Introduction
Prostate cancer (PCa), one of most common
malignancy, is a severe health threatening illness for older men in
the world (1). PCa primarily
begins as being hormone dependent tumour, then it transforms into
being hormone independent tumour and resists to hormone related
therapy as the disease advances (2). Currently, several treatment
approaches are accessible for PC patients, including surgery,
radiation or androgen-deprivation therapy (3–6).
However, it is necessary to discover novel agents to surmount
several adverse effects related with present therapies.
Natural products, derived from plants or microbes,
have developed a crucial resource of anti-cancer treatments.
Piperine, one of the most popular spices used in food and in
traditional Chinese medicine, is a chief plant alkaloid present in
long and black peppers (Piper nigrum Linn and Piper
longum Linn). It has been broadly informed that piperine has an
extensive pharmacological properties, such as antioxidant (7), anti-inflammatory (8,9),
hepatoprotective (10),
antimicrobial (11,12), immunomodulatory and anticancer
activities (13,14). In spite of its broad use and its
tendency to reduce the chances of several cancer types, the
valuable effects of piperine against PCa are poorly understood.
Thus, there is great interest in recognizing therapeutic effect in
the treatment of PCa.
Though there is plenty of data for cancer
preventative and therapeutic effects, the impact of piperine on PCa
cell migration has not yet been fully explored, nor is the
molecular mechanism of piperine-mediated prohibition of cancer cell
migration obvious at this moment. In this work, we investigated the
influence of piperine in prostate carcinoma cell migration, and the
underlying mechanisms of piperine on cell migration. Our results
indicated that piperine depressed the migration process via
downregulating the protein kinase B (Akt)/mammalian target of
rapamycin (mTOR)/matrix metalloproteinase (MMP)-9 signaling pathway
in DU145 cells.
Materials and methods
Cell lines and cell culture
The androgen-independent PCa cell line (DU145) was
purchased from the American Type Culture Collection (ATCC;
Manassas, VA, USA). DU145 cells were cultured in DMEM medium
(HyClone; GE Healthcare Life Sciences, Logan, UT, USA) added with
10% FBS (Invitrogen, Victoria, Australia), 100 IU/ml penicillin and
100 µg/ml streptomycin (nvitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) and all cells were incubated at 37°C in a
humidified incubator at 5% CO2.
Cell viability assay
To explore the cell proliferation, we used the Cell
Counting Kit-8 (CCK-8) (WST-8; Dojindo Molecular Technologies,
Inc., Kumamoto, Japan). Cells (7×103 cells/well) were
planted onto 96-well plates and raised for 24 h. Next cells were
cultured with piperine for 48 h, and subsequently CCK-8 solution
(10 µl) was added into the 96-well plates. After 4 h of culture,
the absorbance at 450 nm was read using a plate reader (Molecular
Devices, Sunnyvale, CA, USA).
Flow cytometry analysis of apoptotic
cells
DU145 cells were planted at a concentration of
1×106/ml onto 6-well culture plates. While the cells
achieved about 70% degree confluence, the medium was exchanged, and
piperine (160 µM) or diluent was supplemented. Subsequently, DU145
cells were trypsinized to obtain a single cell suspension. One
million cells were double-stained with APC-labeled annexin V and
propidium iodide (BD Biosciences, Franklin Lakes, NJ, USA).
Proportion of apoptotic cells was determined by flow cytometry (BD
Biosciences).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from DU145 cells and then
quantitated by an ultraviolet spectrophotometer. Next cDNA was
synthesized from RNA (200 ng) in a 20 µl reverse transcription
reaction (Promega Corporation, Madison, WI, USA). qPCR was worked
as follow: 30 sec at 94°C for denaturation, 30 sec at 54°C for
annealing and 30 sec at 65°C for extension, for a total of 30
cycles. qPCR was completed with the SYBR green real-time PCR kit
(Qiagen, Hilden, Germany) and the ABI 7500 Sequence Detection
System (Applied Biosystems; Thermo Fisher Scientific, Inc.). PCR
products were run on 2% agarose gels to prove that correct
molecular sizes were present. Each sample was analyzed in
triplicate using RT-qPCR. The primers for RT-qPCR as follow: MMP-9,
forward 5′-AATCTCTTCTAGAGACTGGGAAGGAG-3′ and reverse
5′-AGCTGATTGACTAAAGTAGCTGGA-3′; GAPDH, forward
5′-AGAGAGAGGCCCTCAGTTGCT-3′ and reverse
5′-TTGTGAGGGAGATGCTCAGTGT-3′, synthesized by BGI Tech (Shenzhen
Co., Ltd., Shenzhen, China). GAPDH was used as an internal control.
Fold changes were analyzed using the 2−ΔΔCq method.
Western blotting
Total proteins were got by cell lysis in ice-cold
RIPA buffer in DU145 cells. Cells extracts were subjected to a 10%
polyacrylamide gel and blotted onto a polyvinylidene difluoride
(PVDF). The blot was then blocked with 5% non-fat milk in TBST for
2 h and subsequently incubated at 4°C overnight with primary
antibodies anti-MMP-9 (1:1,000), as well as p-Akt, t-Akt, p-mTOR,
mTOR, Bcl-2, Bax, and anti-GAPDH (1:500), and then cultivated with
goat anti-rabbit IgG-HRP. The bands were visualized via ECL Western
blot detection reagents (Thermo Fisher Scientific, Inc.), and the
results were analyzed by Quantity One (Bio-Rad Laboratories, Inc.
Hercules, CA, USA) software.
