Introduction
Osteoarthritis (OA) is a common degenerative joint
disease which is predominantly characterized by irreversible loss
of the cartilage and inflammation of synovium (1). Chondrocytes are the only resident
cells in articular cartilage and their function is an important
factor in determining the pathogenesis of OA (2). Notably, previous studies have
demonstrated that inflammation is induced by pro-inflammatory
cytokines, which also serve a critical role in the development and
progression of OA (3–5). It has also been indicated that
inflammatory responses lead to an increase in apoptosis and
dysfunction of chondrocytes, and as a result, the catabolism,
degeneration and pathogenesis associated with OA are exaggerated.
Further studies have verified that the inflammatory response is
induced by interleukin (IL)-1β (3), a pro-inflammatory and pro-catabolic
cytokine, which leads to upregulation of nitric oxide and
prostaglandin E2 by producing more matrix metalloproteinases and
less extracellular matrix. Therefore, the present study aimed to
identify a promising compound that may inhibit inflammatory
mediator production, and possesses the ability to improve the
viability and function of chondrocytes, therefore impeding the
progress of OA (5).
Autophagy, an evolutionarily conserved
self-protective mechanism (6), is
highly efficient in protecting physiological processes by removing
protein aggregates or unnecessary cellular components. Furthermore,
it has been demonstrated that autophagy is involved in inflammatory
reactions and downregulation of autophagy in macrophages leads to
inflammation (7). In addition, a
previous study reported that resveratrol inhibits endothelial
inflammation via autophagy induction (8). However, the regulatory mechanisms for
autophagy in inflammation are also complex and remain to be fully
elucidated. Sirtuin (SIRT)6, an NAD+-dependent
deacetylase, belongs to class III histone/protein deacetylases and
is a member of the silent information regulator 2 family (9,10).
In the Sirtuin family, SIRT6 and SIRT1 are predominantly localized
in the nucleus and share functional similarities. It has been
reported that SIRT1 mediates human and mice chondrocyte survival in
the context of arthritis. It has also been indicated that knockdown
of SIRT1 in chondrocytes lessens cartilage-specific gene expression
and accelerates hypertrophy and cartilage matrix loss.
Additionally, a previous study revealed that fenofibrate prevented
high glucose-induced inflammatory responses in cardiac myoblasts by
upregulating SIRT1-mediated autophagy (11). Previously, SIRT6 has gained
considerable research attention owing to the newly discovered roles
it serves in autophagy (12) and
osteoarthritis development. There is research that suggests that
overexpression of SIRT6 suppresses inflammatory responses in tumor
necrosis factor (TNF)-α induced synoviocytes and bone destruction
(13). SIRT6 overexpression
induces autophagy via attenuation of insulin-like growth
factor-protein kinase B-mechanistic target of rapamycin signaling.
Conversely, SIRT6 inhibition suppresses autophagy. Furthermore,
SIRT6 has been demonstrated to prevent OA development by reducing
the inflammatory response and chondrocyte senescence (14). However, the roles of SIRT6 in
cartilage and its anti-inflammatory mechanism remain unknown, and
require further investigation. Therefore, it was hypothesized that
the activation of SIRT6 serves a role in the regulation of
autophagy, with subsequent inhibitory effects on inflammation in
chondrocytes and OA progression.
Hydroxytyrosol (HT) is a phenol molecule derived
from olive leaves and olive oil and is part of the traditional
Mediterranean diet (15,16). Previous studies have indicated that
consumption of HT exhibits certain health benefits on various
physiological functions due to its anti-oxidative and
anti-inflammatory pharmacological activities (17). Furthermore, it has been
demonstrated that HT inhibits the inflammatory response in vascular
endothelial cells, macrophages and monocytes (18). It has also been demonstrated that
HT increases the autophagy of chondrocytes and preosteoblast cells
and protects them from cell death and oxidative damage (19,20),
respectively. In addition, the study revealed that overexpression
of SIRT6 could suppress inflammation in a variety of cells
(19,20). However, the roles of SIRT6 and
autophagy in the anti-inflammatory process of HT in chondrocytes
remains unknown. Therefore, elucidating the potential underlying
anti-inflammatory mechanisms of HT and the involvement of SIRT6 and
autophagy may be valuable in treating inflammation-associated
diseases including OA.
Materials and methods
Chemical reagents and antibodies
HT (purity >98%) was purchased from Xi'an
App-Chem Bio (Tech) Co., Ltd. (Shaanxi Sheng, China). Tumor
necrosis factor-α (TNF-α) was an inflammatory induction factor
obtained from PeproTech, Inc. (Rocky Hill, NJ, USA). The antibodies
used in this experiment are included SIRT6, LC3, Beclin1, MCP-1,
β-actin and horse radish peroxidase conjugated anti-rabbit
secondary antibody. SIRT6 antibody (cat. no. 12486, Cell Signaling
Technology, Inc., Danvers, MA, USA) was used as a primary antibody.
Polyclonal anti-microtubule-associated protein 1A/1B-light chain 3
(LC3; cat. no. 12741) and anti-Beclin1 antibodies (cat. no. 3495)
were provided by Cell Signaling Technology, Inc. Antibodies against
monocyte chemoattractant protein 1 (MCP-1; cat. no. NBP2-22115)
were purchased from Novus Biologicals, LLC, Littleton, CO, USA.
Antibodies against β-actin (cat. no. 4970) were purchased from Cell
Signaling Technology, Inc. and horse radish peroxidase conjugated
anti-rabbit secondary antibody was provided by Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA).
