Introduction
Stroke is a life-threatening condition with a high
mortality rate and a high risk of subsequent disability (1,2).
Ischemic stroke is the most common form of stroke, accounting for
~85% of the total number of strokes (3). Despite advances in current stroke
therapies, many patients do not benefit from conventional
treatments.
Ischemic strokes are caused by a blockage of the
arteries responsible for the provision of blood to the brain or
spinal cord, therefore resulting in critically reduced blood flow
to said region(s). Well-functioning collateral circulation has been
demonstrated to improve the clinical prognosis following an
ischemic stroke (4–6). Angiogenesis is the generation of new
blood vessels from pre-existing vasculature (7). A series of studies have revealed that
post-ischemic angiogenesis contributes to the improvement in
neurological functional recovery following a stroke (8). Therefore, angiogenesis has been
suggested to be a promising therapeutic target for ischemic
stroke.
Thymic stromal lymphopoietin (TSLP), a member of the
interleukin 7 cytokine family, is predominantly produced by
epithelial cells, fibroblasts and smooth muscle cells (9,10).
TSLP signals via a TSLP receptor (TSLPR), which is widely
distributed among a number of different immune cells, such as mast
cells, monocytes, dendritic cells and lymphocytes (11–16).
Thus, TSLP has been suggested to be involved in the modulation of
both innate and adaptive immune responses (17–20).
Previously, Xie et al (21)
reported that TSLP is able to modulate the biological behavior of
vascular endothelial cells in vitro, and is also involved in
the angiogenesis of cervical cancer. However, the exact role of
TSLP/TSLPR in angiogenesis following ischemic stroke has not
previously been investigated.
In the present study, the biological role of
TSLP/TSLPR in angiogenesis in rats subjected to middle cerebral
artery occlusion (MCAO), and human umbilical vein endothelial cells
(HUVECs) subjected to oxygen-glucose deprivation (OGD) were
investigated. Furthermore, whether or not the phosphatidylinositol
3 kinase (PI3K)/protein kinase B (AKT) pathway can mediate the
effects of TSLP/TSLPR on angiogenesis following ischemic stroke was
determined.
Materials and methods
Animals
This study was approved by the Ethics Committee of
Hunan Provincial People's Hospital (Changsha, China), and all of
the experiments performed on animals were performed in compliance
with the principles of experimental animal ethics. A total of 48
Sprague-Dawley male rats at the age of 8 weeks, weighing 300–350 g,
were obtained from Shanghai SLAC Laboratory Animal Co., Ltd.
(Shanghai, China). The rats were maintained under controlled
conditions (22±2°C; 55% humidity) with a 12 h light/dark cycle, and
free access to food and fresh water. The permanent middle cerebral
artery occlusion (MCAO) model was established in accordance with
the Longa et al study (22). Briefly, the rats in the MCAO group
were anesthetized via an intraperitoneal injection of 10% chloral
hydrate (300 mg/kg intraperitoneal injection). Subsequently, the
right common carotid artery was exposed through a 2 cm midline
incision in the neck. To occlude the middle cerebral artery, a 4-0
nylon suture with a silicone tip was inserted into the internal
carotid artery until mild resistance was felt. At 6, 12, 24 and 72
h time intervals following MCAO, the rats were sacrificed via an
intraperitoneal injection of pentobarbital (Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany). Rats in the sham group were anesthetized
and underwent surgery without MCAO. The neurological function of
the rats was then tested 2 h post-MCAO in accordance with the Longa
et al study (22), and rats
with scores of between one and three were held for further
experiments. Human TSLP recombinant protein was sourced from Abnova
(Taipei, Taiwan). The MCAO rats (n=6/group) were then given either
10 µg of recombinant TSLP or phosphate-buffered saline (PBS)
intraperitoneally for 24 h. Subsequently, the rats were sacrificed
and cerebral infarct areas were collected.
Cell culture and treatment
HUVECs (American Type Culture Collection, Manassas,
VA, USA) were cultured in a RPMI-1640 (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal
calf serum (Gibco; Thermo Fisher Scientific, Inc.) and 100 U/ml
streptomycin/penicillin at 37°C. Cells cultured under normal
conditions were maintained in a humidified atmosphere of 95% air
and 5% CO2. Cells in the OGD condition were cultured in
the RPMI-1640 without glucose, and maintained in a humidified
atmosphere of 94% N2, 1% O2 and 5%
CO2 for 2 h. Following this, the OGD-treated cells were
cultured in the RPMI-1640 with 5.5 mmol/l glucose under normoxic
conditions for reoxygenation for 24 h. The cells subjected to OGD
were treated with TSLP at a concentration of 20 ng/ml. LY294002 (50
µM; Cell Signaling Technology, Inc., Danvers, MA, USA) was
administered in order to suppress the PI3K/AKT pathway.
ELISA
Tissues of the cerebral infarct area and HUVEC cell
supernatants were collected, and the concentration of TSLP was then
determined by ELISA assay according to the manufacturer's
instructions (DTSLP0, R&D Systems, Inc., Minneapolis, MN,
USA).
Western blot analysis
Total proteins were prepared from the cerebral
infarct area and HUVEC cells using a Total Protein Extraction kit
(Thermo Fisher Scientific, Inc.), and protein concentrations were
then determined using a Bicinchoninic Acid protein assay (Pierce;
Thermo Fisher Scientific, Inc.). Equal masses of protein samples
(50 µg) were subsequently separated on a 10% SDS-PAGE gel and
electrophoretically transferred to nitrocellulose membranes.
Following blocking with Tris-buffered saline 0.1% Tween (TBST)
containing 5% non-fat milk for 2 h at room temperature, the
membranes were then incubated with the primary antibodies at 4°C
overnight. The primary antibodies used in these analyses included
anti-TSLPR (1:500; sc-517429, Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA), vascular endothelial growth factor A (VEGFA;
1:800; sc-507, Santa Cruz Biotechnology, Inc.), angiopoietin 2
(Ang-2; 1:400; sc-74402, Santa Cruz Biotechnology, Inc.),
phosphorylated AKT (p-AKT; Ser 473 1:400; 12694, Cell Signaling
Technology, Inc.), AKT (1:800; 2920, Cell Signaling Technology,
Inc.) and GAPDH (1:1,000; sc-47724, Santa Cruz Biotechnology,
Inc.). Following washing with TBST, the membranes were further
incubated with horseradish peroxidase-conjugated secondary antibody
(1:5,000; sc-2005, Santa Cruz Biotechnology, Inc.) at 37°C for 2 h.