Transwell migration assay
Trypsinized cells suspended in serum-free DMEM
medium were planted onto upper chamber at a concentration of
5×105/well, and 600 µl of medium added with 10% serum
were supplemented to the inferior chamber. After 48 h, the cells
were wiped off of the inserts with a cotton swab. 4%
paraformaldehyde (PFA) was used to fix the residual cells migrating
to the other side of the membrane. Then the cells were stained with
Crystal Violet for 10 min, and subsequently the mean amount of
those cells was analyzed to assess their migrated ability. Images
were captured with the Olympus microscope.
Statistical analysis
All quantitative data were performed as mean ± SEM
and one-way ANOVA followed by post-hoc Tukey's test to analyze the
differences between sets of data. Statistics were completed using
the SPSS 20.0 software (IBM Corp., Armonk, NY, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Piperine repressed cell proliferation
and apoptosis in PCa DU145 cells
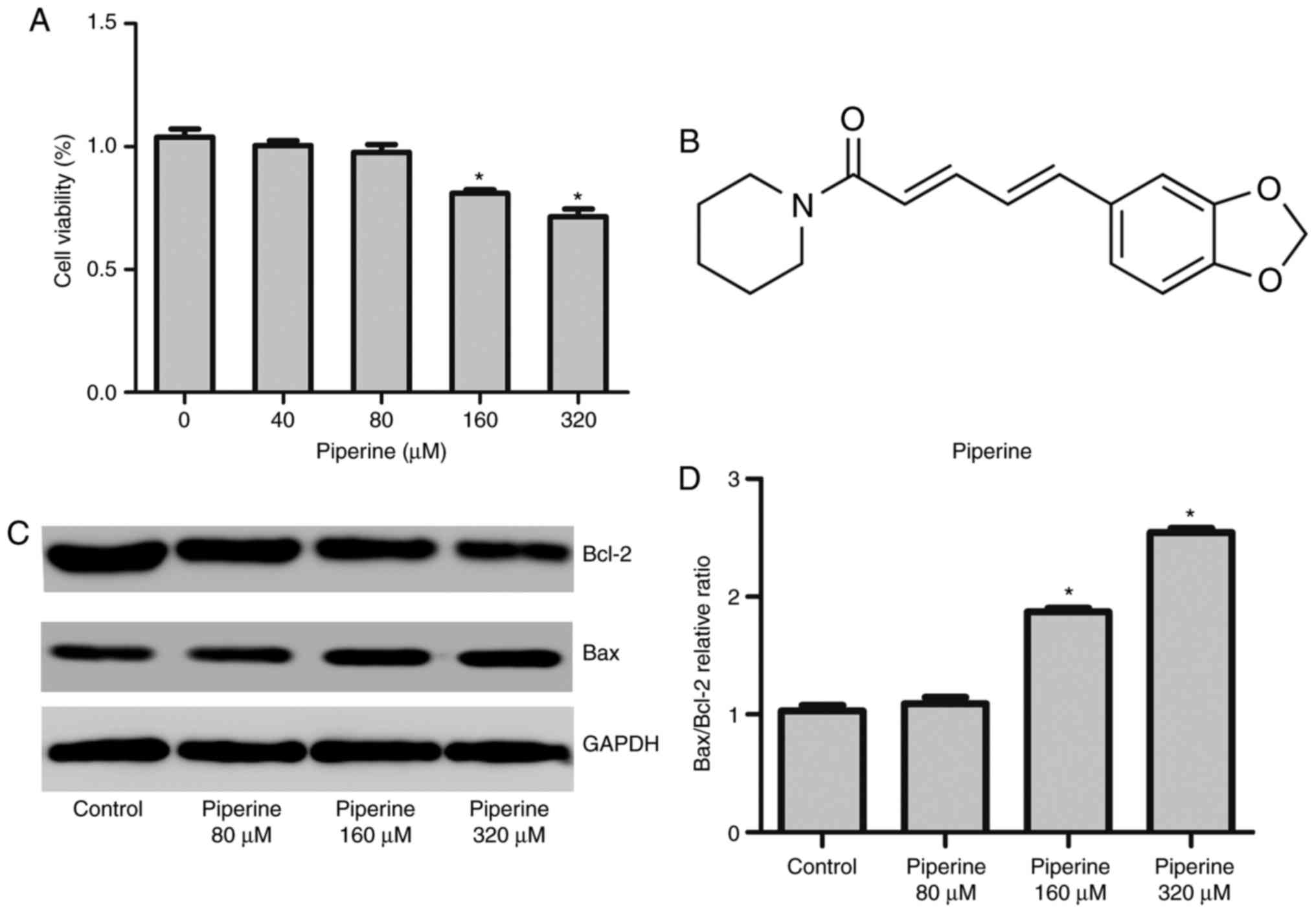
To explore the cytotoxic effects of piperine on
human PCa cells, DU145 cells were pretreated with piperine for 48
h, and subsequently examined the viability by the CCK-8 assay. The
outcome was that piperine treatment remarkably diminished cell
proliferation in a dose-dependent manner (Fig. 1A).
