Introduction
It has previously been demonstrated that foam cell
formation occurs when monocyte-derived macrophages accumulate
atherogenic lipoproteins, and is one of the hallmarks of early
atherosclerosis (1,2). Vascular smooth muscle cell migration
to the vascular intima and pro-inflammatory cytokine secretion are
important steps in the development of atherosclerosis (3,4). The
foam cells derived from macrophages are primarily formed by the
excessive uptake of oxidative low density lipoprotein (5,6). The
reverse cholesterol transporters (RCTs) include ATP-binding
cassette transporter (ABC) A1, ABCG1 and scavenger receptor class B
type I (SR-BI), which mediate the intracellular cholesterol efflux
to high density lipoprotein (HDL) or apolipoprotein (apo) A-I
(7,8). Ample evidence indicates that
increased RCT expression through anti-inflammatory and antioxidant
activity may reduce the accumulation of intracellular cholesterol,
thereby inhibiting the progression of atherosclerosis (9,10).
Curcumin, extracted from Curcuma longa, a widely
used traditional Chinese herb for the treatment of human diseases,
has been reported to exhibit several beneficial effects in the
cardiovascular system, including anti-inflammatory and anti-oxidant
(11,12). Currently, there is little
information regarding the role of curcumin in the setting of
cholesterol efflux. Additionally, accumulating evidence indicates
that heme oxygenase-1 (HO-1) contributes to the effect of
anti-inflammation and anti-oxidation in several cell types and
animal models (13–16). However, it remains unclear whether
HO-1 is involved in the promoting effect of curcumin on cholesterol
efflux. If it were identified that HO-1 was involved in the
promoting effect of curcumin on cholesterol efflux, then whether
the reverse cholesterol transporters (ABCA1, ABCG1 and SR-BI) serve
an important role is worthy of further study.
The present study aimed to explore the effect of
curcumin on cholesterol efflux in macrophages. It was demonstrated
that curcumin enhances SR-BI and ABCA1 expression. It was
additionally observed that curcumin activated nuclear factor,
erythroid 2 like (Nrf) 2-antioxidant response element (ARE) and
upregulated the expression of HO-1, which mediated ABCA1 and SR-BI
expression, and thereby promoted cholesterol efflux.
Materials and methods
Cell culture and treatment
RAW264.7 macrophage cells (American Type Culture
Collection, Manassas, VA, USA) were cultured in Dulbecco's modified
Eagle's medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.), 100 U/ml penicillin and 100 µg/ml streptomycin
at 37°C in a humidified atmosphere containing 5% CO2.
THP-1 cells (American Type Culture Collection) were cultured in
RPMI-1640 (Gibco; Thermo Fisher Scientific, Inc.) and underwent
induced differentiation into macrophages, as previously described
(17). Cells were cultured in
serum-free medium for 24 h, and treated with curcumin
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) or zinc
protoporphyrin (ZnPP; Sigma-Aldrich; Merck KGaA) for 1 h at 37°C.
The purity of curcumin (Sigma-Aldrich; Merck KGaA) was 99% and the
different concentrations of curcumin solution were configured by
PBS.
Preparation of nuclear proteins
Nuclear protein was extracted from cells as
previously described (18).
Protein content was determined using a bicinchoninic assay protein
reagent (Beyotime Institute of Biotechnology, Haimen, China).
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total RNA was extracted using
TRIzol®-reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). RT-PCR was performed by using Invitrogen One step III™
Reverse Transcription PCR kit (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. The PCR
thermocycling conditions were as follows: 30 cycles of 30 sec at
93°C for denaturation, 30 sec at 56°C for annealing, 45 sec at 73°C
for extension. Primer sequences: Forward,
5′-GGGTGACAGAAGAGGCTAAGACC-3′ and reverse,
5′-AGATTCTCCCCTGCAGAGAGAAG-3′ for HO-1; GAPDH forward,
5′-TGCACCACCAACTGCTTAGC-3′ and reverse, 5′-GGCATGGACTGTGGTCATGA-3′.
The products were analyzed in 1.5% agarose gel electrophoresis,
stained with ethidium bromide (Sigma-Aldrich; Merck KGaA) and
images were then captured under ultraviolet light. GAPDH served as
the internal control.
Western blot analysis
Proteins were extracted from cells using
radioimmunoprecipitation lysis buffer (Santa Cruz Biotechnology,
Inc., Dallas, TX, USA) for 30 min at 4°C. The protein content was
determined using a Bicinchoninic Acid Protein Assay kit
(Sigma-Aldrich; Merck KGaA). Total proteins (50 µg per lane) were
separated by 10% SDS-PAGE and transferred onto nitrocellulose
membranes. Membranes were blocked at room temperature for 1 h with
8% skimmed milk in Tris-buffered saline, followed by overnight
incubation at 4°C with primary antibodies and then washed, prior to
incubation for 2 h at room temperature with a goat anti-rabbit
horseradish peroxidase-conjugated IgG secondary antibody (cat. no.
