Introduction
A rotator cuff tear (RCT) is the one of the most
common injuries that causes shoulder pain and dysfunction. Despite
improvements in surgical techniques, firm attachment at the
tendon-bone interface following rotator cuff repair remains a
problem. Previous studies have reported high re-tear rates
following rotator cuff repair (1–5). The
re-tear of a rotator cuff negatively affects the long-term outcomes
of patients compared with patients whose repairs heal without a
recurrent defect (6). Therefore,
improving tendon-bone healing following rotator cuff repair is a
subject of interest.
In previous studies regarding tendon-bone healing
enhancement, hydroxyapatite (HA) has been demonstrated to have
positive effects in anterior cruciate ligament (ACL) reconstruction
models (7,8) as well as RCT repair models (9). Huangfu and Zhao (8) demonstrated that a mixture of
tricalcium phosphate and HA led to better biomechanical results and
more mature histological patterns compared with the control group.
Li et al (7) demonstrated
that an HA coating could induce polyethylene terephthalate
artificial ligament graft osseointegration in the bone tunnel in a
rabbit model. Zhao et al (9) applied
Ca5(PO4)2SiO4(CPS) and
HA at the interface of a rotator cuff repair site following a
chronic RCT, and both CPS and HA bioceramics aided in cell
attachment and proliferation, and accelerated novel bone formation.
Ideally, HA should not only supply material for the reconstruction
of the tendon-bone interface but also offer stimulatory information
for cell proliferation and differentiation.
Tendons attach to the bone across fibrocartilaginous
transition areas that can be divided into four distinct zones:
Tendon, fibrocartilage, mineralized fibrocartilage and bone
(10). The transforming growth
factor β (TGFβ) family serves a key function in connective tissue
development (11–13). Previous studies have investigated
the roles of TGFβ1 and TGFβ3 in the process of healing following
rotator cuff repair. Arimura et al (14) demonstrated that TGFβ1 enhances the
formation of tough fibrous tissues at the healing site by
inhibiting matrix metalloproteinase (MMP)-9 and MMP-13 expression
to increase collagen accumulation but without the growth of
tenogenic lineage cells. TGFβ1 has also been reported to be
effective in enhancing the biomechanical properties of tendons or
ligaments in rabbit and dog models (15,16).
By contrast, Manning et al (13) used an osmotic pump for sustained
delivery of growth factors at the repair site and demonstrated that
TGFβ1 promoted the formation of scar tissue. There was no
improvement in healing with the application of TGFβ3. The role of
TGFβ isoforms remains poorly characterized and requires further
investigation.
The purpose of the present study was to evaluate
whether the interposition of HA materials encapsulated with TGFβ1
could enhance the structural, histological and biomechanical
properties in a rat rotator cuff repair model. The authors
hypothesized that i) the interposition of HA during RCT repair
would enhance the tendon-bone site healing; and ii) TGFβ1 would
promote fibrocartilage formation, and improve the healing structure
in the interface zone. Overall, the present study hypothesized that
HA ceramics with TGFβ1 would have better characteristics compared
with HA alone and demonstrate a better promoting effect in the
interface area.
Materials and methods
Preparation of HA-TGFβ1
HA hierarchically nanostructured microspheres, which
have potential applications in drug delivery and protein adsorption
owing to their relatively large specific surface area, nanoporosity
and hollow structure, were synthesized as reported by Qi et
al (17). Briefly, 0.1110 g
CaCl2 and 0.1963 g creatine phosphate disodium salt
tetrahydrate were dissolved in deionized water (40 ml) with
consistent magnetic stirring at room temperature while the pH value
was maintained at 10 using 1 M sodium hydroxide (NaOH) solution.
The resulting solution was transferred into a 60-ml autoclave-safe
container, sealed and microwave-heated in a microwave oven (MDS-6;
Sineo Microwave Chemistry Technology, Co., Ltd., Shanghai, China)
to 120°C and maintained at this temperature for 10 min. Then,
following cooling to room temperature, the product was collected by
centrifugation at room temperature for 5 min (10,000 × g), followed
by washing several times with deionized water and ethanol and
drying at 60°C for 24 h.
To promote the recovery of the
tendon-bone-interface, human TGFβ1 growth factor was loaded into
prepared HA microspheres. A dried powder of HA (100 mg) was added
into a PBS solution of TGFβ1 (1 µg/ml, 10 ml); afterward, the
resulting suspension was continuously agitated at 37°C for 24 h.
Finally, the growth factor-loaded HA hierarchically nanostructured,
porous microspheres (10 µg/100 mg, HA-TGFβ1) were obtained by
freeze-drying.
Surgical technique
All experimental procedures were approved by the
Institutional Animal Studies Committee of Shanghai Jiao Tong
University Affiliated Sixth Hospital (Shanghai, China; no:
DWLL2017-0304).
A total of 135 male Sprague-Dawley rats (obtained at
350–450 g, 2 months, Shanghai SIPPR-BK Laboratory Animal Co., Ltd.,
Shanghai, China) were used in this study. They were provided with
fresh water and rat chow ad libitum and housed in a specific
pathogen free environment (temperature: 25°C; humidity: 50%; light
and dark cycle: 12-h). The surgical procedure was performed
according to previously published study (9). The animals were randomly divided into
one of three groups. In the control group, the tendon was repaired
to its anatomic footprint using transosseous repair (n=45). In the
experimental groups, 90 rats underwent transosseous repair and were
implanted with either HA ceramic powder (n=45) or HA-TGFβ1 ceramic
powder (n=45) to augment the repair.
