Introduction
Osteosarcoma is a frequently observed primary
malignant tumor that most commonly affects children, adolescents
and young adults (1). Current
therapy for osteosarcoma consists of comprehensive treatment. In
~20% of patients, osteosarcoma is resistant to available therapies,
leading to recurrence and lung metastases (1–3).
Treatment of metastatic osteosarcoma remains a challenge in
oncology. Therefore, a better understanding of the pathogenesis and
biology of osteosarcoma may provide a rational basis for improving
treatment efficacy, particularly for metastatic disease (4).
The 14-3-3 proteins are a group of intracellular
proteins that exists in all eukaryotic organisms. The 14-3-3
protein family has seven isoforms, β, ε, ζ, η, θ, γ and σ, which
serve as scaffolds to interact with various proteins, including
transcription factors, signaling molecules, tumor suppressors,
cytoskeletal proteins and apoptosis factors (5). Interaction with 14-3-3 can alter the
localization, activity, stability, phosphorylation state and
conformation of target proteins (6). More than 200 14-3-3 target proteins
have been identified, including proteins involved in cell
apoptosis, cell cycle progression, signal transduction,
differentiation, senescence and DNA replication (7,8).
Previous studies have indicated that abnormal expression of 14-3-3
is associated with the development and progression of various
tumors (9). Each 14-3-3 isoform
has distinct tissue localization and isoform-specific functions
(10). The majority of 14-3-3
proteins are tumor promoters, whereas 14-3-3σ serves as a tumor
suppressor (11). Loss of 14-3-3σ
expression has been observed in several types of human cancers,
including lung cancer, breast cancer and prostate cancer (12). Expression of 14-3-3σ is
significantly downregulated in cancerous lung tissues and is
associated with the differentiation grade and prognosis of the
patient, whereas increased 14-3-3σ expression significantly
suppresses the proliferation of lung squamous cell carcinoma cells
(13). 14-3-3γ is frequently
upregulated in lung cancer, accompanied by loss of functional p53,
which suggests that the oncogenic activities of 14-3-3γ act
synergistically with the loss of p53 to promote lung tumorigenesis
(14). Mulvey et al
demonstrated that 14-3-3ζ is overexpressed in colorectal cancer,
and 14-3-3ζ knockdown reduces anchorage-independent growth of
colorectal cancer cells (15),
suggesting that 14-3-3ζ may be a putative drug target for the
treatment of colorectal cancer. 14-3-3θ expression is markedly
higher in breast cancer, and elevated 14-3-3θ is correlated with
poor prognosis (16).
Additionally, 14-3-3θ knockdown inhibits the growth and metastasis
of breast cancer, indicating that 14-3-3θ may serve as a candidate
prognostic biomarker and target for new therapies in metastatic
breast cancer (16). Accumulating
evidence suggests that 14-3-3β is important in tumorigenesis and
tumor progression. For example, 14-3-3β is expressed abundantly in
a majority of primary tumors, and elevated 14-3-3β is associated
with subsequent extrahepatic metastasis and decreased survival
rates in patients with hepatocellular carcinoma (17,18).
14-3-3β is overexpressed in astrocytoma, and knockdown of 14-3-3β
inhibits the proliferation of U87 human glioblastoma cells
(19). Overexpression of 14-3-3β
in NIH 3T3 fibroblast cells stimulates cell growth and promotes
tumor formation in nude mice (20). High cytoplasmic levels of 14-3-3β
independently correlate with poor disease-specific survival in
vulvar squamous cell carcinoma (21). These data suggest a crucial role of
14-3-3β in the abnormal growth of tumor cells, and new therapeutic
strategies or drugs aimed at 14-3-3β may have potential for the
treatment of cancer. Nevertheless, there is a paucity of
information regarding 14-3-3β expression and its exact role in
osteosarcoma progression.
The present study demonstrated that 14-3-3β was
highly expressed in human osteosarcoma tissues and osteosarcoma
cell lines. These data indicated that increased 14-3-3β expression
may be associated with the development and progression of
osteosarcoma, and suggested that 14-3-3β may be a novel target in
developing therapeutic applications for the treatment of
osteosarcoma patients.
Materials and methods
Specimens
The current study was reviewed and approved by the
Clinical Research Ethics Committee of The Affiliated Hospital of
Nantong University (Nantong, China). Fresh osteosarcoma tissues and
matched normal tumor-adjacent tissues were collected from 16
patients (11–27 years-old, male 10, female 6) that underwent
resection surgery between January 2009 and June 2015 at The
Affiliated Hospital of Nantong University. All tissue specimens
were immediately frozen in liquid nitrogen and stored at −80°C for
subsequent experiments.
Reagents
All cell culture reagents were from Gibco (Thermo
Fisher Scientific, Inc., Waltham, MA, USA). Human osteoblastic cell
line (hFOB1.19) and osteosarcoma cell lines (U2OS, Saos-2 and MG63)
were obtained from the American Type Culture Collection (Manassas,
VA, USA). Matrigel was purchased from Collaborative Biomedical
Products (Bedford, MA, USA). The iScript cDNA Synthesis kit and the
iTaq Fast SYBR Green Supermix were purchased from Bio-Rad
Laboratories, Inc. (Hercules, CA, USA). Transwell invasion chambers
were purchased from Costar (Thermo Fisher Scientific, Inc.). The
protein extraction kit, propidium iodide (PI), Cell Counting kit-8
(CCK-8) and RNase A were purchased from Beyotime Institute of
Biotechnology (Haimen, China). The enhanced chemiluminescence (ECL)
kit was from Pierce (Thermo Fisher Scientific, Inc.). The primers
were as follows: 14-3-3β (GenBank accession no. GI:197692220),
forward 5′-atg ggc aaa gag tac cgt ga-3′, reverse 5′-tgt tgt ctc
cag atg cca ct-3′; and GAPDH (GenBank accession no. GI:182976),
forward 5′-agg tcg gag tca acg gat tt-3′, reverse 5′-atc tcg ctc
ctg gaa gat gg-3′. Primers were synthesized by Shanghai Generay
Biotech Co., Ltd. (Shanghai, China). 14-3-3β small interfering
(si)RNA (5′-AUUGGAUACGCUGAAUGAAGA-3′) and negative control siRNA
(cat. no. siN05815122147-1-5) were purchased from Guangzhou RiboBio
Co., Ltd. (Guangzhou, China). Rabbit anti-14-3-3β (cat. no.
ab97273) and anti-β-catenin (cat. no. ab23512) polyclonal
antibodies were purchased from Abcam (Cambridge, UK). Mouse
anti-cyclin D1 (cat. no. 554180) monoclonal antibody was purchased
from BD Biosciences (Franklin Lakes, NJ, USA). Rabbit anti-β-actin
(cat. no. 253613) and anti-v-myc avian myelocytomatosis viral
oncogene homolog (c-myc; cat. no. 251334) polyclonal antibodies
were purchased from Abbiotec, LLC (San Diego, CA, USA). Rabbit
anti-matrix metallopeptidase 9 (MMP9; cat. no. ABIN1873732)
polyclonal antibody was purchased from Abnova (Taipei, Taiwan).