Lipofectamine® 2000 was obtained from Invitrogen (Thermo
Fisher Scientific, Inc., Waltham, MA, USA). RNAiso Plus,
PrimeScript RT Master mix and SYBR Premix Ex Taq II were purchased
from Takara Bio, Inc. (Otsu, Japan). A rat IL-1β ELISA kit was
obtained from Cusabio Biotech Co., Ltd (cat. no. KET9001). (Newark,
DE, USA). The mRNA primers were synthesized by Generay Biotech Co.,
Ltd. (Shanghai, China). 3-methyladenine (3-MA) was purchased from
Sigma-Aldrich; Merck KGaA (Darmstadt, Germany). Rat IL-1β and IL-6
enzyme-linked immunosorbent assay (ELISA) kit was obtained from
Cusabio Biotech Co., Ltd. (cat. no. KET9001). All other chemicals
were of high purity and were obtained from commercial sources.
Cell culture
The approval for the use of the animals in this
study was granted by the Animal Ethics Committee of Xi'an Jiaotong
University (Xi'an, China). Adult male Sprague-Dawley rats were
provided by the Experimental Animal Center of Xi'an Jiaotong
University (Animal certificate no. SYXK (shan) 2014–003). A total
of 2 Male Sprague-Dawley rats (200–300 g) were euthanized as
previously described immediately upon receipt (21). The knee cartilages were separated
under microscopy and digested with 0.25% trypsin-EDTA for 30 min
and collagenase II for 4 h at 37°C, and then the chondrocytes were
filtered to produce a single cell suspension. Chondrocytes were
cultured in high-glucose (4.5 g/l) Dulbecco's modified Eagle's
medium (DMEM; Hyclone; GE Healthcare Life Sciences, Logan, UT, USA)
with antibiotics (1% penicillin and streptomycin) and 10% fetal
bovine serum (Hyclone; GE Healthcare Life Sciences) in an incubator
containing 5% CO2 at 37°C, Passages between 2 and 4 were
used for the experiments.
Cell viability assay
A Cell Counting kit (CCK)-8 assay (Roche Applied
Science, Penzberg, Germany) was performed to determine cell
viability. Briefly, chondrocytes were seeded at a density of
2×103 cells well in 96-well microplates. Following
incubation for 24 h, HT was added to the culture medium at
different concentrations (0, 12.5, 25, 50, 100, 200 and 400 µM) and
then treated with TNF-α (5 ng/ml) for 24 h at 37°C. The cells were
then cultured with fresh media for an additional 48 h at 37°C.
CCK-8 solution was added (10 µl well), and the cells were further
incubated for 3 h at 37°C in a 5% (v/v) CO2 atmosphere.
The optical density was measured using a microplate reader (Thermo
Fisher Scientific, Inc.) at a wavelength of 450 nm, with a
background control of media and CKK-8 solution as a blank.
ELISA
Following different treatment conditions, cell
culture supernatants were collected. The sample was centrifuged at
a rate of 200 × g for 10 min at 4°C. Concentrations of
pro-inflammatory cytokines IL-1β and IL-6 were measured using a
high-sensitive ELISA, according to the manufacturer's protocol.
RNA interference
Rat small interfering RNA (siRNA) and negative
control siRNA (NC siRNA) were chemically synthesized by Shanghai
GenePharma Co., Ltd. (Shanghai, China). Chondrocytes were seeded in
6-well plates at the density of 5.0×105/ml and then were
transfected with 20 µM synthesized siRNA targeting rat SIRT6. The
siRNA and Lipofectamine 2000 were separately diluted in serum-free
DMEM and incubated for 5 min at room temperature. Then the two
solutions were gently mixed and incubated for 20 min at room
temperature, prior to addition to the cells. The chondrocytes were
transfected with siRNA-Lipofectamine complexes and incubated for 48
h at 37°C in a CO2 incubator, and then used in
subsequent experiments.
Western blot analysis
Cellular proteins were extracted with
radioimmunoprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology, Haimen, China) containing protease inhibitor
cocktail. The sample concentration was determined by BCA kit
(Beyotime Institute of Biotechnology), after that, 25 µml of
samples were added to each well and electrophoresis was performed
in 10% of the separation gel. Protein samples were separated by
SDS-PAGE under reducing conditions and transferred to
polyvinylidene difluoride membranes. The membranes were blocked
with 5% nonfat dry milk for 4 h at room temperature and then
incubated with antibodies against rabbit anti-SIRT6 (1:1,000),
anti-LC3II/I (1:1,000), anti-Beclin1 (1:1,000), anti-MCP-1 (1:400)
and β-actin (1:1,000) at 4°C overnight. The membranes were then
incubated with a goat anti-rabbit IgG-horseradish peroxidase
antibody (1:5,000 or 1:10,000) for 2 h at room temperature and
subsequently washed three times with TBST buffer solution (1%
Tween-20). The relative intensity of protein bands was quantified
by Quantity One Analysis Software (version 4.62; Bio-Rad
Laboratories, Inc., Hercules, CA, USA) and β-actin was used as an
internal control.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from cells using RNAiso Plus
reagent. cDNAs were synthesized from total RNA using PrimeScript RT
Master mix following the manufacturer's protocol. cDNA was
amplified using the SYBR Premix Ex Taq™ kit. Primers are given in
Table I. The relative gene
expression was quantitatively analyzed by the 2ΔΔCq
method (22). Data were normalized
to the levels of GAPDH mRNA.
 | Table I.Primer sequences used for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primer sequences used for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Primer | Sequence (5′-3′) |
|---|
| Sirtuin 6 | Forward |
5′-GCAGTCTTCCAGTGTGGTGT-3′ |
|
| Reverse |
5′-CCATGGTCCAGACTCCGT-3′ |
| GAPDH | Forward |
5′-GGCACAGTCAAGGCTGAGAATG-3′ |
|
| Reverse |
5′-ATGGTGGTGAAGACGCCAGTA-3′ |
Statistical analysis
Data are presented as the mean ± standard error from
three independent experiments. Statistical significance was
determined using one-way analysis of variance and the Fisher's
least significant difference post hoc test, using SPSS software,
version 19.0 (IBM SPSS, Armonk, NY, USA). P<0.05 was considered
to indicate a statistically significant difference.