Following anesthesia, a longitudinal incision was
made on the anterolateral aspect of the shoulder, splitting the
deltoid muscle. The acromioclavicular joint was divided to allow
visualization of the rotator cuff tendons. The anterior margin of
the supraspinatus tendon was identified adjacent to the biceps
tendon and the posterior margin was determined by the junction with
the infraspinatus tendon fibers. The supraspinatus tendon was
marked with a 5-0 polypropylene suture (Ethicon, Inc., Somerville,
NJ, USA). The tendon was then sharply dissected from its insertion
site at the great tuberosity. The tuberosity was gently
decorticated and debrided of all soft tissue and fibrocartilage
with a scalpel blade until bleeding was noted. A 0.8-mm
anterior-posterior transverse bone tunnel was created at the
humeral head. A small trough was made using an 18-gauge blunt
needle located centrally in the footprint to allow for seating of
the ceramic powder. For the control group, a Mason-Allen stitch
using a 3-0 Ethibond® (Ethicon, Inc.) suture was placed
into the supraspinatus tendon. Suture ends from the tendon were
then passed through the bone tunnels and firmly tied over the
humeral metaphyseal cortex, anatomically repairing the
supraspinatus tendon to its native footprint. For the experimental
groups, 2 mg HA or HA-TGFβ1 powder was implanted into the trough,
while the trough remained empty in the control group. The deltoid
muscle and skin were then closed, and the rats were allowed to
engage in unrestricted cage activity. For 3 days postoperatively,
buprenorphine (0.05 mg/kg) was administered subcutaneously for
postoperative analgesia.
Gross observation
Animals were sacrificed at 2, 4 and 8 weeks
following surgery. Following euthanasia and dissection, all
specimens were evaluated for macroscopic tendon-bone repair,
residual materials, synovial hyperplasia and shoulder range of
motion.
Micro-computed tomography (CT)
analysis
A total of 45 animals (5 animals per group per time
point) were sacrificed for micro-CT analysis at 2, 4 and 8 weeks
following surgery. Their right shoulders were dissected to include
only the supraspinatus tendon-bone complex and the proximal third
of the humerus for fixation in a 1:1 solution of ethanol and
sterile water at room temperature for 24 h. The bone density and
novel bone formation were assessed with micro-CT (eXplore Locus SP;
GE Healthcare, Chicago, IL, USA). Each sample was placed in the
holder surrounded by ethanol solution and scanned using the
conditions of 80 kV, 450 mA and a 0.045-mm effective pixel size.
The images were subjected to a global threshold to distinguish the
bone voxels for each specimen. Following the threshold scan, the 3D
reconstruction images were obtained. A customized 4×4
mm2 cylindrical region of interest (ROI) was centered at
the surface of the repaired supraspinatus tendon-bone footprint
area, which contained the distal portion of the supraspinatus
tendon and the bony footprint. The size of the ROI was determined
based on post-mortem observations that the supraspinatus footprint
measured ~3.5×3.5 mm2. The bone mineral density (BMD)
and bone volume fraction (bone volume/total volume; BV/TV) of the
ROI were calculated.
Histomorphometric analysis
A total of 45 animals were sacrificed for
histomorphometric analysis. Following the necropsies, the tissue
specimens were fixed in 10% neutral buffered formalin at room
temperature for 48 h. The tissue specimens were decalcified with
Immunocal (Wuhan Servicebio Co., Ltd., Wuhan, China) and embedded
in paraffin. Sections (5-µm) of the repaired supraspinatus
tendon-greater tuberosity construct were cut in the coronal plane.
The tissue sections were stained with hematoxylin & eosin,
safranin O/fast green, and picrosirius red. Hematoxylin & eosin
staining were performed at room temperature for 3 h. Safranin
O/fast green were performed at room temperature for 2 h.
Picrosirius red staining were performed at room temperature for 3
h.
Using light microscopy (Leica DM4000B; Leica
Microsystems GmbH, Wetzlar, Germany), tissue sections stained with
safranin O/fast green were analyzed to determine the total area of
novel fibrocartilage formation at the tendon-bone interface of the
RCT. Digital images were acquired by a Leica DFC420C camera (Leica
Microsystems GmbH). The ImageJ software program version 1.51
(National Institutes of Health, Bethesda, MD, USA) was used to
outline the area of metachromasia on the safranin O-stained slides
at a total magnification of ×40 in order to determine the area of
novel fibrocartilage formation. The total area of metachromasia for
each specimen was recorded for analysis.
Using polarized light microscopy, tissue sections
were stained with picrosirius red for semi-quantitative analysis of
the collagen deposition and maturation at the repair site.
Measurements were obtained by rotating the polarization plane until
maximum brightness was obtained to control the variations in
specimen orientation on the slide. All the tissue sections were cut
to a uniform thickness and the light intensities were measured
under the same conditions of illumination and with the same
settings to facilitate comparisons between groups. The digital
images were imported into ImageJ for processing. The images
underwent 8-bit digitization, producing images in which
non-collagenous material was dark (zero) and collagenous material
was depicted by gray scales from 1 to 255. Ten rectangular areas
(50×50 µm) were randomly selected at the tendon near the interface
region and the gray scales were measured and recorded.
Biomechanical testing
A total of 45 animals were sacrificed for
biomechanical testing. The humerus with the attached supraspinatus
tendon was dissected from the surrounding tissues. The
cross-sectional area of the supraspinatus tendon at its insertion
site was measured using a digital caliper. The tendon was then
placed into a custom-designed uniaxial testing system. The tendon
was secured in a screw grip while the humerus was secured into a
vice grip. The specimen was preloaded to 0.1 N and then loaded to
failure at a rate of 14 µm/sec, corresponding to 0.4% strain. The
maximum load at failure and the failure site were recorded. The
ultimate stress at failure was calculated by dividing the ultimate
load-to-failure by the cross-sectional area.
Statistical analysis
Data are presented as the mean ± standard deviation.
Statistical analysis was performed with SPSS 17.0 (SPSS Inc.,
Chicago, IL, USA) Statistical analysis was performed using one-way
analysis of variance with the least significant difference method
as a post hoc test to determine the level of significance.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Macroscopic observation
In all rats, the repaired tendons remained in
continuity with the bones and no evidence of infection was observed
at the surgical site. There was no obvious limit to the shoulder
range of motion. There were no obvious differences in the gross
appearance of tendons following sacrifice. In the HA and HA-TGFβ1
groups, the shoulders contained remnants of the ceramic powder at
the tendon attachment site in the 2- and 4-week group. There were
no remnants in the control group or in the experimental groups at
the 8-week time point.