The target bands were then developed using the super ECL reagent
(Thermo Fisher Scientific, Inc.). The density of bands was analyzed
using Image-Pro Plus, version 6.0 software (Media Cybernetics,
Inc., Rockville, MD, USA).
Cell proliferation assay
The MTT Assay Kit was purchased from Beyotime
Institute of Biotechnology (Shanghai, China). According to the
manufacturer's instructions, 2×103 cells were plated
into the 96-well plates, and treated with 20 ng/ml TSLP. The cells
were allowed to grow for 12, 24, 48 and 72 h time intervals, and
then 10 µl MTT solution (5 mg/ml) was added into each well.
Following incubation at 37°C for 4 h, 10 µl formazan solution was
added into each well and incubated at 37°C for a further 4 h in
order to dissolve the formazan crystals. The absorbance at 570 nm
was then determined using a microplate reader (Multiskan Spectrum;
Thermo Fishers Scientific, Inc.).
Transwell migration assay
A 6-well Transwell system (8 µm; Corning
Incorporated, Corning, NY, USA) was used in the present study to
determine cell migration. Following washing with PBS, the cells
were suspended in RPMI-1640 cell medium without serum at a density
of 5×104 cells/ml in the upper chambers, and 2 ml of
cell suspension was then added to the Transwell plates. RPMI-1640
cell medium with 10% fetal calf serum (1 ml) was then added to the
lower chambers. The plates were incubated at 37°C for 24 h, and the
upper chamber was then fixed in 95% ethanol at room temperature for
15 min and stained with 10% hematoxylin for 15 min at room
temperature. The number of migrated cells was revealed using an
Eclipse TS100 microscope (magnification ×400; Nikon Corporation,
Tokyo, Japan).
Tube formation assay
The BD Matrigel matrix (BD Biosciences, Franklin
Lakes, NJ, USA) was thawed overnight on ice at 4°C, and was
subsequently added to pre-chilled 24-well plates and incubated at
37°C for 1 h for solidification. The cells were then digested at a
density of 4×105 cells/ml, and 50 µl of the cell
suspension was then added to each well. The plates were then
incubated at 37°C, and formation of tube structure was observed 8 h
later using a Leica DFC345 FX microscope. Tube lengths for each
group were then measured using ImageJ software, version 1.45
(GraphPad Software, Inc., La Jolla, CA, USA), in 10 randomly
selected fields.
Statistical analysis
All statistical analyses were performed using SPSS
19.0 (IBM Corp., Armonk, NY, USA), and the data are expressed as
the mean ± standard deviation. Comparisons between two groups were
performed using the Student's t test, and one-way analysis of
variance followed by Fisher's Least Significant Difference test was
used to compare the statistically significant differences between
multiple groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
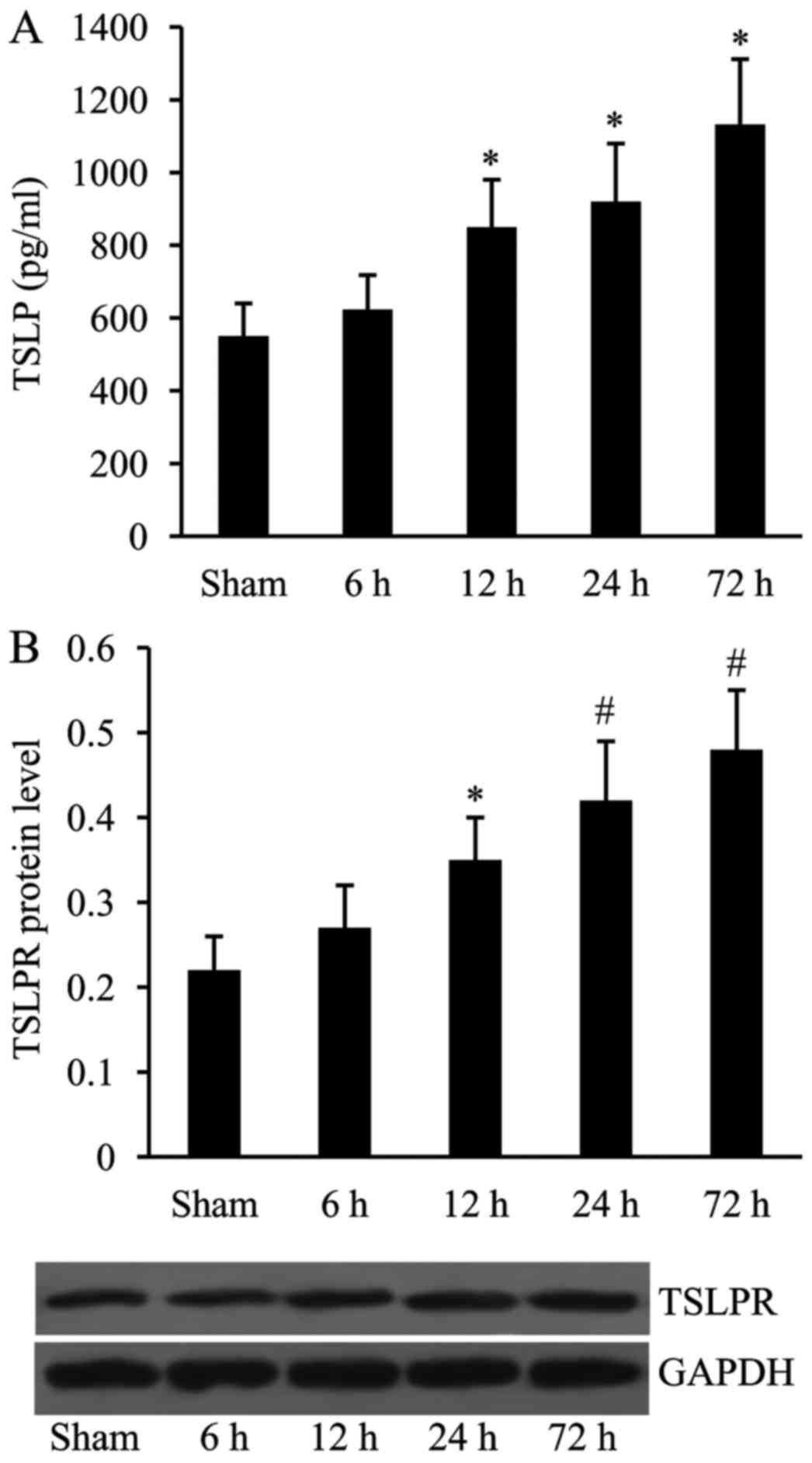
Expression of TSLP and TSLPR is
upregulated following MCAO
The rat MCAO model was constructed and the
expression of TSLP and TSLPR was then examined using ELISA and
western blot analyses at 6, 12, 24 and 72 h time intervals
following MCAO. As presented in Fig.
