus evanescens of the okhotsk sea.
Bull Exp Biol Med. 136:471–473. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mourão PA: Use of sulfated fucans as
anticoagulant and antithrombotic agents: Future perspectives. Curr
Pharm Des. 10:967–981. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Teng H, Yang Y, Wei H, Liu Z, Liu Z, Ma Y,
Gao Z, Hou L and Zou X: Fucoidan suppresses hypoxia-induced
lymphangiogenesis and lymphatic metastasis in mouse
hepatocarcinoma. Mar Drugs. 13:3514–3530. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yoshimoto M, Higaki K, Nanba E and
Ikeguchi M: Anti-proliferation activity of fucoidan in MKN45
gastric cancer cells and downregulation of phosphorylated ASK1, a
cell cycle-regulated kinase. Yonago Acta Med. 58:1–7.
2015.PubMed/NCBI
|
|
7
|
Han YS, Lee JH and Lee SH: Fucoidan
inhibits the migration and proliferation of HT-29 human colon
cancer cells via the phosphoinositide-3 kinase/Akt/mechanistic
target of rapamycin pathways. Mol Med Rep. 12:3446–3452. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Maruyama H, Tamauchi H, Iizuka M and
Nakano T: The role of NK cells in antitumor activity of dietary
fucoidan from Undaria pinnatifida sporophylls (Mekabu). Planta Med.
72:1415–1417. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Riou D, Colliec-Jouault S, du Sel Pinczon
D, Bosch S, Siavoshian S, Le Bert V, Tomasoni C, Sinquin C, Durand
P, Roussakis C, et al: Antitumor and antiproliferative effects of a
fucan extracted from ascophyllum nodosum against a non-small-cell
bronchopulmonary carcinoma line. Anticancer Res. 16:1213–1218.
1996.PubMed/NCBI
|
|
10
|
Koyanagi S, Tanigawa N, Nakagawa H, Soeda
S and Shimeno H: Oversulfation of fucoidan enhances its
anti-angiogenic and antitumor activities. Biochem Pharmacol.
65:173–179. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Teas J: The consumption of seaweed as a
protective factor in the etiology of breast cancer. Med Hypotheses.
7:601–613. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vishchuk OS, Ermakova SP and Zvyagintseva
TN: The fucoidans from brown algae of Far-Eastern seas: Anti-tumor
activity and structure-function relationship. Food Chem.
141:1211–1217. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tsai HL, Tai CJ, Huang CW, Chang FR and
Wang JY: Efficacy of low-molecular-weight fucoidan as a
supplemental therapy in metastatic colorectal cancer patients: A
double-blind randomized controlled trial. Mar Drugs. 15:E1222017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Brini M, Ottolini D, Cali T and Carafoli
E: Calcium in health and disease. Metal Ions Life Sci. 13:81–137.
2013. View Article : Google Scholar
|
|
15
|
Huang W, Lu C, Wu Y, Ouyang S and Chen Y:
T-type calcium channel antagonists, mibefradil and NNC-55-0396
inhibit cell proliferation and induce cell apoptosis in leukemia
cell lines. J Exp Clin Cancer Res. 34:542015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yu W, Wang P, Ma H, Zhang G, Yulin Z, Lu
B, Wang H and Dong M: Suppression of T-type Ca2+ channels inhibited
human laryngeal squamous cell carcinoma cell proliferation running
title: Roles of T-type Ca2+ channels in LSCC cell proliferation.
Clin Lab. 60:621–628. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kao TJ and Millette CF: L-type
voltage-operated Ca(+2) channels modulate transient Ca(+2) influx
triggered by activation of Sertoli cell surface L-selectin. J Cell
Biochem. 101:1023–1037. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
van Tits LJ, Hak-Lemmers HL, Demacker PN,
Stalenhoef AF and Willems PH: Oxidized low-density lipoprotein
induces calcium influx in polymorphonuclear leukocytes. Free Radic
Biol Med. 29:747–755. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang JZ, McBride JW and Yu XJ: L-selectin
and E-selectin expressed on monocytes mediating Ehrlichia
chaffeensis attachment onto host cells. FEMS Microbiol Lett.
