Introduction
Conventional use of cyclosporine A (CsA) following
kidney transplantation is considered to be associated with the
development of chronic allograft nephropathy, which leads to a
gradual and irreversible loss of graft function and is a major
cause of redialysis following renal transplantation (1). At present, the mechanism of
CsA-induced nephrotoxicity (CAN) is not fully understood, however,
results indicate that CsA may lead to an increase in reactive
oxidative metabolites and reduced renal antioxidant capacity
(2). Oxidative stress is a major
trigger of CAN. CsA directly induces endothelial cell membrane
lipid peroxidation and oxidative stress in cells, which enhances
the production of oxygen free radicals (3,4). In
addition, CsA also blocks the formation of nitric oxide, thereby
increasing the damage caused by oxidative stress. Blocking the
expression of nuclear factor-κB (NF-κB) reduces the production of
reactive oxidative metabolites and improves kidney antioxidant
capacity (5,6). Curcumin (Cur) has been reported to
increase the proliferation, reduce the rate of apoptosis and reduce
the Bcl-2-associated X (Bax)/Bcl-2 ratio in human umbilical vein
endothelial cells (7).
Oxidative stress is also associated with the
occurrence of CAN (8).
Histological manifestations of CAN include progressive
glomerulosclerosis, interstitial fibrosis associated with
mononuclear cell infiltration and renal tubular atrophy, while the
primary clinical manifestations are progressive deterioration of
renal function, hypertension and proteinuria (9,10).
These manifestations are consistent with renal injury in which
oxidative stress is the major cause. There is also evidence that
oxidative stress is involved in the glomerular atrophy observed in
interstitial fibrosis of epithelial cells to fibroblasts during the
process of metaplasia (11).
Oxidative stress in chronic graft kidney glomerular atrophy
interstitial degeneration in an animal experimental model has also
been confirmed (12), increased
cell membrane unsaturated fatty acids and cholesterol lipid
peroxidation was observed, while the cell membrane fluidity was
decreased and permeability was increased, affecting the
membrane-associated enzyme involved in the biochemical process and
ion pump function. In addition, oxidative stress may also induce
the oxidation of biological macromolecules, and protein structure
and conformational were altered through the direct effect on the
sensitive amino acids (tryptophan, tyrosine, histidine and
cysteine), or through lipid peroxidation products causing oxidative
damage, leading to cell death or apoptosis, tissue and organ damage
(13). Oxidative stress also leads
to the dysfunction of important intracellular organelles, such as
the mitochondrial inner membrane that functions in the oxidative
phosphorylation process, which leads to mitochondrial dysfunction
(14,15). Studies have demonstrated that
oxidative stress is also an important intracellular messenger,
activating various intracellular signaling pathways (16,17)
and mediating cell stress responses and injury responses. Oxidative
stress is an important pathogenic factor influencing the recovery
of short- and long-term function following renal transplantation
(18–20). The progression of CAN may be
alleviated according to the characteristics of its different stages
and the application of suitable antioxidants.
Cur is a yellow, acidic phenol that is extracted
from Curcuma longa L., also termed Turmeric, is one member
of ginger family (21). Cur is the
major active ingredient that has a pharmacological role. Cur is a
type of plant polyphenol that exhibits a wide range of biological
activities, including antioxidative, anti-inflammatory, anticancer,
anti-atherosclerotic, antimutagenic and immunoregulatory effects
(22). Cur has antioxidant
capacity in neutral and acidic environments, and interferes with
cell signaling at various levels and affects biological enzyme
activity, angiogenesis and cell adhesion (23,24).
It was also reported in a preclinical study that Cur influences
gene transcription and induces apoptosis (25). In rat kidneys, Cur alleviates
damage caused by nephrotoxic substances, including doxorubicin,
cyclosporine, gentamicin, chloroquine and ischemia-reperfusion
injury due to its antioxidant properties (26–28).
The potential of Cur in the prevention and treatment of diabetic
nephropathy is primarily based on its antioxidative (29,30)
and antifibrotic (31) properties;
to the best of our knowledge, its role in inflammatory lesions has
not previously been reported. Cur is reported to inhibit the
activity of lipoxygenase and cyclooxygenase (32), reduce free radicals generated from
the arachidonic acid pathway, suppress xanthine oxidase activity
(33), decrease adenosine
metabolism of free radicals and interfere with nitric oxide
synthase activity (34) to reduce
the arginine metabolism of nitric oxide caused by the generation of
free radicals, thereby limiting oxygen free radical damage and
exhibiting a protective role. Cur may function in the antioxidant
process through various mechanisms (35,36):
Cur and its derivatives inhibit metal ion (Fe2+ and
Cu2+)-induced lipid peroxidation, inhibits oxidative
modification of low density lipoprotein and protects DNA from
oxidative lipid damage; Cur inhibits the production of reactive
oxygen species and scavenging free radicals (including ·OH, DPPH·
and O2−) and peroxide. Cur reduces serum and tissue
lipid peroxide levels and enhances superoxide dismutase (SOD) and
glutathione peroxidase (GSH-Px) activity; Cur inhibits NADPH
oxidase expression and the activation of xanthine oxidase 8; and
Cur inhibits the synthesis of nitric oxide or accelerates its
clearance so that levels in the kidney are low to prevent nitric
oxide toxicity. The effect of oxidative stress on chronic CsA renal
injury in rats and the effect of Cur on renal injury have not been
widely reported. Therefore, the present study aimed to provide an
experimental basis for the further development and application of
the natural active substance, Cur.
Materials and methods
Drugs
Cur, with a purity of 99.8%, was purchased from
Shijiazhuang spring letter Biotechnology Co., Ltd. (Shijiazhuang,
China; http://www.sjzcxswkj.com) and CsA was
obtained from Zhejiang Ruibang Pharmaceutical Co., Ltd. (Wenzhou,
China).