1, compared with the sham at 6 h, the expression levels of both
TSLP and TSLPR were significantly increased at 12, 24 and 72 h time
intervals following MCAO.
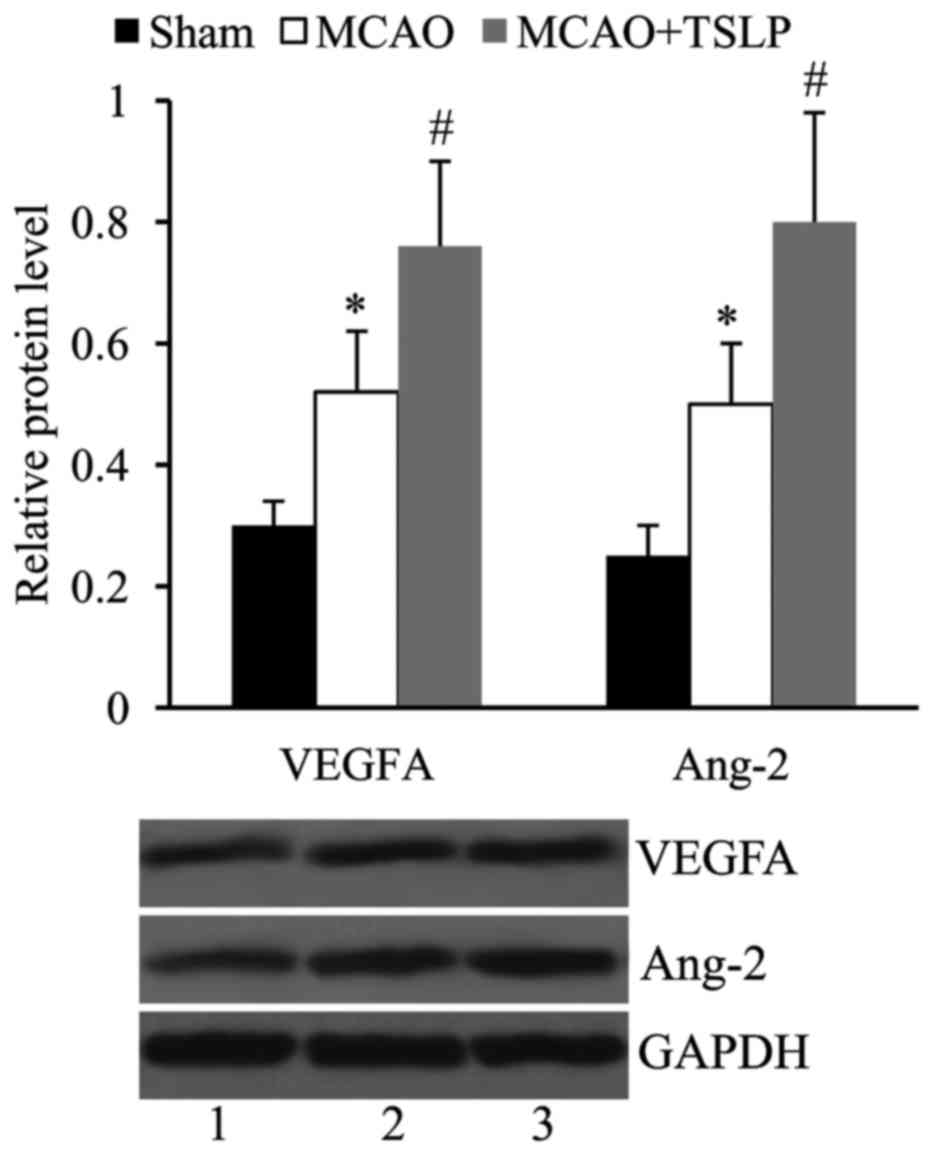
TSLP promotes the expression of VEGFA
and Ang-2 following MCAO
In order to investigate whether TSLP affects
angiogenesis following MCAO, the rats in the MCAO group were
injected with TSLP (10 µg), and the expression levels of VEGFA and
Ang-2 in the cerebral infarct area were determined by western blot
analysis. It was revealed that compared with the sham, the
expression levels of both VEGFA and Ang-2 were significantly
upregulated following MCAO. VEGFA and Ang-2 expression levels were
further increased in the MCAO rats injected with TSLP (Fig. 2).
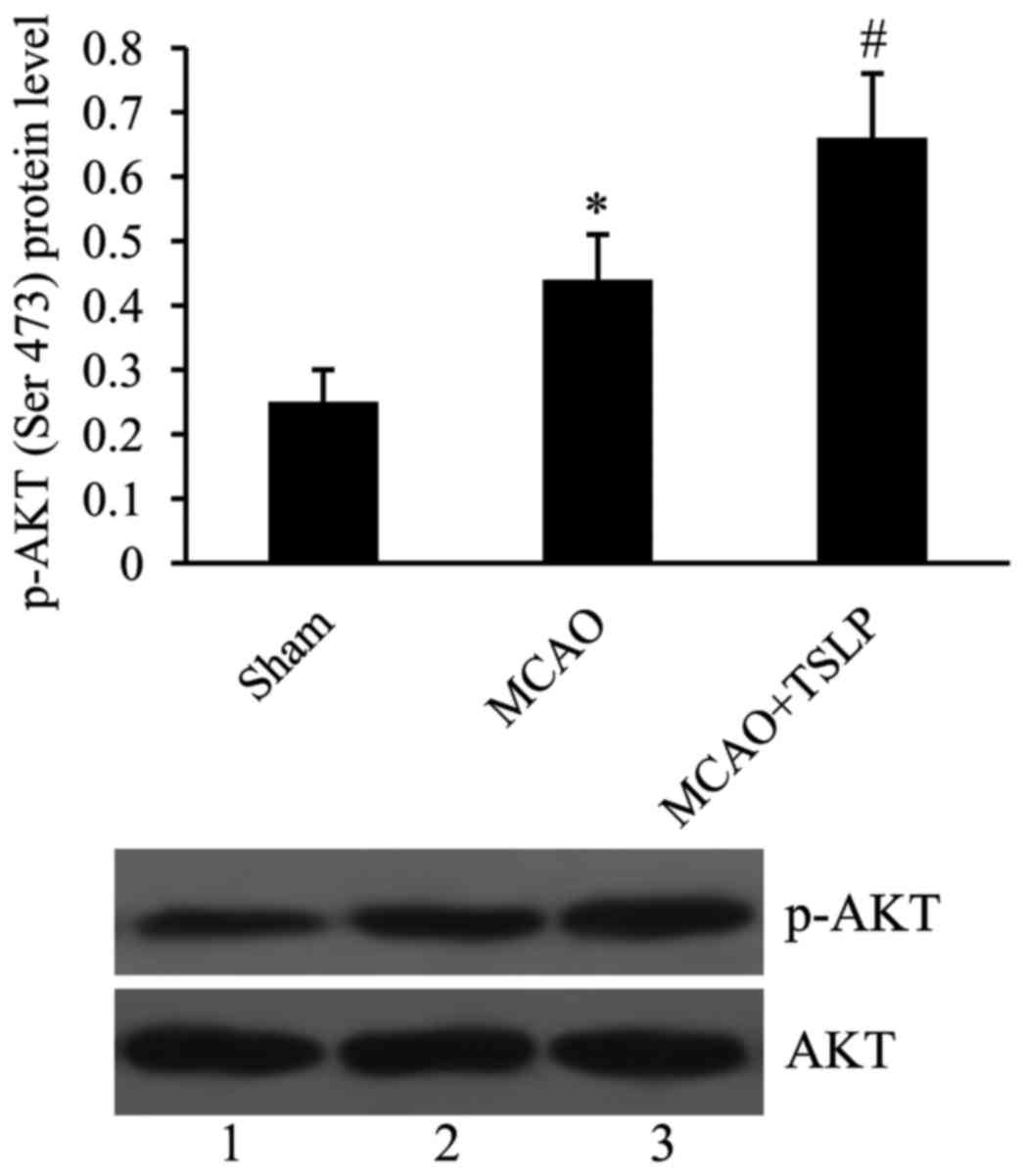
TSLP activates PI3K/AKT signaling
pathway following MCAO