Horseradish peroxidase-conjugated goat anti-rabbit (cat. no. 31460)
and goat anti-mouse (cat. no. 31430) immunoglobulin (Ig)G
polyclonal antibodies were purchased from Invitrogen (Thermo Fisher
Scientific, Inc.).
Cell culture and transfection
Human osteosarcoma U2OS, Saos-2 and MG63 cells were
maintained in RPMI-1640 medium (cat. no. 11875127; Thermo Fisher
Scientific, Inc.) supplemented with 10% fetal bovine serum (FBS;
cat. no. 10099141; Thermo Fisher Scientific, Inc.), 2 mM
L-glutamine and 100 µg/ml penicillin/streptomycin at 37°C in a
humidified 5% CO2 incubator. Human osteoblast-like
hFOB1.19 cells were cultured in Dulbecco's Modified Eagle's Medium
(cat. no. 11965118; Thermo Fisher Scientific, Inc.)/Ham's F-12
nutrient mixture (cat. no. 11765054; v/v: 1:1; Thermo Fisher
Scientific, Inc.) containing 10% FBS and Geneticin (cat. no.
10131027; 400 µg/ml; Thermo Fisher Scientific, Inc.) at 34°C in a
humidified 5% CO2 incubator.
Among the human osteosarcoma cell lines, MG63 cells
were selected for siRNA experiments. MG63 cells were transfected
with 50 nM 14-3-3β siRNA (knockdown group) or negative control
siRNA (control group), using Lipofectamine® 2000
transfection reagent (Thermo Fisher Scientific, Inc.), according to
the manufacturer's instructions. Untransfected MG63 cells served as
the blank control group. At 48 h following transfection, MG63 cells
were washed twice with cold phosphate-buffered saline (PBS),
trypsinised, and collected by centrifugation at 1,200 g × 5 min for
further experiments.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RNA was extracted from 5×107 cells using
1 ml TRIzol™ reagent (Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. cDNA synthesis and PCR
were subsequently performed using the Bio-Rad PCR Detection System
(Bio-Rad Laboratories, Inc.) as previously described (22). PCR was performed with an initial
denaturation step at 95°C for 5 min, followed by 35 cycles of: 95°C
for 3 sec; 60°C for 30 sec; and finally 72°C for 10 min. Relative
mRNA expression was calculated using the 2−ΔΔCq method
(23). All the experiments were
repeated in triplicate.
Western blotting
Proteins were extracted from 5×107 cells
with radioimmunoprecipitation buffer (Beyotime Institute of
Biotechnology) and quantified by a bicinchoninic acid assay kit
(Beyotime Institute of Biotechnology). A total of 40 µg total
proteins were separated on 12% SDS-PAGE and then transferred to
polyvinylidene fluoride membranes. The membranes were blocked in 5%
skimmed milk in TBS for 1 h at ambient temperature and then
incubated with primary antibodies (against the following antigens:
14-3-3β, β-catenin, cyclin D1, c-myc, MMP9 and β-actin) at a
1:2,000 in 5% skimmed milk in TBS + 0.1% Tween-20 (TBST) at 4°C
overnight. Membranes were washed with TBST, then probed with goat
anti-rabbit immunoglobulin (Ig)G or goat anti-mouse IgG diluted at
a 1:2,000 in 5% skimmed milk in TBST for 2 h at ambient
temperature. Finally, the membranes were washed in TBST and
developed using the ECL kit. Band intensities were quantified by
densitometric analysis software Quantity-one v4.62 (Bio-Rad
Laboratories, Inc.). Relative expression levels of target proteins
were normalized to the β-actin loading control. All the experiments
were repeated in triplicate.
Cell viability and cell cycle
analysis
Cell viability was determined with CCK-8, according
to the manufacturer's protocol. Briefly, MG63 cells were
synchronized in G0 by serum deprivation for 24 h. MG63 cells were
then collected by centrifugation at 1,200 × g for 5 min and seeded
at a density of 3,000 cells/well in 96-well microplates. CCK-8
solution (10 µl) and RPMI-1640 (100 µl) were added to each well and
incubated for 2 h at 30°C. The optical density was detected at a
wavelength of 450 nm using a microplate reader. All the experiments
were repeated in triplicate.
For cell cycle analysis, MG63 cells were
synchronized in G0 by culturing in serum-free RPMI-1640 medium for
24 h, and then cultured in complete medium for an additional 24 h.
Following incubation, MG63 cells were collected by centrifugation
at 1,200 g × for 5 min and fixed in 70% cold ethanol (0.5 ml) at
4°C for 30 min. Fixed MG63 cells were washed twice in PBS, and 50
µl a 100 µg/ml stock of RNase was added to 200 µl PI (50 µg/ml
stock) was which added to each cell suspension and incubated for 30
min at room temperature in the dark prior to analysis. Cell cycle
phase distribution was detected by flow cytometry (FACSCalibur; BD
Biosciences, Franklin Lakes, NJ, USA) and CellQuest software
(version 6.0; BD Biosciences) was used to analyze the results, as
described previously (22). Data
were expressed as the fraction of cells in the different cell cycle
phases, and the experiment was repeated 3 times.
Transwell invasion assay
Cell invasion capacity was examined by Transwell
invasion assay. Matrigel was diluted to 100 µg/ml with serum-free,
cold RPMI-1640 culture medium; 100 µl of the diluted Matrigel was
added to the upper chamber of a 24-well Transwell plate and
incubated at 37°C for at least 4–5 h for the Matrigel to solidify.
The solid Matrigel was washed with warmed serum-free culture media,
and 200 µl RPMI-1640 medium containing 1×103 MG63 cells
were added to the Matrigel-coated, upper chamber of each Transwell
plate. The bottom chamber was filled with 600 µl RPMI-1640 medium
containing 10% FBS as a chemoattractant. The Transwell plates were
incubated at 37°C in a humidified 5% CO2 and 95% air
incubator for 48 h. Following incubation, the MG63 cells that
remained on the upper chamber were removed using a cotton swab. The
migrated cells at the bottom surface were fixed with 4%
paraformaldehyde for 15 min at room temperature and stained with a
crystal violet solution for 10 min at room temperature, followed by
observation under a Leica DMI3000 B inverted microscope (Leica
Microsystems GmbH, Wetzlar, Germany) and an average number of cells
was taken by counting six random fields of view per filter.
Experiments were performed in triplicate.