SAB5300163; 1:1,000; Sigma-Aldrich; Merck KGaA) secondary antibody
staining. The rabbit primary antibodies (Anti-ABCA1 cat. no.
ab18180; 1:2,000; ABCG1; cat. no. ab52617; 1:2,000 and SR-BI; cat.
no. ab52629; 1:2,000) were purchased from Abcam (Cambridge, UK).
The other antibodies were purchased from Beyotime Institute of
Biotechnology. Anti-HO-1 (cat. no. AF1333; 1:2,000) and Anti-Nrf2
(cat. no. AF1609; 1:3,000). The bands were visualized using an
Enhanced Chemiluminescence system (GE Healthcare Life Sciences,
Little Chalfont, UK), and the band density was determined by Image
J software version 1.38 (National Institutes of Health, Bethesda,
MD, USA).
siRNA transfection
Macrophages were transfected with Nrf2 siRNA, or
HO-1 siRNA using Lipofectamine 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) for 24 h. Following transfection for 24 h, the
cells were treated with curcumin for another 12 h, then lysed for
western blot analysis.
Transient transfection and luciferase
assay
Cells were seeded in 24-well plates and grown to 70%
confluence. Cells were transfected with pGL-ARE and pRL-TK plasmid
(provided by Dr Li, Southern Medical University, Guangdong, China)
using Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol.
Following transfection for 24 h, the cells were treated with
different concentrations of curcumin (10, 20, 40 µM) for 6 h. Dual
luciferase reporter assay was performed on the lysed cells
co-transfected with pGL-ARE (firefly luciferase) and pRL-TK
(Renilla luciferase). ARE-driven promoter activity was analyzed by
a dual-luciferase reporter assay system (Dual-Glo®
Luciferase Assay System, Promega Corporation, Madison, WI, USA).
ARE luciferase activity was normalized to the Renilla luciferase