In order to investigate whether or not the PI3K/AKT
pathway could be activated by TSLP following MCAO, p-AKT was
investigated using western blot. It was subsequently revealed that
the level of p-AKT was significantly increased in the MCAO group
compared with the control group. Additionally, 10 µg TSLP injection
caused further activation of the PI3K/AKT signaling pathway in the
MCAO rats (Fig. 3).
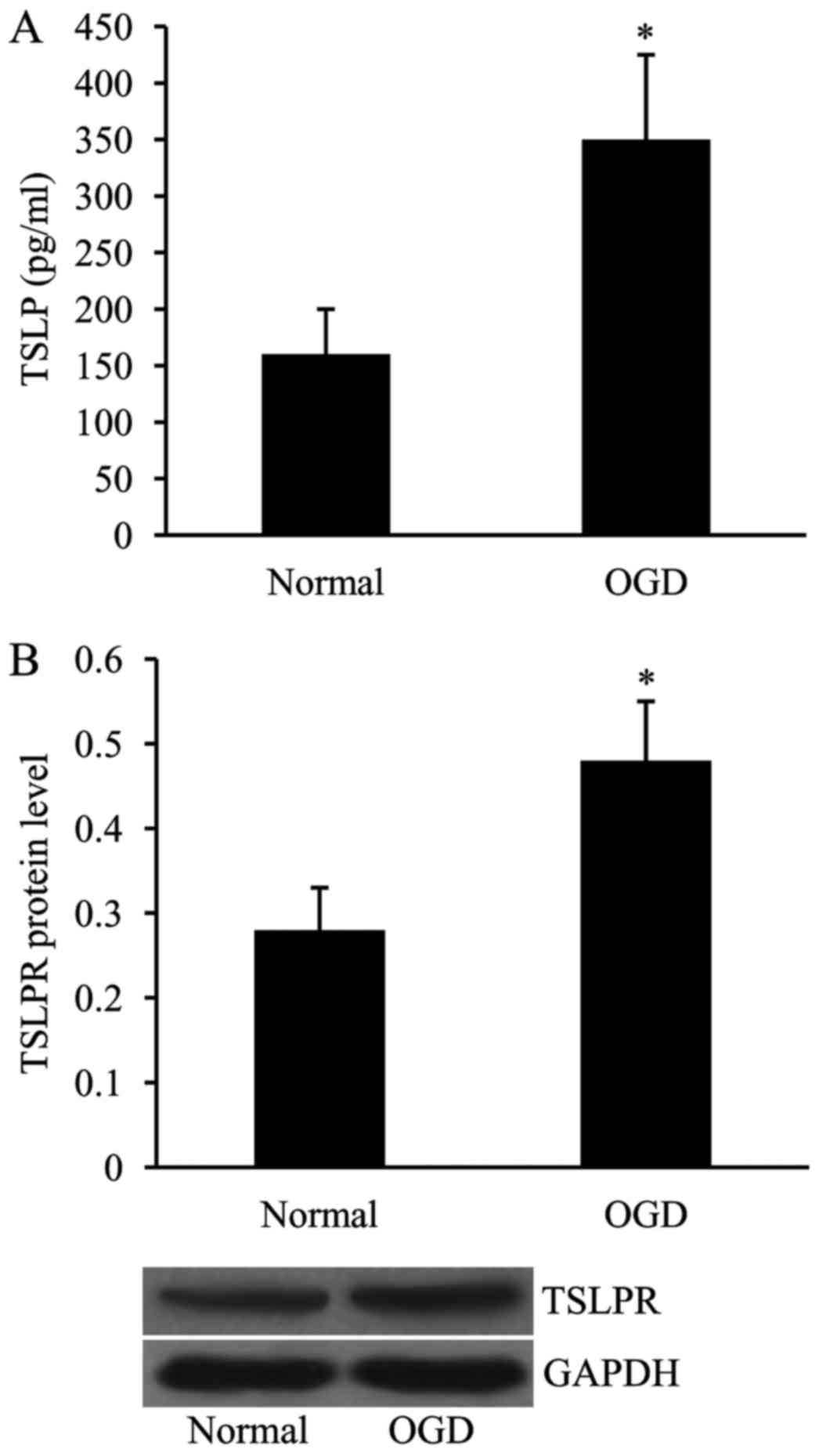
Expression of TSLP and TSLPR is
upregulated in HUVECs subjected to OGD
In order to create the in vitro MCAO model,
the HUVECs were exposed to OGD, and the expression levels of both
TSLP and TSLPR were subsequently examined using ELISA and western
blot analyses, respectively. As demonstrated in Fig. 4, the expression levels of TSLP and
TSLPR in OGD-treated HUVECs, in comparison with the cells cultured
under normal conditions, were significantly increased.