Statistical analysis
SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA)
was used to analyze statistical significance of the data, by
performing one-way analysis of variance and Bonferroni's post-hoc
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Upregulation of 14-3-3β in
osteosarcoma
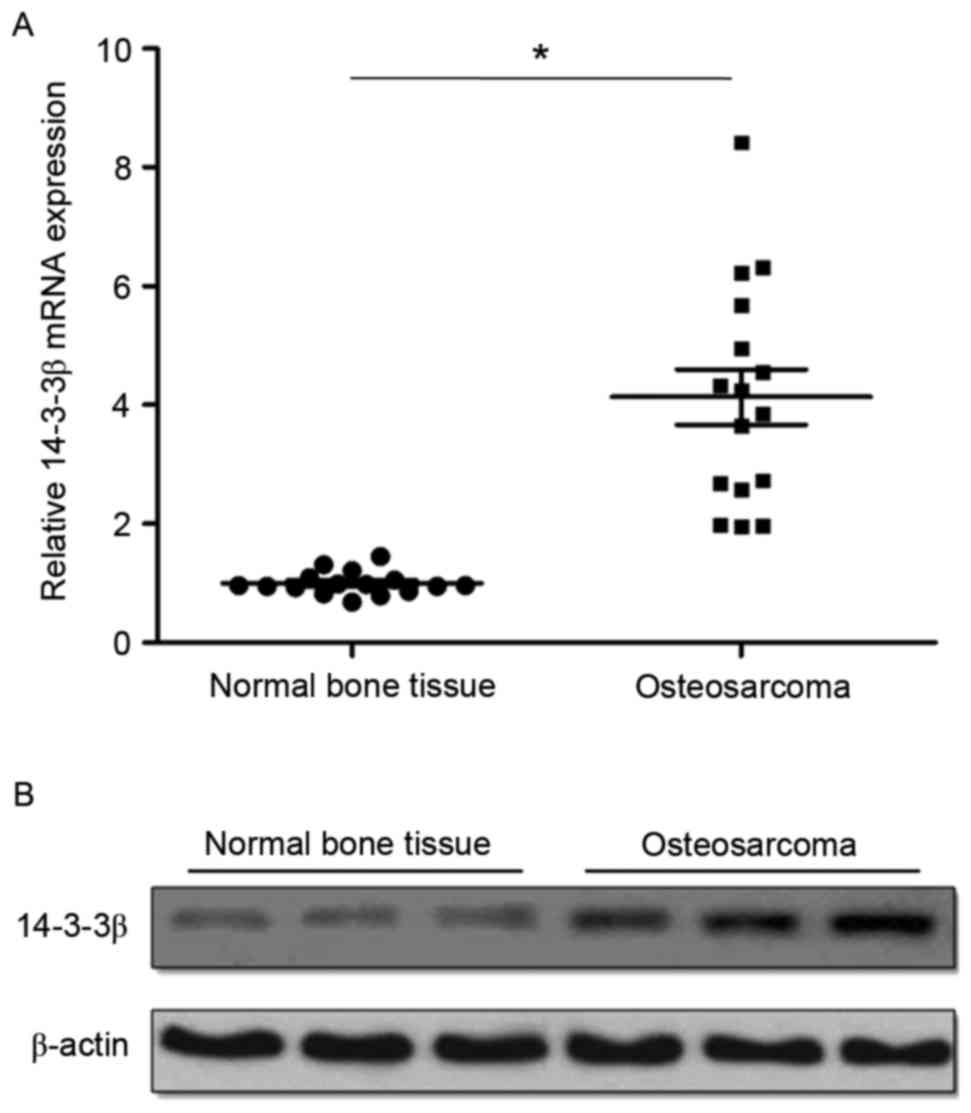
14-3-3β mRNA and protein expression levels were
detected by RT-qPCR and western blotting, respectively. RT-qPCR
analysis demonstrated that 14-3-3β mRNA was significantly
overexpressed in osteosarcoma tissues compared with matched normal
tumor-adjacent bone tissues (Fig.
1A). Western blotting results were similar to RT-qPCR results,
demonstrating that the expression of 14-3-3β protein was also
markedly upregulated in osteosarcoma tissues compared with matched
normal tumor-adjacent bone tissues (Fig. 1B). The levels of 14-3-3β expression
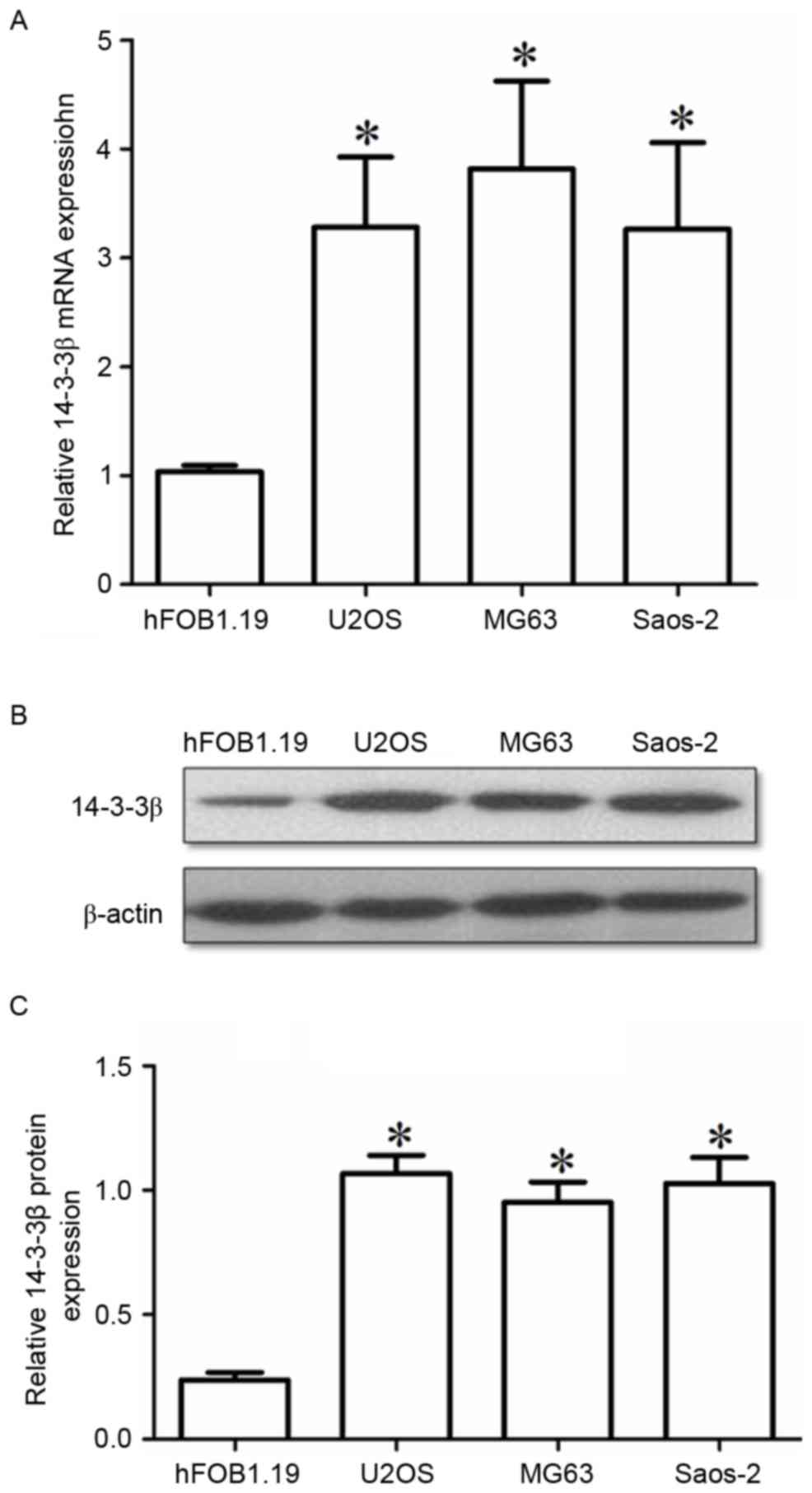
were also examined in three osteosarcoma cell lines: U2OS, Saos-2
and MG63. All 3 cell lines exhibited significantly higher 14-3-3β
mRNA and protein expression compared with the normal osteoblast
hFOB1.19 cells (Fig. 2A and B,
respectively). The present results indicated that 14-3-3β
upregulation may be important in the tumorigenesis and progression
of osteosarcoma.