activity in each sample.
Cholesterol efflux assay
Cholesterol efflux was determined as previously
described (10). Cells (treated
with curcumin) were equilibrated with NBD cholesterol
(Sigma-Aldrich; Merck KGaA; 1 µg/ml) in the presence of apoAI (10
µg/ml). Cells with NBD cholesterol labeling were washed with PBS
and incubated for 6 h with RPMI-1640 medium. Cholesterol from cells
released into the medium with fluorescent label was tested by a
multilabel counter with 485 nm excitation and 535 nm emission.
Statistical analysis
All statistical analyses were performed using SPSS
software version 13.0 (SPSS, Inc., Chicago, IL, USA). All the data
were presented as the mean ± standard deviation of three assays.
Statistical analysis was performed using one-way analysis of
variance followed by Tukey's post hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Curcumin promotes cholesterol efflux
in Raw264.7 cells and THP-1 macrophages and ZnPP (HO-1 inhibitor)
attenuates promoting effect
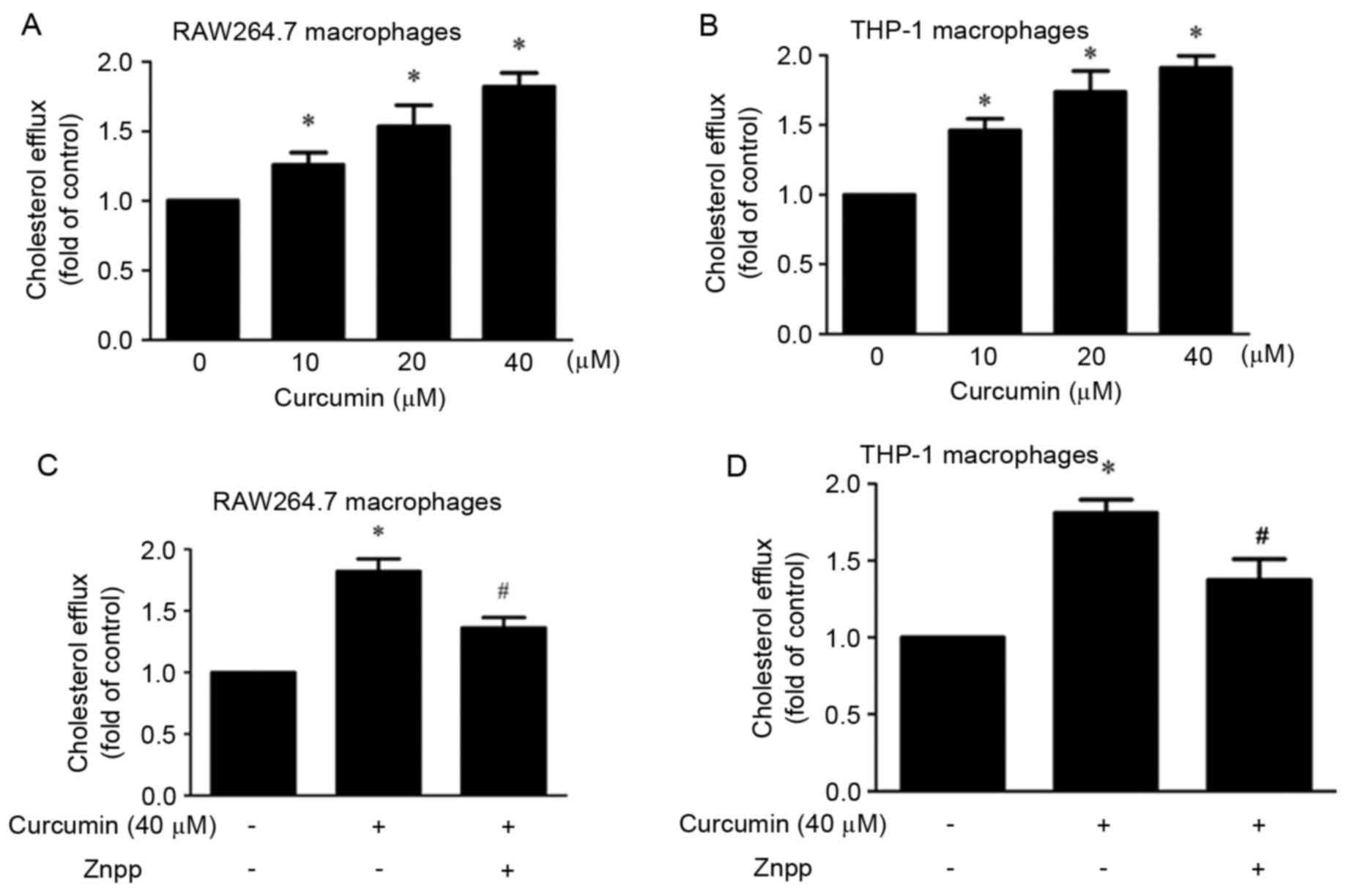
The present study investigated the effect of
curcumin on cholesterol efflux. It was demonstrated that compared
with control group, curcumin significantly promoted cholesterol
efflux in a dose-dependent manner (10, 20 and 40 µM) in macrophages
(Fig. 1A and B). In order to study
whether HO-1 mediated this effect, cells were pre-treated with
ZnPP. The results indicated that the effect was partly blocked by
ZnPP (Fig. 1C and D).
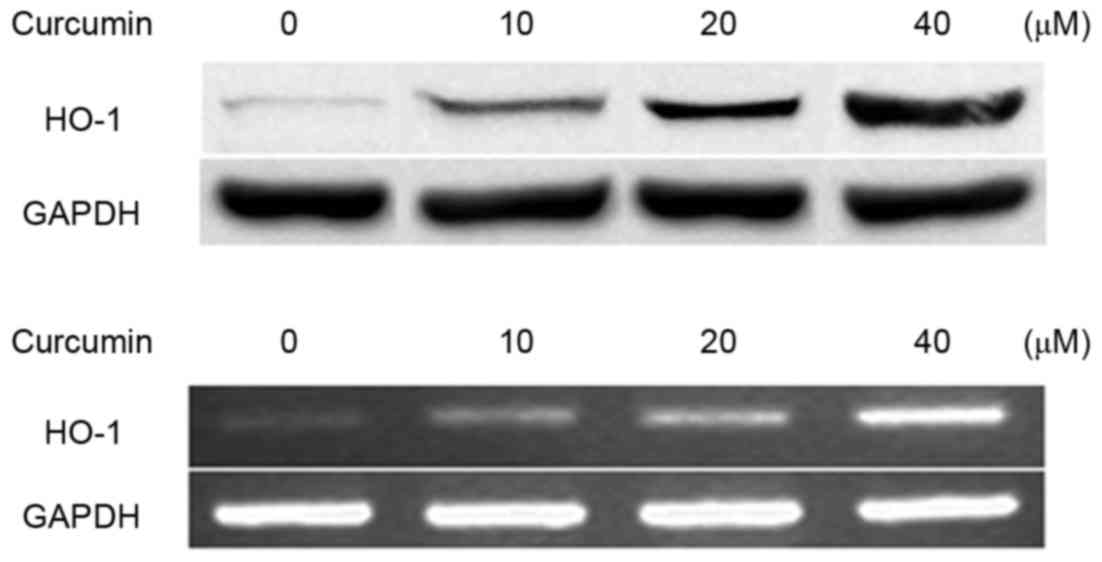
Curcumin increases HO-1 expression in
a dose-dependent manner
The effect of curcumin on the expression of HO-1 was
detected by western blotting and RT-PCR in RAW264.7 macrophages.