The chemical structure of piperine was showed as
Fig. 1B. To uncover the role of
piperine on cell proliferation and apoptosis, the western blotting
and the Annexin V apoptosis detection kit was performed as
previously reported (15). DU145
cells were pretreated with piperine (80, 160 or 320 µM) for 48 h
and subsequently stained with Annexin V and propidium iodide to
visualize the cells numbers via fluorescent microscope. Besides,
piperine treatment group reduced the expression of antiapoptotic
protein Bcl-2 and promoted the expression of proapoptotic protein
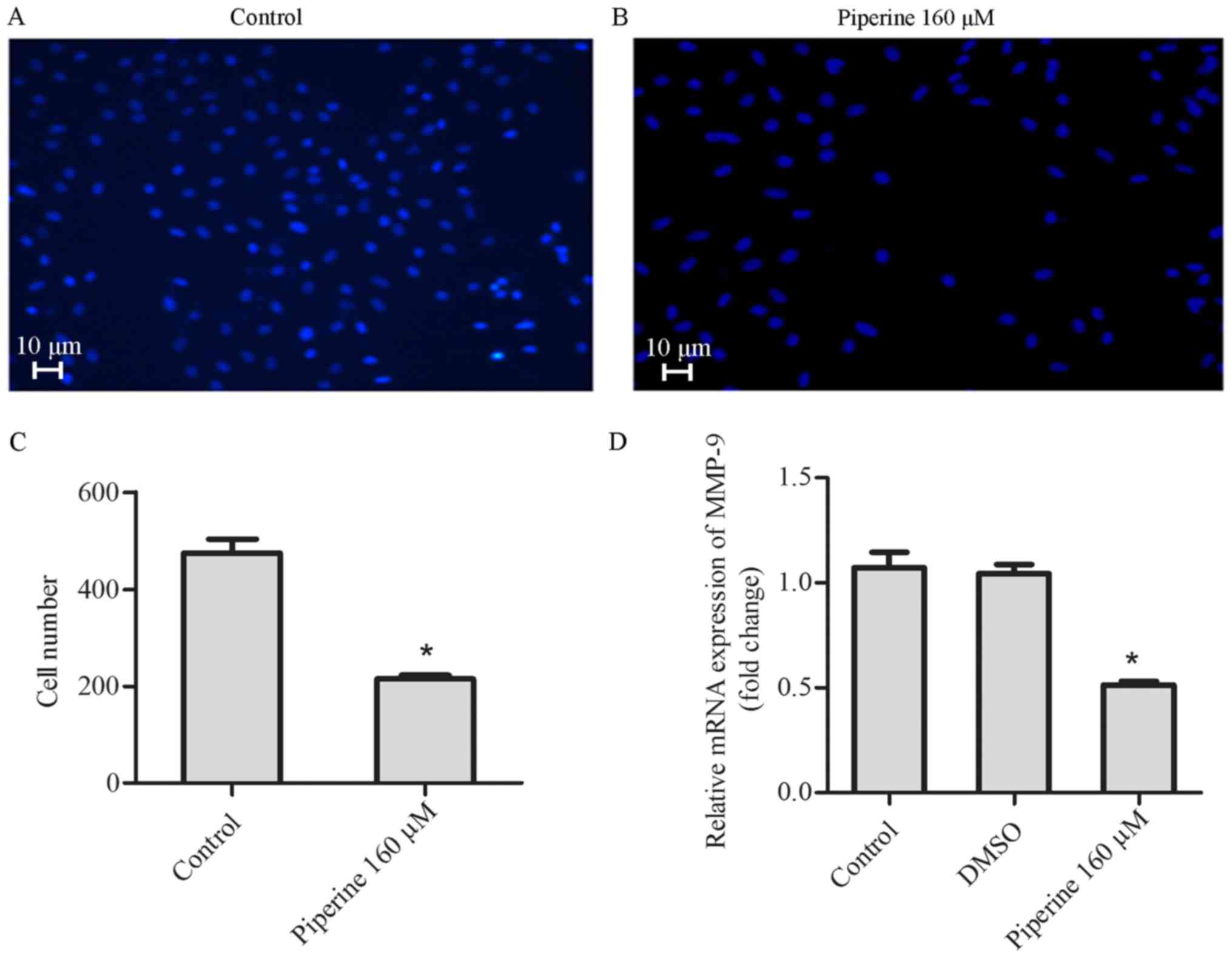
Bax (Fig. 1C and D). We observed
that the apoptotic cells markedly increased in piperine treatment
group compared to the control group (Fig. 2A and B). Based on the above
results, we uncovered that piperine could promote cell apoptosis
and hinder cell proliferation in DU145 cells in a dose-dependent
manner, and the concentration 160 µM was selected for further
mechanistic studies.
Piperine reduced the migration of
DU145 cells
To establish how piperine influences the metastatic
processes, the role of piperine on DU145 cell migration was
performed. In Transwell migration assay, piperine decreased cell
migration significantly after 48 h in the DU145 cell (Fig. 3A-C). In agreement, results of
western blot and qPCR disclosed that piperine could also suppress
the expression of MMP-9 (Figs. 3D
and 4A-B). These consequences
indicated that piperine could inhibit the migration of DU145
cells.