TSLP promotes the in vitro
angiogenesis of HUVECs subjected to OGD
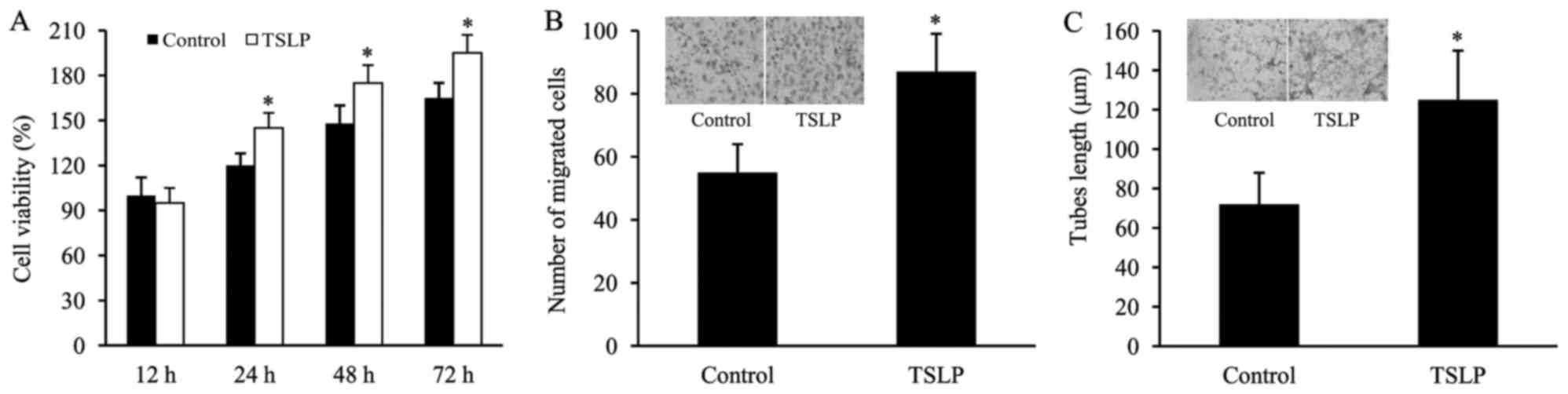
The HUVECs subjected to OGD were treated with TSLP,
and then cell viability, cell migration and tube formation were
examined in order to determine the effect of TSLP on the angiogenic
capacity of OGD-treated HUVECs. It was observed that cell viability
was significantly increased following TSLP treatment (Fig. 5A). Furthermore, in the TSLP group,
the number of migrated cells was significantly increased in
comparison with the control (Fig.
5B). Additionally, TSLP treatment led to an increase in the
tube lengths of OGD-treated HUVECs compared with the control
treatment (Fig. 5C).
TSLP activates PI3K/AKT signaling
pathway in HUVECs subjected to OGD
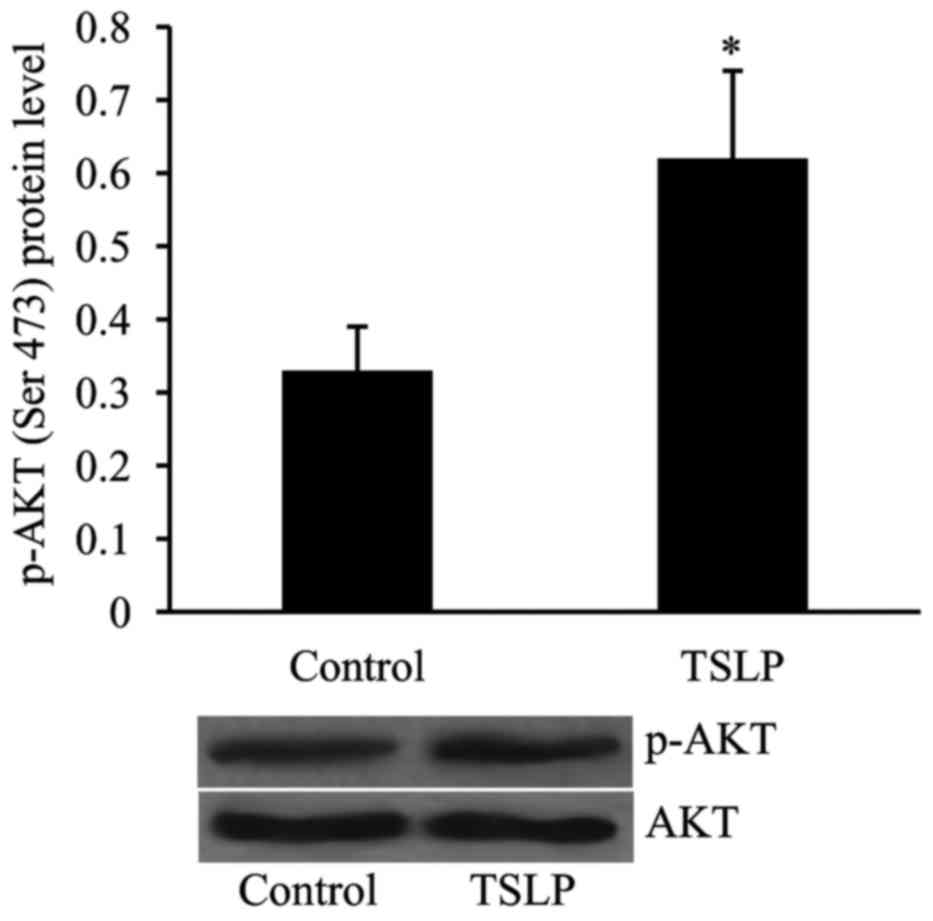
The level of p-AKT was examined in OGD-treated
HUVECs in order to determine whether or not the PI3K/AKT pathway is
involved in mediating the effect of TSLP on angiogenesis in
vitro. As revealed in Fig. 6,
the level of p-AKT was significantly increased in OGD-treated
HUVECs following TSLP treatment compared with the control
group.
PI3K inhibition attenuates the effect
of TSLP on in vitro angiogenesis of HUVECs subjected to OGD
LY294002, a PI3K inhibitor, was used to determine
whether the PI3K/AKT signaling pathway mediates the effect of TSLP
on in vitro angiogenesis of OGD-treated HUVECs. As
demonstrated by western blot analysis, LY294002 inhibited the
expression of p-AKT induced by TSLP in OGD-treated HUVECs (Fig. 7A).