The results demonstrated that curcumin increased HO-1 expression at
the mRNA and protein levels in RAW264.7 macrophages (Fig. 2).
Curcumin upregulates ABCA1 and SR-BI
expression and HO-1 small interfering (si)RNA transfection
attenuates the effect of curcumin on ABCA1 and SR-BI
expression
The results demonstrated that curcumin upregulates
ABCA1 and SR-BI expression, and had no effect on expression of
ABCG1 in RAW264.7 macrophages (Fig.
3A). In order to verify whether HO-1 mediated the effect of
curcumin on ABCA1 and SR-BI expression, HO-1 siRNA (600 nM) was
transfected into macrophages, and it was observed that the
expression of HO-1 induced by curcumin decreased (Fig. 3B). Additionally, HO-1 siRNA
transfection attenuated curcumin upregulation of the expression of
ABCA1 and SR-BI. This result suggested that HO-1 exhibits a key
role in regulating the expression of ABCA1 and SR-BI (Fig. 3C).
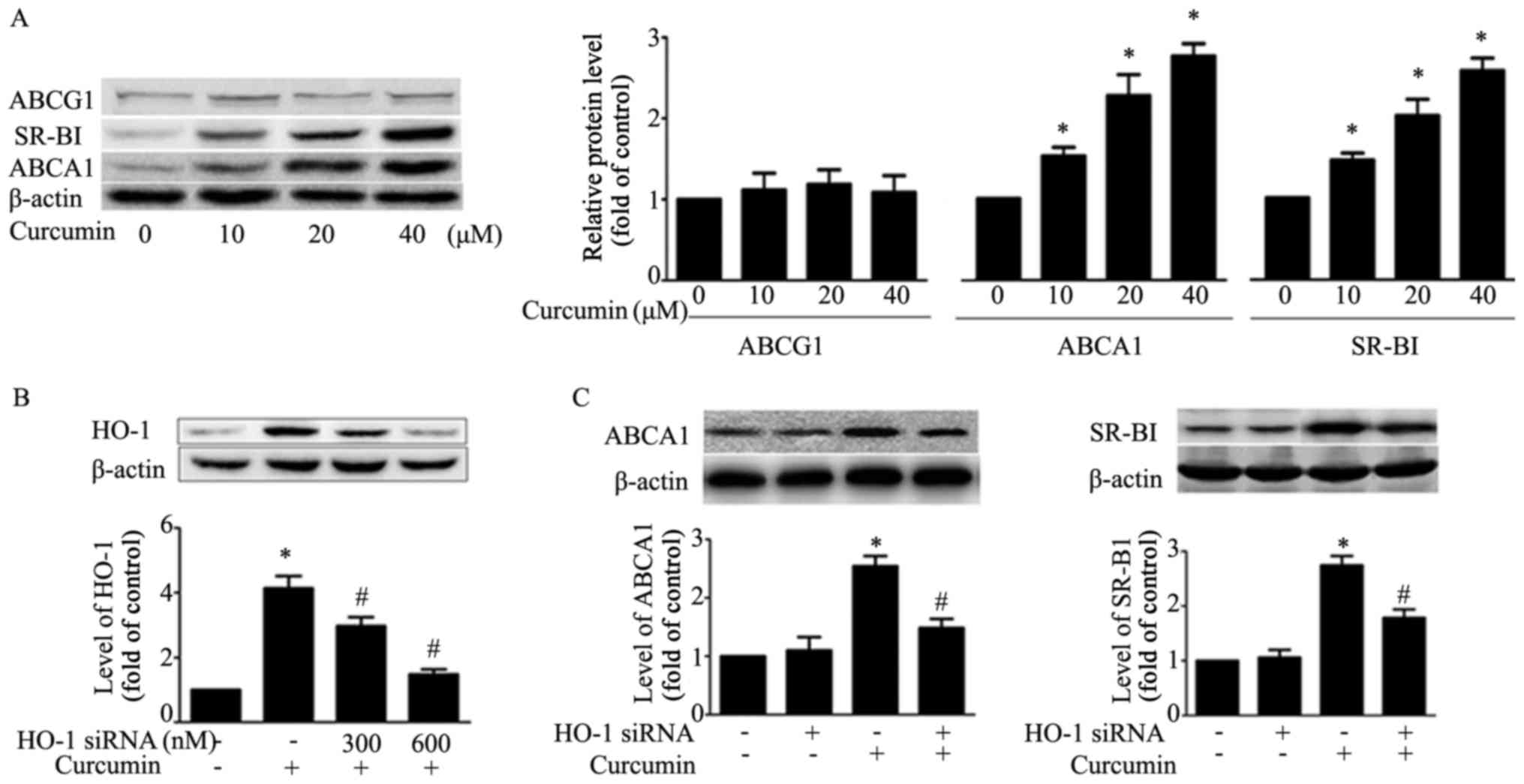 | Figure 3.Effects of curcumin on protein
expression of SR-BI, ABCA1, ABCG1 and HO-1 mediates this effect in
macrophages. (A) Macrophages were treated with indicated
concentrations (0, 10, 20, 40 µM) of curcumin for 12 h and the
protein level of SR-BI, ABCA1 and ABCG1, or β-actin was determined
by western blotting. *P<0.05 vs. untreated group. (B)
Macrophages were transfected with various concentrations of HO-1
siRNA (300, 600 nM) for 24 h, followed by curcumin treatment (40
µM) for an additional 12 h. Protein expression of HO-1 and β-actin
was measured by western blotting. (C) Macrophages were pre-treated
with HO-1 siRNA (600 nM) for 24 h, followed by curcumin for an
additional 12 h. Protein levels of SR-BI, ABCA1 and β-actin was
determined by western blotting. *P<0.05 vs. vehicle-treated
group; #P<0.05 vs. curcumin alone group. Data are
presented as the mean ± standard deviation of three independent
experiments. HO-1, heme oxygenase-1; si, small interfering; SR-BI,
