Introduction
Pancreatic ductal adenocarcinoma (PDAC) is one of
the most aggressive malignancies and may become the second leading
cause of cancer-associated mortality by the year 2030 (1). The majority of PDAC patients are
diagnosed at advanced and aggressive stage, when surgery is not an
option (2). Therefore, systemic
chemotherapy is crucial for PDAC therapy at advanced stages
(3). Although great efforts have
been dedicated into developing efficacious compounds against PDAC
in preclinical and clinical studies, the 5-year median survival
rate remains at ~5% and has not changed significantly during the
past 40 years (4). Thus, novel
therapeutic compounds are urgently required to combat this
aggressive malignancy.
Inula helenium L. (IHL) is a traditional
Chinese medicinal herb that has been used to treat intestinal
tuberculosis associated-enterorrhagia, bronchitis and chronic
enterogastritis, and has recently been identified to possess
anticancer activity (5–7). Sesquiterpene lactones are a family of
compounds that being identified responsible for the anticancer
activity of IHL (8–10). However, existing methods have
proved to be insufficient to extract sesquiterpene lactones from
IHL. Ineffective components in the IHL crude extract would
undermine the anti-cancer activity (11–13).
In addition, the anti-proliferative activity of IHL against PDAC
cells has yet to be reported.
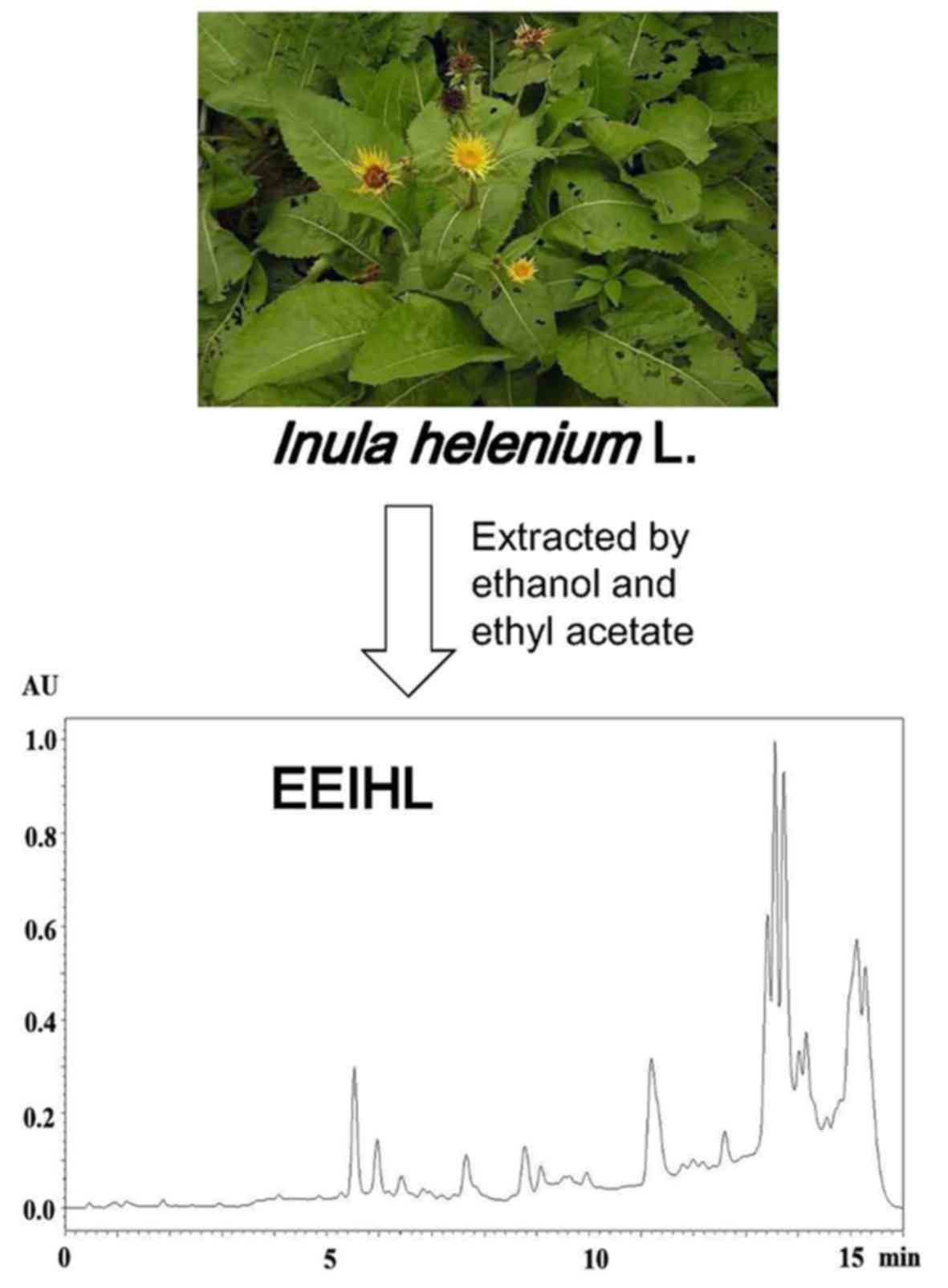
The present study optimized the extraction process
of IHL. The dried rhizome and root of IHL was first extracted with
95% ethanol, and yielded extracts were partitioned by ethyl acetate
to give the final product, ethyl acetate extract of IHL (EEIHL). It
was identified that EEIHL induced CFPAC-1 cell cycle arrest in the
G0/G1 phase, and induced
mitochondria-dependent apoptosis. In addition, EEIHL demonstrated
potent anti-migration activity compared with isoalantolactone,
possibly through downregulating the phosphorylation of signal
transducer and activator of transcription (STAT)3 and AKT, thus
indicating that EEIHL may possess anti-proliferative activity
against PDAC cells.
Materials and methods
Extraction and characterization of
EEIHL
Inula helenium L. was obtained from the
Bozhou traditional Chinese medicine market (Anhui, China). The
dried rhizome and root of Inula helenium L. (10.0 kg) were
crushed into coarse powder. The powder was soaked with 95% ethanol
for 48 h, and extracted with 20-fold 95% ethanol at room
temperature by percolation. The combined extract was concentrated
in a vacuum to give a crude extract. The crude extract was
suspended in 1.5 l warm water and partitioned with ethyl acetate
(3×1.5 l). Subsequent to evaporation in reduced pressure, 370.16 g
EEIHL was yielded (yield, 3.7%). To verify that EEIHL contained
bioactive compounds, a vanillin-sulfuric acid colorimetry assay was
applied to establish sesquiterpene lactones in EEIHL (14).
High performance liquid chromatography
(HPLC) chromatogram of EEIHL
EEIHL was run through an Acquity UPLC system with a
BEH C18 column (25°C, 2.1×100 mm, 1.7 µm; Waters Corporation,
Milford, MA, USA). CH3CN and H2O were used as
the mobile phase, and a gradient program was applied as follows:
0–2.5 min, 30% of CH3CN; 2.5–4 min, 30–45% of
CH3CN; 4–7 min, 45% of CH3CN; 7–8 min, 45–60%
of CH3CN; 8–9 min, 60% of CH3CN; 9–10 min,
60–85% of CH3CN; 10–12 min, 85% of CH3CN;
12–13 min, 85–30% of CH3CN; and maintained at 30% of
CH3CN for the next 3 min. The sample solution was 5 mg
of EEIHL dissolved in 1.0 ml MeOH, and the injection volume was 1
µl with the flow rate of 0.3 ml/min. The detection wavelength was
set at 210 nm.