14-3-3β knockdown inhibits cell
viability in MG63 cells
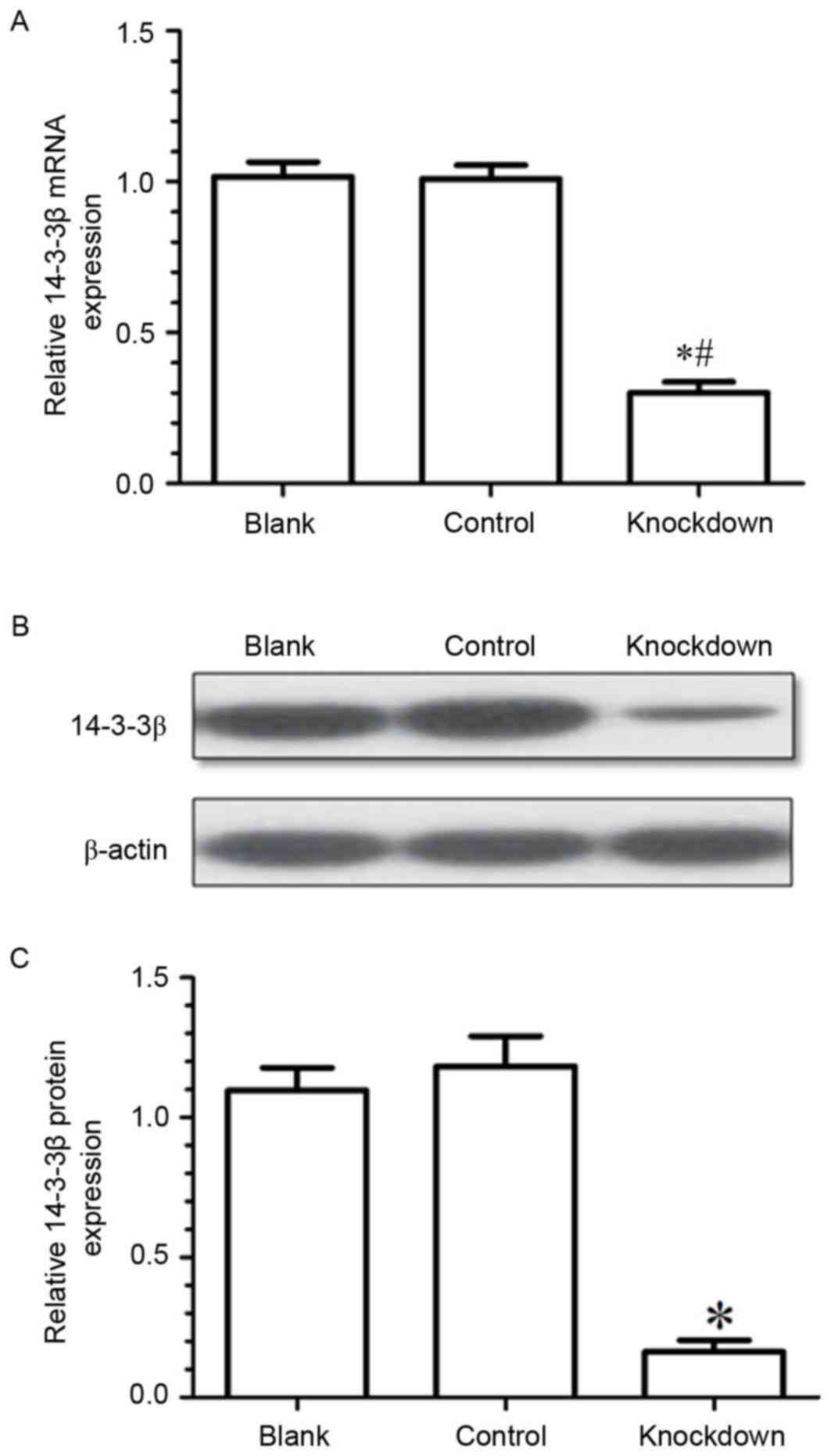
To explore the effects of 14-3-3β on the biological
behavior of osteosarcoma cells, the expression of 14-3-3β mRNA in
MG63 cells was silenced by siRNA transfection. Results from RT-qPCR
and western blotting demonstrated that 14-3-3β mRNA and protein
expression levels were significantly downregulated in the knockdown
group compared with the control (negative control siRNA) and the
blank (untransfected cells) groups (Fig. 3). The present data demonstrated
that the expression of 14-3-3β had been effectively suppressed by
siRNA in the MG63 osteosarcoma cells.