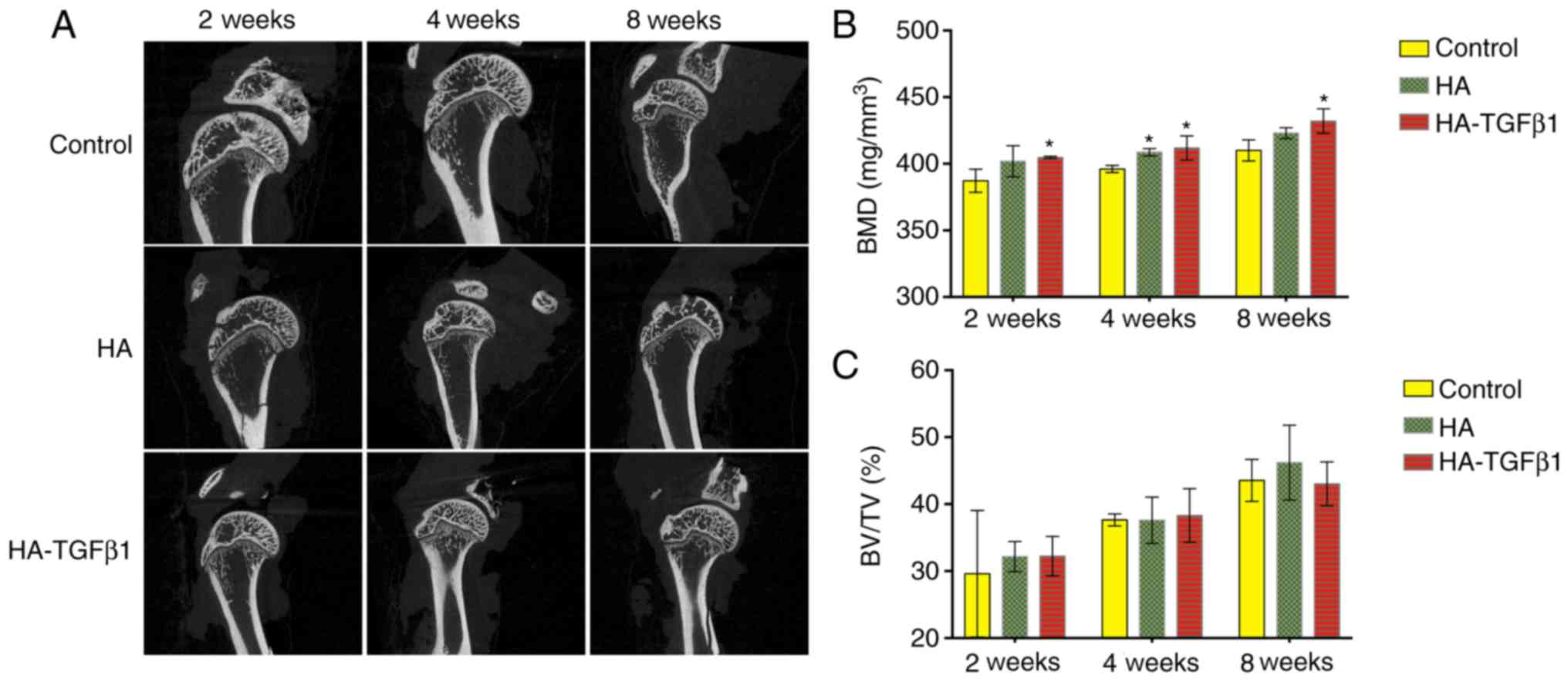
Micro-CT analysis
The BMD values of the HA-TGFb1 groups were
significantly higher compared with the control group at all time
points [2 weeks: 387.20±8.68 mg/mm3 (control) vs.
401.93±11.70 mg/mm3 (HA), and 404.73±0.87
mg/mm3 (HA-TGF β1), P=0.025; 4 weeks: 396.07±2.63
mg/mm3 (control) vs. 408.67±2.71 mg/mm3 (HA),
P=0.040, and 411.87±9.1 mg/mm3 (HA-TGFβ1, P=0.045); 8
weeks: 410.00±7.88 mg/mm3 (control) vs. 423.00±4.01
mg/mm3 (HA), and 432.03±9.17 mg/mm3
(HA-TGFβ1), P=0.034]. However, there were no significant
differences between the experimental groups within the same time
point (Fig. 1).
The same trend was not observed for the value of
BV/TV. No significant differences in BV/TV values were observed
among the control and experimental groups (Fig. 1). Otherwise, some remnants of
powder at the tendon-bone insertion site were observed at 2 and 4
weeks following surgical repair in the experimental groups
only.