227:303–309. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Meisenberg A, Kaschuba D, Balfanz S,
Jordan N and Baumann A: Molecular and functional profiling of
histamine receptor-mediated calcium ion signals in different cell
lines. Anal Biochem. 486:96–101. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pulli I, Blom T, Löf C, Magnusson M,
Rimessi A, Pinton P and Törnquist K: A novel chimeric aequorin
fused with caveolin-1 reveals a sphingosine kinase 1-regulated
Ca2+ microdomain in the caveolar compartment. Biochim
Biophys Acta. 1853:2173–2182. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sun J, Wang J, Chen P, Feng X, Du W and
Liu BF: A chemical signal generator for resolving temporal dynamics
of single cells. Anal Bioanal Chem. 400:2973–2981. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Park KS, Lee HY, Lee SY, Kim MK, Kim SD,
Kim JM, Yun J, Im DS and Bae YS: Lysophosphatidylethanolamine
stimulates chemotactic migration and cellular invasion in SK-OV3
human ovarian cancer cells: Involvement of pertussis
toxin-sensitive G-protein coupled receptor. FEBS Lett.
581:4411–4416. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mousli M, Bronner C, Landry Y, Bockaert J
and Rouot B: Direct activation of GTP-binding regulatory proteins
(G-proteins) by substance P and compound 48/80. FEBS Lett.
259:260–262. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Higashijima T, Uzu S, Nakajima T and Ross
EM: Mastoparan, a peptide toxin from wasp venom, mimics receptors
by activating GTP-binding regulatory proteins (G proteins). J Biol
Chem. 263:6491–6494. 1988.PubMed/NCBI
|
|
26
|
Davies-Cox EV, Laffafian I and Hallett MB:
Control of Ca2+ influx in human neutrophils by inositol
1,4,5-trisphosphate (IP3) binding: Differential effects of
micro-injected IP3 receptor antagonists. Biochem J. 355:139–143.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tortorici G, Zhang BX, Xu X and Muallem S:
Compartmentalization of Ca2+ signaling and
Ca2+ pools in pancreatic acini. Implications for the
quantal behavior of Ca2+ release. J Biol Chem.
269:29621–29628. 1994.PubMed/NCBI
|
|
28
|
Hill SJ, Ganellin CR, Timmerman H,
Schwartz JC, Shankley NP, Young JM, Schunack W, Levi R and Haas HL:
International Union of Pharmacology. XIII. Classification of
histamine receptors. Pharmacol Rev. 49:253–278. 1997.PubMed/NCBI
|
|
29
|
Bootman MD, Berridge MJ and Taylor CW:
All-or-nothing Ca2+ mobilization from the intracellular
stores of single histamine-stimulated HeLa cells. J Physiol.
450:163–178. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Thorn P: Ca2+ influx during
agonist and Ins(2,4,5)P3-evoked Ca2+ oscillations in
HeLa epithelial cells. J Physiol. 482:275–281. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sauve R, Simoneau C, Parent L, Monette R
and Roy G: Oscillatory activation of calcium-dependent potassium
channels in HeLa cells induced by histamine H1 receptor
stimulation: A single-channel study. J Membr Biol. 96:199–208.
1987. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Volpi M and Berlin RD: Intracellular
elevations of free calcium induced by activation of histamine H1
receptors in interphase and mitotic HeLa cells: Hormone signal
transduction is altered during mitosis. J Cell Biol. 107:2533–2539.
1988. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Koibuchi Y, Ichikawa A, Nakagawa M and
Tomita K: Histamine release induced from mast cells by active
components of compound 48/80. Eur J Pharmacol. 115:163–170. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Khakh BS, Burnstock G, Kennedy C, King BF,
North RA, Séguéla P, Voigt M and Humphrey PP: International union
of pharmacology. XXIV. Current status of the nomenclature and
properties of P2X receptors and their subunits. Pharmacol Rev.
53:107–118. 2001.PubMed/NCBI
|
|
35
|
Abbracchio MP, Burnstock G, Boeynaems JM,
Barnard EA, Boyer JL, Kennedy C, Knight GE, Fumagalli M, Gachet C,
Jacobson KA and Weisman GA: International union of pharmacology
LVIII: Update on the P2Y G protein-coupled nucleotide receptors:
From molecular mechanisms and pathophysiology to therapy.
Pharmacolo Rev. 58:281–341. 2006. View Article : Google Scholar
|
|
36
|
Gavazzo P, Morelli E and Marchetti C:
Susceptibility of insulinoma cells to cadmium and modulation by
L-type calcium channels. Biometals. 18:131–142. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wu H, Gao SB, Sakurai T and Terakawa S:
Fucoidan suppresses endocytosis in cultured HeLa cells. Chin J
Integr Med. Aug 18–2011.(Epub ahead of print). View Article : Google Scholar
|