Proton nuclear magnetic resonance
(1H-NMR) analysis of EEIHL
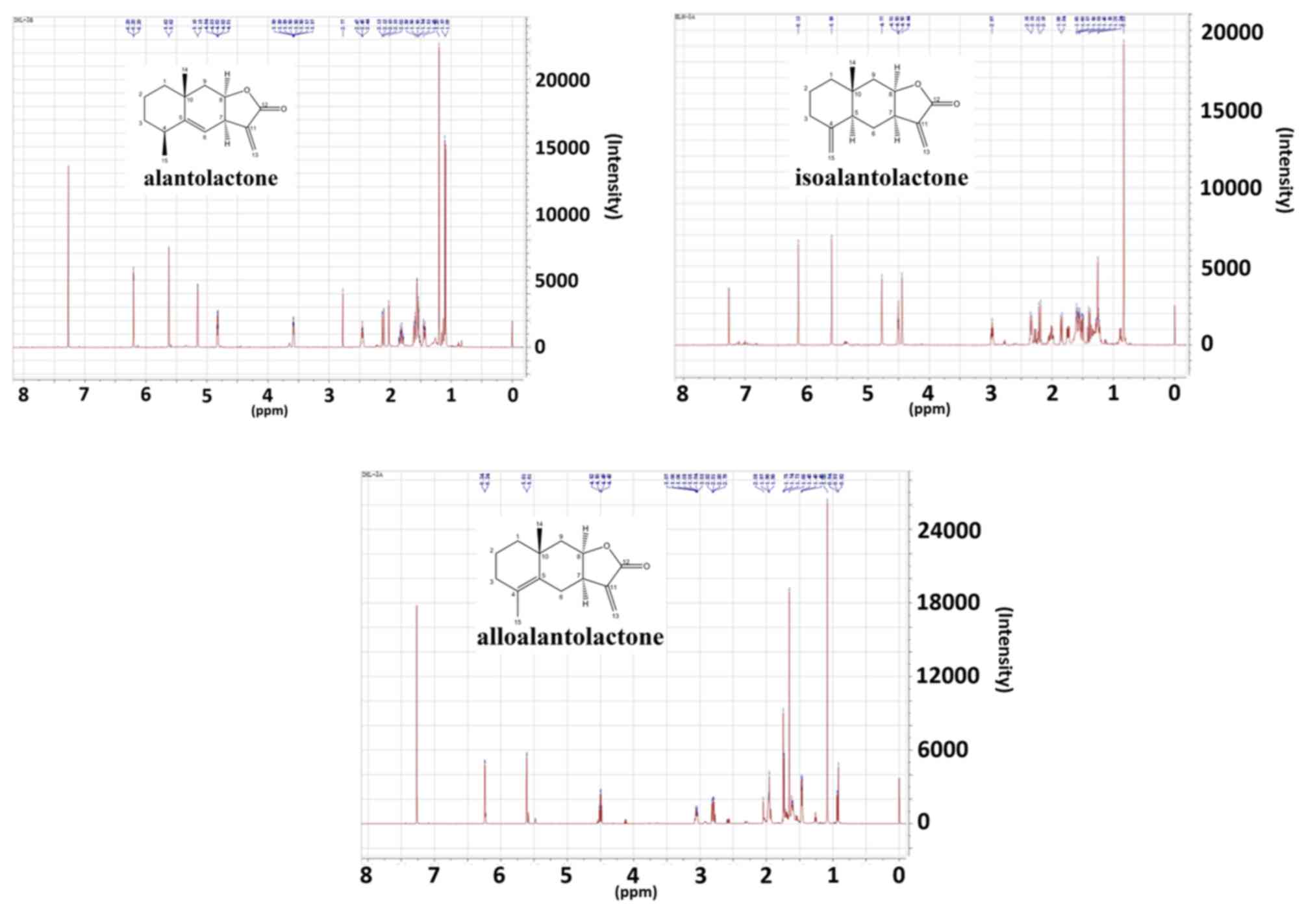
The presence of alantolactone, isoalantolactone and
alloalantolactone was verified by 1H-NMR. A total of 10
mg of sample was dissolved in CDCl3, and then detected
by NMR. Tetramethylsilane was used as internal standard. The
1H-NMR was performed with a Bruker Ultra Shield Plus 600
MHz spectrometer (Bruker Corporation, Billerica, MA, USA).
Materials and cell culture
RPMI-1640 medium and fetal bovine serum (FBS) were
purchased from Gibco (Thermo Fisher Scientific, Inc., Waltham, MA,
USA). The Cycletest Plus DNA Reagent kit was purchased from BD
Biosciences (Franklin Lakes, NJ, USA). DAPI was purchased from
Beyotime Biotechnology (Shanghai, China). The Annexin V-FITC
Apoptosis kit was purchased from BestBio Company (Shanghai, China).
A Mitochondrial Membrane Potential Assay kit was purchased from
Signalway Antibody LLC (College Park, MD, USA).
The primary antibodies against cyclin-dependent
kinase (CDK)2 (cat. no. ab32147), phosphorylated (p)-CDK2 (cat. no.
ab76146), CDK6 (cat. no. ab124821), cyclin D1 (cat. no. ab134175),
p-Rb (cat. no. ab184796), cyclin E1 (cat. no. ab135380), Bcl-2
(cat. no. ab32124), Bax (cat. no. ab32503), Bim (cat. no. ab32158),
Mcl-1 (cat. no. ab32087), poly(ADP-ribose) polymerase (PARP; cat.
no. ab191217), caspase-3 (cat. no. ab13847), X-linked inhibitor of
apoptosis protein (XIAP; cat. no. ab21278), AKT (cat. no. ab8805),
p-AKT (cat. no. ab38449), signal transducer and activator of
transcription 3 (STAT3; cat. no. ab68153), p-STAT3 (cat. no.
ab76315) and β-actin (cat. no. ab8226) were purchased from Abcam
(Cambridge, MA, USA). Primary antibodies were diluted at
1:1,000.
CFPAC-1 human PDAC cells were purchased from the
Cell Bank of the Chinese Academy of Sciences (Shanghai, China).
Cells were cultured with RPMI-1640 medium containing 10% FBS and 1%
penicillin/streptomycin at 37°C in a 5% CO2 humidified
atmosphere. EEIHL was dissolved in DMSO at 100 mg/ml.
Cell viability assay
The rate of cell proliferation was measured by a
Cell Counting Kit-8 (CCK-8) assay (BestBio Company). Cells were
cultured in 96-well plates at a concentration of
7×103/well. Cells were cultured for 24 h and treated
with the indicated concentrations of EEIHL. At 48 h of treatment,
the supernatant was removed, 100 µl of CCK-8 solution was added to
each well and the plates were incubated for a further 2 h at 37°C.
Cell viability was quantified by a Multiskan Spectrum
spectrophotometer (Thermo Fisher Scientific, Inc.) by the optical
density (OD) at 450 nm. Cell viability was calculated as
[(OD450 of treated cells/OD450 of control
cells) ×100%]. Three independent experiments were performed.
Colony formation assay
CFPAC-1 cells were seeded into 6-well plates at a
density of 1×103/well and incubated for 24 h. The cells
were then treated with the indicated concentrations of EEIHL.
Following 24 h of treatment, the supernatant was removed and cells
were cultured for a further two weeks. Then the cells were fixed
with 4% paraformaldehyde for 15 min and stained with Giemsa
solution for 15 min at room temperature. Visible colonies were
imaged with a ChemiDoc XPS system (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA).
Cell cycle analysis
Exponentially growing cells were seeded in 6-well
plates (2×105/well) and cultured overnight in a 5%
CO2 atmosphere at 37°C. Following treatment with EEIHL,
CFPAC-1 cells were harvested and washed twice with cold PBS and
then fixed in 70% cold ethanol at 4°C overnight. Cells were stained
with the Cycletest Plus DNA Reagent kit (BD Biosciences), according
to the manufacturer's protocol. Cell cycle distribution was
analyzed using a flow cytometer (BD Biosciences). Data were
collected by BD FACSDiva software version 8.0.1 (BD Biosciences)
and analyzed by ModFit LT for Windows version 4.1.7 (Verity
Software House, Topsham, ME, USA). Three independent experiments
were performed.