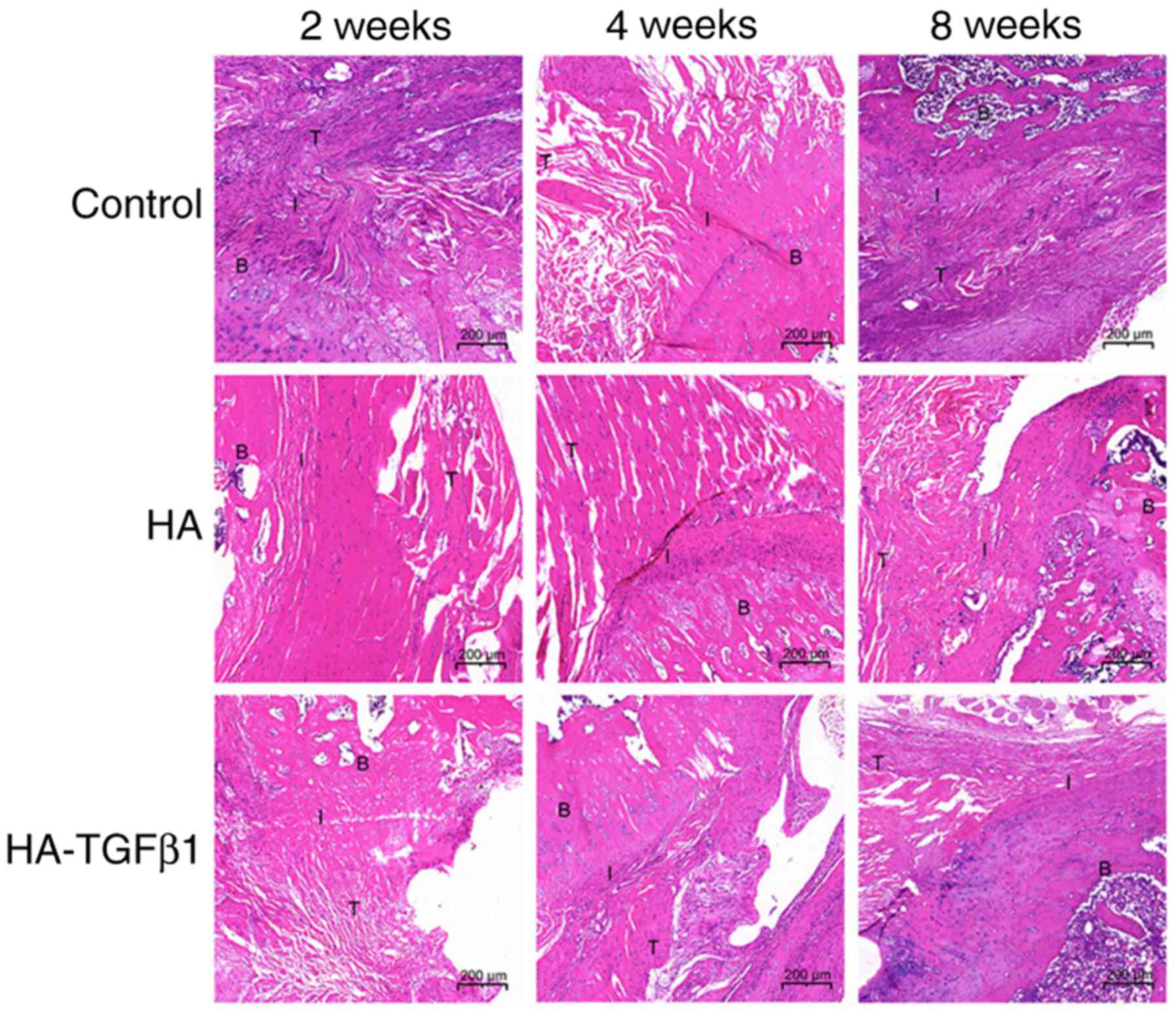
Histological analysis
In the 2-week groups, the specimens in the HA and
HA-TGFβ1 groups demonstrated that the tendon-bone interface had
more organized collagen fibers compared with those in the control
group. However, a mass of inflammatory cells, primarily consisting
of polymorphonuclear leukocytes, was present in the HA group. In
addition, chondrocytes and novel fibrocartilage formation was
observed in the HA-TGFβ1 group (Fig.
2).
In the 4-week groups, better-oriented collagen
fibers, fewer inflammatory cells and more bone ingrowth into the
interface area could be observed in all groups compared with those
at 2 weeks. In addition, a larger area of fibrocartilage was
observed in the HA and HA-TGFβ1 groups compared with the control
group (Fig. 2).
The specimens in the 8-week control group
demonstrated better-organized fibers in the gap tissue. The fibers
were in the same direction of tensile pull of the tendon compared
with those in the 4-week control group. In addition, the
fibrocartilage in the tendon-bone interface of the experimental
groups was remodeled into a more natural appearance and increased
novel bone formation was observed (Fig. 2).
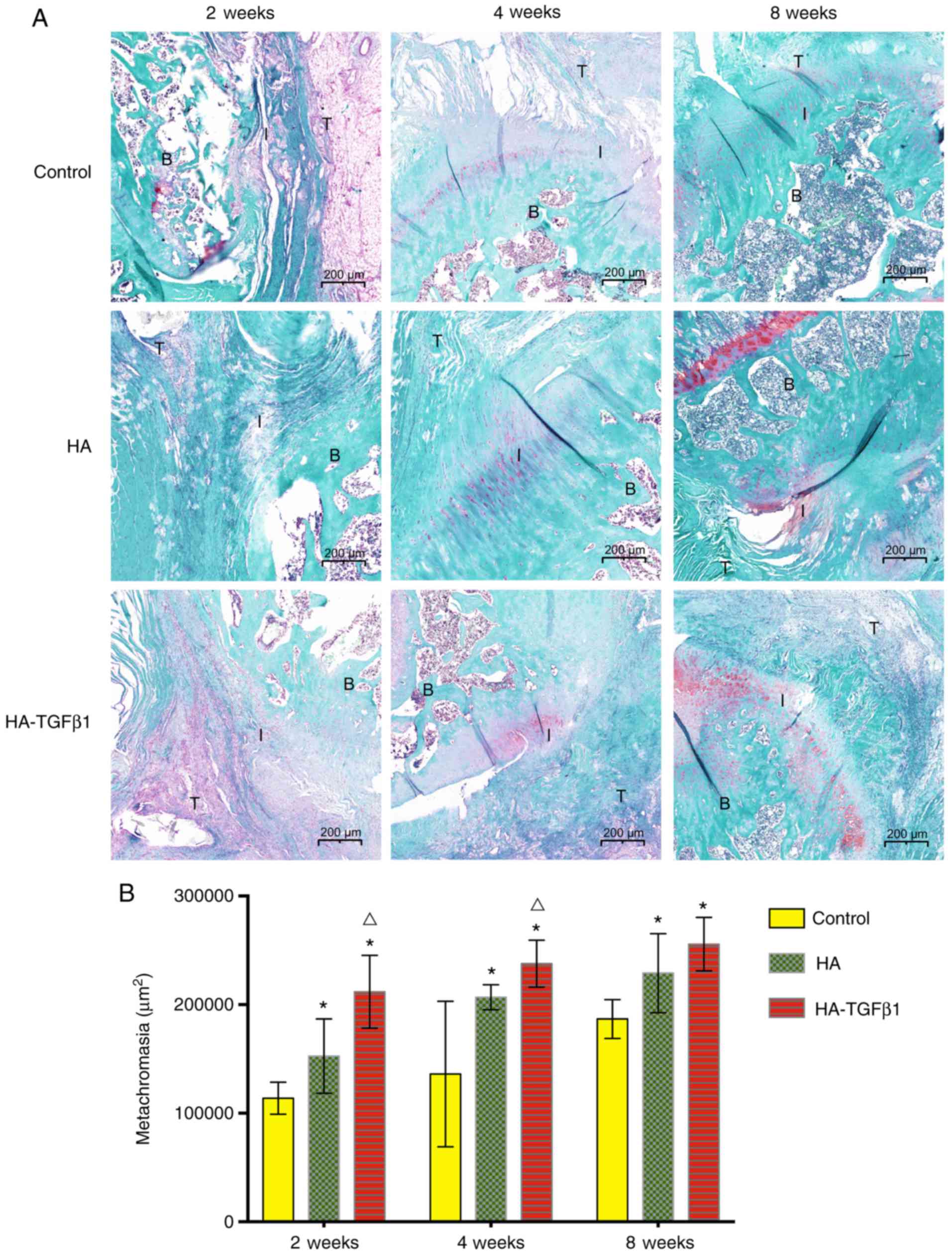