Primary reagents
Rabbit anti-human Bax primary antibody and
horseradish peroxidase-conjugated goat anti-rabbit secondary
antibody were purchased from Cell Signaling Technology, Inc.
(Danvers, MA, USA). Rabbit anti-human Bcl-2 primary antibody and
β-actin primary antibody were purchased from Abcam (Cambridge, UK).
SOD (cat no. A001-3), malondialdehyde (MDA; cat no. A003-1), GSH-Px
(cat no. A005), reactive oxygen species (ROS; cat no. E004) and
catalase (CAT; cat no. A007-1-1) detection kits were all purchased
from Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
Other reagents were of domestic and analytical grade.
HK-2 human renal cells culture
HK-2 cells were purchased from Beijing Zhongyuan
Jinqiao Biotechnology Co., Ltd. (Beijing, China). The base medium
for this cell line was provided by Invitrogen; Thermo Fisher
Scientific, Inc. (Waltham, MA, USA) as part of a kit: Keratinocyte
serum free medium (K-SFM; kit cat no. 17005-042). This kit was
supplied with each of the two additives required to grow this cell
line (bovine pituitary extract (BPE) and human recombinant
epidermal growth factor (EGF). The following components were added
the base medium: 0.05 mg/ml BPE-provided with the K-SFM kit; 5
ng/ml EGF-provided with the K-SFM kit. Atmosphere: air, 95%; carbon
dioxide (CO2), 5%, 37.0°C and humidity 70–80%.
Detection of oxidative stress in HK-2
human renal cells
HK-2 cells (2×104 cells/well) were
assigned to the following groups for 48 h at 37°C in a 5%
CO2 incubator: Control (equivalent volume of saline), 50
µM Cur, 2 µM CsA, 10 µM Cur + 2 µM CsA, 50 µM Cur + 2 µM CsA, 100
µM Cur + 2 µM CsA and 5 µM N-acetyl-L-cysteine (NAC; Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany). Cells were washed with PBS,
collected in a test tube with 1 ml normal saline and lysed with an
ultrasonic crusher. After centrifugation at 12,000 × g for 10 min
at 4°C, SOD activity and MDA levels in the supernatant were
measured by SOD and MDA kits, a GSH-Px detection kit was used to
measure GSH-Px activity, an ROS detection kit was used to measure
ROS levels and a CAT detection kit was used to determine CAT
activity.
MTT assay
HK-2 cells in logarithmic growth phase were
inoculated in 96-well plates (5×103 cells per well),
added with different concentrations of Cur (0.1, 1, 10, 100 and
1,000 µM) and then co-incubated with or without (2 µM) for 24 h at
37°C. After incubating at 37°C for 4 h with 20 µl MTT (5 mg/ml),
200 µl DMSO was added. After 10 min vibration, the absorbance value
of the mixture was measured at 560 nm using a microplate
reader.
Western blot analysis
HK-2 cells in the control and different treatment
groups were washed three times with cold PBS and lysed with RIPA
lysis buffer (Beyotime Institute of Biotechnology, Haimen, China;
cat. no. P0013B) on ice. Lysates were collected and centrifuged at
12,000 × g for 10 min at 4°C, the supernatants were collected and
protein concentration was determined by a BCA assay. A 10% SDS-PAGE
gel was prepared and samples were loaded (80 µg protein per lane),
and run on the gel. After 2 h of SDS-PAGE, proteins were
transferred to polyvinylidene difluoride membranes. Subsequently,
blocking with 5% non-fat milk was performed for 1 h at room
temperature and the membranes were incubated with diluted primary
antibodies, including rabbit anti-human Bax (cat. no. 5023; 1:1,500
dilution), anti-β actin antibody (cat no. ab227387; 1:2,000
dilution) and Bcl-2 (cat. no. ab194583; 1:2,000 dilution)
antibodies overnight at 4°C. The membrane was washed three times
with 1X TBS 0.1% Tween-20 and then the horseradish
peroxidase-conjugated goat anti-rabbit secondary antibody (cat. no.
7074; 1:4,000) was added and incubated with the membrane at room
temperature for 2 h. Grey scale and area of the protein band were
analyzed by gel imaging system (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) with enhanced chemiluminescent kit (SuperSignal;
Pierce, Thermo Fisher Scientific, Inc.; cat no. 34094). The protein
expression level was indicated by the integral grey value (D,
density) using Image J software (National Institutes of Health,
USA).
Apoptosis assay
HK-2 cells treated with medium or different drugs
for 48 h were collected and treated according to the Annexin V-FITC
Apoptosis Detection kit (Vazyme Biotech; cat no. a211-01). After
washing twice with PBS, cells were suspended with 1X binding
buffer, added with 5 µl of fluorescein isothiocyanate labeled
Annexin V and 5 µl of PI, and then mixed gently. After incubation
at room temperature for 15 min, the cells were analyzed by flow
cytometry. A total of 20,000 cells were analyzed each time.
CAN rats model
Male Sprague-Dawley rats (n=60; 7 weeks of age),
clean grade and weighing 200–250 g, were purchased from the
Experimental Animal Center of Changzhou Cavens Laboratory Animal
Co., Ltd. (Changzhou, China) and kept under controlled conditions
(temperature 23±1.5°C, relative humidity 40–60%, 0.03–0.04%
CO2, 12/12 light/dark cycle)- at the Animal Center of
Ningbo University (Ningbo, China). Rats were allowed free access to
drinking water and food. The present study was approved by the
animal ethical committee of the Medical School of Ningbo
university. The rats were randomly divided into four groups
according to their body weight, with n=6 per group: Control group,
Cur group (30 mg/kg/day) (37)-,
CsA group (20 mg/kg/day) and Cur (30 mg/kg/day) + CsA (20
mg/kg/day) group, all of which were administered for 21 days. The
control group received 0.9% sodium chloride injection (4 ml/kg/day,
intragastric) + vegetable oil (2 ml/kg/day, subcutaneous), the Cur
group received Cur (30 mg/kg/day, intragastric) + vegetable oil (2
ml/kg/day, subcutaneous), the CsA group received 0.9% sodium
chloride injection (4 ml/kg/day, intragastric) + CsA (20 mg/kg/day
dissolved in vegetable oil, subcutaneous) and the CsA + Cur group
received Cur (30 mg/kg/day, intragastric) + CsA (20 mg/kg/day
dissolved in vegetable oil, subcutaneous).