Apoptosis assay
Exponentially growing cells were seeded in 6-well
plates (2×105/well) and cultured overnight in a 5%
CO2 atmosphere at 37°C. Following treatment with EEIHL
for 48 h, the cells were harvested and washed with PBS. Then the
cells were stained with the Annexin V-FITC Apoptosis kit, according
to the manufacturer's protocol, and analyzed with a flow cytometer
(BD Biosciences). Three independent experiments were performed.
DAPI staining
CFPAC-1 cells (8×104 cells/well) were
cultured in 24-well plates. Following exposure to EEIHL, cells were
fixed with 4% paraformaldehyde for 20 min, and stained with DAPI
for 15 min at room temperature. Following washing with PBS, cells
were observed under a fluorescence microscope (Ti-E; Nikon
Corporation, Tokyo, Japan).
Detection of mitochondrial membrane
potential
Mitochondrial membrane potential was visualized by
the 5,5′,6,6′-tetrachloro-1,1′,3,3′ tetraethyl-imidacarbocyanine
iodide (JC-1) stain from the Mitochondrial Membrane Potential Assay
kit. Cells were seeded into 6-well plates at a density of
2×105/well, and cultured for 24 h. Following treatment,
the cells were collected, washed with PBS, and incubated with JC-1
for 15 min at 37°C. Subsequent to washing, cells were immediately
analyzed using a flow cytometer (BD Biosciences). Three independent
experiments were performed.
Caspase-3 activity assay
CFPAC-1 cells (2×105 cells/well, 6-well
plate) were incubated with EEIHL for 48 h. Cells were washed with
PBS and lysed in cell lysis buffer included in the Caspase-3 assay
kit. Caspase-3 activity in cell lysates were determined
colorimetrically using a BioVision colorimetric caspase assay kit
(BioVision, Inc., Milpitas, CA, USA). Chromophore conjugated
peptides, including DEVD-p-nitroanilide (pNA) and VEID-pNA, were
substrates for caspase-3, releasing pNA on caspase activity, which
was quantified according to the manufacturer's protocol.
Western blot analysis
Following treatment with different concentrations of
EEIHL, total proteins (40 µg) were extracted using
radioimmunoprecipitation assay lysing buffer (RIPA; cat. no.
P0013B; Beyotime Institute of Biotechnology, Shanghai, China) and
subjected to 12% SDS-PAGE prior to being transferred onto PVDF
membranes (Bio-Rad Laboratories, Inc.). The membranes were blocked
with 5% non-fat milk at room temperature for 1 h, and then
incubated with specific primary antibodies overnight at 4°C.
Subsequent to washing in TBST, membranes were incubated with the
secondary antibodies (dilution 1:5,000; HRP Goat Anti-Mouse cat.
no. A21010 and HRP Goat Anti-Rabbit cat. no. A21020; Abbkine,
Wuhan, Hubei, China) at room temperature for a further 1 h. The
protein bands were visualized using an ECL system WBKLS0050 (EMD
Millipore, Billerica, MA, USA) and analyzed using Bio-Rad
Laboratories Quantity One software version 4.6.2 (Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
Wound healing assay
Exponentially growing cells were seeded in 6-well
plates (2×105/well) and cultured overnight in a 5%
CO2 atmosphere at 37°C. Following a 24-h incubation, a
1-ml pipette tip was used to gently scratch the monolayer at the
center of the well. Detached cells were washed away with PBS, and
the remaining cells were cultured with serum-free medium and the
indicated doses of treatment. Cells were incubated for 24 h prior
to the capture of images (DS-Ri2; Nikon Corporation).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from cells with TRIzol (Life
Technologies; Thermo Fisher Scientific, Inc.), precipitated with
isopropyl alcohol, and rinsed with 70% ethanol. Single-strand cDNA
was prepared from the purified RNA using PrimeScript RT Master Mix
(cat. no. RR036A; Takara Biotechnology Co., Ltd., Dalian, Liaoning,
China), followed by qPCR with SYBR-Green (Qiagen GmbH, Hilden,
Germany) in the 7900HT Fast Real-Time PCR system (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The thermocycling
parameters were 94°C for 30 sec followed by 40 cycles of 58°C for
30 sec and 72°C for 30 sec, with one final cycle of 72°C for 90
sec. Three independent experiments were performed. The relative
expression of target genes against the reference gene was obtained
from 2−ΔΔCq values (15).
The primers used were: E-Cadherin, forward,
5′-TTCTGCTGCTCTTGCTGTTT-3′, reverse, 5′-TGGCTCAAGTCAAAGTCCTG-3′;
Snail, forward, 5′-GAAAGGCCTTCAACTGCAAA-3′, reverse,
5′-TGACATCTGAGTGGGTCTGG-3′; GAPDH, forward,
5′-GAGTCAACGGATTTGGTCGT-3′, reverse,
5′-TTGATTTTGGAGGGATCTCG-3′.
Statistical analysis
The results are expressed as the mean ± standard
deviation of at least three independent experiments. A two-sided
Student's t test or one-way analysis of variance followed by a post
hoc Fisher's least significant difference test was used to analyze
the differences among groups. Graphs were prepared using SigmaPlot
software version 12.0 (Systat Software, Inc., San Jose, CA, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Characterization of EEIHL
To extract sesquiterpene lactones from crude IHL
roots efficiently, ethanol and ethyl acetate were used to partition
IHL and produce the final product, EEIHL. A vanillin-sulfuric acid
colorimetry assay was then applied to establish sesquiterpene
lactones in EEIHL. The color of the mixture was purple or
purple-red, indicating the existence of sesquiterpene lactones in
EEIHL, as previously described (16). These three compounds were further
confirmed by analysis with HPLC (Fig.
1). In addition, 1H-NMR spectra were used to
identify the presence and structure of alantolactone,
isoalantolactone and alloalantolactone (Fig. 2).