Results
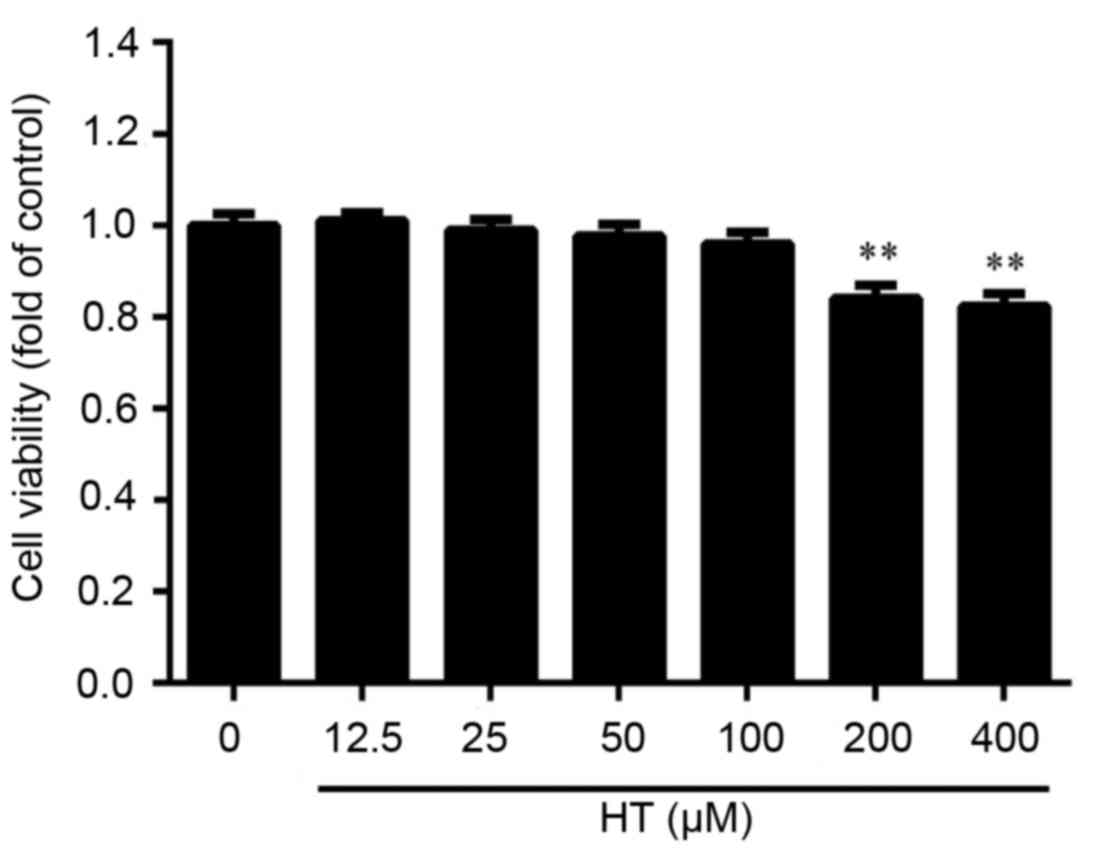
Effect of HT on cell viability in
TNF-α-induced chondrocytes
Initial experiments were performed in an
anti-inflammatory cell model using HT in TNF-α-treated
chondrocytes. It is hypothesized that high concentrations of HT may
exhibit cytotoxicity on chondrocytes. Cultured chondrocytes were
exposed to increasing concentrations of HT (12.5, 25, 50, 100, 200
and 400 µM) for 24 h. Cell viability was examined using CCK-8
assay. As presented in Fig. 1,
cell viability of chondrocytes was significantly reduced following
HT treatment at concentrations of 200 and 400 µM. At 50 µM HT
treatment, there was ~100% cell viability and no significant
cytotoxicity observed, and therefore, dosages around this
concentration were used in subsequent experiments to determine the
anti-inflammatory effect of HT on TNF-α-induced cell
inflammation.
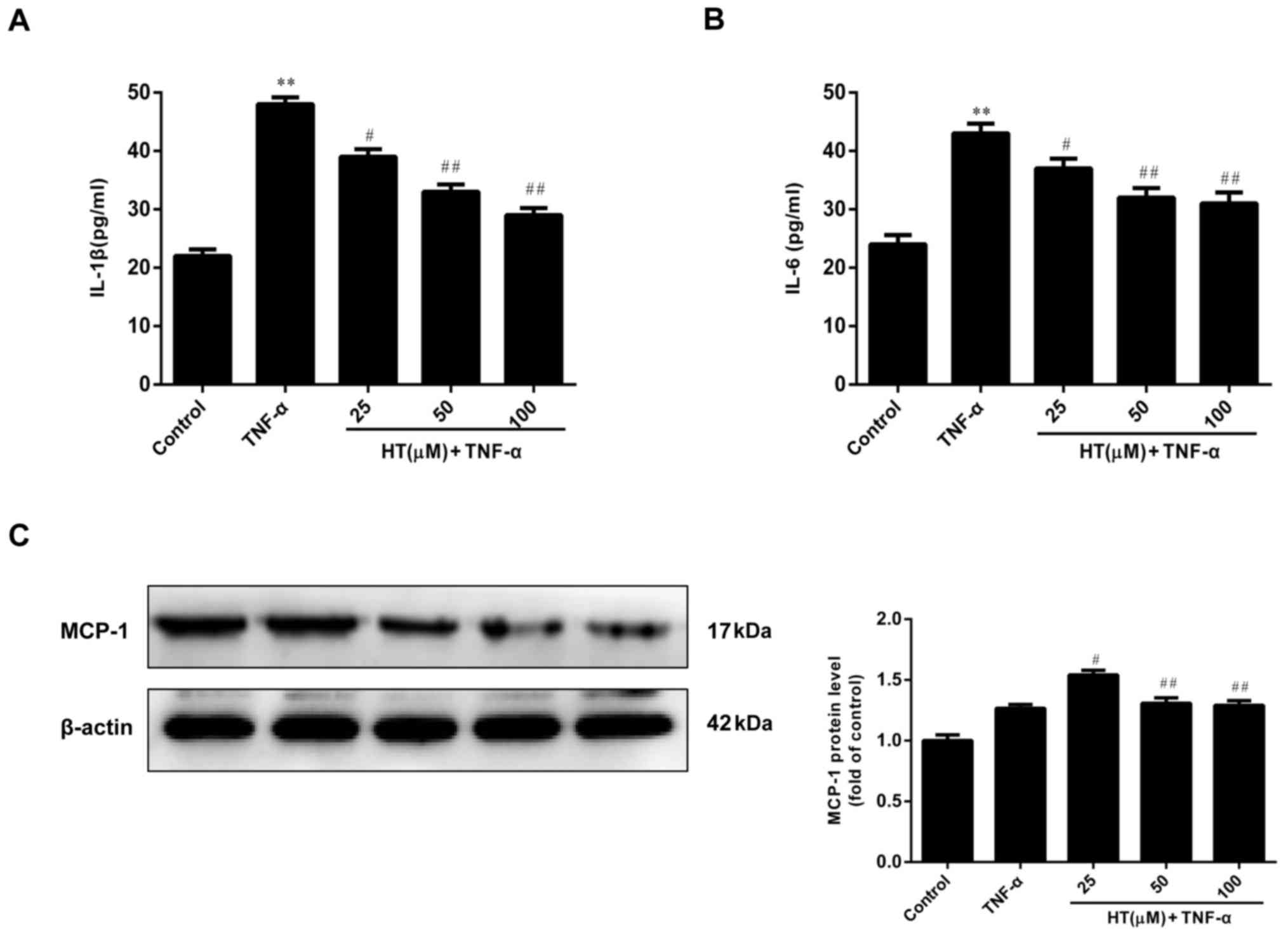
HT inhibits the TNF-α-induced
inflammatory response in chondrocytes
HT has been demonstrated to exert anti-inflammatory
properties, as verified by in vitro studies (17,19).
HT modulates inflammatory responses in murine peritoneal
macrophages and human hepatoma HepG2 and Hep3B cell lines. These
previous observations encouraged the authors of the present study
to determine the effect of HT on inflammatory responses in
TNF-α-induced chondrocytes.
As demonstrated in Fig.
2A-C, TNF-α (5 ng/ml) alone markedly increased the levels of
IL-1β, IL-6 compared with the control, and the MCP-1 protein level
was also slightly elevated compared with the control, and exposure