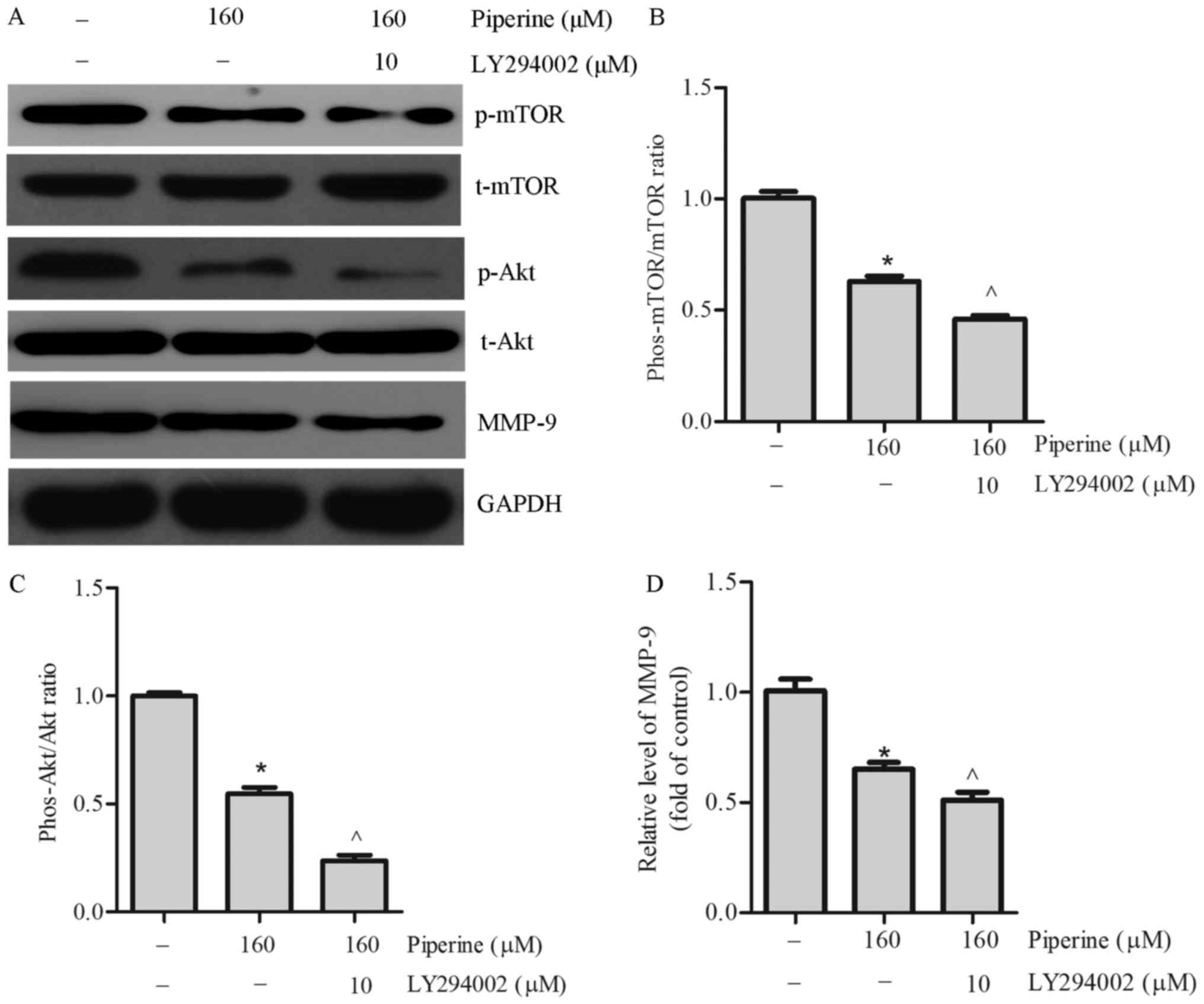
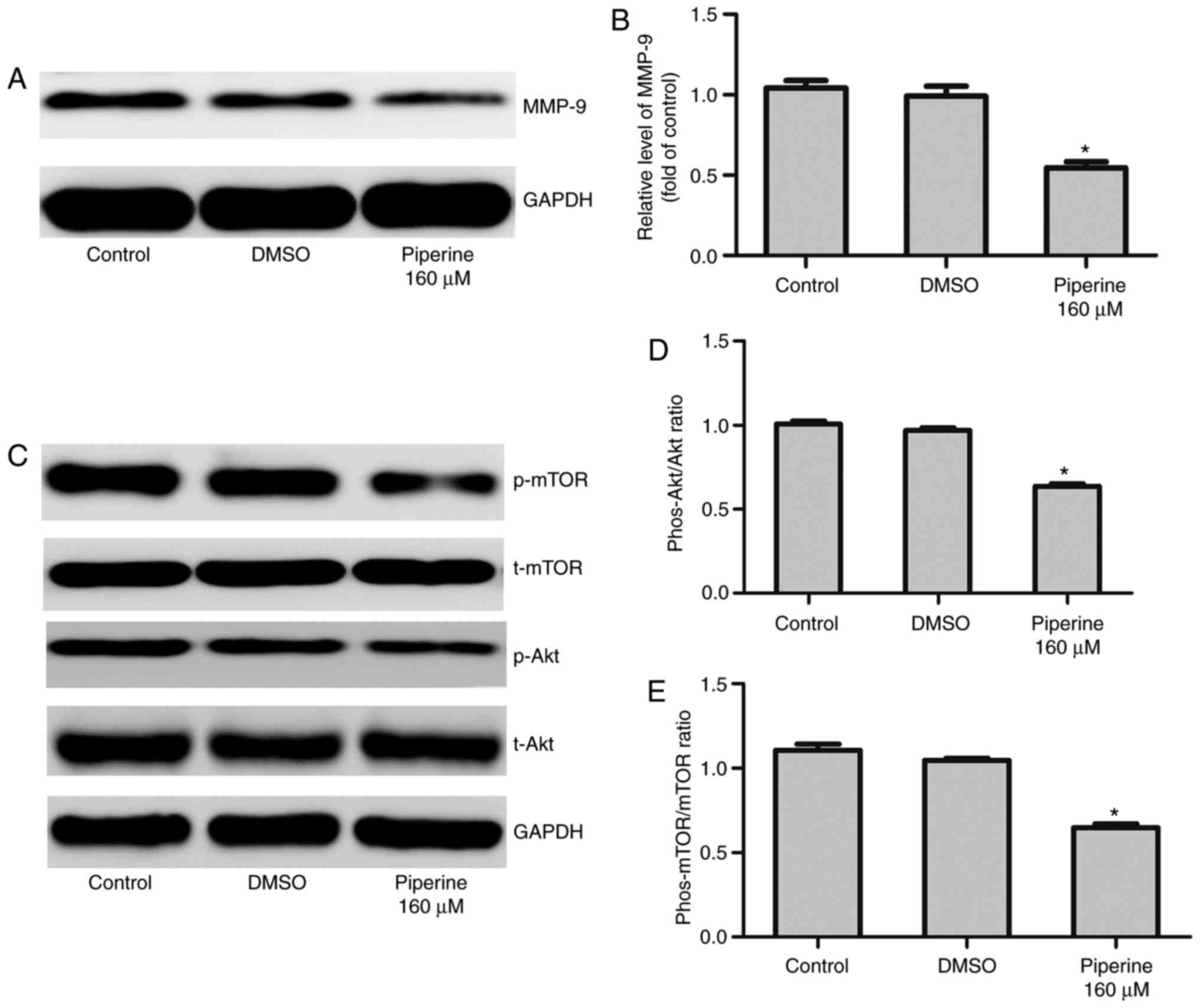 | Figure 4.Piperine significantly decreases
p-AKT, p-mTOR and MMP-9 expression in DU145 cells. Cells were
cultured with piperine (160 µM) for 48 h. (A) Western blotting was
performed to determine the indicated protein levels of (B) MMP-9,
(C) Akt, p-Akt, p-mTOR and mTOR. Densitometric measurements of
protein analysis for the ratio of phosphorylated to total (D) Akt
and (E) mTOR were also evaluated. Data are presented as the mean ±
standard deviation (n=3). *P<0.05 vs. control group. p-/phos-,
phosphorylated; t-, total; Akt, protein kinase B; MMP-9, matrix
metalloproteinase-9; mTOR, mammalian target of rapamycin; DMSO,
dimethylsulfoxide. |
Piperine inhibited DU145 cells
migration via Akt/mTOR signaling pathway