scavenger receptor class B type I; ABC, ATP-binding cassette
transporter. |
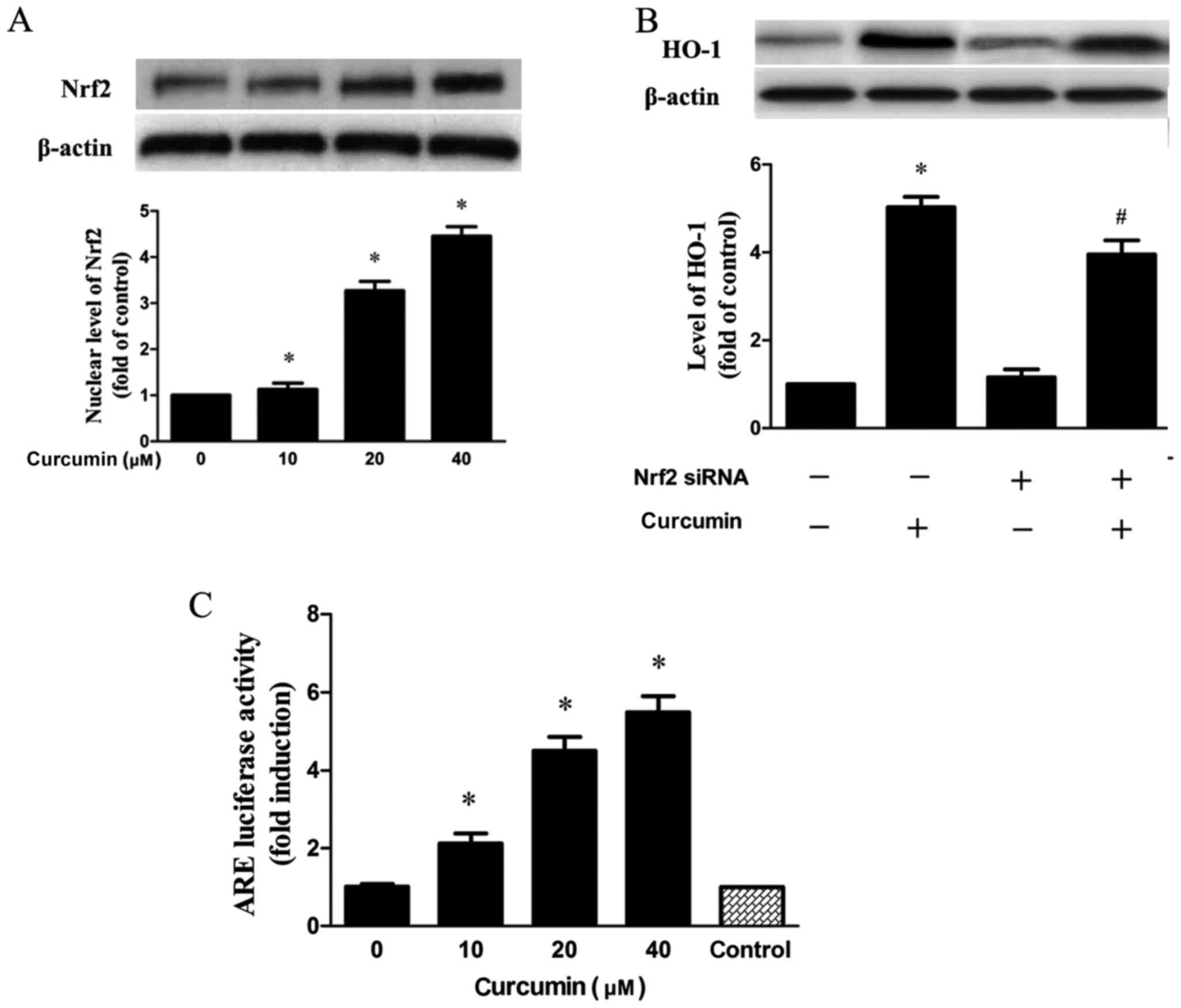
Nrf2-ARE pathway mediates
curcumin-induced HO-1 expression
Nrf2 has been verified to exhibit a key role in HO-1
expression (19). As demonstrated
in the results, Nrf2 expression in the nucleus was dose-dependently
increased when cells were treated with curcumin (Fig. 4A). In addition, transfection with
siRNA Nrf2 significantly inhibited the expression of HO-1 induced
by curcumin (Fig. 4B). Next,
RAW264.7 cells treated with curcumin were transfected with pGL-ARE
and pRL-TK plasmids containing ARE promoter region and the
luciferase structural gene. It was demonstrated that curcumin
significantly elevated Nrf2-driven luciferase activity in a
concentration-dependent manner (Fig.
4C).
Discussion
An important reason for the progression of
atherosclerosis is that monocytes differentiate into macrophages
which accumulate cholesterol in the blood vessel wall to form foam
cells. HDL is an important lipoprotein for cholesterol reverse
transport, which is the process of removing cholesterol from the
peripheral cells (20). It has
previously been demonstrated that ABCA1, SR-BI and ABCG1 exhibit
key roles in cholesterol efflux (7–9).
Therefore, SR-BI, ABCA1 and ABCG1 may be used as therapeutic
targets to prevent and cure atherosclerosis.
It has been verified that curcumin has a protective
effect in atherosclerosis (21).
However, the underlying molecular and cellular mechanisms of the
cholesterol metabolism have not been fully elucidated. The present
study investigated the effect of curcumin on cholesterol efflux in
macrophages. Firstly, it was demonstrated that curcumin promoted
cholesterol efflux from macrophages, which was consistent with
several previous reports (22,23).
HO-1 is an inducible antioxidant enzyme that mediates heme