Renal function test
On the 22nd day, 24 h urine of rats was collected by
a metabolic cage. The rats were anesthetized with ether and 3.0–4.0
ml blood was collected to obtain serum by centrifugation at 1,400 ×
g for 30 min at 4°C. Serum/urine creatinine (Crea) and blood urea
nitrogen (BUN) levels were measured with a Hitachi Model 7060
automatic biochemical analyzer, and creatinine clearance (Ccr) was
calculated as follows: Ccr=[urine Crea concentration ×1 h urine
volume (ml)]/serum Crea concentration.
Histopathological examination
On the 22nd day, rat kidneys were fixed with 5%
formaldehyde at 4°C for 48 h to prepare paraffin blocks and tissue
sections (5 µm) following dehydration. For histological
investigation, the deparaffinized and rehydrated rat kidney tissue
sections were stained with hematoxylin for 15 min and 1% eosin for
3 min at room temperature by the Hematoxylin and Eosin Staining kit
(Beyotime Institute of Biotechnology; cat no. C0105). For periodic
acid-Schiff (PAS) staining, the slides were immersed in periodic
acid solution for 10 min, and then immersed in Schiff's solution
for 30 min at 20°C after being rinsed with distilled water 4 times
using a Periodic Acid Schiff (PAS) Staining kit (cat no. ab150680).
After rinsing slides under hot running tap water, the slides were
stained with hematoxylin for 3 min, rinsed in running tap water for
3 min and applied the bluing reagent for 30 sec. Finally, the
distilled water rinsed slides were dehydrated through graded
alcohols. Expression of NF-κB in kidney tissue was detected by
immunohistochemistry. In brief, tissues in paraffin sections were
treated with the following procedures, including dewaxing,
incubation with anti-NF-kB p65 antibody (cat no. ab16502; 1:1,000
dilution) for overnight at 4°C and horse radish
peroxidse-conjugated goat anti-rabbit secondary antibody (CST; cat
no. 7074) for 2 h at room temperature, stained with DAB and mounted
with anti-fade oil. All sections were observed at ×200
magnification on a light microscope (37).
Statistical analysis
All statistical analyses were performed with PASW
Statistics 18.0 software (SPSS, Inc., Somers, USA). Data are
presented as the mean ± standard deviation. One-way analysis of
variance and the Tukey post-hoc test were used to analyze the
significance between the groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
Cur alleviates oxidative stress in
CsA-treated HK-2 cells
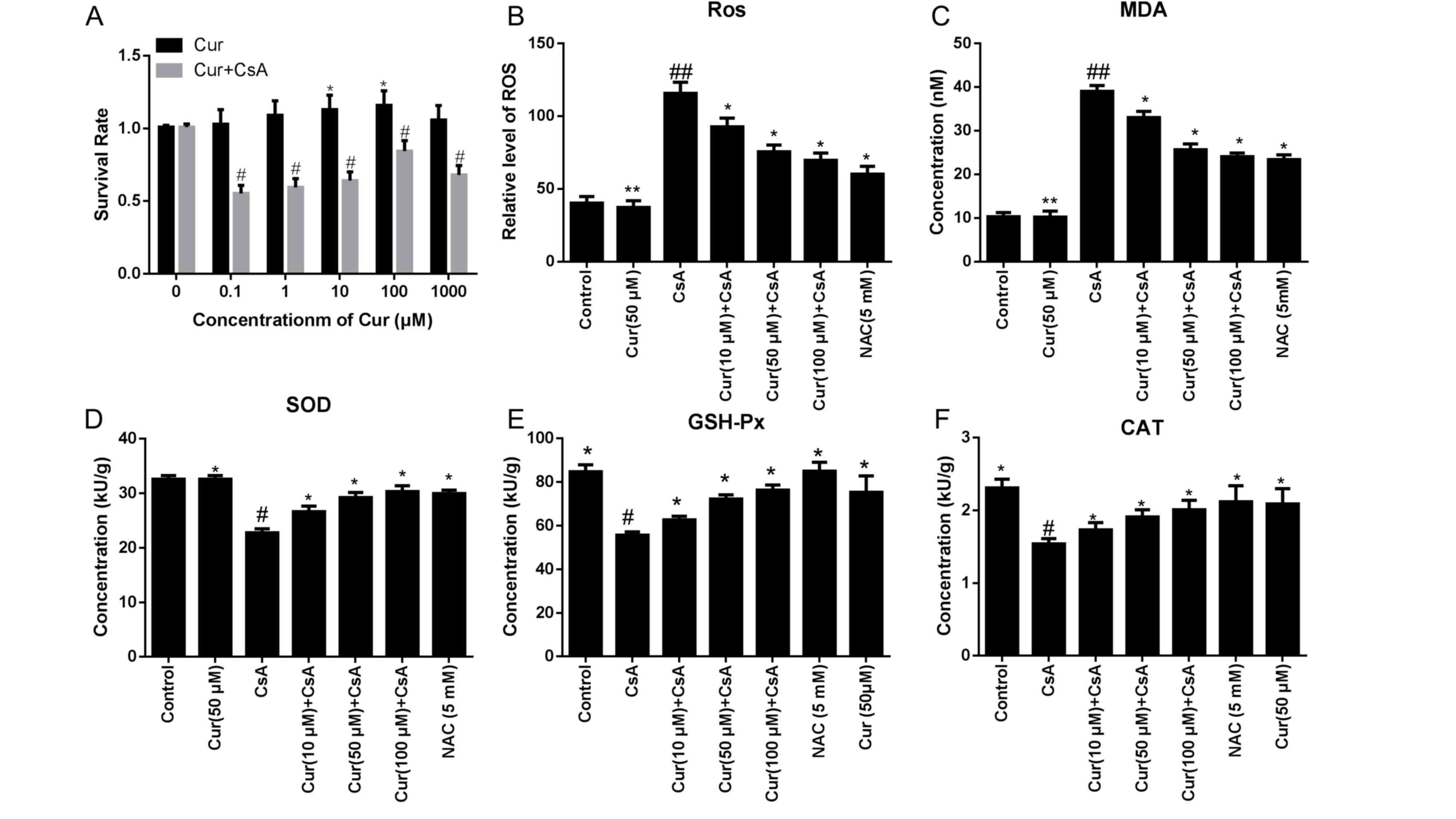
In vitro, a concentration of 100 µM Cur improved
cell viability when treated with CsA, compared with lower
concentrations of Cur (Fig. 1A).