Numerous reports indicate that Akt/mTOR signaling
pathway perform a vital effect in regulating MMP-9 expression in
DU145 cells (16). To recognize
the influence of Akt/mTOR signaling pathway, western blotting was
performed. We noticed that piperine pretreatment remarkably
decreased the expression of phosphorylated mTOR (p-mTOR) and
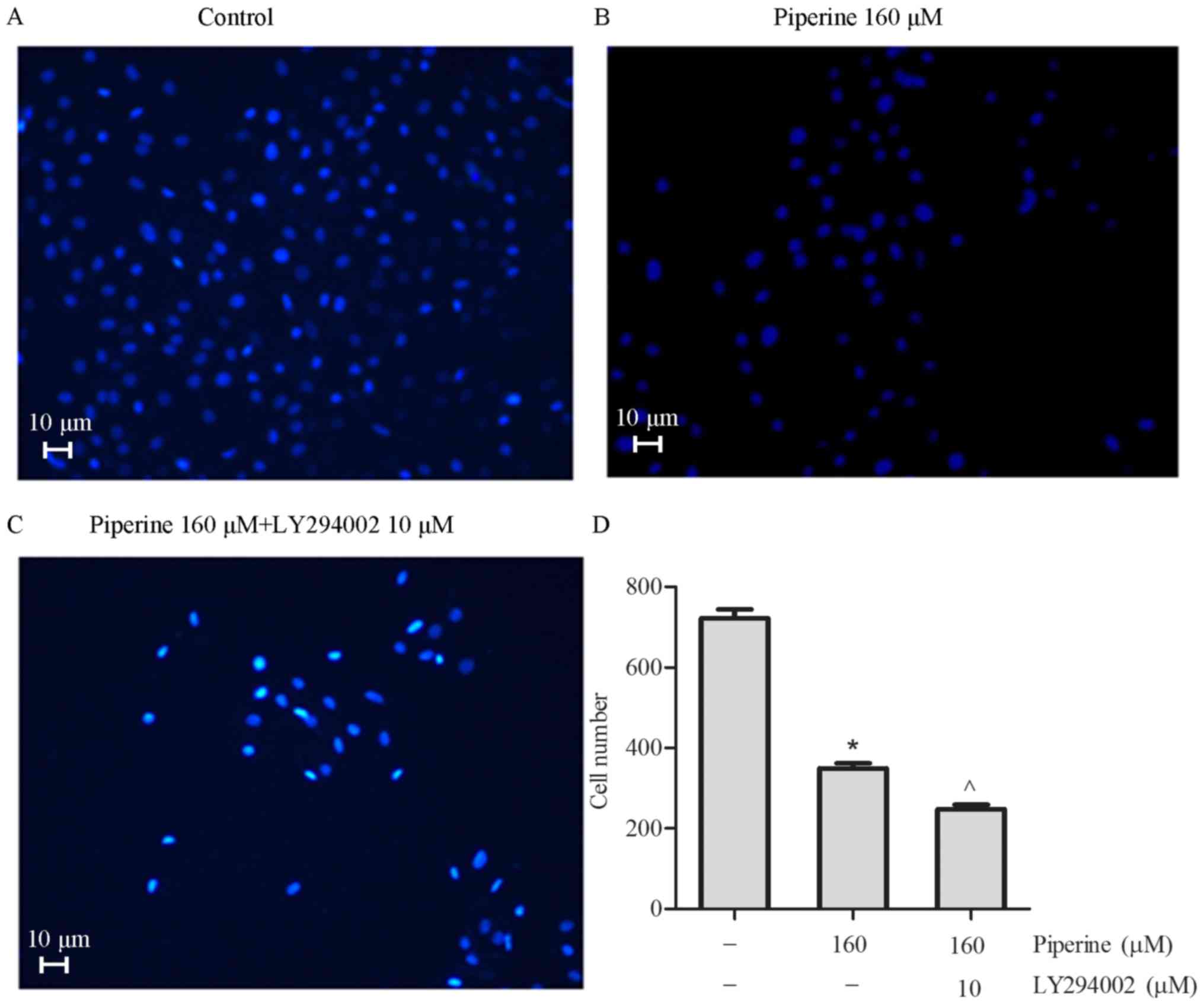
phosphorylated Akt (p-Akt) in DU145 cells (Fig. 4C-E). To further exam the effect of
piperine in the DU145 cells metastasis process, LY294002, an Akt
inhibitor, was chosen to combine with piperine for mechanism and
functional research. In cells pretreatment with LY294002 and
piperine group relative to the piperine alone group, cell migration
was significantly reduced in the DU145 cells (Fig. 5), as well as the protein levels of
p-mTOR, p-Akt, and MMP-9 (Fig.