EEIHL inhibited the proliferation of
CFPAC-1 cells
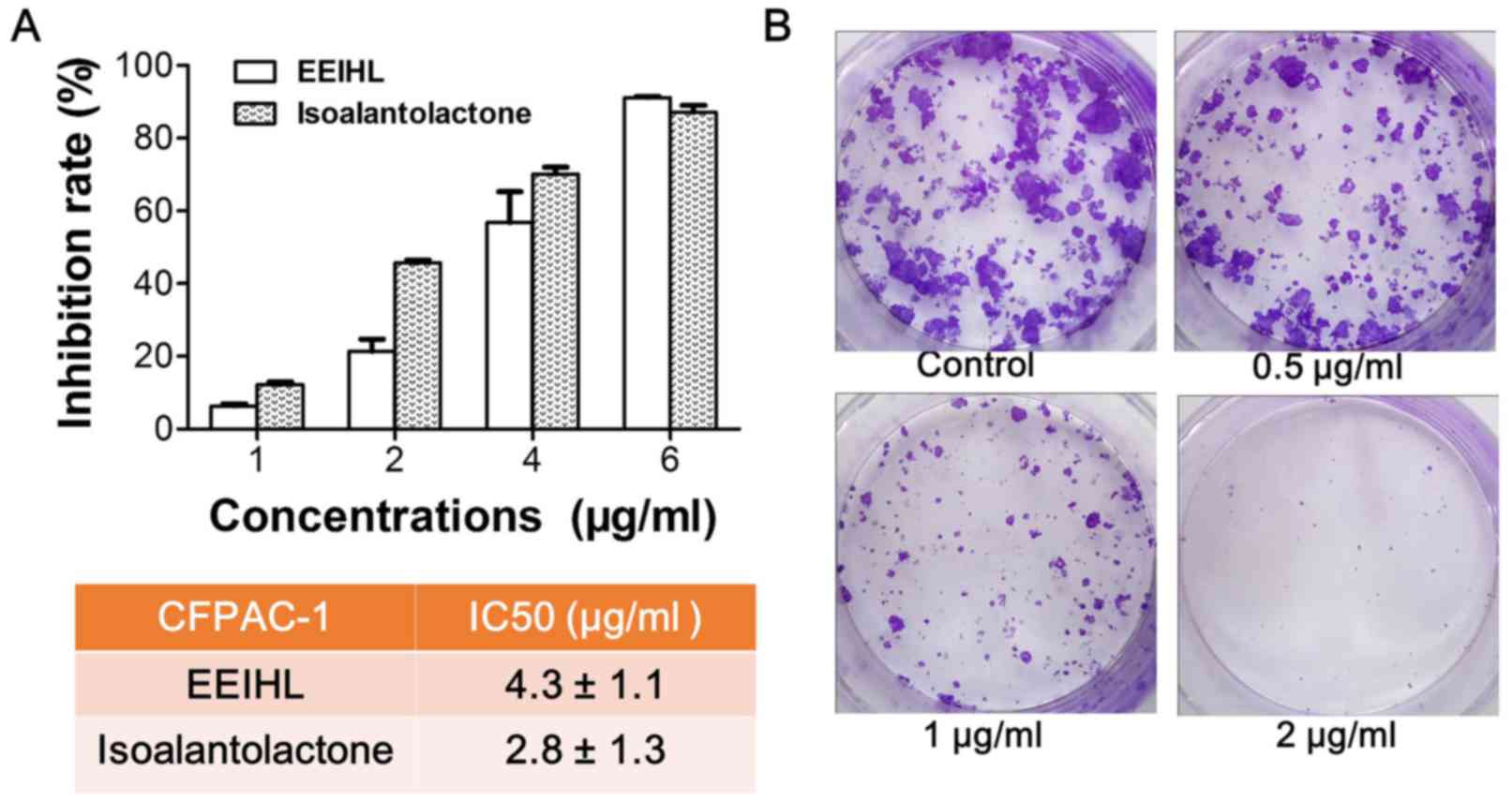
The anti-proliferative activity of EEIHL against
CFPAC-1 PDAC cells was determined by CCK-8 assay and colony
formation assay. As shown in Fig.
3A, EEIHL and isoalantolactone dose-dependently inhibited the
proliferation of CFPAC-1 cells at 48 h treatment. The inhibition
rate was >90% following treatment with 6 µg/ml of EEIHL for 48
h, and the half maximal inhibitory concentration (IC50)
was 4.3 and 2.8 µg/ml for EEIHL and isoalantolactone, respectively.
In addition, a relatively low concentration of EEIHL inhibited the
formation of CFPAC-1 colonies (Fig.
3B). When cells were treated with 2 µg/ml of EEIHL for 24 h and
cultured for a further 2 weeks, the number of CFPAC-1 colonies was
diminished. At concentrations of 1 or 2 µg/ml, EEIHL demonstrated
anti-proliferation activity after 24 h treatment. Therefore, EEIHL
potently inhibited the proliferation and colony formation of
CFPAC-1 cells.
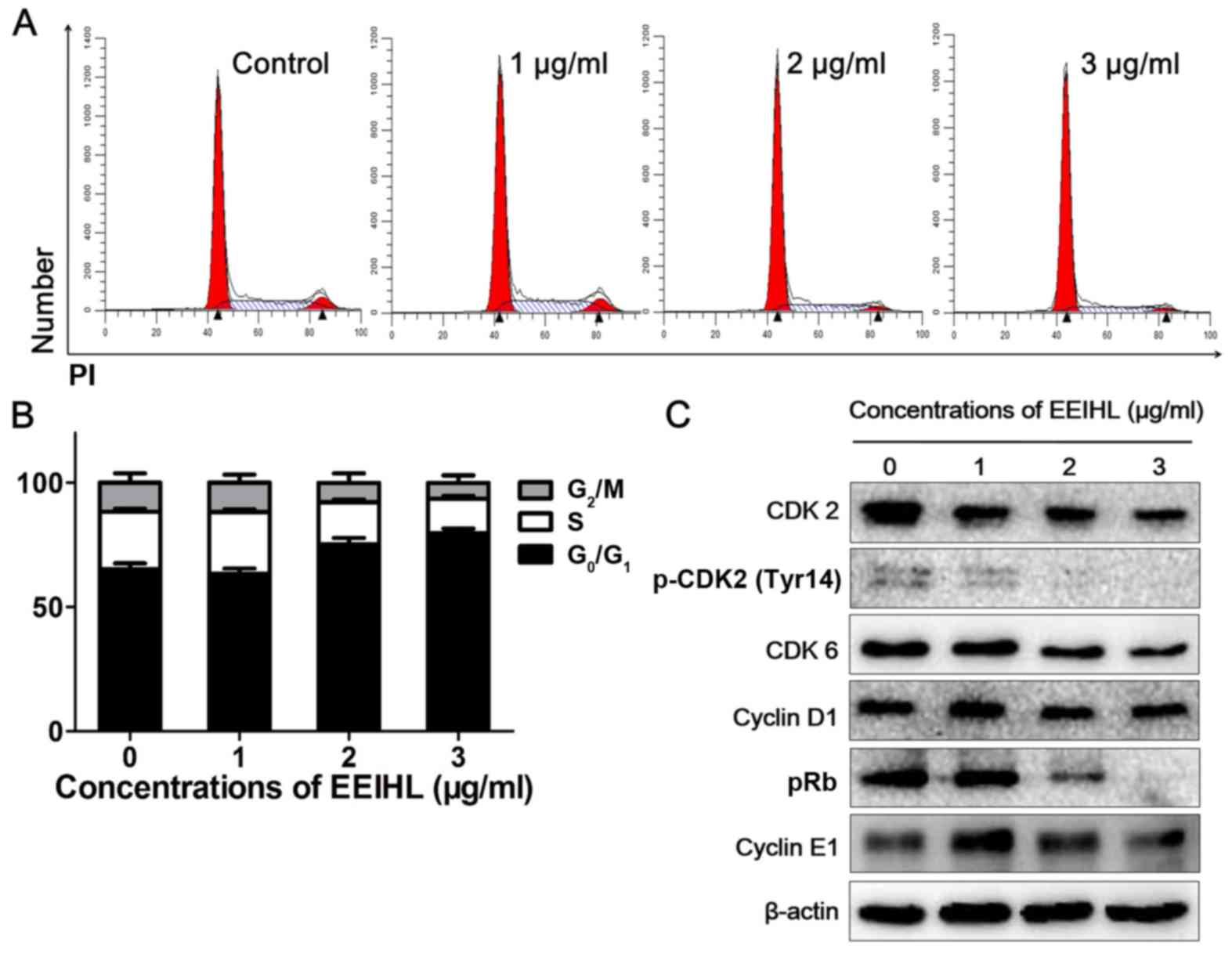
Low concentrations of EEIHL caused
CFPAC-1 cell-cycle arrest at the G0/G1
phase
Following treatment with EEIHL for 24 h, CFPAC-1
cells were stained with propidium iodide (PI) followed by cell
cycle analysis. As shown in Fig. 4A
and B, 2 µg/ml of EEIHL treatment resulted in a 15% increase in
the proportion of cells in the G0/G1 phase.
The protein expression of total CDK2 and p-CDK2 gradually decreased
following treatment with EEIHL (Fig.