of chondrocytes to increasing concentrations of HT (25 to 100 µM)
resulted in a reduction in expression of these proteins, suggesting
that HT exhibited an anti-inflammatory effect in a dose-dependent
manner. Therefore, HT had a concentration-dependent inhibitory
effect on the release levels of IL-1β, IL-6 and MCP-1 proteins in
TNF-α-stimulated chondrocytes.
HT inhibits the inflammatory response
of chondrocytes via autophagy
To investigate the effect of HT on autophagy in
chondrocytes, the conversion of LC3I to LC3II and the expression of
the Beclin1 protein were measured using western blotting and the
results were demonstrated in Fig. 3A
and B. Chondrocytes treated with HT exhibited statistically
significant increased expression levels of LC3-II (50 and 100 µM
HT) and cleaved Beclin1 (25, 50 and 100 µM) compared with
chondrocytes cultured with TNF-α alone, suggesting that HT may
promote autophagy in chondrocytes under TNF-α-induced inflammatory
conditions.
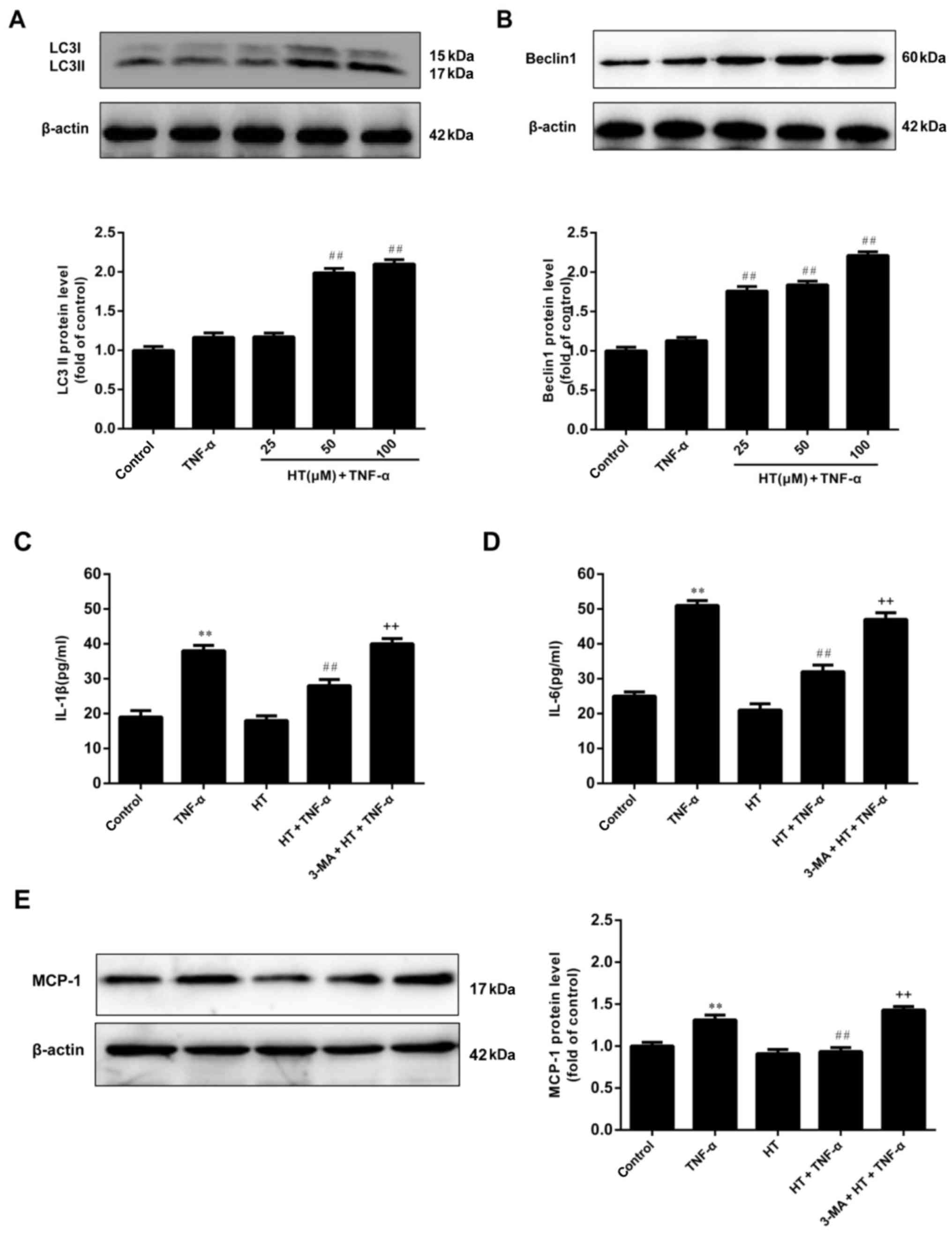 | Figure 3.HT promotes autophagy in TNF-α-induced
chondrocytes. Chondrocytes were pretreated with HT at different
concentrations (25, 50 and 100 µM) for 1 h and then stimulated with
TNF-α (5 ng/ml) for 24 h. The protein expression of (A) LC3II and
(B) Beclin1 were assessed by western blot analysis. Next, the
chondrocytes were pretreated with an autophagy inhibitor, 3-MA (5
mM), for 1 h, and then incubated with HT (50 µM) for 1 h, followed
by stimulation with TNF-α for 24 h. The concentration of (C) IL-1β
and (D) IL-6 in cell supernatants were determined by ELISA. (E) The
expression levels of MCP-1 were detected by western blot analysis.
