Introduction
Bladder cancer (BC) is the fourth most common cancer
in men and the seventh most common solid tumor in women worldwide,
with an estimated 430,000 new cases diagnosed in 2012 (1,2).
While the incidence rate is stable or declining in men, it exhibits
an increasing trend in women (3).
BC has a complex biological behavior, with frequent relapse and
metastasis (4). Previous data
shows that about one-third of initial BC cases will exhibit local
progression and distant metastasis, and the 5-year survival rate is
<62% (5). However, the
mechanism underlying BC is not clear, and the mechanisms of
occurrence, recurrence and metastasis are still unknown. Therefore,
it is of great value to explore the molecular mechanisms involved
in the apoptosis, proliferation, metastasis and invasion of BC for
the improvement of prevention, diagnosis and therapy.
The histopathology and molecular pathways in BC
pathogenesis have been described. Somatic copy number alterations
in multiple regions have been identified in previous studies,
including amplification of PPARG and E2F3, with loss of CDKN2A and
RB1 (6,7). The Cancer Genome Atlas (TCGA) project
reported that potential therapeutic targets had been identified in
69% of the bladder tumors investigated; 42% of the tumors were
reported to have targets in the phosphatidylinositol-3-OH
kinase/AKT/mTOR pathway, and 45% were reported to have targets in
the RTK/MAPK pathway (8). So far,
knowledge of the molecular biology of BC has lagged behind that of
other cancers. No molecular or gene-targeting agents have been
approved for the treatment of the disease. Therefore, understanding
the molecular mechanism of BC is vital for the development of more
precise diagnostic and effective therapeutic strategies.
With the continuous development of bioinformatics
and molecular biology, it is possible to explore the mechanism of
carcinogenesis and development at the molecular level. In previous
decades, a large number of important signaling pathways in
tumorigenesis were identified through analysis of the expression
profiles of gene microarrays. This technology has also been used
for genomic analysis, which may aid in the discovery of key genes
that are interrelated with tumorigenesis (9).
In the present study, a gene expression profile
(GSE7476) was downloaded from the Gene Expression Omnibus database
(GEO). The differentially expressed genes (DEGs) between the
controls and BC samples were analyzed. Gene Ontology (GO) and Kyoto
Encyclopedia of Genes and Genomes (KEGG) analyses in the DAVID
database were applied to analyze the functional enrichment and
significant pathways associated with the DEGs. In addition, we
constructed a PPI network to identify the critical DEGs and
significant modules. This study aimed to investigate the
involvement of genes critical to BC, and to promote the development
of novel targeted agents for BC therapeutic intervention.
Materials and methods
Microarray data
Gene expression profiles for BC (GSE7476) were
downloaded from the GEO on the NCBI website (http://www.ncbi.nlm.nih.gov/geo). The probe-level data
were converted into the corresponding gene symbols to detect the
expression of gene transcript levels, according to the annotation
information downloaded from the platform GPL570 (Affymetrix Human
Genome U133 Plus 2.0 Array), which contains 54,675 probes. The gene
expression profiles consisted of 12 urothelial samples from
patients with prostatic hyperplasia or renal failure with no
evidence of bladder malignancy, and 43 tumor samples from different
BC risk groups. The mean age of the BC risk groups, which consisted
of 39 males and 4 females, was 77 years (10). In total, 15 low-grade superficial
tumor samples, 13 high-grade superficial tumor samples and 15
high-grade muscle-invasive tumors samples were assigned to the BC
risk group (10). The healthy
control (HC) group, which comprised 12 males, had a median age of
59 years (10). The datasets from
the 12 HC and 43 BC samples were analyzed.
Data processing and screening of
DEGs
The CEL file data of GSE7476, downloaded from the
GEO database, were read using the affy package in the R programming
language (R). The original probe-level data were converted into
gene symbols. Then, the expression values of multiple probes for
the same gene were transformed into a single value by taking the
mean expression value. The RMA method (robust multi-array average)
was applied to carry out data pre-processing, including background
correction, normalization and expression calculation of the
original array data. The Limma package in R (11) was used to identify the DEGs between
BC and HC samples. The Benjamini-Hochberg (BH) method (12) was introduced to adjust the raw
P-values into a false discovery rate (FDR) to avoid the multi-test
problem, which might produce too many false positive results.