The levels of ROS and MDA were significantly downregulated in a
dose-dependent manner following treatment with Cur, compared with
the CsA alone group, as demonstrated in Fig. 1B and C. The ROS level in the CsA
alone group was 115.67±7.66 KU/g, while levels in the 100 µM Cur +
CsA group were 71.67±5.35 KU/g (P<0.01; Fig. 1B). MDA levels in the CsA alone
group were 39.01±1.36, while levels in the 100 µM Cur + CsA group
were 23.85±1.22 nM (P<0.01; Fig.
1C). In addition, the activity of SOD was increased in the 100
µM Cur + CsA group (22.76±0.73 KU/g in the CsA alone group vs.
32.6±0.66 KU/g in the 100 µM Cur + CsA group; P<0.05; Fig. 1D) and the GSH-Px activity was also
increased following treatment with 100 µM Cur (55.65±1.45 in the
CsA alone group vs. 76.04±2.07 KU/g in the 100 µM Cur + CsA group;
P<0.05; Fig. 1E). Furthermore,
CAT activity was increased in the 100 µM Cur-treated group compared
with the CsA alone group (1.54±0.07 in the CsA alone group vs.
2.31±0.12 KU/g in the 100 µM Cur + CsA group; P<0.05; Fig. 1F). CsA-induced increases in ROS and
MDA, and decreases in SOD, GSH-Px and CAT activity, were
dose-dependently reversed by treatment with Cur (Fig. 1B-F).
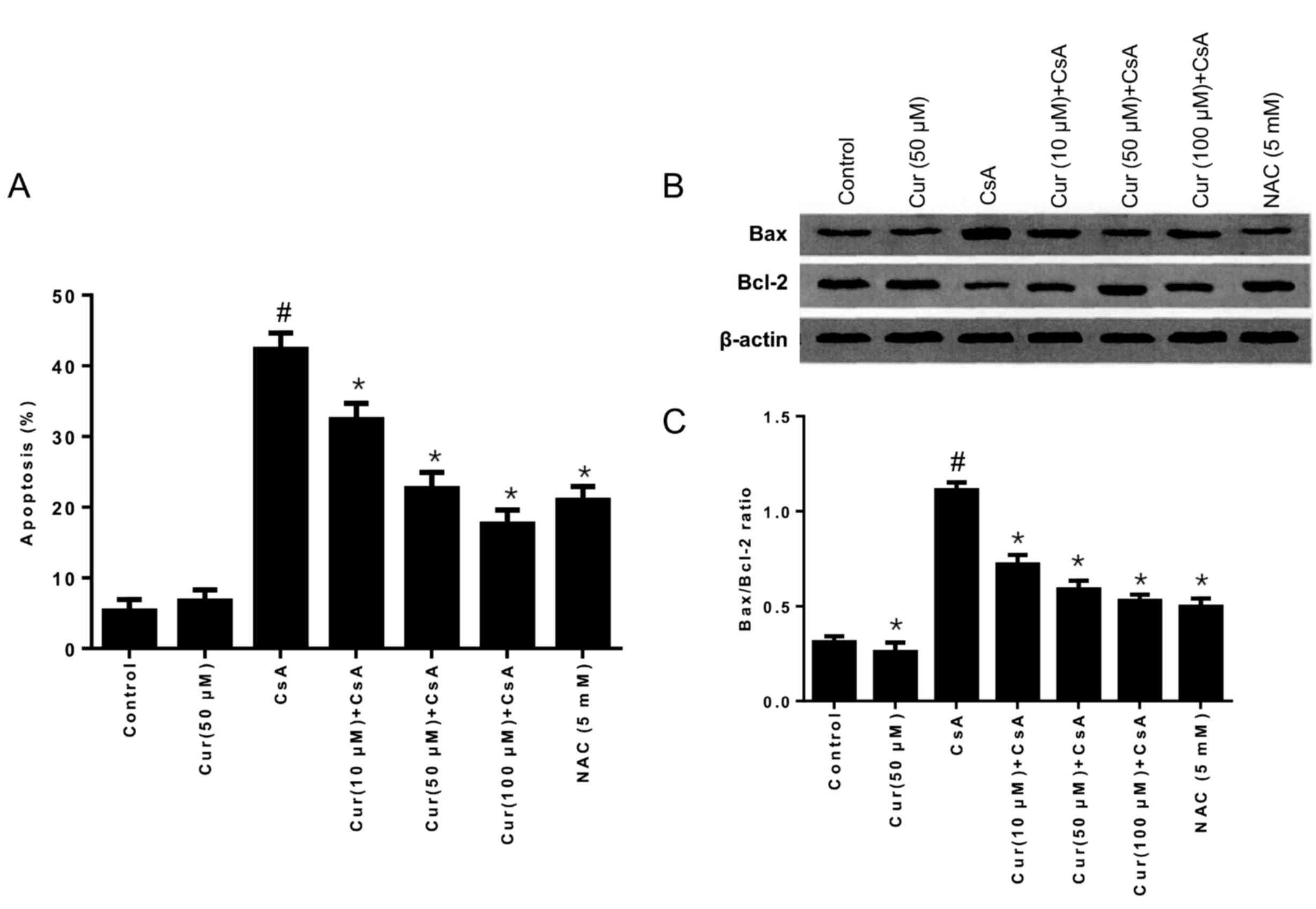
Effects of Cur on Bax and Bcl-2
protein expression
The results in Fig.