The results are expressed as the mean ± standard error (n=3).
**P<0.01 vs. control group. ##P<0.01 vs. TNF-α
group. ++P<0.01 vs. HT+TNF-α group. HT,
hydroxytyrosol; TNF-α, tumor necrosis factor-α; 3-MA,
3-methyladenine; IL-1β, interleukin-1β; IL-6, interleukin-6; MCP-1,
monocyte chemoattractant protein 1. |
Based on these findings, HT may inhibit inflammatory
responses and promote autophagy in a dose-dependent manner.
However, whether the anti-inflammatory effects of HT are dependent
on activation of autophagy remains unclear. To determine whether
the inflammatory response is mediated by autophagy induction in
TNF-α-treated chondrocytes, the effect of 3-MA, an autophagy
inhibitor, on TNF-α-induced chondrocyte inflammation was evaluated.
It was demonstrated in Fig. 3C, D and
E that the anti-inflammatory effect of HT was significantly
blocked by pretreatment of cells with 3-MA. These results suggested
that the inflammatory response of TNF-α-induced chondrocytes was
inhibited through autophagy.
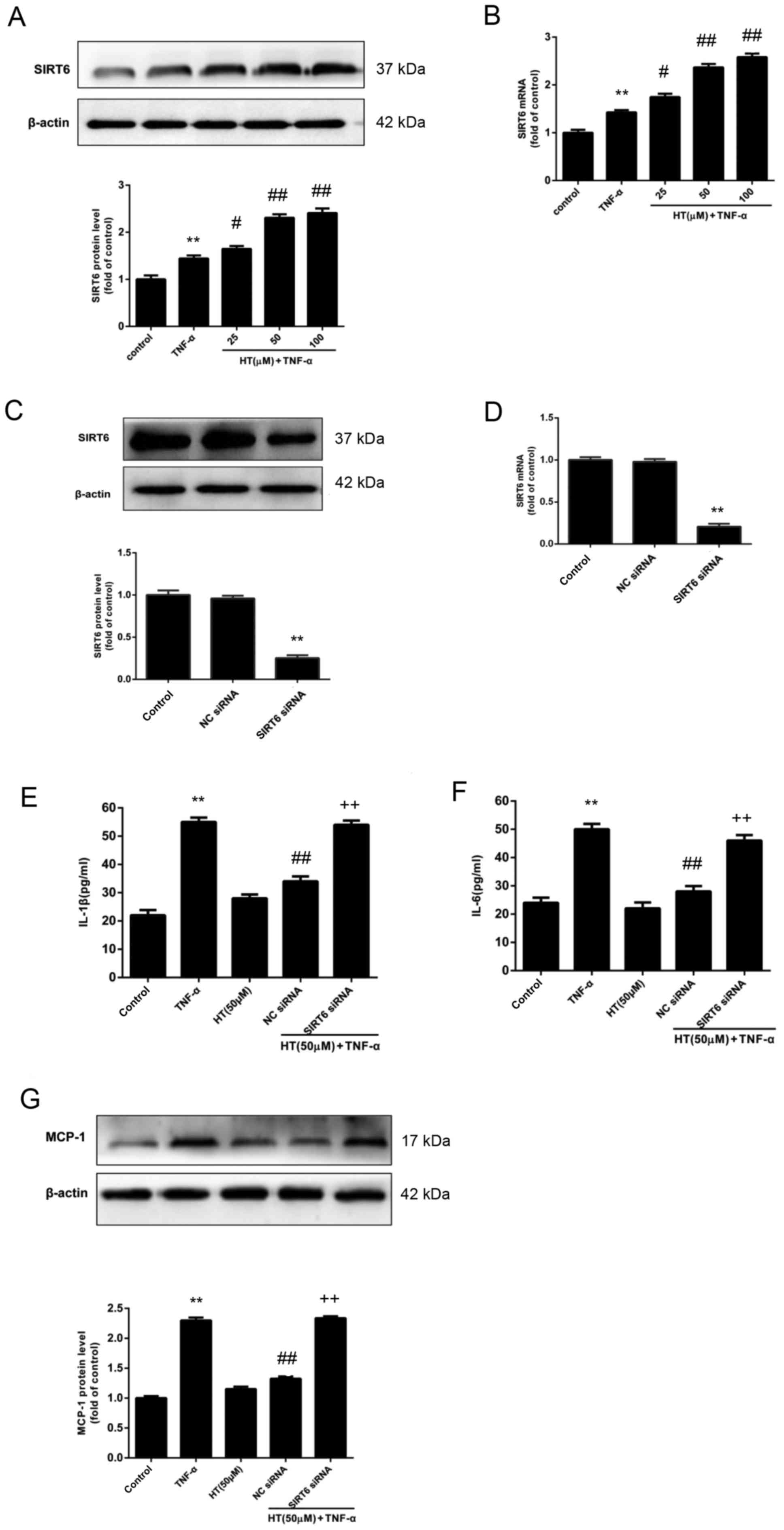
HT inhibits TNF-α-induced inflammatory
response in chondrocytes through SIRT6
The aim was to determine the regulatory role of
SIRT6 on the inflammatory response in chondrocytes in the presence
of HT. To do this, the expression levels of SIRT6 in chondrocyte
cells were determined. The protein and mRNA expression levels of
SIRT6 were determined by western blot analysis (Fig. 4A) and RT-qPCR (Fig. 4B). Fig. 4A and B revealed that HT enhanced
the expression of SIRT6 at the protein and mRNA level in
chondrocytes compared with cells stimulated with TNF-α alone.