P<0.05 and |log2 fold change (FC)|≥1 were set as the
thresholds for identifying DEGs.
Functional and pathway enrichment
analysis of DEGs
GO and KEGG analyses were applied for the functional
annotation and pathway analysis, using the Database for Annotation
Visualization and Integrated Discovery (DAVID; https://david.ncifcrf.gov/) (13). The human genome was selected as the
background parameter. P<0.05 and a count ≥2 were set as the
thresholds to indicate a statistically significant difference.
PPI network construction and analysis
of modules
PPI analyses may be helpful in identifying the
generic organizational principles of functional networks, and to
provide novel insights into protein function (14). In order to reveal the functional
associations between proteins on a genome-wide scale, the STRING
database (http://string-db.org/) online software
(15,16) was used to construct a PPI
network.
PPI networks were created after all DEGs were
imported into the Cytoscape plugin. Confidence score ≥0.4 was set
as the cut-off criterion. Molecular Complex Detection (MCODE)
(17) was then applied to conduct
module analysis in the resulting PPI network with the following
parameters: Node score cutoff, ≥2; degree cutoff, ≥2; max depth,
100; and K-core, ≥2.
Results
Data preprocessing and DEG
screening
The RNA was isolated from the tissue from BC and HC
samples, respectively, for use in the microarray studies. A total
of 20,487 gene symbols were discerned and the gene expression
matrix of the samples was obtained. Based on the R analysis, a
total of 1,173 DEGs were identified in BC compared with HC samples,