6A-D). Collectively, these outcomes imply that Akt/mTOR
signaling pathway was partially correlated to the anti-metastasis
function of piperine.
Discussion
In our study, the involvement of piperine and its
molecular mechanism in DU145 cells was investigated. Piperine was
exhibited to promote apoptosis and to inhibit cell proliferation in
DU145 cells as demonstrated by CCK8 and flow cytometry. At the
molecular level, the results demonstrated that piperine suppressed
Akt and mTOR activation. In addition, PI3K/Akt/mTOR was identified
to be a vital molecular target of piperine (16,17).
Akt and mTOR invalidation, using inhibitor, reduced the MMP9
expression in DU145 tumor cells. Collectively, these data suggested
that piperine may be beneficial in the treatment of PCa.
Currently, it is thought that piperine could present
as a natural source anticancer drug due to its reported capability
to repress carcinogenesis, angiogenesis, metastasis, and tumor
growth (18–20); nonetheless, the impact of piperine
on human PCa cells has not completely been understood. Ouyang et
al showed that piperine represses the proliferation of human
PCa cells through promotion of autophagy and cell cycle arrest
(21). A recent study displays
that piperine treatment significantly diminished Prostate Specific
Antigen (PSA) levels in LNCaP cells, also promoted apoptosis in
hormone dependent PCa cells (LNCaP), and decreased the cell
migration of LNCaP and PC-3 cells in Boyden chamber assay (22). Furthermore, our data revealed that
piperine could significantly repress the proliferation and induce
apoptosis of DU145 cells. Besides, our data also exhibited that the
downregulation of MMP-9 expression resulted in inhibition of
migration in DU145 cells. Accordingly, these findings implied that
piperine acted a vital role in treatment of human PCa cells.
Numerous genetic changes occur during the process of
carcinogenesis. Identifying vital proteins, such as PI3K, Akt, and
ERK MAPK involved in these processes is crucial for comprehending
carcinogenesis and creating new therapies (23–25).
The PI3K/Akt signaling pathway is occupied in cell migration and
invasion (26,27). Recent researches indicated that the
signaling pathway PI3K/Akt/mTOR is essential in the metastasis of
PCa (28,29). Besides, MMPs, a group of
zinc-dependent endopeptidases, are involved in the degradation of
the extracellular matrix under normal physiological conditions and
during the metastatic process (30). MMP-9 is a vital effector molecule
that boosts tumor cell invasion via type-IV collagen
degradation-dependent extracellular matrix remodeling (31). In order to exam if piperine targets
the PI3K/Akt/mTOR signaling-mediated cellular events in DU145
cells, the effect of piperine on these signaling molecules was
explored by immunoblotting. In accordance with these reports
(9,32), we detected that piperine caused a
decrease in cellular levels of Akt and mTOR. Through our work, a
relation emerges between MMP-9 expression and levels of
phosphorylated Akt and mTOR, which alters cell migration. The
expression of phosphorylated Akt, phosphorylated mTOR, and MMP-9
were remarkable diminished in pretreatment with Akt inhibitor
LY294002 group. Besides, inhibition of Akt activity also led to a
decrease in migration, measured in a transwell migration assay.
Taken together, our result suggested that piperine repressed the
migration and the expression of MMP-9 via Akt/mTOR signaling
pathway in DU145 cells partially.
We acknowledge that this study has some limitations.
First, we just detected the effect of piperine in DU145 cells.
Future studies should investigate the effect of piperine in other
PCa cell lines. Second, our result suggested that piperine
significantly reduced the expression of MMP-9 via Akt/mTOR
signaling pathway in DU145 cells; however, more definitive target
protein, such as EMT-related proteins including E-cadherin, TTF-1,
are still needed. Similarly, there are several apoptosis-related
proteins, and we studied only pro-apoptotic protein Bax and
anti-apoptotic protein Bcl-2, and did not directly assess other
apoptosis-related proteins, which would be the subject of our
future research. Finally, in vivo experiments using knock
out mouse models are necessary to further validate the influence of
piperine in PCa.