Next, it was investigated whether the
anti-inflammatory effect of HT may be regulated by SIRT6 expression
in chondrocytes, and it was demonstrated that the expression of
SIRT6 protein and mRNA were reduced to ~30% by SIRT6-specific siRNA
in chondrocytes, compared with chondrocytes treated with NC siRNA
(Fig. 4C and D, respectively). In
support with the hypothesis that SIRT6 regulates the
anti-inflammatory effect of HT in chondrocytes, siRNA knockdown of
SIRT6 was associated with significantly increased release of the
proinflammatory cytokines IL-1β and IL-6 (P<0.01; Fig. 4E and F). The results also revealed
that knockdown of SIRT6 increased the protein expression levels of
MCP-1 compared with the NC siRNA+HT+TNF-α group (P<0.01;
Fig. 4G). Taken together, these
findings suggested that HT inhibited the TNF-α-induced inflammatory
response through SIRT6 activation in chondrocytes.
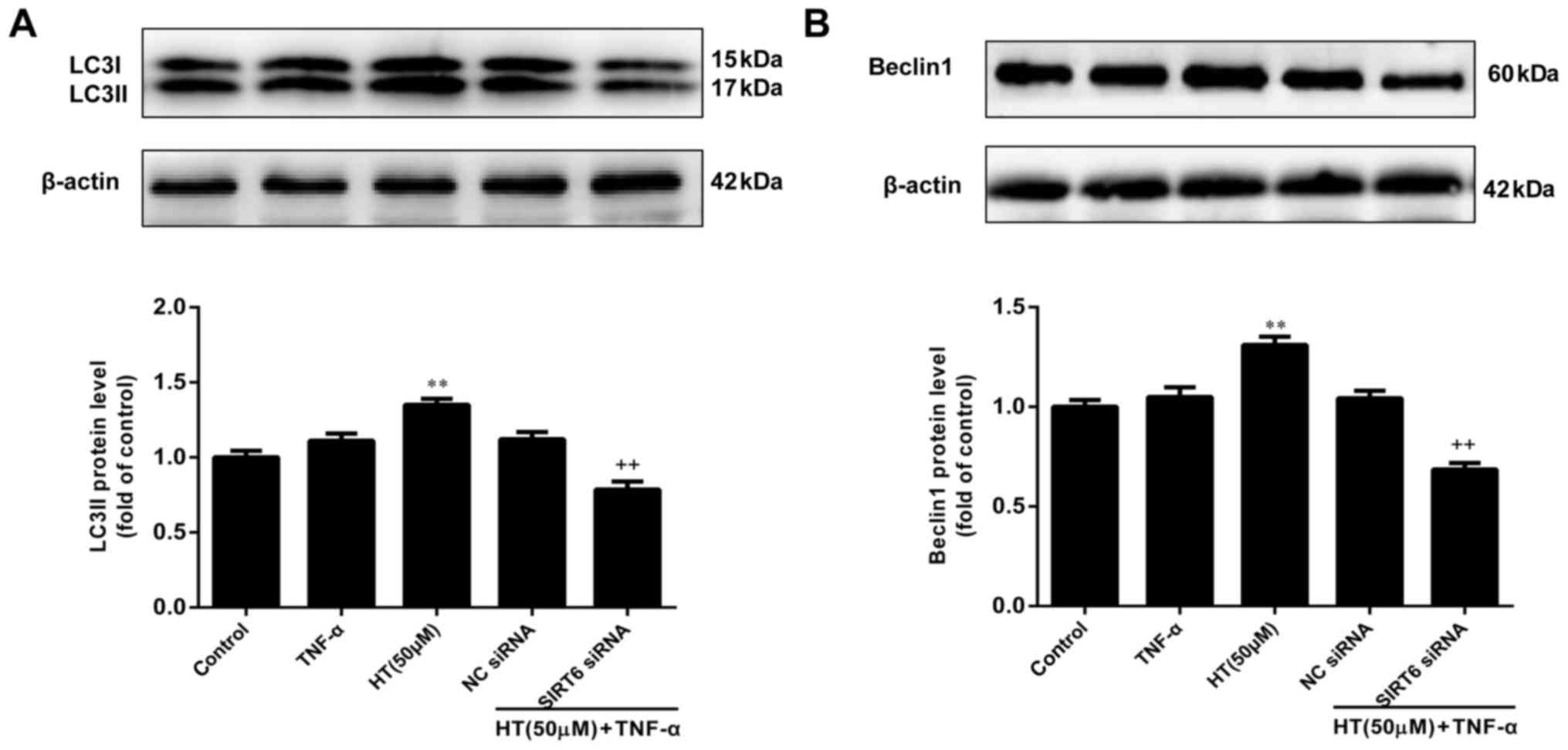
HT inhibits TNF-α-induced inflammatory
response in chondrocytes through SIRT6-mediated autophagy
The final aim of the present study was to determine
whether HT regulates autophagy through SIRT6 in TNF-α-induced
chondrocytes. To assess this, chondrocytes were transfected with
SIRT6-specific siRNA to decrease SIRT6 expression and subsequently,
western blotting was performed to determine the effect on LC3-1/II
and Beclin1 expression levels. As presented in Fig. 5A and B, knockdown of SIRT6 reduced
the levels of Beclin-1 and LC3-II in chondrocytes compared with the
NC siRNA group. These results suggested that knockdown of SIRT6
significantly suppressed the expression of LC3 and Beclin1
(P<0.01). Therefore, it was concluded that HT regulated the
inflammatory response through SIRT6-meditated autophagy in
TNF-α-induced chondrocytes.