including 859 upregulated genes and 314 downregulated genes.
P<0.05 and |FC|≥2.0 were set as the threshold criteria. The top
10 upregulated DEGs and top 10 downregulated DEGs are listed in
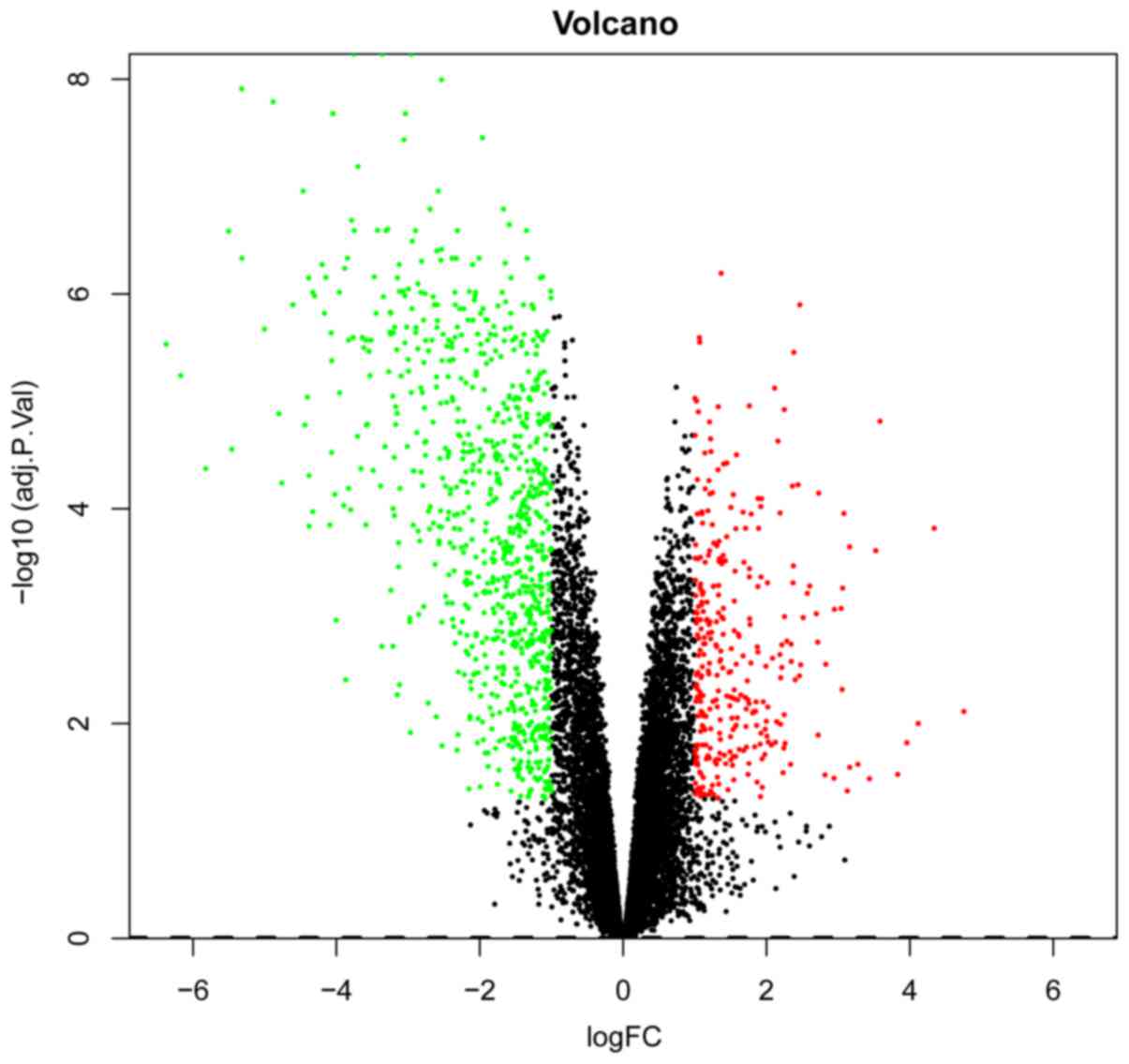
Table I. A volcano plot of the
DEGs is presented in Fig. 1.
 | Table I.Top ten upregulated and downregulated
differentially expressed genes between bladder cancer and normal
tissues. |
Table I.
Top ten upregulated and downregulated
differentially expressed genes between bladder cancer and normal
tissues.
| A, The top 10
upregulated differentially expressed genes |
|---|
|
|---|
| Gene symbol | logFC | AveExpr | t | P-value | adj.P.Val | B |
|---|
| DPP3 | 1.367807192 | 6.823383737 | −15.62834787 | 1.34E-09 | 6.41E-07 | 12.55923385 |
| PAFAH1B3 | 2.465494694 | 7.611594612 | −14.08412651 | 4.65E-09 | 1.26E-06 | 11.35210194 |
| TFPT | 1.064195524 | 6.593293201 | −12.69386019 | 1.58E-08 | 2.55E-06 | 10.14074068 |
| RANGAP1 | 1.069859311 | 6.672884639 | −12.47693921 | 1.93E-08 | 2.81E-06 | 9.939786046 |
| IGFBP3 | 2.382533743 | 10.63365369 | −12.1139983 | 2.73E-08 | 3.49E-06 | 9.59578721 |
| PVRL4 | 2.113260416 | 7.562648025 | −11.09448808 | 7.52E-08 | 7.52E-06 | 8.574037157 |
| SEC61A1 | 1.001492679 | 8.052241965 | −10.79227308 | 1.03E-07 | 9.34E-06 | 8.254355505 |
| MTFP1 | 1.022940775 | 6.246895347 | −10.71273652 | 1.12E-07 | 9.91E-06 | 8.168871656 |
| ESRP1 | 1.760663741 | 8.297155088 | −10.59987707 | 1.27E-07 | 1.10E-05 | 8.046588009 |
| ABRACL | 1.327112508 | 7.101266084 | −10.57221373 | 1.30E-07 | 1.12E-05 | 8.016436726 |
|
| B, The top 10
downregulated differentially expressed genes |
|
| Gene symbol | logFC | AveExpr | t | P-value | adj.PVal | B |