Metachromasia
At all time points (2, 4 and 8 weeks) following
surgery, HA-TGFβ1 powder and HA powder significantly increased the
area of glycosaminoglycan staining at the interface area compared
with the control group [2 weeks: 113,928.2±14,736.7 µm2
(control) vs. 152,623±34,236 µm2 (HA) P=0.049 and
211,810±33,514 µm2 (HA-TGFβ1) P<0.05; 4 weeks:
136,111±67,093 µm2 (control) vs. 206,826±11,452
µm2 (HA) P=0.049 and 237,829±21,610 µm2
(HA-TGFβ1) P=0.012; 8 weeks: 186,792±17,931 µm2
(control) vs. 229,105±36,367 µm2 (HA) P=0.031 and
255,690±24,626 µm2 (HA-TGFβ1) P=0.002]. The group
implanted with HA-TGFβ1 powder demonstrated a significantly larger
area of metachromasia (P<0.05) and a larger area of newly formed
fibrocartilage in most specimens compared with the HA group at 2
and 4 weeks postoperatively. However, at 8 weeks, there were no
significant differences between the HA and HA-TGFβ1 groups
(Fig. 3).
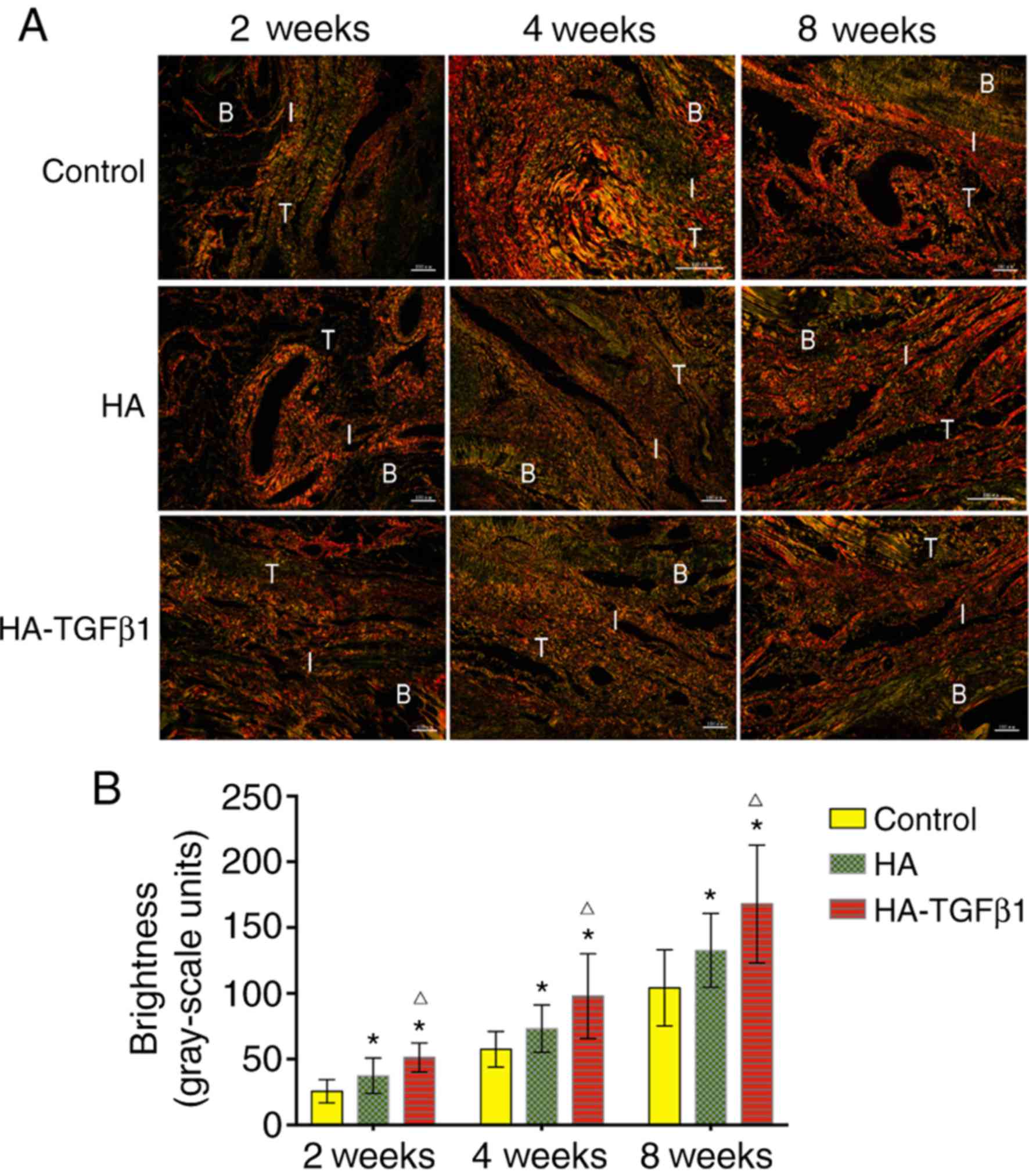
Collagen organization
According to birefringence under polarized light at
the tendon-bone-interface, the collagen organization in the
experimental groups was improved significantly compared to that in
the control group [2 weeks: 25.7±8.7 grayscale units (control) vs.