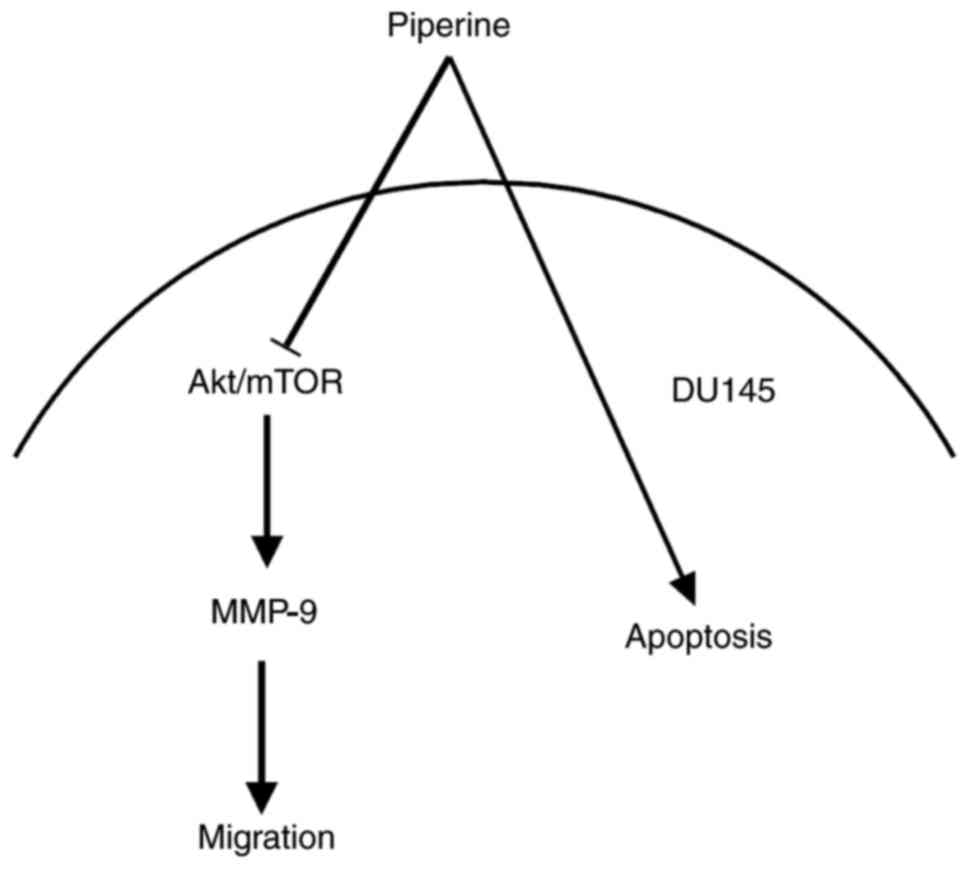
In conclusion, our data reveal that piperine
inhibits PCa cells migration via suppressing Akt/mTOR and
downregulating MMP-9, and that the Akt/mTOR signaling seem to be
the upstream regulators of MMP-9 protein (the schematic model is
shown in Fig. 7). In brief, it is
indicated that piperine represses metastasis through inhibiting the
Akt/mTOR/MMP-9 signaling pathway in DU145 cells partially, and that
it may be a potential anticancer drug candidate in future
therapeutic medications in PCa patients.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Science Fund
of Northwest University (grant no. 2016023).
Availability of data and materials
The analyzed datasets generated during the study are
available from the corresponding author on reasonable request.
Authors' contributions
YZ designed the study, and YZ and YY performed the
experiments and analysis.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cooperberg MR and Chan JM: Epidemiology of
prostate cancer. World J Urol. 35:8492017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shetty AV, Thirugnanam S, Dakshinamoorthy
G, Samykutty A, Zheng G, Chen A, Bosland MC, Kajdacsy-Balla A and
Gnanasekar M: 18α-glycyrrhetinic acid targets prostate cancer cells
by down-regulating inflammation-related genes. Int J Oncol.
39:635–640. 2011.PubMed/NCBI
|
|
3
|
Sharifi N, Gulley JL and Dahut WL:
Androgen deprivation therapy for prostate cancer. JAMA.
294:238–244. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pan H, Jansson KH, Beshiri ML, Yin J, Fang
L, Agarwal S, Nguyen H, Corey E, Zhang Y, Liu J, et al: Gambogic
acid inhibits thioredoxin activity and induces ROS-mediated cell
death in castration-resistant prostate cancer. Oncotarget.
8:77181–77194. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dias A, Kote-Jarai Z, Mikropoulos C and
Eeles R: Prostate cancer germline variations and implications for
screening and treatment. Cold Spring Harb Perspect Med. Nov
3–2017.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen Z, Zheng Y, Ji G, Liu X, Li P, Cai L,
Guo Y and Yang J: Accuracy of dynamic contrast-enhanced magnetic
resonance imaging in the diagnosis of prostate cancer: Systematic
review and meta-analysis. Oncotarget. 8:77975–77989.
2017.PubMed/NCBI
|
|
7
|
Mittal R and Gupta RL: In vitro
antioxidant activity of piperine. Methods Find Exp Clin Pharmacol.
22:271–274. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pan H, Xu LH, Huang MY, Zha QB, Zhao GX,
Hou XF, Shi ZJ, Lin QR, Ouyang DY and He XH: Piperine metabolically
regulates peritoneal resident macrophages to potentiate their
functions against bacterial infection. Oncotarget. 6:32468–32483.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Doucette CD, Greenshields AL, Liwski RS
and Hoskin DW: Piperine blocks interleukin-2-driven cell cycle
progression in CTLL-2 T lymphocytes by inhibiting multiple signal
transduction pathways. Toxicol Lett. 234:1–12. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sethiya NK, Shah P, Rajpara A, Nagar PA
and Mishra SH: Antioxidant and hepatoprotective effects of mixed
micellar lipid formulation of phyllanthin and piperine in carbon
tetrachloride-induced liver injury in rodents. Food Funct.
6:3593–3603. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Toyoda T, Shi L, Takasu S, Cho YM,
Kiriyama Y, Nishikawa A, Ogawa K, Tatematsu M and Tsukamoto T:
Anti-inflammatory effects of capsaicin and piperine on helicobacter
pylori-induced chronic gastritis in mongolian gerbils.
Helicobacter. 21:131–142. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sharma S, Kalia NP, Suden P, Chauhan PS,
Kumar M, Ram AB, Khajuria A, Bani S and Khan IA: Protective
efficacy of piperine against mycobacterium tuberculosis.