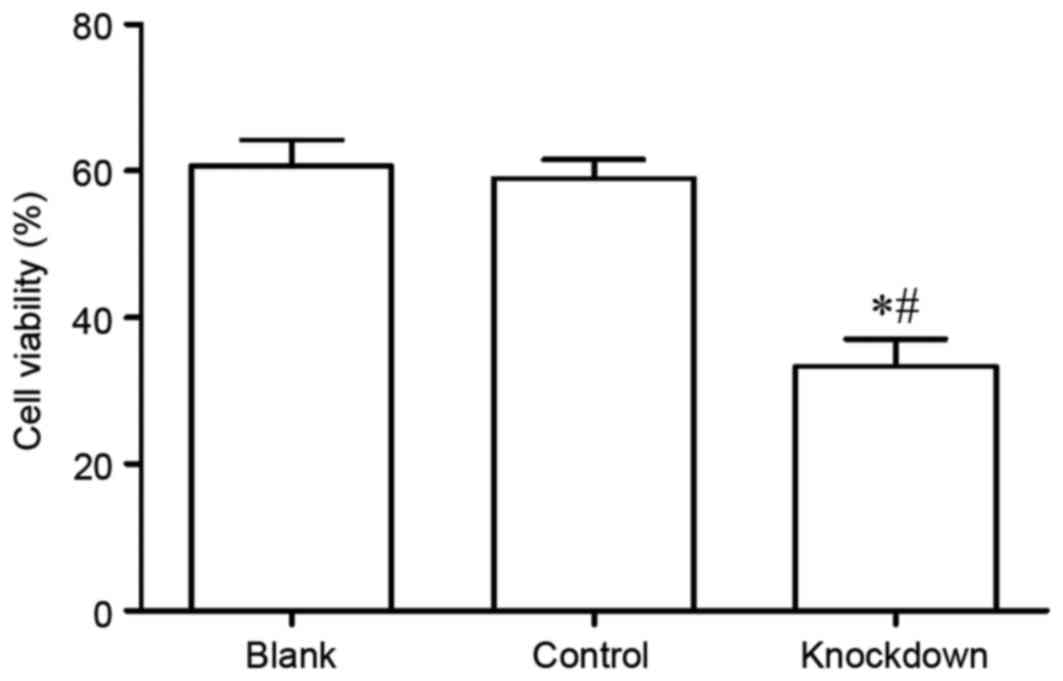
Subsequently, the viability of MG63 cells was
assayed using the CCK-8 assay. The results demonstrated that MG63
cell viability in the 14-3-3β knockdown group was significantly
decreased compared with the control and the blank group (Fig. 4). The data suggest that 14-3-3β may
be important in MG63 cell viability.
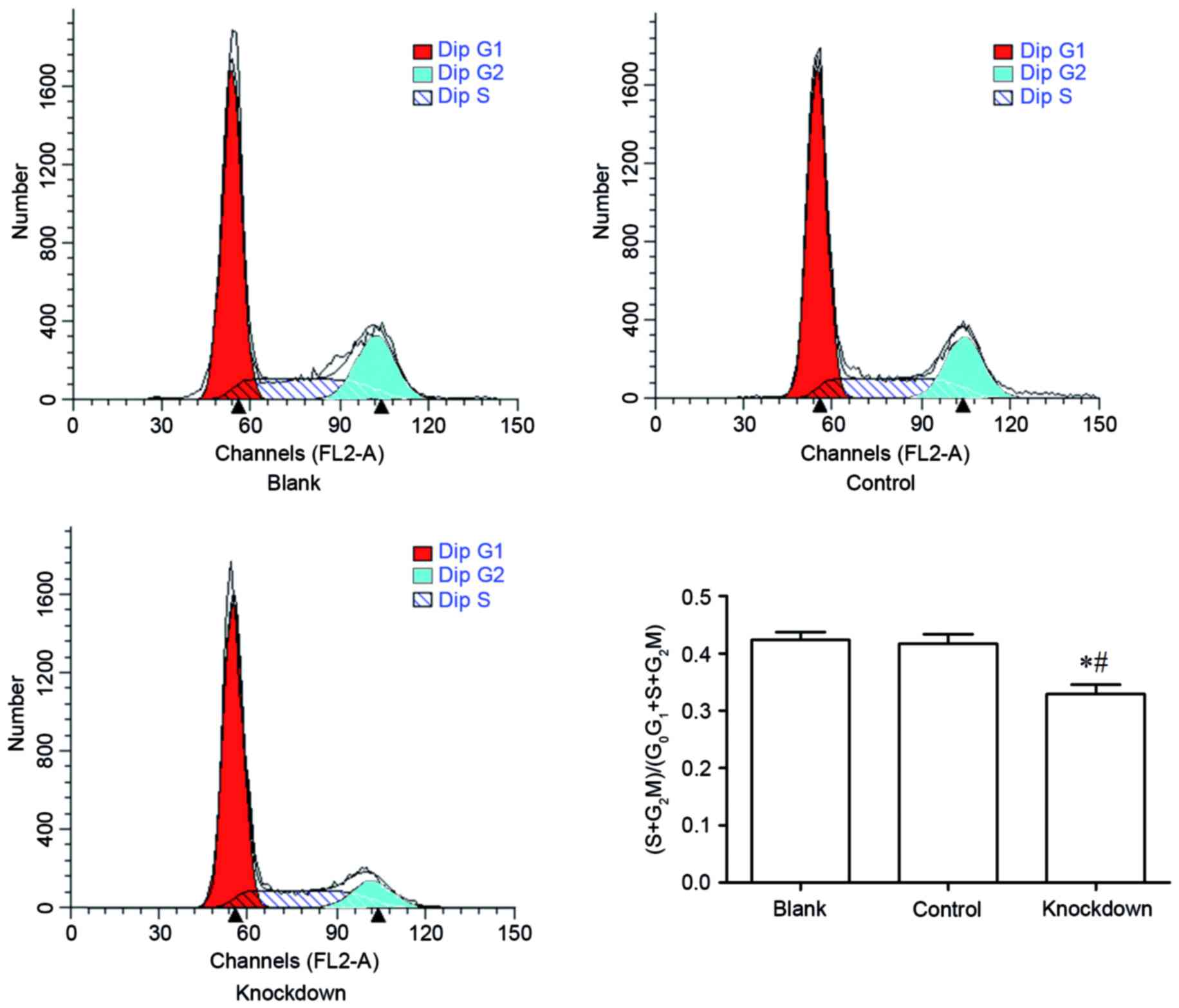
14-3-3β knockdown suppresses cell
cycle progression in MG63 cells
The effect of 14-3-3β knockdown in cell cycle phase
distribution was assessed by flow cytometry. The percent of MG63
cells at the S phase and the G2-M phase in the 14-3-3β knockdown
group was significantly decreased compared with the control and the
blank group (Fig. 5). By contrast,
the percent of MG63 cells at the G0-G1 phase in the 14-3-3β
knockdown group was increased compared with the control and the
blank group (Fig. 5). These
findings demonstrated that 14-3-3β knockdown inhibited cell cycle
progression in MG63 osteosarcoma cells.