|
| SCARA5 | −3.36100537 | 4.768657761 | 30.97352259 | 3.13E-13 | 5.84E-09 | 19.86271169 |
| LINC01082 | −3.753683567 | 5.294466313 | 29.16744989 | 6.59E-13 | 5.84E-09 | 19.29373062 |
| OLFML1 | −2.95576203 | 4.434015195 | 28.55851363 | 8.55E-13 | 5.84E-09 | 19.08956481 |
| TMEM100 | −2.533343457 | 3.70073062 | 26.68801945 | 1.97E-12 | 1.01E-08 | 18.41959434 |
| MIR100HG | −5.32118364 | 4.747251266 | 25.79518302 | 3.01E-12 | 1.23E-08 | 18.07515932 |
| CFD | −4.881864287 | 8.124954765 | 24.85704639 | 4.74E-12 | 1.62E-08 | 17.69452213 |
| SLIT2 | −4.050964305 | 5.040374213 | 23.90645097 | 7.67E-12 | 2.09E-08 | 17.28787436 |
| PRDM6 | −3.036050363 | 4.838278315 | 23.58237834 | 9.07E-12 | 2.09E-08 | 17.14414695 |
| MRGPRF | −4.045214224 | 5.668562294 | 23.55835407 | 9.18E-12 | 2.09E-08 | 17.13338551 |
| LRFN5 | −1.962030571 | 3.960439049 | 22.38800885 | 1.72E-11 | 3.51E-08 | 16.59060679 |
Functional and pathway enrichment
analysis of DEGs
To further explore the systematic characterization
and biological functions of the identified DEGs, functional
annotation and pathway analysis, including GO and KEGG, were
performed using DAVID.
In this study, the three GO categories [cellular
component (CC), biological process (BP) and molecular function MF)]
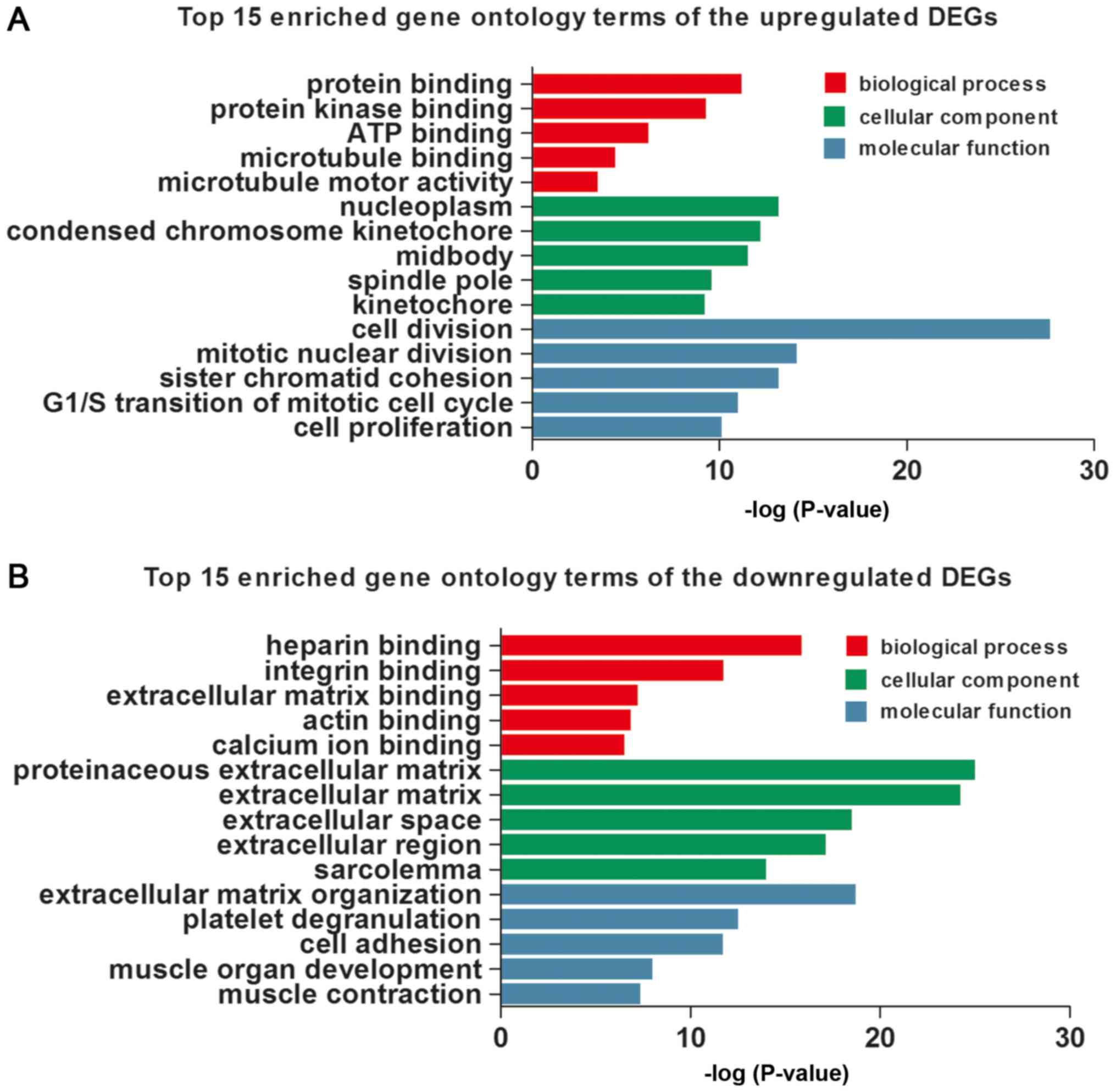
were detected, respectively, using DAVID. The top 15 GO terms of
the upregulated and downregulated DEGs are shown in Table II and Fig. 2, respectively. The upregulated DEGs
were involved in the different GO terms, such as ‘cell division’
(ontology: BP), ‘nucleoplasm’ (ontology: CC) and ‘protein binding’