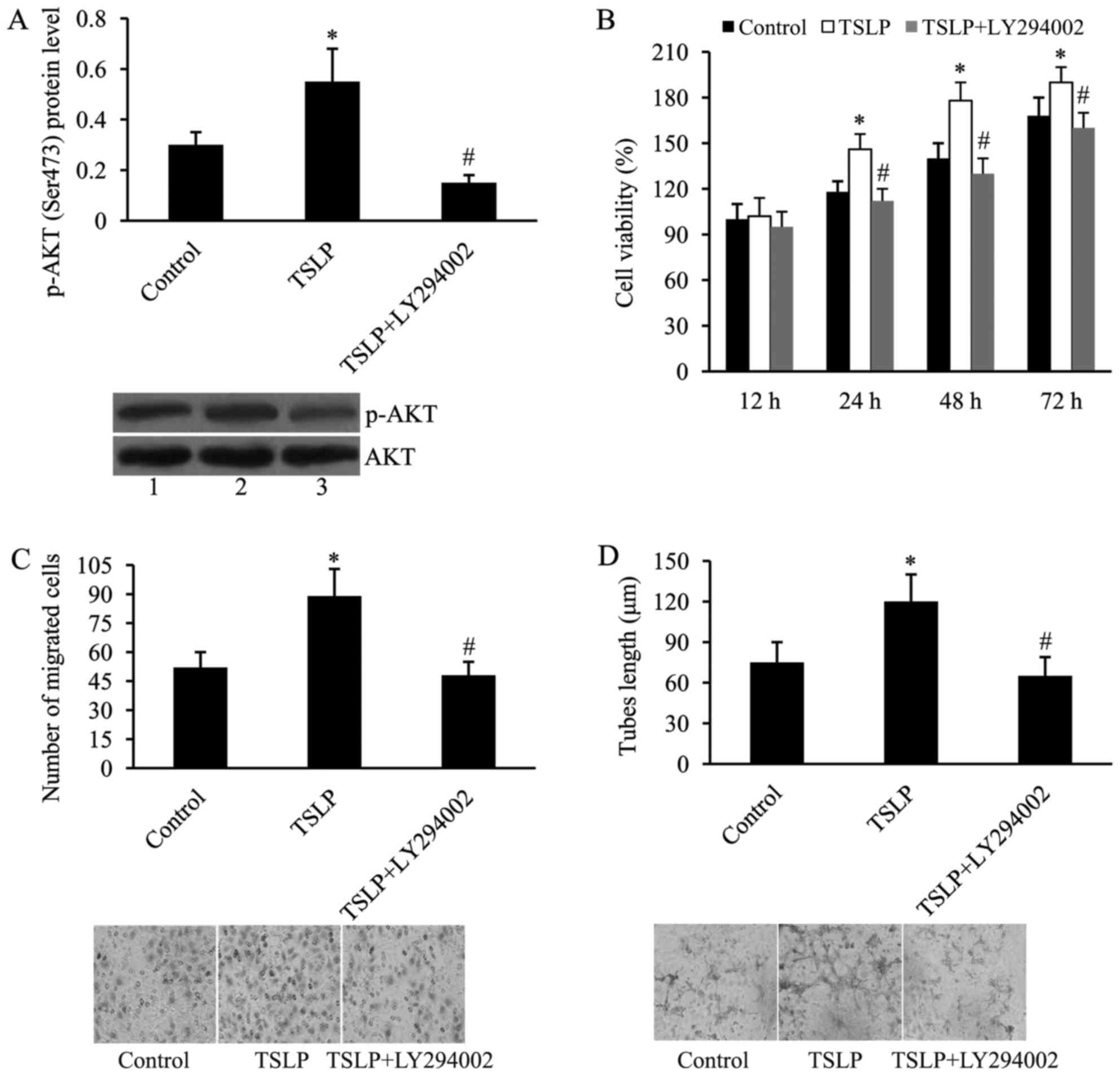 | Figure 7.PI3K inhibition attenuates the effect
of TSLP on in vitro angiogenesis of HUVECs subjected to OGD.
(A) LY294002 attenuates TSLP-induced PI3K/AKT activation in
OGD-treated HUVECs. AKT served as the control. Lane 1, control;
lane 2, TSLP; lane 3, TSLP + LY294002; (B) LY294002 attenuates
TSLP-induced cell proliferation of OGD-treated HUVECs; (C) LY294002
attenuates TSLP-induced cell migration of OGD-treated HUVECs; and
(D) LY294002 attenuates TSLP-induced tubes length of OGD-treated
HUVECs. n=3. *P<0.05 vs. control; #P<0.05 vs. TSLP
group. TSLP, thymic stromal lymphopoietin; PI3K,
phosphatidylinositol 3 kinase; HUVECs, human umbilical vein
endothelial cells; OGD, oxygen-glucose deprivation; AKT, protein
kinase B; p-AKT, phosphorylated AKT; LY294002, PI3K inhibitor. |
Additionally, cell viability was investigated using
MTT. It was subsequently revealed that the effect of TSLP on cell
proliferation was reversed by the addition of LY294002 (Fig. 7B). In addition, TSLP-induced cell
migration was suppressed by LY294002 (Fig. 7C). Tube lengths were also assessed
using the tube formation assay. As demonstrated by Fig. 7D, the effect of TSLP on tube
formation of OGD-treated HUVECs was attenuated by the addition of
LY294002.
Discussion
OGD and MCAO are in vitro and in vivo
cerebral ischemia models (23–26).
HUVECs are commonly used as a laboratory model system for the study
of angiogenesis. Previous studies have used OGD-treated HUVECs to
investigate stroke (27–29). In this study, an in vitro
cerebral ischemia model was established by exposing HUVECs to OGD.
The present study demonstrated that TSLP/TSLPR promote angiogenesis
following ischemic stroke in both animal experiments and cultured
cell experiments. Furthermore, it was confirmed that TSLP/TSLPR may
exert effects on angiogenesis via the PI3K/AKT signaling
pathway.
Several studies have previously reported that TSLP
is involved in the pathogenesis of atherosclerosis, diabetes,
obesity and asthma (30–32). Recently, Kitic et al
(33) reported that TSLP is also
expressed in the central nervous system, and that microglial cells
express TSLPR. The expression of TSLP in the central nervous system
varies among different pathological conditions (33). However, whether TSLP/TSLPR are
involved in the pathogenesis of ischemic stroke remains unknown. In
the present study, a rat MCAO model was established, and it was
revealed that the expression levels of TSLP and TSLPR were
significantly increased in the infarct area between 12 and 72 h
following MCAO. An in vitro MCAO model was constructed by
exposing the HUVECs to OGD, and it was revealed that the expression
levels of TSLP and TSLPR were significantly increased in HUVECs
subjected to OGD compared with those cultured under normal
conditions. These results suggest that TSLP/TSLPR may be involved
in the pathogenesis of ischemic stroke.
Angiogenesis is a key neurorestorative event in
response to ischemia (8).
Following ischemic stroke, angiogenesis occurs in the ischemic
boundary zone and improves neurological function (34,35).