4C), whereas the protein level of cyclin D1 or cyclin E1
remained unchanged. Notably, pRb was downregulated following EEIHL
treatment, indicating that EEIHL may downregulate CDK2 by affecting
pRb.
High concentrations of EEIHL caused
mitochondrial-dependent apoptosis in CFPAC-1 cells
As the EEIHL concentration was increased, CFPAC-1
cells displayed an increasing extent of mitochondrial-dependent
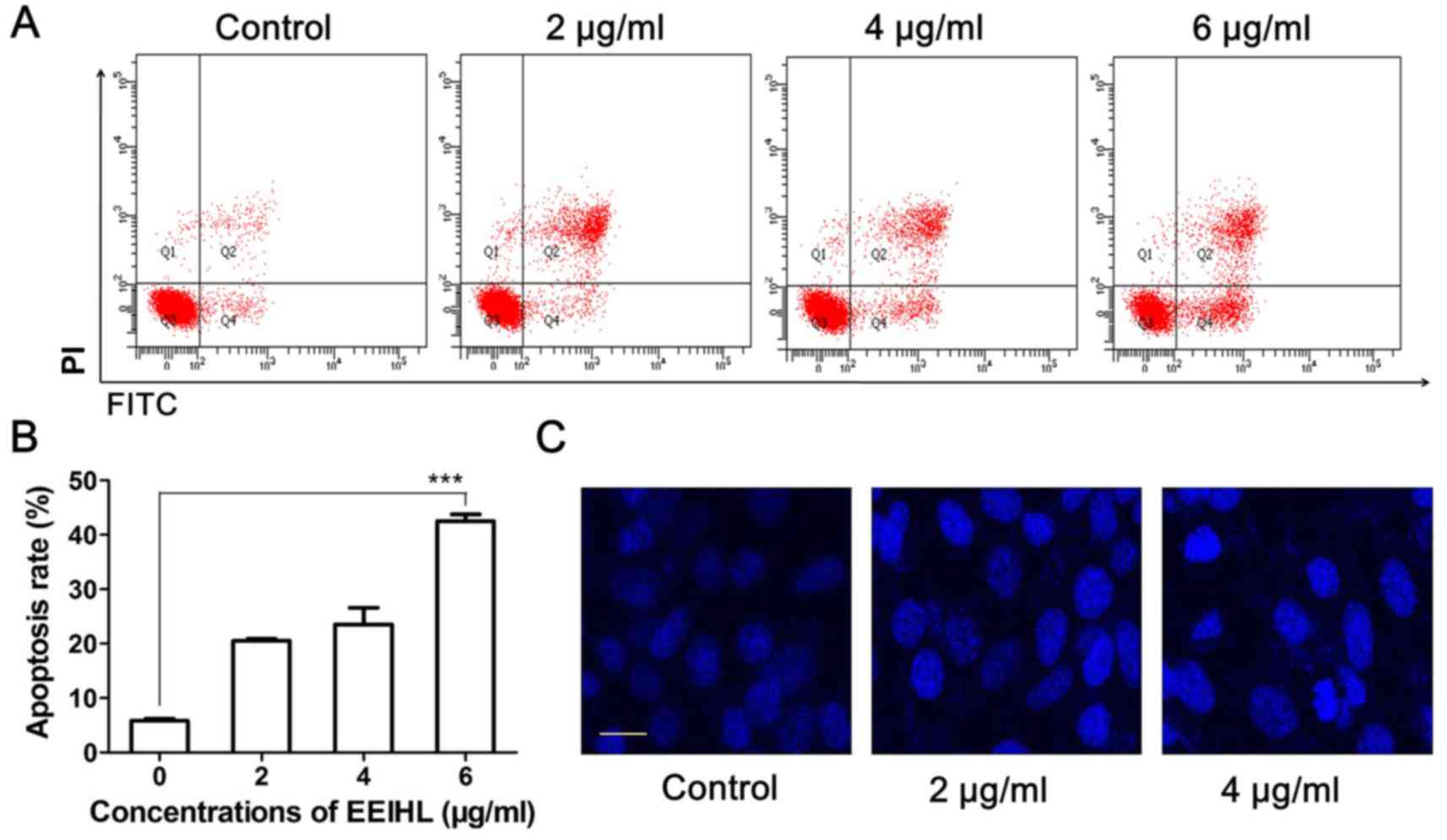
apoptosis. As shown in Fig. 5A and
B, 4 and 6 µg/ml of EEIHL treatment induced 27 and 48%
apoptosis, respectively, while untreated cells displayed an
apoptosis rate of 6%. Meanwhile, 4 µg/ml of EEIHL resulted in
shrunken nuclei and intensified DAPI fluorescence (Fig. 5C).
To explore the role of the mitochondria in
EEIHL-induced apoptosis, the JC-1 stain was applied to determine
the mitochondrial membrane potential (Fig. 6). Following treatment with
increasing concentrations of EEIHL, depolarized mitochondrial
membrane potential gradually increased to 10.8% (Fig. 6A and B). Proteins determining
mitochondrial fate were then analyzed by western blot (Fig. 6D). In accord with the depolarized
mitochondrial membrane potential, pro-apoptotic protein Bim was
evidently elevated, whereas anti-apoptotic proteins Bcl-2 and Mcl-1
were downregulated, suggesting the crucial role of the mitochondria
in EEIHL induced apoptosis. Furthermore, as indicated by the
appearance of cleaved PARP and decreased levels of XIAP, EEIHL
induced caspase-dependent apoptosis; this was verified by
determining the caspase activity following EEIHL treatment
(Fig. 6C). In addition, EEIHL
induced the decreased expression of p-AKT and p-STAT3, suggesting
alternative pathways involved in the anti-proliferation effect of
EEIHL (Fig. 6D).