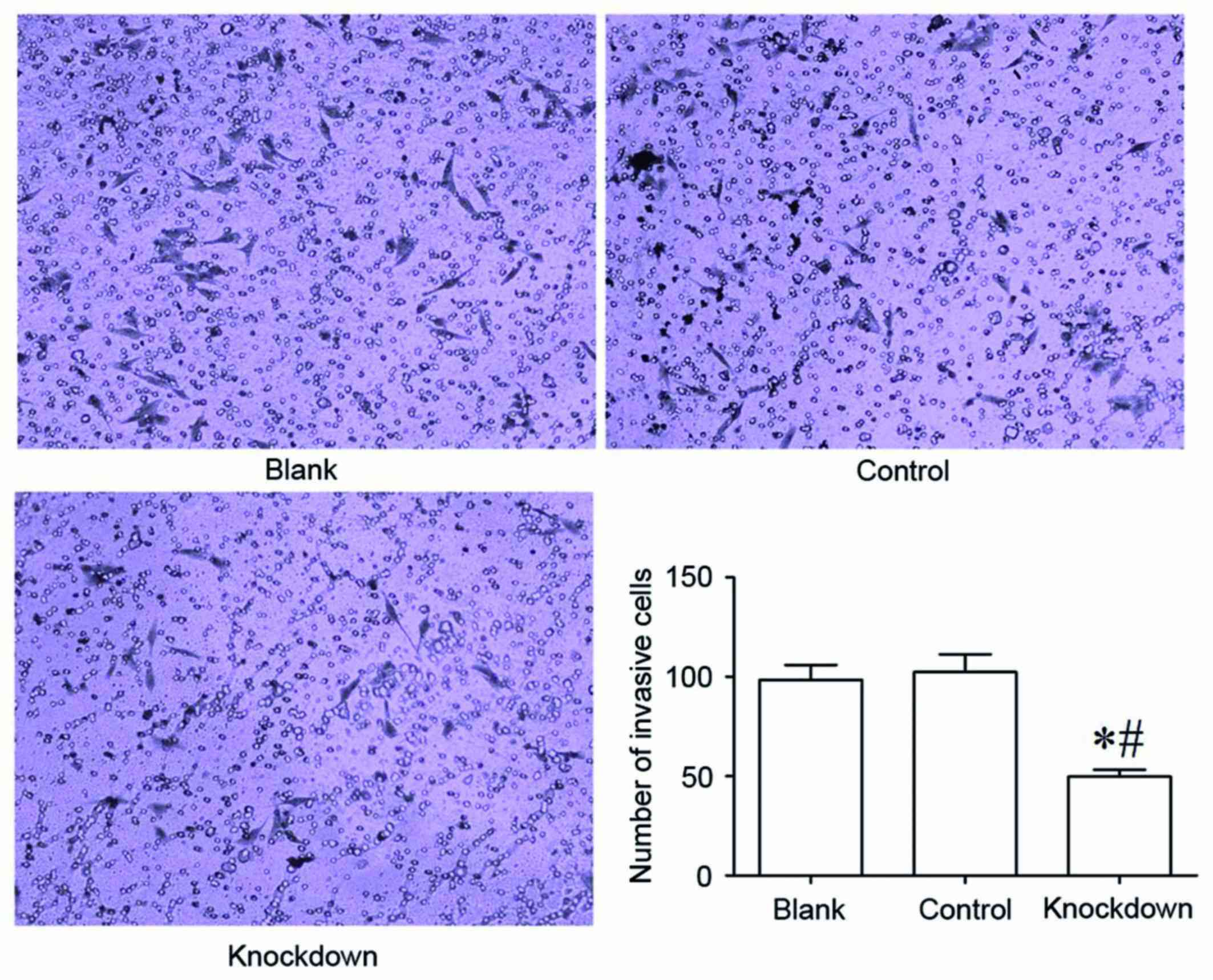
14-3-3β knockdown inhibits cell
invasion in MG63 cells
The Transwell invasion chamber assay was used to
determine the invasive ability of MG63 osteosarcoma cells. The
results demonstrated that the 14-3-3β knockdown group had
significantly fewer MG63 cells invaded into the Matrigel membrane
compared with the control and the blank group (Fig. 6). The number of invaded MG63 cells
did not differ significantly between the control group and the
blank group (Fig. 6). These data
suggested that 14-3-3β downregulation suppressed the invasive
ability of MG63 osteosarcoma cells.
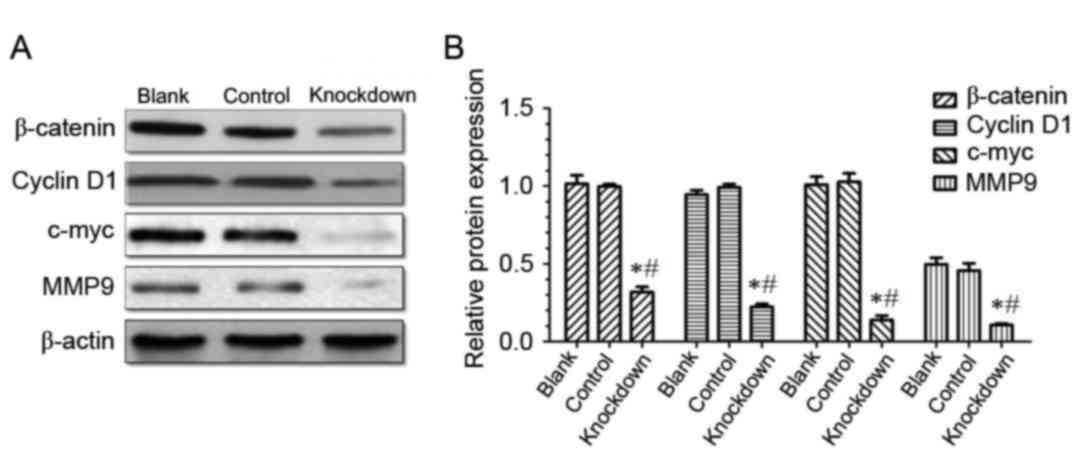
14-3-3β knockdown suppresses the
expression of β-catenin, cyclinD1, c-myc and MMP9 in MG63
cells
The present study demonstrated that 14-3-3β is
upregulated in osteosarcoma tissues and cell lines, and that
14-3-3β knockdown inhibited osteosarcoma MG63 cell viability,
proliferation and invasion. Western blotting demonstrated that the
level of β-catenin expression was significantly downregulated in
the 14-3-3β knockdown group compared with the control and the blank
group (Fig. 7), which suggests
that 14-3-3β may regulate the expression of β-catenin.
β-catenin is a positive regulator of the canonical
Wnt signaling pathway, therefore, the hypothesis that 14-3-3β may
act through the canonical Wnt pathway was tested. Protein
expression levels of cyclin D1, c-myc and MMP9, which are known
target genes of the canonical Wnt pathway, were determined by
western blotting. The results demonstrated that expression levels
of cyclin D1, c-myc and MMP9 were significantly decreased in the
14-3-3β knockdown group compared with the control and the blank
group (Fig. 7). These data
indicated that 14-3-3β may suppress proliferation and invasion of
osteosarcoma cells through the inhibition of the canonical Wnt
signaling pathway.