2A demonstrate that the rate of apoptosis was gradually
decreased with increasing concentrations of Cur in HK-2 cells,
compared with the CsA alone group, which indicated that Cur may
inhibit CsA-induced cell apoptosis. The ratio of Bax/Bcl-2 is an
important indicator in the balance between cell apoptosis and
survival. The ratio of Bax/Bcl-2 was decreased when CsA was
combined with Cur treatment, compared with the CsA alone group, as
demonstrated in Fig. 2B and C,
which indicates that levels of the anti-apoptotic protein Bcl-2
were higher compared with the proapoptotic protein Bax, thus
indicating that Cur may improve cell survival in CsA-treated HK-2
cells.
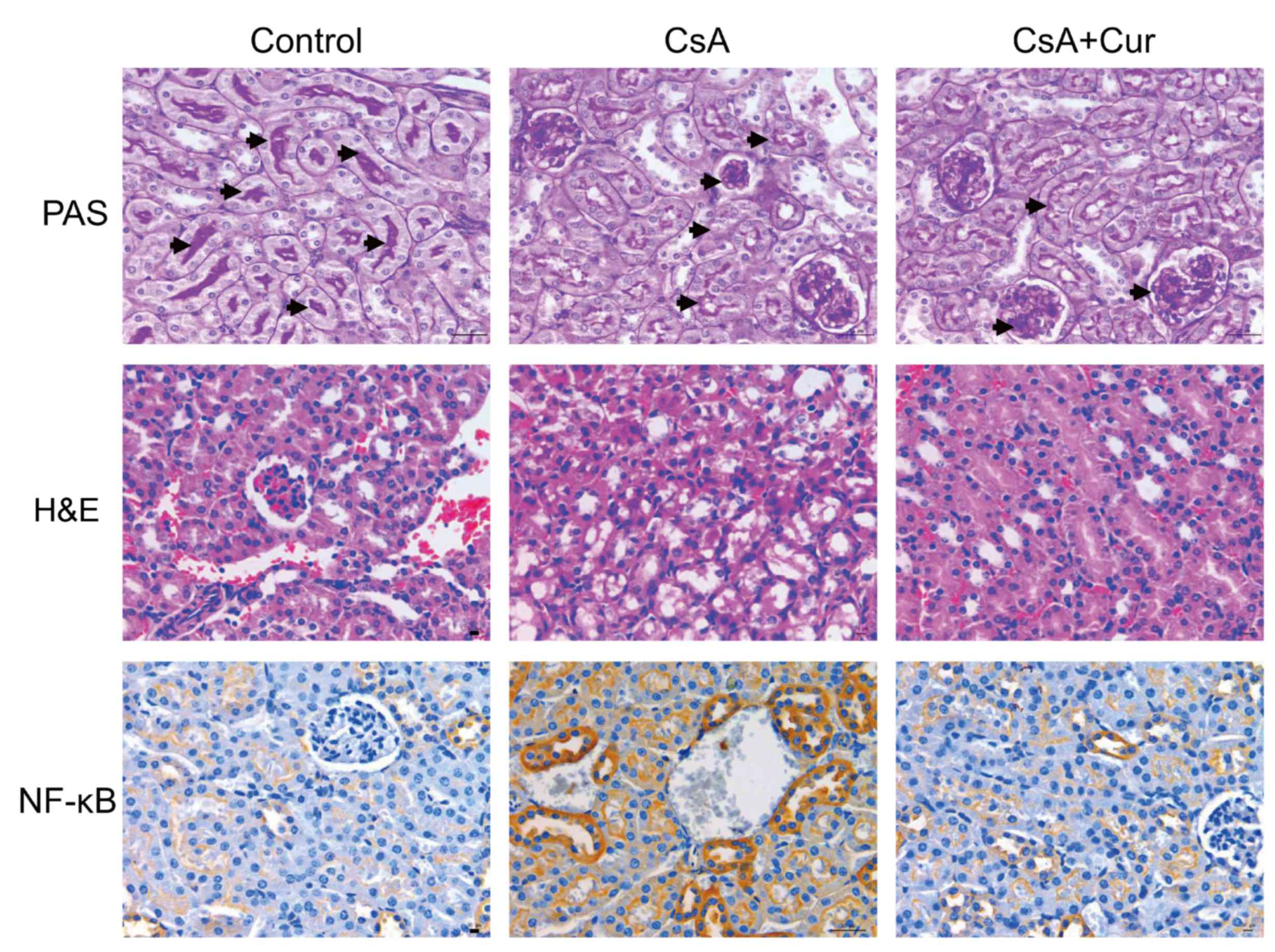
Effect of Cur on CsA-induced CAN model
in rats
To further evaluate the protective effect of Cur in
a CsA-induced rat nephrotoxicity model, renal histopathological
analysis was performed. Histopathological results are presented in
Fig. 3. Hematoxylin and eosin
staining demonstrated that the tissue sections of the control group
exhibited normal structure. By contrast, the kidneys of rats
treated with CsA exhibited marked histological changes, cellular
edema, vacuolar deformation and dissolution. These changes were
reduced when CsA was combined with Cur treatment. The nucleus
presented blue and the glomerular basement membrane was purple. The
glomerular volume was markedly increased in the CsA group, with
mesangial matrix hyperplasia (positive PAS staining), glomerular
swelling, an irregular morphology of certain glomeruli, lobulated
capillary loops and a markedly increased renal capsular space.