37.4±13.4 grayscale units (HA) P=0.027 and 51.4±11.1 grayscale
units (HA-TGFβ1) P<0.05; 4 weeks: 57.6±13.5 grayscale units
(control) vs. 73.3±17.9 grayscale units (HA) P=0.04 and 97.9±32.3
grayscale units (HA-TGFβ1) P=0.003; 8 weeks: 104.3±29.0 grayscale
units (control) vs. 132.7±28.0 grayscale units (HA), P=0.039 and
168.1±44.8 grayscale units (HA-TGFβ1), P=0.001]. In addition,
collagen organization was significantly improved in the HA-TGFβ1
group compared with the HA group at 2, 4, and 8 weeks (2 weeks:
P=0.021; 4 weeks: P=0.049; 8 weeks: P=0.048). These results
demonstrated the beneficial effect of HA and TGFβ1 on collagen
production at the tendon-bone interface (Fig. 4).
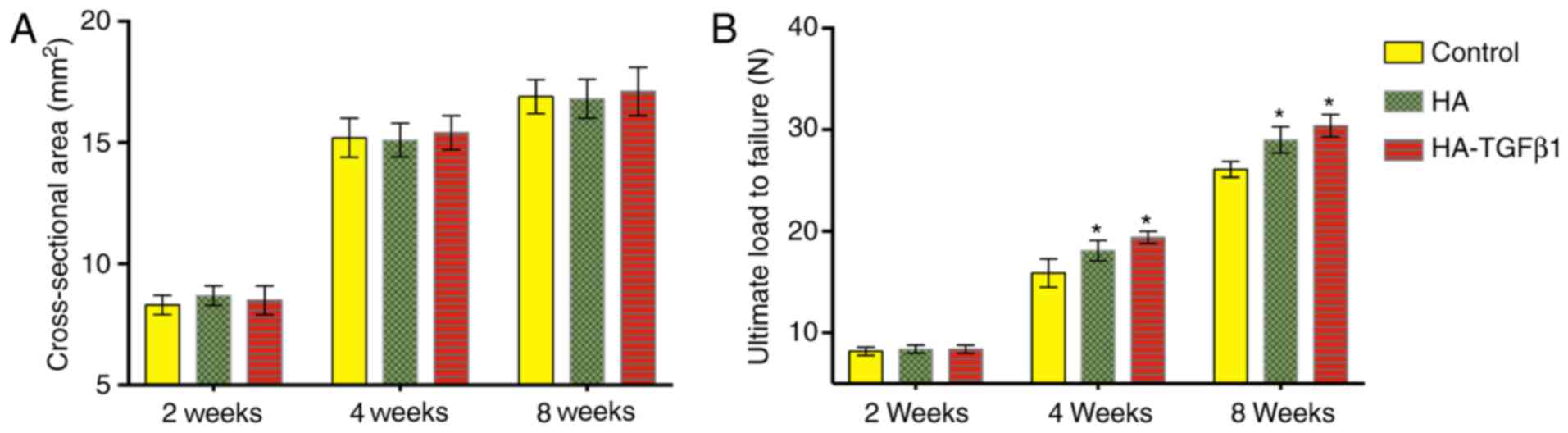
Ultimate load-to-failure
There were no significant differences in the
cross-sectional area of the healing enthesis between the control
group and the experimental groups at 2, 4, or 8 weeks following
surgery (Fig. 5).
At 2 weeks following surgery, no significant
differences in the ultimate load-to-failure were observed (control:
8.2±0.4 N; HA: 8.4±0.4 N; HA-TGFβ1: 8.4±0.3 N). At 4 weeks, the
ultimate load-to-failure forces in the experimental groups were
significantly increased compared with the control group (control:
15.9±1.4 N; HA: 18.1±1.0 N, P=0.02; HA-TGFβ1: 19.4±0.6 N, P=0.001).
At 8 weeks following surgery, the ultimate load-to-failure forces
were significantly increased in the HA group and the HA-TGFβ1 group
compared with the control group (control: 26.1±0.8 N; HA: 29±1.3 N,
P=0.002; HA-TGFβ1: 30.4±1.1 N, P<0.05). No significant
differences in ultimate load-to-failure force were observed between
the HA and HA-TGFβ1 groups at all time points postoperatively
(Fig. 5).