Discussion
14-3-3 is a family of highly conserved proteins that
are involved in a number of cellular processes (24). The majority of 14-3-3 proteins have
been demonstrated to be essential in regulating apoptosis,
proliferation and oncogenic transformation (11). 14-3-3β exhibits oncogenic
potential, and increased expression of 14-3-3β is detected in
multiple types of carcinomas (18). However, the expression pattern and
the exact role of 14-3-3β in osteosarcoma remain unclear.
In the present study, the expression of 14-3-3β was
demonstrated to be significantly increased in osteosarcoma tissues
and cell lines, compared with normal tissues and normal osteoblast
cells, respectively. These findings are similar to the upregulated
expression of 14-3-3β in hepatocellular carcinoma, astrocytoma,
lung cancer, colorectal cancer, gastric cancer and vulvar squamous
cell carcinoma (17–19,21).
These data indicate that the upregulation of 14-3-3β may be
essential in tumorigenesis and progression of osteosarcoma. To
further explore the effect of 14-3-3β on the biological behavior of
osteosarcoma cells, 14-3-3β expression was silenced in MG63
osteosarcoma cells by siRNA. The results demonstrated that cell
viability was significantly inhibited following 14-3-3β knockdown
in MG63 cells. The number of MG63 cells at the S and
G2-M phases of the cell cycle was lower in the 14-3-3β
knockdown cell group compared with the control group, which
suggests that 14-3-3β silencing inhibited MG63 cell proliferation.
Therefore, 14-3-3β overexpression may be responsible for cell cycle
deregulation, a key feature of carcinogenesis. The present data are
similar to previous findings for other types of cancer, which have
demonstrated that 14-3-3β knockdown inhibits proliferation of
hepatocellular carcinoma cells and astrocytoma cells (19,25).
Activation of the Wnt/β-catenin signaling pathway promotes cellular
proliferation, whereas Wnt inhibition reduces proliferation
(26,27). Downregulation of 14-3-3β
significantly decreases nuclear localization of β-catenin, followed
by a decrease in the activity of Wnt/β-catenin signaling (28). Therefore, β-catenin nuclear
translocation, induced by overexpression of 14-3-3β, activates the
transcription of oncogenes, including cyclin D1 and c-myc (19,29).
In the present study, β-catenin expression was significantly
downregulated following 14-3-3β knockdown in osteosarcoma cells,
which suggests that 14-3-3β may regulate expression of β-catenin.
Since β-catenin is a positive regulator of the canonical Wnt
signaling pathway, it was hypothesized that 14-3-3β may inhibit
proliferation of osteosarcoma cells through Wnt. To test this
hypothesis, expression of cyclin D1 and c-myc, which are known
targets downstream of the canonical Wnt signaling pathway, were
determined. The results demonstrated that expression of cyclin
D1and c-myc was significantly inhibited following 14-3-3β
knockdown, suggesting that 14-3-3β may regulate growth and
proliferation in osteosarcoma cells through Wnt/β-catenin
signaling.
Previous studies have demonstrated that
overexpression of 14-3-3β is significantly correlated with
metastasis, increased invasive ability and poor prognosis in many
types of cancer, including hepatocellular carcinoma and gastric
cancer (18,30). Tang et al (18) demonstrated that elevated expression
of 14-3-3β in hepatocellular carcinoma cell lines led to enhanced
cell migration and invasion, as well as upregulation of MMP2 and
MMP9 expression. In the current study, 14-3-3β knockdown suppressed
MG63 osteosarcoma cell invasion, suggesting that 14-3-3β
overexpression may enhance osteosarcoma cell invasion. This is in
agreement with a previous study, which demonstrated that targeted
depletion of 14-3-3β reduces lung cancer cell migration and
invasion (31). Activation of
Wnt/β-catenin signaling promotes the invasive activities of several
types of cancer cells (32,33),
while suppression of Wnt/β-catenin signaling inhibits cancer cell
invasion (34,35). In the present study, 14-3-3β
inhibited β-catenin expression, as well as osteosarcoma cell
invasion, suggesting that 14-3-3β may regulate cell invasion
through the canonical Wnt signaling pathway. The expression of
MMP9, which is a target gene of the canonical Wnt pathway and a
promoter of cell invasion, was further explored. The results
demonstrated that MMP9 expression levels were significantly
decreased following 14-3-3β knockdown, compared with the control
siRNA-transfected cells.
In conclusion, the present study demonstrated that
14-3-3β silencing was able to suppress proliferation and migration
of osteosarcoma cells, possibly through the inactivation of the
canonical Wnt/β-catenin signaling pathway. These findings suggest
that the increased expression of endogenous 14-3-3β observed in
osteosarcoma cells compared with normal tissues, may be important
in regulating proliferation and migration of osteosarcoma cells,
and that 14-3-3β may be valuable as a prognostic marker and/or
therapeutic target for osteosarcoma.
Acknowledgements
The present study was supported by the Natural
Science Foundation of Jiangsu Province (grant no. BK20131199), the
fifty-fifth batch funding of the China Postdoctoral Science
Foundation (grant no. 2014M551640), Nantong Science and Technology
Innovation Program (grant no. MS22016008) and by the Project of
Jiangsu provincial Health and Family Planning Commission (grant no.
H201524).
References
|
1
|
Wan J and Zhang X, Liu T and Zhang X:
Strategies and developments of immunotherapies in osteosarcoma.
Oncol Lett. 11:511–520. 2016.PubMed/NCBI
|
|
2
|
Endo-Munoz L, Evdokiou A and Saunders NA:
The role of osteoclasts and tumour-associated macrophages in
osteosarcoma metastasis. Biochim Biophys Acta. 1826:434–442.
2012.PubMed/NCBI
|
|
3
|
Lamora A, Talbot J, Bougras G, Amiaud J,
Leduc M, Chesneau J, Taurelle J, Stresing V, Le Deley MC, Heymann
MF, et al: Overexpression of smad7 blocks primary tumor growth and
lung metastasis development in osteosarcoma. Clin Cancer Res.
20:5097–5112. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Poletajew S, Fus L and Wasiutyński A:
Current concepts on pathogenesis and biology of metastatic
osteosarcoma tumors. Ortop Traumatol Rehabil. 13:537–545. 2011.(In
English, Polish). View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang X, Cao W, Lin H, Zhang W, Lin W, Cao
L, Zhen H, Huo J and Zhang X: Isoform-specific expression of 14-3-3
proteins in human astrocytoma. J Neurol Sci. 276:54–59. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Freeman AK and Morrison DK: 14-3-3
Proteins: Diverse functions in cell proliferation and cancer
progression. Semin Cell Dev Biol. 22:681–687. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tzivion G, Gupta VS, Kaplun L and Balan V:
14-3-3 proteins as potential oncogenes. Semin Cancer Biol.