Discussion
Pro-inflammatory cytokines serve critical roles in
the development of inflammation (3,4).
Chondrocytes stimulated with TNF-α may lead to the release of
inflammatory mediators, including IL-1β and IL-6. These
inflammatory mediators amplify inflammation and accelerate the
pathophysiology of OA. Previous studies have suggested that
elevated IL-1β in chondrocytes may lead to cartilage destruction
and pain (22). Therefore,
inhibition of IL-1β and IL-1β-induced inflammatory mediators may
possess significant clinical value in the treatment of OA. It has
been reported that HT regulates the inflammatory response. In the
present study, it was revealed that HT significantly inhibited
IL-1β and IL-6 production in TNF-α induced chondrocytes.
A previous study revealed that members of the
Sirtuin family participate in inflammation (22). SIRT6 may suppress inflammatory
responses in collagen-induced arthritis (12). Additionally, it has been revealed
that SIRT6-induced autophagy is involved in oxidative
stress-induced neuronal damage (13,14).
Although previous reports highlight SIRT6 as a negative regulator
of vascular inflammation (13),
the association between autophagy and the regulatory role of SIRT6
in the anti-inflammatory process has yet to be identified.
Therefore, the present study hypothesized that HT may inhibit the
inflammatory response of chondrocytes via SIRT6-mediated autophagy.
Firstly, it was demonstrated that HT promoted autophagy in
TNF-α-induced chondrocytes. This was consistent with previous
studies that revealed that HT may increase autophagy and protect
chondrocytes from oxidative stress-induced DNA damage and cell
death (18). In addition, the
present study demonstrated that HT upregulated the expression of
SIRT6 at the protein and mRNA levels in TNF-α-stimulated
chondrocytes. Knockdown of SIRT6 reduced the effect of HT on
autophagy in chondrocytes by decreasing the expression levels of
LC3II and Beclin1. These results suggested that HT regulates
autophagy in chondrocytes through SIRT6, verifying that HT may
possess effective anti-inflammatory activities in TNF-α induced
chondrocytes and thus prevent the progression of OA.
To the best of the author's knowledge, this may be
the first study to demonstrate that HT prevents TNF-α-induced
chondrocyte inflammation by enhancing SIRT6 mediated autophagy
activation. The present study provided a potential mechanism
associating autophagy with the onset and progression of OA
(23,24). SIRT6-mediated autophagy activation
may be applied for the treatment of inflammatory diseases. In
addition, drugs used in OA knees have failed to prevent disease
progression and exhibit side effects. Local administration of
glucocorticoids (GCs) has been used to treat OA. GCs inhibit local
immune response through the receptor. Therefore, they serve an
important role in alleviating the symptoms of patients. However,
there is an amount of evidence demonstrating that GCs have a
degenerative effect on certain collagen tissue, including bones,
tendons and skin (25). Therefore,
it is of increasing interest to identify plant-derived compounds
for the treatment of OA (26,27).
In conclusion, the results of the present study
suggested that HT inhibits the inflammatory response of
chondrocytes through SIRT6-mediated autophagy. This may provide
novel insights into the association between HT, autophagy and
inflammation in chondrocytes. Therefore, modulating autophagy
represents an attractive future therapeutic target for treating
diseases caused by inflammation. However, the present study did not
confirm whether HT directly regulates SIRT6, which can affect
autophagy to inhibit inflammatory response. Further investigation
will allow better characterization of the molecular mechanisms
underlying these effects. A further study will investigate which
receptors or signal molecules are involved in the regulatory
process of HT on SIRT6.
References
|
1
|
Roos EM and Arden NK: Strategies for the
prevention of knee osteoarthritis. Nat Rev Rheumatol. 12:92–101.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang MC, Egan B and Wang JX: Epigenetic
mechanisms underlying the aberrant catabolic and anabolic
activities of osteoarthritic chondrocytes. Int J Biochem Cell Biol.
67:101–109. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Honorati MC, Cattini L and Facchini A:
IL-17, IL-1beta and TNF-alpha stimulate VEGF production by
dedifferentiated chondrocytes. Osteoarthritis Cartilage.