NF-κB-positive cell cytoplasm or nuclei were stained brown. The
results demonstrated that NF-κB-positive cells, and therefore
inflammation, were markedly increased in the CsA group compared
with the control group, which was reduced when CsA was combined
with Cur treatment.
Renal function in Cur-treated CAN
rats
As demonstrated in Table I, renal failure was induced by
continuous administration of CsA (20 mg/kg/day) for 21 days. The
serum Crea and BUN were significantly elevated and the Ccr rate was
significantly reduced (P<0.05) in the CsA alone group compared
with the control group. Cur (30 mg/kg/day) alone did not
significantly reduce renal function in rats compared with the
control group, however, Cur significantly improved CsA-induced
renal failure.
 | Table I.Effect of Cur on CsA-induced renal
dysfunction in rats. |
Table I.
Effect of Cur on CsA-induced renal
dysfunction in rats.
| Group | BUN, mmol/l | Serum Crea,
µmol/l | Ccr, ml/min |
|---|
| Cur |
4.77±0.52 |
53.12±5.32 |
0.22±0.08 |
| CsA |
10.47±1.45c |
68.33±10.12b |
0.17±0.11a |
| Cur+CsA |
6.68±0.91b,e |
57.98±8.96a,d |
0.18±0.09d |
| Control |
4.88±0.71 |
49.62±6.23 |
0.22±0.06 |
Discussion
CsA is a cyclic polypeptide composed of 11 amino
acids. As a potent immunosuppressive agent, CsA specifically acts
on lymphocytes and inhibits the synthesis and release of
lymphokines, such as interleukin-2 (38). Quiescent lymphocytes are in the G0
phase of the cell cycle and the early G1 phase. Animal experiments
have demonstrated that CsA extended the survival time of allogeneic
organ transplantation and inhibited the cell-mediated immune
response (39). CsA-induced
dose-dependent renal toxicity is the primary reason for its limited
clinical application, which results in renal tubular atrophy,
vacuolar degeneration and renal failure (40). In the present study, rats were
given CsA for 21 consecutive days, and the results demonstrated
marked nephrotoxicity, with increased levels of Crea and BUN,
reduced Ccr and marked vacuolar degeneration and tubular atrophy in
the kidney. A previous study demonstrated that CsA-induced
increases in ROS, leading to cell membrane lipid peroxidation, is
one potential mechanism of CsA-induced nephrotoxicity, while
another study reported that CsA induced in vivo inducible
nitric oxide synthase expression, resulting in high concentrations
of nitric oxide and ROS generation, and increased free radical
activity (2). Peroxynitrite, a
powerful free radical, has been reported to regulate the
tricarboxylic acid cycle, mitochondrial function, electron transfer
and affect DNA synthesis, resulting in increased pathological
damage (34,41). The results of the present study,
from experiments on HK-2 cells, also confirmed that CsA treatment
significantly increased the levels of MDA and Bax protein
expression, and decreased SOD activity and Bcl-2 expression. Cur is
an excellent hydrogen or neutron donor and, during redox reactions
generated as a result of excessive free radicals, the body converts
free radicals into phenolic oxygen free radicals, which protects
the organism against free radical damage. In the body, Cur reacts
with free radicals to generate more stable phenolic oxygen free
radicals, thereby inactivating free radicals (27). In addition, Cur has been reported
to exhibit a protective effect against CsA-induced nephrotoxicity
in rats, as demonstrated by histological alterations and
Glutathione S-transferase immune expression (42). Tirkey et al (42) reported that, through its
antioxidant activity, Cur effectively salvaged CsA-induced
nephrotoxicity. Furthermore, Hu et al (43) demonstrated that the protective
effect of Cur may be mediated by inhibiting the hypermethylation of
the klotho promoter. The present study confirmed that Cur
significantly increased renal antioxidant capacity and decreased
the Bax/Bcl-2 ratio in CsA-treated HK-2 cells, which may be
associated with the improvements in CsA-induced renal failure and
renal tubular deformation and cell vacuolization following Cur
treatment in rats. In addition, Tirkey et al (42) demonstrated that Cur markedly
reduced elevated levels of thiobarbituric acid reactive substances,
significantly attenuated renal dysfunction and increased the levels
of antioxidant enzymes in CsA-treated rats and normalized the
altered renal morphology. The development of renal diseases has
been previously associated with NF-κB activation. NF-κB regulates
the transcription of inflammatory factors in mesangial and tubular
epithelial cells; therefore, it has a central role in the
development and progression of renal disease (44). Wang et al (45) demonstrated that limb ischemic
preconditioning-induced renoprotection in contrast-induced
nephropathy may be dependent on increased renalase expression via
activation of the tumor necrosis factor-α/NF-κB pathway. Renalase
may contribute to the renal protective effect of delayed ischaemic
preconditioning via the regulation of hypoxia-inducible factor-1α
(45,46). In conclusion, the present study
demonstrated that Cur exhibited a protective effect on CsA-induced
renal dysfunction, and the underlying mechanism may be associated
with the antioxidant capacity of Cur in experimental animals.
However, further investigation is required to determine whether Cur
may be used as a clinical adjuvant to reduce renal toxicity induced
by CsA.