Discussion
The current study investigated whether the
interposition of HA encapsulated with TGFβ1 could enhance the
healing of the tendon-bone interface and promote bone and collagen
formation following RCT repair in an acute rotator cuff injury
model. The results demonstrate that the application of bioceramics
may promote novel bone formation at the repair site. Micro-CT
analysis revealed that HA bioceramics alone and with TGFβ1
exhibited obvious osteogenic activity and osteoconductivity at 2, 4
and 8 weeks post-surgery. The local application of the growth
factor encapsulated into the HA was associated with the improvement
of fibrocartilage and collagen formation at the tendon-bone
interface compared with rotator cuff repair alone at all time
points. This demonstrated a notable effect of this material in
tendon-bone healing. Furthermore, the application of HA bioceramics
alone improved the area of fibrocartilage and collagen organization
compared with the control group. These results indicate that HA is
a suitable drug carrier material in tendon-bone healing. At the 2
weeks after surgery there were improvements in the area of
fibrocartilage and in collagen formation with HA-TGFβ1 bioceramics
compared with HA bioceramics or repair only, which indicated that
local delivery of this growth factor could enhance the mature
healing enthesis at the tendon-bone repair site.
HA has been used as an osteoconductive material for
bone growth. HA-based biomaterials are the most common materials
used in modern bone substitution (18–21).
In addition, a previous study revealed that tendon-bone healing
depends upon the bone ingrowth into the healing interface (18). Zhao et al (9) demonstrated that the interposition of
HA aids in cell attachment and proliferation, and accelerates novel
bone formation. HA was selected for its osteoconductive character
as the delivery vehicle for TGFβ1. HA was demonstrated to be an
ideal augmentative matrix for rotator cuff repair with regards to
cytocompatibility, osteogenic activity and osteoconductivity,
according to a previous study (9).
In the present study, HA was observed to be a suitable
osteoconductive material, as it promoted increased bone formation
at the footprint. HA could also enhance healing by increasing the
area of fibrocartilage and improving collagen organization at the
tendon-bone insertion. HA alone could serve a non-negligible role
in the tendon-bone healing process.
A previous study demonstrated the potential of TGFβ1
in the application of tendon-bone healing. TGFβ1 could
significantly increase the binding strength of the graft to the
tunnel wall following ACL reconstruction (16). In addition, the perpendicular
collagen fibers connecting the tendon to the bone were richly
regenerated in the TGFβ1 group. Kovacevic et al (22) combined TGFβ3 with an injectable
Ca-P matrix and demonstrated that it may improve healing following
rotator cuff repair. A recent study demonstrated that upregulating
TGFβ expression in bone mesenchymal stem cells (BMSCs) can promote
tendon-to-bone healing following ACL reconstruction by regulating
the TGFβ1 mitogen-activated protein kinase (MAPK) 1 signaling
pathway (23). In addition, TGFβ1
is the most important fibrosis inducer (24), which could explain the increase in
collagen fibers. Furthermore, TGFβ1 can also induce BMSC migration
to bone resorption locations and promote bone resorption as well as
formation (25). The micro-CT
results of the present study also support this theory. The bone
formation at the repair site increased in the HA-TGFβ1-treated
repair group compared with repair only. In vitro research
has indicated that MAPK subtypes regulate chondrogenesis of rat
BMSCs by interaction with the TGFβ1/mothers against decapentaplegic
homolog 3 signaling pathway (26).
Using the rat rotator cuff repair model, the present study
demonstrated that TGFβ1 delivery with an HA matrix could increase
bone and fibrocartilage formation and improve collagen organization
at the tendon-bone interface following rotator cuff repair.
There were several limitations of the present study.
First, the acute injury and repair rat model is different from RCT
and repair in humans. However, previous studies have validated the
relevance of this animal model (27,28).
Second, the present study only investigated one dose of TGFβ1; this
dose was selected according to a previous study in a rat rotator
cuff repair model, where each animal received 2.75 µg of TGFβ mixed
with Ca-P matrix (22). Third,
this is a pilot study using a small (size) animal model with
limited evaluation tools and a limited observation period. Some
differences observed may be relatively small. However, most
differences were significant. In a future study, the authors plan
to choose a large animal model that can simulate the human
condition more accurately and to investigate the effect of multiple
doses of TGFβ1, as well as multiple growth factors, on the healing
process.
Local delivery of TGFβ1 in HA ceramic powder at the
tendon-bone interface during rotator cuff repair was demonstrated
to strengthen the healing enthesis, increase bone and
fibrocartilage formation, and improve collagen organization
compared with repair alone. In addition, the HA matrix itself was
demonstrated to enhance the tendon-bone healing process. Further
research should focus on loading HA ceramics with multiple growth
factors. Augmentation of the repair combined with drug delivery may
improve the clinical outcome of rotator cuff repair by enhancing
the tendon-bone healing.
Acknowledgements
Financial support from the National Natural Science
Foundation of China (grant nos. 81271961 and 81572106) is
gratefully acknowledged.
References
|
1
|
Domb BG, Glousman RE, Brooks A, Hansen M,
Lee TQ and ElAttrache NS: High-tension double-row footprint repair
compared with reduced-tension single-row repair for massive rotator
cuff tears. J Bone Joint Surg Am. 90 Suppl 4:S35–S39. 2008.
View Article : Google Scholar
|
|
2
|
Nelson CO, Sileo MJ, Grossman MG and
Serra-Hsu F: Single-row modified mason-allen versus double-row
arthroscopic rotator cuff repair: A biomechanical and surface area
comparison. Arthroscopy. 24:941–948. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ozbaydar M, Elhassan B, Esenyel C, Atalar
A, Bozdag E, Sunbuloglu E, Kopuz N and Demirhan M: A comparison of
single-versus double-row suture anchor techniques in a simulated
repair of the rotator cuff: An experimental study in rabbits. J
Bone Joint Surg Br. 90:1386–1391. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Boileau P, Brassart N, Watkinson DJ,
Carles M, Hatzidakis AM and Krishnan SG: Arthroscopic repair of
full-thickness tears of the supraspinatus: does the tendon really
heal? J Bone Joint Surg Am. 87:1229–1240. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gerber C, Fuchs B and Hodler J: The
results of repair of massive tears of the rotator cuff. J Bone
Joint Surg Am. 82:505–515. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zumstein MA, Jost B, Hempel J, Hodler J
and Gerber C: The clinical and structural long-term results of open
repair of massive tears of the rotator cuff. J Bone Joint Surg Am.