12:683–691. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lianxu C, Hongti J and Changlong Y:
NF-kappa Bp65-specific siRNA inhibits expression of genes of COX-2,
NOS-2 and MMP-9 in rat IL-1 beta-induced and TNF-alpha-induced
chondrocytes. Osteoarthritis Cartilage. 14:367–376. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tetlow LC, Adlam DJ and Woolley DE: Matrix
metalloproteinase and proinflammatory cytokine production by
chondrocytes of human osteoarthritic cartilage: Associations with
degenerative changes. Arthritis Rheum. 44:585–594. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Doria A, Gatto M and Punzi L: Autophagy in
human health and disease. N Engl J Med. 368:18452013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Netea-Maier RT, Plantinga TS, van de
Veerdonk FL, Smit JW and Netea MG: Modulation of inflammation by
autophagy: Consequences for human disease. Autophagy. 12:245–260.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen ML, Yi L, Jin X, Liang XY, Zhou Y,
Zhang T, Xie Q, Zhou X, Chang H, Fu YJ, et al: Resveratrol
attenuates vascular endothelial inflammation by inducing autophagy
through the cAMP signaling pathway. Autophagy. 9:2033–2045. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kawahara TL, Michishita E, Adler AS,
Damian M, Berber E, Lin M, McCord RA, Ongaigui KC, Boxer LD, Chang
HY and Chua KF: SIRT6 links histone H3 lysine 9 deacetylation to
NF-kappa B-dependent gene expression and organismal life span.
Cell. 136:62–74. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Michishita E, Park JY, Burneskis JM,
Barrett JC and Horikawa I: Evolutionarily conserved and
nonconserved cellular localizations and functions of human SIRT
proteins. Mol Biol Cell. 16:4623–4635. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang J, Cheng Y, Gu J, Wang S, Zhou S,
Wang Y, Tan Y, Feng W, Fu Y, Mellen N, et al: Fenofibrate increases
cardiac autophagy via FGF21/SIRT1 and prevents fibrosis and
inflammation in the hearts of Type 1 diabetic mice. Clin Sci
(Lond). 130:625–641. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Takasaka N, Araya J, Hara H, Ito S,
Kobayashi K, Kurita Y, Wakui H, Yoshii Y, Yumino Y, Fujii S, et al:
Autophagy Induction by SIRT6 through attenuation of insulin-like
growth factor signaling is involved in the regulation of human
bronchial epithelial cell senescence. J Immunol. 192:958–968. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu Y, Chen L, Wang Y, Li W, Lin Y, Yu D,
Zhang L, Li F and Pan Z: Overexpression of Sirtuin 6 suppresses
cellular senescence and NF-κB mediated inflammatory responses in
osteoarthritis development. Sci Rep. 5:176022015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nagai K, Matsushita T, Matsuzaki T,
Takayama K, Matsumoto T, Kuroda R and Kurosaka M: Depletion of
SIRT6 causes cellular senescence, DNA damage, and telomere
dysfunction in human chondrocytes. Osteoarthritis Cartilage.
23:1412–1420. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Granados-Principal S, Quiles JL,
Ramirez-Tortosa CL, Sanchez-Rovira P and Ramirez-Tortosa MC:
Hydroxytyrosol: From laboratory investigations to future clinical
trials. Nutr Rev. 68:191–206. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bulotta S, Celano M, Lepore SM, Montalcini
T, Pujia A and Russo D: Beneficial effects of the olive oil
phenolic components oleuropein and hydroxytyrosol: Focus on
protection against cardiovascular and metabolic diseases. J Transl
Med. 12:2192014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Luo C, Li Y, Wang H, Cui Y, Feng ZH, Li H,
Li Y, Wang Y, Wurtz K, Weber P, et al: Hydroxytyrosol promotes
superoxide production and defects in autophagy leading to
anti-proliferation and apoptosis on human prostate cancer cells.
Curr Cancer Drug Targets. 13:625–639. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cetrullo S, D'Adamo S, Guidotti S, Borzì
RM and Flamigni F: Hydroxytyrosol prevents chondrocyte death under
oxidative stress by inducing autophagy through sirtuin 1-dependent
and -independent mechanisms. Biochim Biophys Acta. 1860:1181–1191.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gao J, Zou X, Yang L, Feng Z and Liu J:
Hydroxytyrosol protects against acrolein induced preosteoblast cell
toxicity: Involvement of Nrf2/Keap1 pathway. J Funct Foods.
19:28–38. 2015. View Article : Google Scholar
|
|
20
|
Aparicio-Soto M, Sánchez-Fidalgo S,
González-Benjumea A, Maya I, Fernández-Bolaños JG and
Alarcón-de-la-Lastra C: Naturally occurring hydroxytyrosol
derivatives: Hydroxytyrosyl acetate and 3,4-dihydroxyphenylglycol
modulate inflammatory response in murine peritoneal macrophages.
Potential utility as new dietary supplements. J Agric Food Chem.
63:836–846. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Feng G, Wan Y, Balian G, Laurencin C and
Li X: Adenovirus-mediated expression of growth and differentiation
factor-5 promotes chondrogenesis of adipose stem cells. Growth
Factors. 26:132–142. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lin J, Sun B, Jiang C, Hong H and Zheng Y:
Sirt2 suppresses inflammatory responses in collagen-induced
arthritis. Biochem Biophys Res Commun. 441:897–903. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sasaki H, Takayama K, Matsushita T, Ishida
K, Kubo S, Matsumoto T, Fujita N, Oka S, Kurosaka M and Kuroda R:
Autophagy modulates osteoarthritis-related gene expression in human
chondrocytes. Arthritis Rheum. 64:1920–1928. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shen C, Cai GQ, Peng JP and Chen XD:
Autophagy protects chondrocytes fromglucocorticoids-induced
apoptosis via ROS/Akt/FOXO3 signaling. Osteoarthritis Cartilage.
23:2279–2287. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Caramés B, Olmer M, Kiosses WB and Lotz
MK: The relationship of autophagy defects to cartilage damage
during joint aging in a mouse model. Arthritis Rheumatol.
67:1568–1576. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu N, Wang WB, Zhao Z, Zhang T and Song
YW: Autophagy in human articular chondrocytes is cytoprotective
following glucocorticoid stimulation. Mol Med Rep. 9:2166–2172.
2014. View Article : Google Scholar : PubMed/NCBI
|