Acknowledgements
This present study was funded by Ningbo Yinzhou
Science and Technology Bureau Project [grant no. 2011 (111)].
References
|
1
|
Jorga A, Holt DW and Johnston A:
Therapeutic drug monitoring of cyclosporine. Transplant Proc. 36 2
Suppl:pp. 396S–403S. 2004; View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tedesco D and Haragsim L: Cyclosporine: A
review. J Transplant. 2012:2303862012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhong Z, Arteel GE, Connor HD, Yin M,
Frankenberg MV, Stachlewitz RF, Raleigh JA, Mason RP and Thurman
RG: Cyclosporin A increases hypoxia and free radical production in
rat kidneys: Prevention by dietary glycine. Am J Physiol.
275:F595–F604. 1998.PubMed/NCBI
|
|
4
|
Pérez de Lema G, Arribas I, Prieto A,
Parra T, de Arriba G, Rodríguez-Puyol D and Rodríguez-Puyol M:
Cyclosporin A-induced hydrogen peroxide synthesis by cultured human
mesangial cells is blocked by exogenous antioxidants. Life Sci.
62:1745–1753. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mariee AD and Abd-Ellah MF: Protective
effect of docosahexaenoic acid against cyclosporine A-induced
nephrotoxicity in rats: A possible mechanism of action. Ren Fail.
33:66–71. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tavares P, Fontes Ribeiro CA and Teixeira
F: Cyclosporin effect on noradrenaline release from the sympathetic
nervous endings of rat aorta. Pharmacol Res. 47:27–33. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wan Q and Liu ZY: Effect of curcumin on
human umbillcal veln endothellal cell injury induced by lipocalin
2. Chin J Arterioscler. 21:229–233. 2016.(In Chinese).
|
|
8
|
Djamali A, Reese S, Yracheta J, Oberley T,
Hullett D and Becker B: Epithelial-to-mesenchymal transition and
oxidative stress in chronic allograft nephropathy. Am J Transplant.
5:500–509. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shrestha BM and Haylor J: Biological
pathways and potential targets for prevention and therapy of
chronic allograft nephropathy. Biomed Res Int. 2014:4824382014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Maryniak RK, First MR and Weiss MA:
Transplant glomerulopathy: Evolution of morphologically distinct
changes. Kidney Int. 27:799–806. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kang HM, Ahn SH, Choi P, Ko YA, Han SH,
Chinga F, Park AS, Tao J, Sharma K, Pullman J, et al: Defective
fatty acid oxidation in renal tubular epithelial cells has a key
role in kidney fibrosis development. Nat Med. 21:37–46. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fonseca I, Reguengo H, Almeida M, Dias L,
Martins LS, Pedroso S, Santos J, Lobato L, Henriques AC and
Mendonça D: Oxidative stress in kidney transplantation:
Malondialdehyde is an early predictive marker of graft dysfunction.
Transplantation. 97:1058–1065. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Andersen JK: Oxidative stress in
neurodegeneration: Cause or consequence? Nat Med. 10 Suppl:S18–S25.
2004. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Priya DK, Gayathri R, Gunassekaran GR and
Sakthisekaran D: Protective role of sulforaphane against oxidative
stress mediated mitochondrial dysfunction induced by benzo(a)pyrene
in female Swiss albino mice. Pulm Pharmacol Ther. 24:110–117. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wei Y, Clark SE, Thyfault JP, Uptergrove
GM, Li W, Whaley-Connell AT, Ferrario CM, Sowers JR and Ibdah JA:
Oxidative stress-mediated mitochondrial dysfunction contributes to
angiotensin II-induced nonalcoholic fatty liver disease in
transgenic Ren2 rats. Am J Pathol. 174:1329–1337. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Evans JL, Goldfine ID, Maddux BA and
Grodsky GM: Oxidative stress and stress-activated signaling
pathways: A unifying hypothesis of type 2 diabetes. Endocr Rev.
23:599–622. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yu JH and Kim H: Oxidative stress and
inflammatory signaling in cerulein pancreatitis. World J
Gastroenterol. 20:17324–17329. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Romero F, Herrera J, Nava M and
Rodríguez-Iturbe B: Oxidative stress in renal transplantation with
uneventful postoperative course. Transplant Proc. 31:pp. 2315–2316.
1999; View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ruiz MC, Moreno JM, Ruiz N, Vargas F,
Asensio C and Osuna A: Effect of statin treatment on oxidative
stress and renal function in renal transplantation. Transplant
Proc. 38:pp. 2431–2433. 2006; View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vostálová J, Galandáková A, Svobodová AR,
Kajabová M, Schneiderka P, Zapletalová J, Strebl P and Zadražil J:
Stabilization of oxidative stress 1 year after kidney
transplantation: Effect of calcineurin immunosuppressives. Ren
Fail. 34:952–959. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nelson KM, Dahlin JL, Bisson J, Graham J,
Pauli GF and Walters MA: The essential medicinal chemistry of
curcumin. J Med Chem. 60:1620–1637. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gupta SC, Patchva S, Koh W and Aggarwal
BB: Discovery of curcumin, a component of golden spice, and its
miraculous biological activities. Clin Exp Pharmacol Physiol.
39:283–299. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sharma M, Manoharlal R, Puri N and Prasad
R: Antifungal curcumin induces reactive oxygen species and triggers
an early apoptosis but prevents hyphae development by targeting the
global repressor TUP1 in Candida albicans. Biosci Rep. 30:391–404.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rahmani AH, Al Zohairy MA, Aly SM and Khan
MA: Curcumin: A potential candidate in prevention of cancer via
modulation of molecular pathways. Biomed Res Int. 2014:7616082014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Prakobwong S, Gupta SC, Kim JH, Sung B,
Pinlaor P, Hiraku Y, Wongkham S, Sripa B, Pinlaor S and Aggarwal
BB: Curcumin suppresses proliferation and induces apoptosis in
human biliary cancer cells through modulation of multiple cell
signaling pathways. Carcinogenesis. 32:1372–1380. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fujisawa S, Atsumi T, Ishihara M and
Kadoma Y: Cytotoxicity, ROS-generation activity and
radical-scavenging activity of curcumin and related compounds.