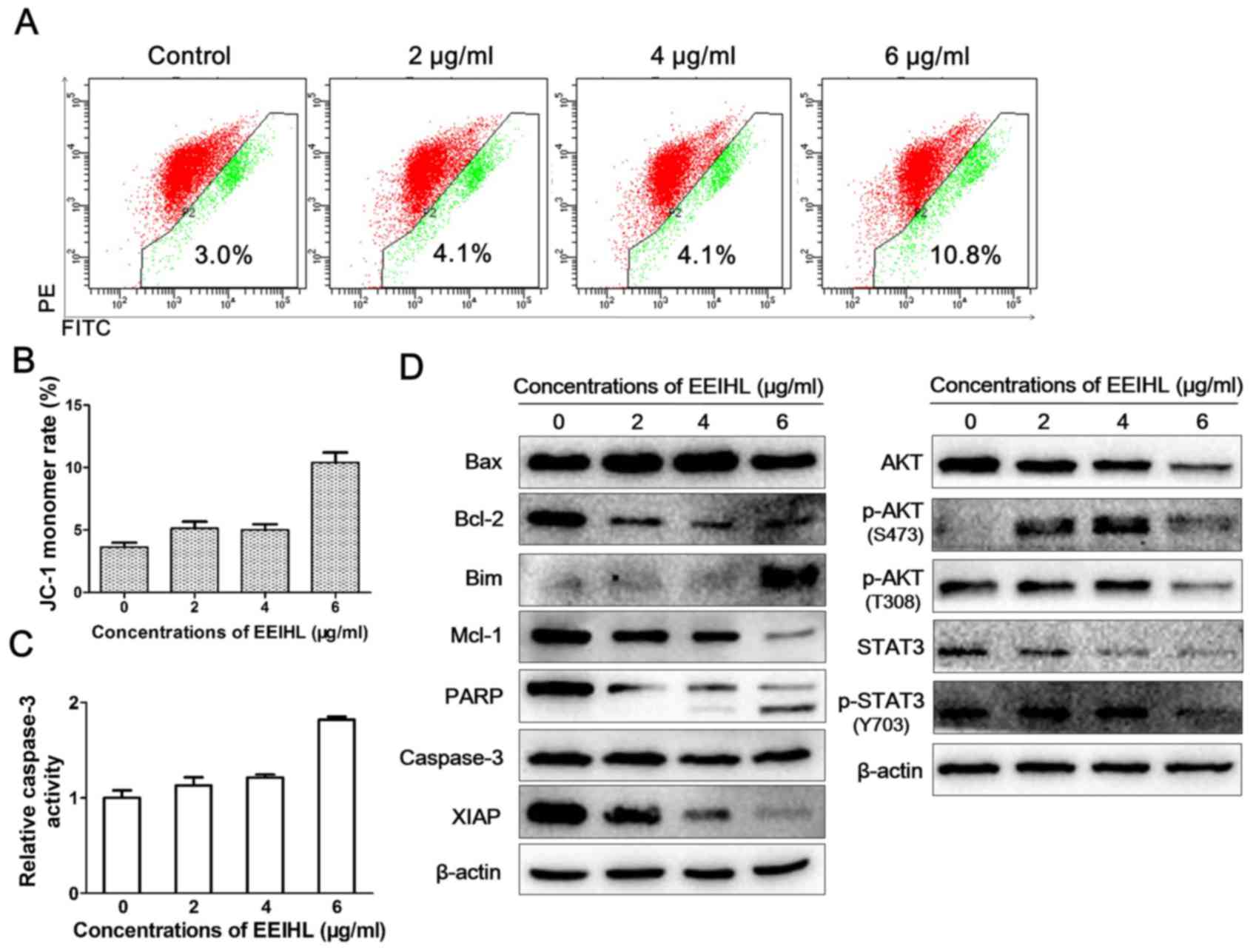 | Figure 6.EEIHL promoted
mitochondrion-dependent apoptosis. (A) CFPAC-1 cells were seeded
into 6-well plates at a density of 2×105/well, and
cultured for 24 h. Following treatment with EEIHL for 24 h, the
JC-1 stain was used to observe the depolarized mitochondria
membrane potential by flow cytometry. (B) Quantitative analysis of
depolarized mitochondria membrane potential. (C) CFPAC-1 cells were
seeded into 6-well plates at the density of 2×105/well,
and cultured for 24 h. Following treatment with EEIHL for 48 h,
caspase-3 activity in cell lysates were determined with a
colorimetric assay. (D) Following treatment with indicated
concentrations of EEIHL for 48 h, cells were lysed and proteins
were extracted for western blot assay. EEIHL, ethyl acetate extract
of Inula helenium L.; JC-1, 5,5′,6,6′-tetrachloro-1,1′,3,3′
tetraethyl-imidacarbocyanine iodide; Bax, Bcl-2-like protein 4;
Bim, Bcl-2-like protein 11; Mcl-1, Bcl-2-like protein 3; PARP,
poly(ADP-ribose) polymerase; XIAP, X-linked inhibitor of apoptosis
protein; STAT, signal transducer and activator of transcription;
p-, phosphorylated. |
EEIHL inhibited CFPAC-1 cell
migration
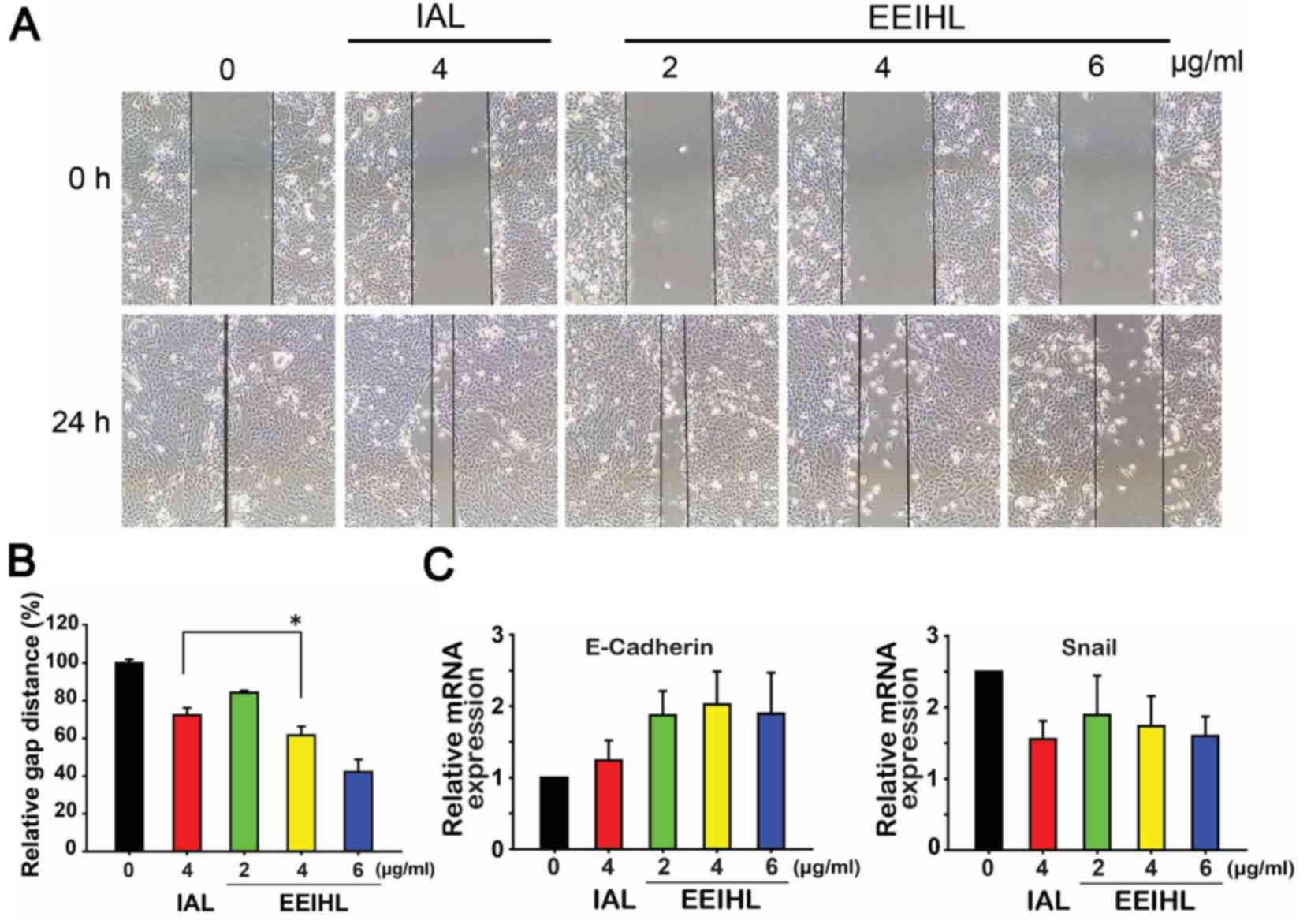
Cell migration was measured using a wound healing
assay. When treated with 2, 4 or 6 µg/ml of EEIHL, the migration
ability of CFPAC-1 cells was gradually attenuated (Fig. 7A and B). In comparison with
isoalantolactone, 4 µg/ml of EEIHL was significantly more potent in
impeding CFPAC-1 cell migration. Snail is a transcriptional factor
that promotes the repression of E-cadherin, which serves a key role
in cell adhesion; the dysregulated transcription of Snail and
E-cadherin is associated with the epithelial-mesenchymal transition
(EMT) and tumor metastasis. As shown in Fig. 7C, the mRNA level of E-cadherin
increased following EEIHL treatment to a greater extent than
treatment with isoalantolactone, indicating the inhibitory effect
of EEIHL on cell mobility. Meanwhile, the mRNA level of Snail was
significantly downregulated, suggesting that EEIHL may also inhibit
the Snail-induced regulation of EMT.