degradation to iron and carbon monoxide, which have therapeutic
effects in atherosclerotic vascular diseases and inflammation
(24). However, the role of HO-1
regarding curcumin promotion of cholesterol efflux is largely
unknown. Therefore, cells were pre-treated with ZnPP (a HO-1
inhibitor), and it was observed that the curcumin-induced effect of
increased cholesterol efflux was partially abolished. In addition,
the results indicated that curcumin significantly increased HO-1
expression in a dose-dependent manner. Overall, the data suggested
that increased HO-1 expression induced by curcumin partly
contributed to the increased cholesterol efflux.
Data from previous studies suggest that
HO-1-dependent mechanisms mediate the regulation of cholesterol
homeostasis by other active agents (25–27).
However, the effect of HO-1 on the expression of SR-BI and ABCA1
remains to be fully elucidated. The results of the present study
demonstrated that curcumin increased SR-BI and ABCA1 expression in
macrophages. In accordance with these results, it has been reported
that curcumin upregulates SR-BI and ABCA1 expression in macrophages
(22,23). The results of the experiments
additionally indicated that the deletion of the HO-1 gene led to an
inhibitory effect on the curcumin-induced increase in ABCA1 and
SR-BI expression. The HO-1 gene may be activated by various factors
at the transcription level, including Nrf2, nuclear factor-κB and
activator protein-1 (28). Of
these factors, Nrf2 is important in upregulating HO-1 expression.
Typically, Nrf2 and its inhibitor Kelch Like ECH Associated Protein
(Keap)1 are located in the cytoplasm. Once stimulated, Nrf2 is
separated from Keap1, and translocates to the nucleus, where it
combines with ARE, leading to the promotion of the expression of
HO-1 (29). Nuclear Nrf2
accumulation is a key step in the activation of the Nrf2-ARE
pathway as a transcription factor (30).