Anticancer Res. 24:563–569. 2004.PubMed/NCBI
|
|
27
|
Calabrese V, Bates TE, Mancuso C,
Cornelius C, Ventimiglia B, Cambria MT, Di Renzo L, De Lorenzo A
and Dinkova-Kostova AT: Curcumin and the cellular stress response
in free radical-related diseases. Mol Nutr Food Res. 52:1062–1073.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Trujillo J, Chirino YI, Molina-Jijón E,
Andérica-Romero AC, Tapia E and Pedraza-Chaverrí J: Renoprotective
effect of the antioxidant curcumin: Recent findings. Redox Biol.
1:448–456. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pan Y, Wang Y, Cai L, Cai Y, Hu J, Yu C,
Li J, Feng Z, Yang S, Li X and Liang G: Inhibition of high
glucose-induced inflammatory response and macrophage infiltration
by a novel curcumin derivative prevents renal injury in diabetic
rats. Br J Pharmacol. 166:1169–1182. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim BH, Lee ES, Choi R, Nawaboot J, Lee
MY, Lee EY, Kim HS and Chung CH: Protective effects of curcumin on
renal oxidative stress and lipid metabolism in a rat model of type
2 diabetic nephropathy. Yonsei Med J. 57:664–673. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pang XF, Zhang LH, Bai F, Wang NP, Garner
RE, McKallip RJ and Zhao ZQ: Attenuation of myocardial fibrosis
with curcumin is mediated by modulating expression of angiotensin
II AT1/AT2 receptors and ACE2 in rats. Drug Des Devel Ther.
9:6043–6054. 2015.PubMed/NCBI
|
|
32
|
Huang MT, Lysz T, Ferraro T, Abidi TF,
Laskin JD and Conney AH: Inhibitory effects of curcumin on in vitro
lipoxygenase and cyclooxygenase activities in mouse epidermis.
Cancer Res. 51:813–819. 1991.PubMed/NCBI
|
|
33
|
Shen L and Ji HF: Insights into the
inhibition of xanthine oxidase by curcumin. Bioorg Med Chem Lett.
19:5990–5993. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ben P, Liu J, Lu C, Xu Y, Xin Y, Fu J,
Huang H, Zhang Z, Gao Y, Luo L and Yin Z: Curcumin promotes
degradation of inducible nitric oxide synthase and suppresses its
enzyme activity in RAW 264.7 cells. Int Immunopharmacol.
11:179–186. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tzankova V, Gorinova C, Kondeva-Burdina M,
Simeonova R, Philipov S, Konstantinov S, Petrov P, Galabov D and
Yoncheva K: Antioxidant response and biocompatibility of
curcumin-loaded triblock copolymeric micelles. Toxicol Mech
Methods. 27:72–80. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sun YM, Zhang HY, Chen DZ and Liu CB:
Theoretical elucidation on the antioxidant mechanism of curcumin: A
DFT study. Org Lett. 4:2909–2911. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hao CX, Su YJ, Wang QJ, et al: Inhibition
effect of curcumin on AP-1 DNA binding activity increased in the
kidney of experimental diabetic rats. J Med Pest Control.
27:417–418. 2011.(In Chinese).
|
|
38
|
Shin GT, Khanna A, Ding R, Sharma VK,
Lagman M, Li B and Suthanthiran M: In vivo expression of
transforming growth factor-beta1 in humans: Stimulation by
cyclosporine. Transplantation. 65:313–318. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Siemionow M, Ozer K, Izycki D, Unsal M and
Klimczak A: A new method of bone marrow transplantation leads to
extention of skin allograft survival. Transplant Proc. 37:pp.
2309–2314. 2005; View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sereno J, Rodrigues-Santos P, Vala H,
Rocha-Pereira P, Alves R, Fernandes J, Santos-Silva A, Carvalho E,
Teixeira F and Reis F: Transition from cyclosporine-induced renal
dysfunction to nephrotoxicity in an in vivo rat model. Int J Mol
Sci. 15:8979–8997. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Buffoli B, Pechánová O, Kojsová S,
Andriantsitohaina R, Giugno L, Bianchi R and Rezzani R: Provinol
prevents CsA-induced nephrotoxicity by reducing reactive oxygen
species, iNOS, and NF-kB expression. J Histochem Cytochem.
53:1459–1468. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tirkey N, Kaur G, Vij G and Chopra K:
Curcumin, a diferuloylmethane, attenuates cyclosporine-induced
renal dysfunction and oxidative stress in rat kidneys. BMC
Pharmacol. 5:152005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hu Y, Mou L, Yang F, Tu H and Lin W:
Curcumin attenuates cyclosporine A-induced renal fibrosis by
inhibiting hypermethylation of the klotho promoter. Mol Med Rep.
14:3229–3236. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhang H and Sun SC: NF-κB in inflammation
and renal diseases. Cell Biosci. 5:632015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wang F, Yin J, Lu Z, Zhang G, Li J, Xing
T, Zhuang S and Wang N: Limb ischemic preconditioning protects
against contrast-induced nephropathy via renalase. EBioMedicine.
9:356–365. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wang F, Zhang G, Xing T, Lu Z, Li J, Peng
C, Liu G and Wang N: Renalase contributes to the renal protection
of delayed ischaemic preconditioning via the regulation of
hypoxia-inducible factor-1α. J Cell Mol Med. 19:1400–1409. 2015.
View Article : Google Scholar : PubMed/NCBI
|