90:2423–2431. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li H, Ge Y, Wu Y, Jiang J, Gao K, Zhang P,
Wu L and Chen S: Hydroxyapatite coating enhances polyethylene
terephthalate artificial ligament graft osseointegration in the
bone tunnel. Int Orthop. 35:1561–1567. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huangfu X and Zhao J: Tendon-bone healing
enhancement using injectable tricalcium phosphate in a dog anterior
cruciate ligament reconstruction model. Arthroscopy. 23:455–462.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhao S, Peng L, Xie G, Li D, Zhao J and
Ning C: Effect of the interposition of calcium phosphate materials
on tendon-bone healing during repair of chronic rotator cuff tear.
Am J Sports Med. 42:1920–1929. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gulotta LV and Rodeo SA: Growth factors
for rotator cuff repair. Clin Sports Med. 28:13–23. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim HM, Galatz LM, Das R, Havlioglu N,
Rothermich SY and Thomopoulos S: The role of transforming growth
factor beta isoforms in tendon-to-bone healing. Connect Tissue Res.
52:87–98. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pryce BA, Watson SS, Murchison ND,
Staverosky JA, Dünker N and Schweitzer R: Recruitment and
maintenance of tendon progenitors by TGFbeta signaling are
essential for tendon formation. Development. 136:1351–1361. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Manning CN, Kim HM, Sakiyamaelbert SE,
Sakiyama-Elbert S, Galatz LM, Havlioglu N and Thomopoulos S:
Sustained delivery of transforming growth factor beta three
enhances tendon-to-bone healing in a rat model. J Orthop Res.
29:1099–1105. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Arimura H, Shukunami C, Tokunaga T,
Karasugi T, Okamoto N, Taniwaki T, Sakamoto H, Mizuta H and Hiraki
Y: TGF-β1 improves biomechanical strength by extracellular matrix
accumulation without increasing the number of tenogenic lineage
cells in a rat rotator cuff repair model. Am J Sports Med.
45:2394–2404. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Anaguchi Y, Yasuda K, Majima T, Tohyama H,
Minami A and Hayashi K: The effect of transforming growth
factor-beta on mechanical properties of the fibrous tissue
regenerated in the patellar tendon after resecting the central
portion. Clin Biomech. 20:959–965. 2005. View Article : Google Scholar
|
|
16
|
Yamazaki S, Yasuda K, Tomita F, Tohyama H
and Minami A: The effect of transforming growth factor-beta1 on
intraosseous healing of flexor tendon autograft replacement of
anterior cruciate ligament in dogs. Arthroscopy. 21:1034–1041.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Qi C, Zhu YJ, Lu BQ, Zhao XY, Zhao J, Chen
F and Wu J: Hydroxyapatite hierarchically nanostructured porous
hollow microspheres: Rapid, sustainable microwave-hydrothermal
synthesis by using creatine phosphate as an organic phosphorus
source and application in drug delivery and protein adsorption.
Chemistry. 19:5332–5341. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Galatz LM, Sandell LJ, Rothermich SY, Das
R, Mastny A, Havlioglu N, Silva MJ and Thomopoulos S:
Characteristics of the rat supraspinatus tendon during
tendon-to-bone healing after acute injury. J Orthop Res.
24:541–550. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Canullo L, Heinemann F, Gedrange T, Biffar
R and Kunert-Keil C: Histological evaluation at different times
after augmentation of extraction sites grafted with a
magnesium-enriched hydroxyapatite: Double-blinded randomized
controlled trial. Clin Oral Implants Res. 24:398–406. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hamerschmidt R, Santos RF, Araújo JC,
Stahlke HJ Jr, Agulham MA, Moreira AT and Mocellin M:
Hydroxyapatite granules used in the obliteration of mastoid
cavities in rats. Braz J Otorhinolaryngol. 77:315–321. 2011.(In
English, Portuguese). View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gredes T, Gedrange T, Hinuber C, Gelinsky
M and Kunert-Keil C: Histological and molecular-biological analyses
of poly(3-hydroxybutyrate) (PHB) patches for enhancement of bone
regeneration. Ann Anat. 199:36–42. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kovacevic D, Fox AJ, Bedi A, Ying L, Deng
XH, Warren RF and Rodeo SA: Calcium-phosphate matrix with or
without TGF-β3 improves tendon-bone healing after rotator cuff
repair. Am J Sports Med. 39:811–819. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang R, Xu B and Xu HG: Up-regulation of
TGF-β promotes tendon-to-bone healing after anterior cruciate
ligament reconstruction using bone marrow-derived mesenchymal stem
cells through the TGF-β/MAPK signaling pathway in a new zealand
white rabbit model. Cell Physiol Biochem. 41:213–226. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li B, Shao Q, Ji D, Li F and Chen G:
Mesenchymal stem cells mitigate cirrhosis through BMP7. Cell
Physiol Biochem. 35:433–440. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tang Y, Wu X, Lei W, Pang L, Wan C, Shi Z,
Zhao L, Nagy TR, Peng X, Hu J, et al: TGF-beta1-induced migration
of bone mesenchymal stem cells couples bone resorption with
formation. Nat Med. 15:757–765. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li J, Zhao Z, Liu J, Huang N, Long D, Wang
J, Li X and Liu Y: MEK/ERK and p38 MAPK regulate chondrogenesis of
rat bone marrow mesenchymal stem cells through delicate interaction
with TGF-beta1/Smads pathway. Cell Prolif. 43:333–343. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Carpenter JE, Thomopoulos S, Flanagan CL,
DeBano CM and Soslowsky LJ: Rotator cuff defect healing: A
biomechanical and histologic analysis in an animal model. J
Shoulder Elbow Surg. 7:599–605. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Soslowsky LJ, Carpenter JE, Debano CM,
Banerji I and Moalli MR: Development and use of an animal model for
investigations on rotator cuff disease. J Shoulder Elbow Surg.
5:383–392. 1995. View Article : Google Scholar
|