(ontology: MF) (Table IIA and
Fig. 2A). The most significantly
downregulated DEGs were related to the GO terms ‘extracellular
matrix organization’ (ontology: BP), ‘proteinaceous extracellular
matrix’ (ontology: CC) and ‘heparin binding’ (ontology: MF)
(Table IIB and Fig. 2B).
 | Table II.The top 15 enrichedgene ontology
terms of up-regulated DEGs and downregulated DEGs. |
Table II.
The top 15 enrichedgene ontology
terms of up-regulated DEGs and downregulated DEGs.
| A, The top 15
enriched gene ontology terms of the upregulated DEGs |
|---|
|
|---|
| Category | Term | Count | P-value |
|---|
| BP | Cell division | 47 | 2.13E-28 |
| BP | Mitotic nuclear
division | 28 | 7.12E-15 |
| BP | sSster chromatid
cohesion | 19 | 6.92E-14 |
| BP | G1/S transition of
mitotic cell cycle | 17 | 9.83E-12 |
| BP | Cell
proliferation | 28 | 7.54E-11 |
| CC | Nucleoplasm | 97 | 6.57E-14 |
| CC | Condensed
chromosome kinetochore | 17 | 6.24E-13 |
| CC | Midbody | 19 | 3.04E-12 |
| CC | Spindle pole | 16 | 2.50E-10 |
| CC | Kinetochore | 14 | 5.65E-10 |
| MF | Protein
binding | 198 | 6.15E-12 |
| MF | Protein kinase
binding | 27 | 4.95E-10 |
| MF | ATP binding | 51 | 5.69E-07 |
| MF | Microtubule
binding | 14 | 3.52E-05 |
| MF | Microtubule motor
activity |
8 | 3.11E-04 |
|
| B, The top 15
enriched gene ontology terms of the down-regulated DEGs |
|
| Category | Term | Count | P-value |
|
| BP | Extracellular
matrix organization | 45 | 1.92E-19 |
| BP | Platelet
degranulation | 27 | 2.96E-13 |
| BP | Cell adhesion | 58 | 1.86E-12 |
| BP | Muscle organ
development | 20 | 9.72E-09 |
| BP | Muscle
contraction | 21 | 4.34E-08 |
| CC | Proteinaceous