VEGFA is an essential molecule in both physiological and
pathological angiogenesis. This growth factor induces
proliferation, differentiation and migration of vascular
endothelial cells. Following ischemic stroke, elevated levels of
VEGFA promote capillary formation and increase blood flow to the
area surrounding the infarction (36). Ang-2 may facilitate endothelial
cell migration and proliferation in co-ordination with VEGFA, thus
acting as an angiogenic signal (37). A previous study reported that TSLP
stimulates the proliferation and activation of HUVECs, and
upregulates the expression of angiogenesis-associated molecules,
CD62E and CD105 (21). Consistent
with these findings, the in vitro results of the present
study demonstrated that TSLP treatment promoted cell proliferation
and migration, and induced tube formation of OGD-treated HUVECs. In
addition, the in vivo results revealed that the expression
levels of angiogenic molecules, VEGFA and Ang-2 were increased
following MCAO. TSLP injection further upregulated VEGFA and Ang-2
expression in the infarct area. These results suggest that TSLP
promotes angiogenesis following ischemic stroke in both in
vivo and in vitro conditions.
The PI3K/AKT signaling pathway participates in
various cellular activities, such as cell proliferation, apoptosis,
differentiation and inflammatory responses. Additionally, the
activation of the PI3K/AKT signaling pathway has been revealed to
be implicated in the occurrence and development of angiogenesis
(38,39). Previous evidence has demonstrated
that TSLP/TSLPR functions via activation of the PI3K/AKT pathway in
order to induce platelet activation (40). In the present study, it was
investigated whether the PI3K/AKT signaling pathway mediates the
effects of TSLP/TSLPR on angiogenesis following ischemic stroke.
Consistent with the aforementioned study (40), TSLP was demonstrated to activate
the PI3K/AKT signaling pathway in OGD-treated HUVECs. LY294002, a
PI3K inhibitor, was used in this study to suppress the PI3K/AKT
signaling pathway. Subsequently, the effects of TSLP on in
vitro angiogenesis of OGD-treated HUVECs were attenuated by
LY294002.
In conclusion, the findings of this study suggest a
novel mechanism underlying the role of TSLP/TSLPR in angiogenesis
following ischemic stroke. This study, to the best of our
knowledge, is the first to demonstrate that TSLP/TSLPR promote
angiogenesis following ischemic stroke in vivo and in
vitro, and that these effects are mediated, at least partially,
via the activation of the PI3K/AKT signaling pathway. Therefore,
TSLP/TSLPR may be potential therapeutic targets for ischemic stroke
treatment. Further studies are required to confirm the effects of
TSLP/TSLPR on angiogenesis in clinical stroke patients, examine the
expression of TSLP/TSLPR in brain tissues and investigate
angiogenesis using imaging tests in infarct areas following
TSLP/TSLPR treatment.
References
|
1
|
GBD 2013 Mortality and Causes of Death
Collaborators, . Global, regional, and national age-sex specific
all-cause and cause-specific mortality for 240 causes of death,
1990–2013: A systematic analysis for the global burden of disease
study 2013. Lancet. 385:117–171. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Feigin VL, Forouzanfar MH, Krishnamurthi
R, Mensah GA, Connor M, Bennett DA, Moran AE, Sacco RL, Anderson L,
Truelsen T, et al: Global and regional burden of stroke during
1990–2010: Findings from the global burden of disease study 2010.
Lancet. 383:245–254. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang WZ: Neurology. 4th. People's Medical
Publishing House; Beijing: pp. 1302001
|
|
4
|
Bang OY, Saver JL, Buck BH, Alger JR,
Starkman S, Ovbiagele B, Kim D, Jahan R, Duckwiler GR, Yoon SR, et
al: Impact of collateral flow on tissue fate in acute ischaemic
stroke. J Neurol Neurosurg Psychiatry. 79:625–629. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Miteff F, Levi CR, Bateman GA, Spratt N,
McElduff P and Parsons MW: The independent predictive utility of
computed tomography angiographic collateral status in acute
ischaemic stroke. Brain. 132:2231–2238. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liebeskind DS, Cotsonis GA, Saver JL, Lynn
MJ, Turan TN, Cloft HJ and Chimowitz MI; Warfarin-Aspirin
Symptomatic Intracranial Disease (WASID) Investigators, :
Collaterals dramatically alter stroke risk in intracranial
atherosclerosis. Ann Neurol. 69:963–974. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Velazquez OC, Snyder R, Liu ZJ, Fairman RM
and Herlyn M: Fibroblast-dependent differentiation of human
microvaseular endothelial cells into capillary-like 3-dimensional
networks. FASEB J. 16:1316–1318. 2002.PubMed/NCBI
|
|
8
|
Arai K, Jin G, Navaratna D and Lo EH:
Brain angiogenesis in developmental and pathological processes:
Neurovascular injury and angiogenic recovery after stroke. FEBS J.
276:4644–4652. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lin J, Chang W, Dong J, Zhang F, Mohabeer
N, Kushwaha KK, Wang L, Su Y, Fang H and Li D: Thymic stromal
lymphopoietin over-expressed in human atherosclerosis: Potential
role in Th17 differentiation. Cell Physiol Biochem. 31:305–318.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhao H, Li M, Wang L, Su Y, Fang H, Lin J,
Mohabeer N and Li D: Angiotensin II induces TSLP via an AT1
receptor/NF-KappaB pathway, promoting Th17 differentiation. Cell
Physiol Biochem. 30:1383–1397. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yu K, Zhu P, Dong Q, Zhong Y, Zhu Z, Lin
Y, Huang Y, Meng K, Ji Q, Yi G, et al: Thymic stromal lymphopoietin
attenuates the development of atherosclerosis in ApoE-/- mice. J Am
Heart Assoc. 2:e0003912013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Blagoev M, Nielsen MM, Angrist M,
Chakravarti A and Pandey A: Cloning of rat thymic stromal
lymphopoietin receptor (TSLPR) and characterization of genomic
structure of murine Tslpr gene. Gene. 284:161–168. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pandey A, Ozaki K, Baumann H, Levin SD,
Puel A, Farr AG, Ziegler SF, Leonard WJ and Lodish HF: Cloning of a
receptor subunit required for signaling by thymic stromal
lymphopoietin. Nat Immunol. 1:59–64. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang W, Wang J, Wang Q, Chen G, Zhang J,
Chen T, Wan T, Zhang Y and Cao X: Identification of a novel type I
cytokine receptor CRL2 preferentially expressed by human dendritic
cells and activated monocytes. Biochem Biophys Res Commun.