In conclusion, the results indicated that curcumin
enhanced Nrf2 expression in the nucleus. When cells were
transfected with Nrf2 siRNA, curcumin-induced HO-1 expression was
significantly reduced. Curcumin increased ARE luciferase activity
in a concentration dependent manner, which suggested that curcumin
exhibits the ability to induce transcription of different ARE
associated genes. Overall, the results indicated that HO-1
participates in the upregulation of SR-BI and ABCA1 expression
induced by curcumin via the Nrf2-ARE pathway in macrophages. The
present study introduced a novel research direction for the key
role of HO-1 in the curcumin-induced promotion of cholesterol
efflux in macrophages. These results suggested that curcumin
activated the Nrf2-ARE pathway and upregulated HO-1 expression,
which mediated SR-BI and ABCA1 expression and thereby promoted
cholesterol efflux. Therefore, the present study may provide a
positive direction for research in atherosclerosis and it may
provide a potential therapeutic molecular target for
atherosclerosis. Further investigation is required to investigate
various in vivo models in the future.
Acknowledgements
Not applicable.
Funding
The present study was partly supported by a research
grant from science and technology strategic cooperation project of
Luzhou Municipal People's Government-Luzhou Medical College (grant
no. 2013LZLY-J53).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors’ contributions
YZ and JF conceived and designed the experiments,
YZ, JF, ZF and JL performed the experiments, YZ, JF, ZF and JL
analyzed the data and YZ and JF wrote the manuscript. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Choudhury RP, Lee JM and Greaves DR:
Mechanisms of disease: Macrophage-derived foam cells emerging as
therapeutic targets in atherosclerosis. Nat Clin Pract Cardiovasc
Med. 2:309–315. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yuan Y, Li P and Ye J: Lipid homeostasis
and the formation of macrophage-derived foam cells in
atherosclerosis. Protein Cell. 3:173–181. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lusis AJ: Atherosclerosis. Nature.
407:233–241. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Berliner JA and Heinecke JW: The role of
oxidized lipoproteins in atherogenesis. Free Radic Biol Med.
20:707–727. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li AC and Glass CK: The macrophage foam
cell as a target for therapeutic intervention. Nat Med.
8:1235–1242. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kleemann R, Zadelaar S and Kooistra T:
Cytokines and atherosclerosis: A comprehensive review of studies in
mice. Cardiovasc Res. 79:360–376. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cheng LC, Su KH, Kou YR, Shyue SK, Ching
LC, Yu YB, Wu YL, Pan CC and Lee TS: α-Lipoic acid ameliorates foam
cell formation via liver X receptor α-dependent upregulation of
ATP-binding cassette transporters A1 and G1. Free Radic Biol Med.
50:47–54. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ji A, Meyer JM, Cai L, Akinmusire A, de
Beer MC, Webb NR and van der Westhuyzen DR: Scavenger receptor
SR-BI in macrophage lipid metabolism. Atherosclerosis. 217:106–112.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen CY, Shyue SK, Ching LC, Su KH, Wu YL,
Kou YR, Chiang AN, Pan CC and Lee TS: Wogonin promotes cholesterol
efflux by increasing protein phosphatase 2B-dependent
dephosphorylation at ATP-binding cassette transporter-A1 in
macrophages. J Nutr Biochem. 22:1015–1021. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lu KY, Ching LC, Su KH, Yu YB, Kou YR,
Hsiao SH, Huang YC, Chen CY, Cheng LC, Pan CC and Lee TS:
Erythropoietin suppresses the formation of macrophage foam cells:
Role of liver X receptor alpha. Circulation. 121:1828–1837. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Seo KI, Choi MS, Jung UJ, Kim HJ, Yeo J,
Jeon SM and Lee MK: Effect of curcumin supplementation on blood
glucose, plasma insulin, and glucose homeostasis related enzyme
activities in diabetic db/db mice. Mol Nutr Food Res. 52:995–1004.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Olszanecki R, Jawień J, Gajda M, Mateuszuk
L, Gebska A, Korabiowska M, Chłopicki S and Korbut R: Effect of
curcumin on atherosclerosis in apoE/LDLR-double knockout mice. J
Physiol Pharmacol. 56:627–635. 2005.PubMed/NCBI
|
|
13
|
Zhong Y, Liu T, Lai W, Tan Y, Tian D and
Guo Z: Heme oxygenase-1-mediated reactive oxygen species reduction
is involved in the inhibitory effect of curcumin on
lipopolysaccharide-induced monocyte chemoattractant protein-1
production in RAW264.7 macrophages. Mol Med Rep. 7:242–246. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Youn GS, Kwon DJ, Ju SM, Choi SY and Park
J: Curcumin ameliorates TNF-α-induced ICAM-1 expression and
subsequent THP-1 adhesiveness via the induction of heme oxygenase-1
in the HaCaT cells. BMB Rep. 46:410–415. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim AN, Jeon WK, Lee JJ and Kim BC:
Up-regulation of heme oxygenase-1 expression through
CaMKII-ERK1/2-Nrf2 signaling mediates the anti-inflammatory effect
of bisdemethoxycurcumin in LPS-stimulated macrophages. Free Radic
Biol Med. 49:323–331. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cremers NA, Lundvig DM, van Dalen SC,
Schelbergen RF, van Lent PL, Szarek WA, Regan RF, Carels CE and
Wagener FA: Curcumin-induced heme oxygenase-1 expression prevents
H2O2-induced cell death in wild type and heme oxygenase-2 knockout
adipose-derived mesenchymal stem cells. Int J Mol Sci.