Tuberculosis (Edinb). 94:389–396. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Greenshields AL, Doucette CD, Sutton KM,
Madera L, Annan H, Yaffe PB, Knickle AF, Dong Z and Hoskin DW:
Piperine inhibits the growth and motility of triple-negative breast
cancer cells. Cancer Lett. 357:129–140. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Do MT, Kim HG, Choi JH, Khanal T, Park BH,
Tran TP, Jeong TC and Jeong HG: Antitumor efficacy of piperine in
the treatment of human HER2-overexpressing breast cancer cells.
Food Chem. 141:2591–2599. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang J, Sikka S, Siveen KS, Lee JH, Um
JY, Kumar AP, Chinnathambi A, Alharbi SA, Basappa, Rangappa KS, et
al: Cardamonin represses proliferation, invasion, and causes
apoptosis through the modulation of signal transducer and activator
of transcription 3 pathway in prostate cancer. Apoptosis.
22:158–168. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kou B, Liu W, He W, Zhang Y, Zheng J, Yan
Y, Zhang Y, Xu S and Wang H: Tetrandrine suppresses metastatic
phenotype of prostate cancer cells by regulating Akt/mTOR/MMP-9
signaling pathway. Oncol Rep. 35:2880–2886. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Berrak O, Arisan ED, Obakan-Yerlikaya P,
Coker-Gürkan A and Palavan-Unsal N: mTOR is a fine tuning molecule
in CDK inhibitors-induced distinct cell death mechanisms via
PI3K/AKT/mTOR signaling axis in prostate cancer cells. Apoptosis.
21:1158–1178. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Deepak V, Kruger MC, Joubert A and Coetzee
M: Piperine alleviates osteoclast formation through the
p38/c-Fos/NFATc1 signaling axis. Biofactors. 41:403–413. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Doucette CD, Hilchie AL, Liwski R and
Hoskin DW: Piperine, a dietary phytochemical, inhibits
angiogenesis. J Nutr Biochem. 24:231–239. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fofaria NM, Kim SH and Srivastava SK:
Piperine causes G1 phase cell cycle arrest and apoptosis in
melanoma cells through checkpoint kinase-1 activation. PLoS One.
9:e942982014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ouyang DY, Zeng LH, Pan H, Xu LH, Wang Y,
Liu KP and He XH: Piperine inhibits the proliferation of human
prostate cancer cells via induction of cell cycle arrest and
autophagy. Food Chem Toxicol. 60:424–430. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Samykutty A, Shetty AV, Dakshinamoorthy G,
Bartik MM, Johnson GL, Webb B, Zheng G, Chen A, Kalyanasundaram R
and Munirathinam G: Piperine, a bioactive component of pepper spice
exerts therapeutic effects on androgen dependent and androgen
independent prostate cancer cells. PLoS One. 8:e658892013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ai XL, Chi Q, Qiu Y, Li HY, Li DJ, Wang JX
and Wang ZY: Gap junction protein connexin43 deregulation
contributes to bladder carcinogenesis via targeting MAPK pathway.
Mol Cell Biochem. 428:109–118. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
He Q, Ren X, Chen J, Li Y, Tang X, Wen X,
Yang X, Zhang J, Wang Y, Ma J and Liu N: miR-16 targets fibroblast
growth factor 2 to inhibit NPC cell proliferation and invasion via
PI3K/AKT and MAPK signaling pathways. Oncotarget. 7:3047–3058.
2016.PubMed/NCBI
|
|
25
|
Zhang D, Sun G, Zhang H, Tian J and Li Y:
Long non-coding RNA ANRIL indicates a poor prognosis of cervical
cancer and promotes carcinogenesis via PI3K/Akt pathways. Biomed
Pharmacother. 85:511–516. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li C, Wang C, Xing Y, Zhen J and Ai Z:
CD133 promotes gallbladder carcinoma cell migration through
activating Akt phosphorylation. Oncotarget. 7:17751–17759.
2016.PubMed/NCBI
|
|
27
|
Selvaraj N, Budka JA, Ferris MW, Jerde TJ
and Hollenhorst PC: Prostate cancer ETS rearrangements switch a
cell migration gene expression program from RAS/ERK to PI3K/AKT
regulation. Mol Cancer. 13:612014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Marques RB, Aghai A, de Ridder CMA,
Stuurman D, Hoeben S, Boer A, Ellston RP, Barry ST, Davies BR,
Trapman J and van Weerden WM: High efficacy of combination therapy
using PI3K/AKT inhibitors with androgen deprivation in prostate
cancer preclinical models. Eur Urol. 67:1177–1185. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen H, Zhou L, Wu X, Li R, Wen J, Sha J
and Wen X: The PI3K/AKT pathway in the pathogenesis of prostate
cancer. Front Biosci (Landmark Ed). 21:1084–1091. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Koutroulis I, Zarros A and Theocharis S:
The role of matrix metalloproteinases in the pathophysiology and
progression of human nervous system malignancies: A chance for the
development of targeted therapeutic approaches? Expert Opin Ther
Targets. 12:1577–1586. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Khan MN, Choi JS, Lee MC, Kim E, Nam TJ,
Fujii H and Hong YK: Anti-inflammatory activities of methanol
extracts from various seaweed species. J Environ Biol. 29:465–469.
2008.PubMed/NCBI
|
|
32
|
Yaffe PB, Power Coombs MR, Doucette CD,
Walsh M and Hoskin DW: Piperine, an alkaloid from black pepper,
inhibits growth of human colon cancer cells via G1 arrest and
apoptosis triggered by endoplasmic reticulum stress. Mol Carcinog.
54:1070–1085. 2015. View
Article : Google Scholar : PubMed/NCBI
|