Discussion
Inula helenium L. is a traditional medicinal
herb that may possess potent anti-cancer activity (5–7). As
previously reported, sesquiterpene lactones are a class of chemical
compounds responsible for the anti-cancer activity of IHL (17). Among those sesquiterpene lactones,
alantolactone and isoalantolactone are the most extensively studied
compounds obtained from the ethanol extracts of IHL (18,19).
However, there remain other sesquiterpene lactones that may also
exhibit anti-cancer activity but have yet to be intensively studied
(20). For instance,
isocostunolide was identified as inducing caspase-dependent
apoptosis in melanoma cells, and its IC50 value was
comparable to alantolactone or isoalantolactone (21). The anti-PDAC activity and potential
mechanisms of sesquiterpene lactones are not fully understood. The
present study optimized the separation strategy by extracting IHL
with 95% ethanol and partitioning with ethyl acetate, aiming to
maximize the content of the sesquiterpene lactones that exhibit
anti-cancer activity.
Sesquiterpene lactones extracted from IHL have been
reported to inhibit proliferation through inducing apoptosis,
causing G1 phase arrest or activating reactive oxygen
species (ROS) in several types of cancer, including breast cancer,
leukemia and lung squamous cell carcinoma (22–24).
To the best of our knowledge, there have been few studies regarding
the effect on pancreatic cancer, apart from Khan et al
(25) who identified that
isoalantolactone could induce ROS-mediated apoptosis in PANC-1
cells. In the present study, EEIHL exhibited anti-proliferative
activity against CFPAC-1 cells in a dose-dependent manner, without
affecting the ROS level (data not shown) (26). Comparing the inhibitory effect of
EEIHL with isoalantolactone, the 48 h IC50 value of
EEIHL on CFPAC-1 cells was 4.3 µg/ml, a similar level to
isoalantolactone (2.8 µg/ml). However, the inhibitory effect of
impurities could not be excluded in this preliminary study. Future
studies, should isolate the mixture of alantolactone,
isoalantolactone and alloalantolactone, and further quantify the
ratio of these three major components with antitumor activity, with
the aim to determine the exact mechanisms of action of
EEIHL-induced apoptosis. Additionally, one cell line is
insufficient to represent the phenotype of metastatic pancreatic
cancer. Accordingly, the authors of the present study are working
on using additional pancreatic cell lines, including BxPC-3 and
Capan-1, and HPDE6-C7 immortal human pancreatic duct epithelial
cells, to demonstrate the specific antitumor effect of EEIHL on
pancreatic cancer cells.
Low concentrations of EEIHL induced CFPAC-1 cell
cycle arrest at the G0/G1 phase rather than
in S phase as in a previous study (27), indicating a distinct mechanism of
action in CFPAC-1 cells. CFPAC-1 cells harbor allelic deletion in
the SMAD4 gene, inducing the inactivation of SMAD4. Loss of SMAD4
causes alterations to multiple kinase pathways, including the p38
and AKT pathways, and increases chemoresistance in vitro
(28,29). The results of the present study
indicated that high concentrations of EEIHL can downregulate the
phosphorylation of AKT and STAT3, affecting mitochondrial apoptotic
proteins, including Bim, Mcl-1 and Bcl-2. AKT is directly
phosphorylated by PDK1 at T308, and forms an activation loop
between SIN1, mTORC2 and p-AKT (T308), leading to the
phosphorylation of AKT S473 by mTORC2 (30,31).
In addition, it has been reported that STAT/AKT pathway inhibition
could cause apoptosis in prostate cancer (32). In the present study, it was
identified that p-AKT (T308) and p-STAT3 (Y703) decreased following
exposure to 6 µg/ml EEIHL, indicating STAT3/AKT inhibition
following EEIHL treatment. To fully clarify the
STAT3/AKT-associated mechanisms of EEIHL-induced apoptosis, it will
be necessary to transfect cells with a hyper-phosphorylated STAT3
plasmid and knockdown AKT in a future study.
The present study compared the anti-migration
activity of EEIHL with isoalantolactone by using a scratch wound
healing assay. At a dose of 4 µg/ml, a 24-h treatment with EEIHL
demonstrated an anti-migration effect comparable to
isoalantolactone. Additionally, EEIHL treatment significantly
increased the mRNA level of E-cadherin, decreased the transcription
of Snail, and downregulated the phosphorylation of STAT3 and AKT.
Saitoh et al (33)
identified that STAT3 acted as a mediator that could enhance the
induction of Snail at the mRNA level. It was previously identified
that the STAT3/AKT pathway mediated EMT and its downstream target,
Snail, in hepatocellular carcinoma (34,35).
The function of STAT3/AKT/Snail in regulating EMT and thus, cell
migration, has been established (36). Therefore, it was assumed that EEIHL
could interfere with the phosphorylation of STAT3/AKT proteins, and
downregulate the transcription of Snail, reducing the mobility of
CFPAC-1 cells.
In conclusion, EEIHL produced anti-cancer activity
in a concentration-dependent manner. Low concentrations of EEIHL
induced G0/G1 phase arrest in CFPAC-1 cells,
whereas high concentrations of EEIHL induced apoptosis, possibly
via the downregulation of the phosphorylation of STAT3 and AKT. In
addition, the inhibition of the STAT3/AKT pathway may affect
downstream mRNA transcription, including of Snail and E-cadherin,
leading to a reduced cell migration ability.
Acknowledgements
The present study was funded by Hangzhou Major
Science and Technology Project (grant no. 20172016A01; to Nengming
Lin), Zhejiang Provincial Program for the Cultivation of High-level
Innovative Health talents (grant no., 2010-190-4; to Nengming Lin)
and the National Natural Science Foundation of China (grant no.,
81603144; to Bo Zhang).
References
|
1
|
Rahib L, Smith BD, Aizenberg R, Rosenzweig
AB, Fleshman JM and Matrisian LM: Projecting cancer incidence and
deaths to 2030: The unexpected burden of thyroid, liver, and
pancreas cancers in the United States. Can Res. 74:2913–2921. 2014.
View Article : Google Scholar
|
|
2
|
Bailey P, Chang DK, Nones K, Johns AL,
Patch AM, Gingras MC, Miller DK, Christ AN, Bruxner TJ, Quinn MC,
et al: Genomic analyses identify molecular subtypes of pancreatic
cancer. Nature. 531:47–52. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Garrido-Laguna I and Hidalgo M: Pancreatic
cancer: From state-of-the-art treatments to promising novel
therapies. Nat Rev Clin Oncol. 12:319–334. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vincent A, Herman J, Schulick R, Hruban RH
and Goggins M: Pancreatic cancer. Lancet. 378:607–620. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Seca AM, Grigore A, Pinto DC and Silva AM:
The genus Inula and their metabolites: From ethnopharmacological to
medicinal uses. J Ethnopharmacol. 154:286–310. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Stojanović-Radić Z, Comić LJ, Radulović N,
Blagojević P, Denić M, Miltojević A, Rajković J and
Mihajilov-Krstev T: Antistaphylococcal activity of Inula helenium
L. root essential oil: Eudesmane sesquiterpene lactones induce cell
membrane damage. Eur J Clin Microbiol Infect Dis. 31:1015–1025.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Qiu J, Luo M, Wang J, Dong J, Li H, Leng
B, Zhang Q, Dai X, Zhang Y, Niu X and Deng X: Isoalantolactone
protects against Staphylococcus aureus pneumonia. FEMS Microbiol
Lett. 324:147–155. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ghantous A, Gali-Muhtasib H, Vuorela H,
Saliba NA and Darwiche N: What made sesquiterpene lactones reach
cancer clinical trials? Drug Discov Today. 15:668–678. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jiang HL, Chen J, Jin XJ, Yang JL, Li Y,
Yao XJ and Wu QX: Sesquiterpenoids, alantolactone analogues, and
seco-guaiene from the roots of Inula helenium. Tetrahedron.