16:203–213. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Niemantsverdriet M, Wagner K, Visser M and
Backendorf C: Cellular functions of 14-3-3 zeta in apoptosis and
cell adhesion emphasize its oncogenic character. Oncogene.
27:1315–1319. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee CH, Ou WB, Mariño-Enriquez A, Zhu M,
Mayeda M, Wang Y, Guo X, Brunner AL, Amant F, French CA, et al:
14-3-3 fusion oncogenes in high-grade endometrial stromal sarcoma.
Proc Natl Acad Sci USA. 109:pp. 929–934. 2012; View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Qi W, Liu X, Qiao D and Martinez JD:
Isoform-specific expression of 14-3-3 proteins in human lung cancer
tissues. Int J Cancer. 113:359–363. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu Y, Tian RF, Li YM, Liu WP, Cao L, Yang
XL, Cao WD and Zhang X: The expression of seven 14-3-3 isoforms in
human meningioma. Brain Res. 1336:98–102. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wilker EW, van Vugt MA, Artim SA, Huang
PH, Petersen CP, Reinhardt HC, Feng Y, Sharp PA, Sonenberg N, White
FM and Yaffe MB: 14-3-3sigma controls mitotic translation to
facilitate cytokinesis. Nature. 446:329–332. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sun N, Wu Y, Huang B, Liu Q, Dong Y, Ding
J and Liu Y: Decreased expression of 14-3-3 σ, an early event of
malignant transformation of respiratory epithelium, also
facilitates progression of squamous cell lung cancer. Thorac
Cancer. 6:715–721. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Radhakrishnan VM, Putnam CW, Qi W and
Martinez JD: P53 suppresses expression of the 14-3-3 gamma
oncogene. BMC Cancer. 11:3782011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mulvey HE, Chang A, Adler J, Del Tatto M,
Perez K, Quesenberry PJ and Chatterjee D: Extracellular
vesicle-mediated phenotype switching in malignant and non-malignant
colon cells. BMC Cancer. 15:5712015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li N, Wang H, Fan J, Tong C, Yang J, Wei
H, Yi J and Ling R: Overexpression of 14-3-3θ promotes tumor
metastasis and indicates poor prognosis in breast carcinoma.
Oncotarget. 5:249–257. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu TA, Jan YJ, Ko BS, Chen SC, Liang SM,
Hung YL, Hsu C, Shen TL, Lee YM, Chen PF, et al: Increased
expression of 14-3-3β promotes tumor progression and predicts
extrahepatic metastasis and worse survival in hepatocellular
carcinoma. Am J Pathol. 179:2698–2708. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tang Y, Lv P, Sun Z, Han L and Zhou W:
14-3-3β promotes migration and invasion of human hepatocellular
carcinoma cells by modulating expression of MMP2 and MMP9 through
PI3K/Akt/NF-κB pathway. PLoS One. 11:e01460702016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gong F, Wang G, Ye J, Li T, Bai H and Wang
W: 14-3-3β regulates the proliferation of glioma cells through the
GSK3β/β-catenin signaling pathway. Oncol Rep. 30:2976–2982. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Takihara Y, Matsuda Y and Hara J: Role of
the beta isoform of 14-3-3 proteins in cellular proliferation and
oncogenic transformation. Carcinogenesis. 21:2073–2077. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang Z, Nesland JM, Suo Z, Trope CG and
Holm R: The prognostic value of 14-3-3 isoforms in vulvar squamous
cell carcinoma cases: 14-3-3β and ε are independent prognostic
factors for these tumors. PLoS One. 6:e248432011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhu J, Liu F, Wu Q and Liu X: Activin A
regulates proliferation, invasion and migration in osteosarcoma
cells. Mol Med Rep. 11:4501–4507. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim JO, Kim SR, Lim KH, Kim JH, Ajjappala
B, Lee HJ, Choi JI and Baek KH: Deubiquitinating enzyme USP37
regulating oncogenic function of 14-3-3γ. Oncotarget.
6:36551–36576. 2015.PubMed/NCBI
|
|
25
|
Wu YJ, Jan YJ, Ko BS, Liang SM and Liou
JY: Involvement of 14-3-3 proteins in regulating tumor progression
of hepatocellular carcinoma. Cancers (Basel). 7:1022–1036. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cheng AS, Lau SS, Chen Y, Kondo Y, Li MS,
Feng H, Ching AK, Cheung KF, Wong HK, Tong JH, et al: EZH2-mediated
concordant repression of Wnt antagonists promotes
β-catenin-dependent hepatocarcinogenesis. Cancer Res. 71:4028–4039.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Stewart DJ: Wnt signaling pathway in
non-small cell lung cancer. J Natl Cancer Inst. 106:djt3562014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cao L, Lei H, Chang MZ, Liu ZQ and Bie XH:
Down-regulation of 14-3-3β exerts anti-cancer effects through
inducing ER stress in human glioma U87 cells: Involvement of
CHOP-Wnt pathway. Biochem Biophys Res Commun. 462:389–395. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen H, Liu L, Ma B, Ma TM, Hou JJ, Xie
GM, Wu W, Yang FQ and Chen YG: Protein kinase A-mediated 14-3-3
association impedes human Dapper1 to promote dishevelled
degradation. J Biol Chem. 286:14870–14880. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ma Y, Li YF, Wang T, Pang R, Xue YW and
Zhao SP: Identification of proteins associated with lymph node
metastasis of gastric cancer. J Cancer Res Clin Oncol.
140:1739–1749. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Okayama A, Miyagi Y, Oshita F, Nishi M,
Nakamura Y, Nagashima Y, Akimoto K, Ryo A and Hirano H: Proteomic
analysis of proteins related to prognosis of lung adenocarcinoma. J
Proteome Res. 13:4686–4694. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cai J, Feng D, Hu L, Chen H, Yang G, Cai
Q, Gao C and Wei D: FAT4 functions as a tumour suppressor in
gastric cancer by modulating Wnt/β-catenin signalling. Br J Cancer.
113:1720–1729. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ye GD, Sun GB, Jiao P, Chen C, Liu QF,
Huang XL, Zhang R, Cai WY, Li SN and Wu JF: OVOL2, an inhibitor of
WNT signaling, reduces invasive activities of human and mouse
cancer cells and is down-regulated in human colorectal tumors.
Gastroenterology. 150:659–671.e16. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fan Y and Guo Y: Knockdown of eIF3D
inhibits breast cancer cell proliferation and invasion through
suppressing the Wnt/β-catenin signaling pathway. Int J Clin Exp
Pathol. 8:10420–10427. 2015.PubMed/NCBI
|
|
35
|
Deng Z, Wang L, Hou H, Zhou J and Li X:
Epigenetic regulation of IQGAP2 promotes ovarian cancer progression
via activating Wnt/β-catenin signaling. Int J Oncol. 48:153–160.
2015.PubMed/NCBI
|