15:17974–17999. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang S, Zhou H, Feng T, Wu R, Sun X, Guan
N, Qu L, Gao Z, Yan J, Xu N, et al: β-Glucan attenuates
inflammatory responses in oxidized LDL-induced THP-1 cells via the
p38 MAPK pathway. Nutr Metab Cardiovasc Dis. 24:248–255. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhong Y, Yu W, Feng J, Fan Z and Li J:
Curcumin suppresses tumor necrosis factor-α-induced matrix
metalloproteinase-2 expression and activity in rat vascular smooth
muscle cells via the NF-κB pathway. Exp Ther Med. 7:1653–1658.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Scapagnini G, Vasto S, Abraham NG, Caruso
C, Zella D and Fabio G: Modulation of Nrf2/ARE pathway by food
polyphenols: A nutritional neuroprotective strategy for cognitive
and neurodegenerative disorders. Mol Neurobiol. 44:192–201. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rosenson RS, Brewer HB Jr, Davidson WS,
Fayad ZA, Fuster V, Goldstein J, Hellerstein M, Jiang XC, Phillips
MC, Rader DJ, et al: Cholesterol efflux and atheroprotection:
Advancing the concept of reverse cholesterol transport.
Circulation. 125:1905–1919. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wongcharoen W and Phrommintikul A: The
protective role of curcumin in cardiovascular diseases. Int J
Cardiol. 133:145–151. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu T, Li C, Sun H, Luo T, Tan Y, Tian D
and Guo Z: Curcumin inhibits monocyte chemoattractant protein-1
expression and enhances cholesterol efflux by suppressing the c-Jun
N-terminal kinase pathway in macrophage. Inflamm Res. 63:841–850.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhao JF, Ching LC, Huang YC, Chen CY,
Chiang AN, Kou YR, Shyue SK and Lee TS: Molecular mechanism of
curcumin on the suppression of cholesterol accumulation in
macrophage foam cells and atherosclerosis. Mol Nutr Food Res.
56:691–701. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Takahashi T, Morita K, Akagi R and Sassa
S: Heme oxygenase-1: A novel therapeutic target in oxidative tissue
injuries. Curr Med Chem. 11:1545–1561. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu Z, Wang J, Huang E, Gao S, Li H, Lu J,
Tian K, Little PJ, Shen X, Xu S and Liu P: Tanshinone IIA
suppresses cholesterol accumulation in human macrophages: Role of
heme oxygenase-1. J Lipid Res. 55:201–213. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li XY, Wang C, Xiang XR, Chen FC, Yang CM
and Wu J: Porphyromonas gingivalis lipopolysaccharide increases
lipid accumulation by affecting CD36 and ATP-binding cassette
transporter A1 in macrophages. Oncol Rep. 30:1329–1336. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li XY, Kong LX, Li J, He HX and Zhou YD:
Kaempferol suppresses lipid accumulation in macrophages through the
downregulation of cluster of differentiation 36 and the
upregulation of scavenger receptor class B type I and ATP-binding
cassette transporters A1 and G1. Int J Mol Med. 31:331–338. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim JW, Li MH, Jang JH, Na HK, Song NY,
Lee C, Johnson JA and Surh YJ: 15-Deoxy-Delta(12,14)-prostaglandin
J(2) rescues PC12 cells from H2O2-induced apoptosis through
Nrf2-mediated upregulation of heme oxygenase-1: Potential roles of
Akt and ERK1/2. Biochem Pharmacol. 76:1577–1589. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kobayashi M and Yamamoto M: Molecular
mechanisms activating the Nrf2-keap1 pathway of antioxidant gene
regulation. Antioxid Redox Signal. 7:385–394. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ma Q, Kinneer K, Bi Y, Chan JY and Kan YW:
Induction of murine NAD(P)H:quinone oxidoreductase by
2,3,7,8-tetrachlorodibenzo-p-dioxin requires the CNC (cap ‘n’
collar) basic leucine zipper transcription factor Nrf2 (nuclear
factor erythroid 2-related factor 2): Cross-interaction between AhR
(aryl hydrocarbon receptor) and Nrf2 signal transduction. Biochem
J. 377:205–213. 2004. View Article : Google Scholar : PubMed/NCBI
|