67:9193–9198. 2011. View Article : Google Scholar
|
|
10
|
Zhang S, Won YK, Ong CN and Shen HM:
Anti-cancer potential of sesquiterpene lactones: Bioactivity and
molecular mechanisms. Curr Med Chem. 5:239–249. 2005.
|
|
11
|
Wang J, Zhao YM, Tian YT, Yan CL and Guo
CY: Ultrasound-assisted extraction of total phenolic compounds from
Inula helenium. ScientificWorldJournal. 2013:1575272013.PubMed/NCBI
|
|
12
|
Park EJ, Kim YM, Park SW, Kim HJ, Lee JH,
Lee DU and Chang KC: Induction of HO-1 through p38 MAPK/Nrf2
signaling pathway by ethanol extract of Inula helenium L. reduces
inflammation in LPS-activated RAW 264.7 cells and CLP-induced
septic mice. Food Chem Toxicol. 55:386–395. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Guo CY, Wang J, Hou Y, Zhao YM, Shen LX
and Zhang DS: Orthogonal test design for optimizing the extraction
of total flavonoids from Inula helenium. Pharmacogn Mag. 9:192–195.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ahmed AF, Al-Qahtani JH, Al-Yousef HM,
Al-Said MS, Ashour AE, Al-Sohaibani M and Rafatullah S:
Proanthocyanidin-rich date seed extract protects against chemically
induced hepatorenal toxicity. J Med Food. 18:280–289. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Du J, Shi HR, Ren F, Wang JL, Wu QH, Li X
and Zhang RT: Inhibition of the IGF signaling pathway reverses
cisplatin resistance in ovarian cancer cells. BMC Cancer.
17:8512017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ding YL, Wang YZ, Zhang J, Zhang QZ, Zhang
JY and Jin H: Application of the vanillin sulfuric acid
colorimetry-ultraviolet spectrometry on quality evaluation of Panax
notoginseng. Guang Pu Xue Yu Guang Pu Fen Xi. 33:471–475. 2013.(In
Chines). PubMed/NCBI
|
|
17
|
Konishi T, Shimada Y, Nagao T, Okabe H and
Konoshima T: Antiproliferative sesquiterpene lactones from the
roots of Inula helenium. Biol Pharm Bull. 25:1370–1372. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Weng Z, Gao H, Hu J, Fan Y, Wang H and Li
L: Isoalantolactone induces autophagic cell death in SKOV3 human
ovarian carcinoma cells via upregulation of PEA-15. Oncol Rep.
35:833–840. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Di W, Khan M, Rasul A, Sun M, Sui Y, Zhong
L, Yang L, Zhu Q, Feng L and Ma T: Isoalantolactone inhibits
constitutive NF-κB activation and induces reactive oxygen
species-mediated apoptosis in osteosarcoma U2OS cells through
mitochondrial dysfunction. Oncol Rep. 32:1585–1593. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zaima K, Wakana D, Demizu Y, Kumeta Y,
Kamakura H, Maruyama T, Kurihara M and Goda Y: Isoheleproline: A
new amino acid-sesquiterpene adduct from Inula helenium. J Nat Med.
68:432–435. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen CN, Huang HH, Wu CL, Lin CP, Hsu JT,
Hsieh HP, Chuang SE and Lai GM: Isocostunolide, a sesquiterpene
lactone, induces mitochondrial membrane depolarization and
caspase-dependent apoptosis in human melanoma cells. Cancer Lett.
246:237–252. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhao YM, Wang J, Liu HB, Guo CY and Zhang
WM: Microwave-assisted Extraction of Alantolactone and
Isoalantolactone from Inula helenium. Indian J Pharm Sci.
77:116–120. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cai H, Meng X, Li Y, Yang C and Liu Y:
Growth inhibition effects of isoalantolactone on K562/A02 cells:
Caspase-dependent apoptotic pathways, S phase arrest, and
downregulation of Bcr/Abl. Phytother Res. 28:1679–1686. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang J, Cui L, Feng L, Zhang Z, Song J,
Liu D and Jia X: Isoalantolactone inhibits the migration and
invasion of human breast cancer MDA-MB-231 cells via suppression of
the p38 MAPK/NF-κB signaling pathway. Oncol Rep. 36:1269–1276.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Khan M, Ding C, Rasul A, Yi F, Li T, Gao
H, Gao R, Zhong L, Zhang K, Fang X and Ma T: Isoalantolactone
induces reactive oxygen species mediated apoptosis in pancreatic
carcinoma PANC-1 cells. Int J Biol Sci. 8:533–547. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang M, Hou Y, Shen Y, Guo X, Shang D and
Zhang D: Probucol reverses homocysteine induced inflammatory
monocytes differentiation and oxidative stress. Eur J Pharmacol.
818:67–73. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yao Y, Xia D, Bian Y, Sun Y, Zhu F, Pan B,
Niu M, Zhao K, Wu Q, Qiao J, et al: Alantolactone induces G1 phase
arrest and apoptosis of multiple myeloma cells and overcomes
bortezomib resistance. Apoptosis. 20:1122–1133. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen YW, Hsiao PJ, Weng CC, Kuo KK, Kuo
TL, Wu DC, Hung WC and Cheng KH: SMAD4 loss triggers the phenotypic
changes of pancreatic ductal adenocarcinoma cells. BMC Cancer.
14:1812014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang X, Cao J, Pei Y, Zhang J and Wang Q:
Smad4 inhibits cell migration via suppression of JNK activity in
human pancreatic carcinoma PANC-1 cells. Oncol Lett. 11:3465–3470.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang G, Murashige DS, Humphrey SJ and
James DE: A positive feedback loop between Akt and mTORC2 via SIN1
phosphorylation. Cell Rep. 12:937–943. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sarbassov DD, Guertin DA, Ali SM and
Sabatini DM: Phosphorylation and Regulation of Akt/PKB by the
Rictor-mTOR Complex. Science. 307:1098–1101. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Guo Y, Zang Y, Lv L, Cai F, Qian T, Zhang
G and Feng Q: IL-8 promotes proliferation and inhibition of
apoptosis via STAT3/AKT/NF-κB pathway in prostate cancer. Mol Med
Rep. 16:9035–9042. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Saitoh M, Endo K, Furuya S, Minami M,
Fukasawa A, Imamura T and Miyazawa K: STAT3 integrates cooperative
Ras and TGF-β signals that induce Snail expression. Oncogene.
35:1049–1057. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yu M, Xue H, Wang Y, Shen Q, Jiang Q,
Zhang X, Li K, Jia M, Jia J, Xu J and Tian Y: miR-345 inhibits
tumor metastasis and EMT by targeting IRF1-mediated mTOR/STAT3/AKT
pathway in hepatocellular carcinoma. Int J Oncol. 50:975–983. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kim HN, Narayanan NK, Lasano S and
Narayanan B: Modulation of PGE2-induced EP4 expression on snail
signaling and the impact on epithelial-mesenchymal transition:
Significance of EP4 antagonism. Anticancer Res. 31:4347–4357.
2011.PubMed/NCBI
|
|
36
|
Shi Y, Zuo D, Wang X, Han M and Wu Y:
shRNA-mediated silencing of TARBP2 inhibits NCI-H1299 non-small
cell lung cancer cell invasion and migration via the JNK/STAT3/AKT
pathway. Mol Med Rep. 14:3725–3730. 2016. View Article : Google Scholar : PubMed/NCBI
|