281:878–883. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tonozuka Y, Fujio K, Sugiyama T, Nosaka T,
Hirai M and Kitamura T: Molecular cloning of a human novel type I
cytokine receptor related to delta1/TSLPR. Cytogenet Cell Genet.
93:23–25. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Reche PA, Soumelis V, Gorman DM, Clifford
T, Liu MR, Travis M, Zurawski SM, Johnston J, Liu YJ, Spits H, et
al: Human thymic stromal lymphopoietin preferentially stimulates
myeloid cells. J Immunol. 167:336–343. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Soumelis V, Reche PA, Kanzler H, Yuan W,
Edward G, Homey B, Gilliet M, Ho S, Antonenko S, Lauerma A, et al:
Human epithelial cells trigger dendritic cell mediated allergic
inflammation by producing TSLP. Nat Immunol. 3:673–680. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
He R and Geha RS: Thymic stromal
lymphopoietin. Ann N Y Acad Sci. 1183:13–24. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ziegler SF and Artis D: Sensing the
outside world: TSLP regulates barrier immunity. Nat Immunol.
11:289–293. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ma P, Bian F, Wang Z, Zheng X,
Chotikavanich S, Pflugfelder SC and Li DQ: Human corneal
epithelium-derived thymic stromal lymphopoietin links the innate
and adaptive immune responses via TLRs and Th2 cytokines. Invest
Ophthalmol Vis Sci. 50:2702–2709. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xie F, Meng YH, Liu LB, Chang KK, Li H, Li
MQ and Li DJ: Cervical carcinoma cells stimulate the angiogenesis
through TSLP promoting growth and activation of vascular
endothelial cells. Am J Reprod Immunol. 70:69–79. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Longa EZ, Weinstein PR, Carlson S and
Cummins R: Reversible middle cerebral artery occlusion without
craniectomy in rats. Stroke. 20:84–91. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Goldberg MP and Choi DW: Combined oxygen
and glucose deprivation in cortical cell culture: Calcium-dependent
and calcium-independent mechanisms of neuronal injury. J Neurosci.
13:3510–3524. 1993.PubMed/NCBI
|
|
24
|
Brint S, Jacewicz M, Kiessling M, Tanabe J
and Pulsinelli W: Focal brain ischemia in the rat: Methods for
reproducible neocortical infarction using tandem occlusion of the
distal middle cerebral and ipsilateral common carotid arteries. J
Cereb Blood Flow Metab. 8:474–485. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tamura A, Graham DI, McCulloch J and
Teasdale GM: Focal cerebral ischaemia in the rat: 1. Description of
technique and early neuropathological consequences following middle
cerebral artery occlusion. J Cereb Blood Flow Metab. 1:53–60. 1981.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tamura A, Graham DI, McCulloch J and
Teasdale GM: Focal cerebral ischaemia in the rat: 2. Regional
cerebral blood flow determined by [14C]iodoantipyrine
autoradiography following middle cerebral artery occlusion. J Cereb
Blood Flow Metab. 1:61–69. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li L, Zhang B, Tao Y, Wang Y, Wei H, Zhao
J, Huang R and Pei Z: DL-3-n-butylphthalide protects endothelial
cells against oxidative/nitrosative stress, mitochondrial damage
and subsequent cell death after oxygen glucose deprivation in
vitro. Brain Res. 1290:91–101. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Urbanek T, Kuczmik W, Basta-Kaim A and
Gabryel B: Rapamycin induces of protective autophagy in vascular
endothelial cells exposed to oxygen-glucose deprivation. Brain Res.
1553:1–11. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dong W, Xiao S, Cheng M, Ye X and Zheng G:
Minocycline induces protective autophagy in vascular endothelial
cells exposed to an in vitro model of ischemia/reperfusion-induced
injury. Biomed Rep. 4:173–177. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ying S, O'Connor B, Ratoff J, Meng Q,
Mallett K, Cousins D, Robinson D, Zhang G, Zhao J, Lee TH and
Corrigan C: Thymic stromal lymphopoietin expression is increased in
asthmatic airways and correlates with expression of Th2-attracting
chemokines and disease severity. J Immunol. 174:8183–8190. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Besin G, Gaudreau S, Ménard M, Guindi C,
Dupuis G and Amrani A: Thymic stromal lymphopoietin and thymic
stromal lymphopoietin-conditioned dendritic cells induce regulatory
T-cell differentiation and protection of NOD mice against diabetes.
Diabetes. 57:2107–2117. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Turcot V, Bouchard L, Faucher G, Garneau
V, Tchernof A, Deshaies Y, Pérusse L, Marceau S, Biron S,
Lescelleur O, et al: Thymic stromal lymphopoietin: An immune
cytokine gene associated with the metabolic syndrome and blood
pressure in severe obesity. Clin Sci(Lond). 123:99–109. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kitic M, Wimmer I, Adzemovic M, Kögl N,
Rudel A, Lassmann H and Bradl M: Thymic stromal lymphopoietin is
expressed in the intact central nervous system and upregulated in
the myelin-degenerative central nervous system. Glia. 62:1066–1074.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu J, Wang Y, Akamatsu Y, Lee CC, Stetler
RA, Lawton MT and Yang GY: Vascular remodeling after ischemic
stroke: Mechanisms and therapeutic potentials. Prog Neurobiol.
115:138–156. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Krupinski J, Kaluza J, Kumar P, Kumar S
and Wang JM: Role of angiogenesis in patients with cerebral
ischemic stroke. Stroke. 25:1794–1798. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Risau W: Mechanisms of angiogenesis.
Nature. 386:671–674. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
37
|
Biel NM and Siemann DW: Targeting the
Angiopoietin-2/Tie-2 axis in conjunction with VEGF signal
interference. Cancer Lett. 380:525–533. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Everaert BR, Van Craenenbroeck EM, Hoymans
VY, Haine SE, Van Nassauw L, Conraads VM, Timmermans JP and Vrints
CJ: Current perspective of pathophysiological and interventional
effects on endothelial progenitor cell biology: Focus on
PI3K/AKT/eNOS pathway. Int J Cardiol. 144:350–366. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tokunaga E, Oki E, Egashira A, Sadanaga N,
Morita M, Kakeji Y and Maehara Y: Deregulation of the Akt pathway
in human cancer. Curr Cancer Drug Targets. 8:27–36. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang B, Peng Y, Dong J, Lin J, Wu C, Su Y,
Fang H, Wang L, Huang K and Li D: Human platelets express
functional thymic stromal lymphopoietin receptors: A potential role
in platelet activation in acute coronary syndrome. Cell Physiol
Biochem. 32:1741–1750. 2013. View Article : Google Scholar : PubMed/NCBI
|