extracellular matrix | 60 | 9.31E-26 |
| CC | Extracellular
matrix | 62 | 5.68E-25 |
| CC | Extracellular
space | 132 | 2.99E-19 |
| CC | Extracellular
region | 145 | 6.81E-18 |
| CC | Sarcolemma | 26 | 1.01E-14 |
| MF | Heparin
binding | 37 | 1.33E-16 |
| MF | Integrin
binding | 26 | 1.77E-12 |
| MF | Extracellular
matrix binding | 11 | 5.86E-08 |
| MF | Actin binding | 34 | 1.34E-07 |
| MF | Calcium ion
binding | 62 | 2.85E-07 |
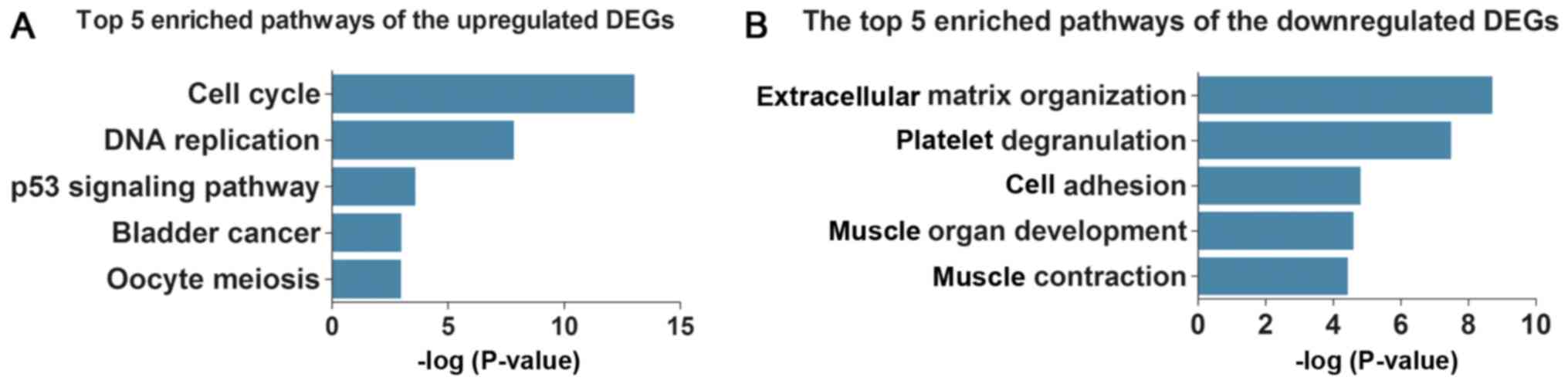
Subsequently, KEGG pathway analysis demonstrated
that the upregulated DEGs were enriched in five key pathways
(Table IIIA and Fig. 3A), including ‘cell cycle’, ‘DNA
replication’ and ‘p53 signaling pathway’, whereas the downregulated
DEGs were enriched in five different pathways (Table IIIB and Fig. 3B), including ‘complement and
coagulation cascades’, ‘focal adhesion’ and ‘hypertrophic
cardiomyopathy (HCM)’.
 | Table III.The top five enriched pathways of
upregulated differentially expressed genes and downregulated
differentially expressed genes. |
Table III.
The top five enriched pathways of
upregulated differentially expressed genes and downregulated
differentially expressed genes.
| A, The top 5
enriched pathways of the upregulated DEGs |
|---|
|
| Pathway ID | Name | Count | P-value | Genes |
|---|
| hsa04110 | Cell cycle | 21 | 9.58E-14 | CDC7, CDC6, CDK1,
SKP2, TTK, ESPL1, CDC20, PTTG1, MCM2, MCM4, MCM5, CCNB1, CCNE1,
CCND1, MAD2L1, MCM7, CCNB2, PLK1, PCNA, BUB1B, CCNA2 |
| hsa03030 | DNA
replication | 10 | 1.47E-08 | RFC5, PRIM1, RFC4,
MCM7, PCNA, MCM2, RNASEH2A, MCM4, MCM5, FEN1 |
| hsa04115 | p53 signaling
pathway | 8 | 2.53E-04 | BID, CCNB1, CCNE1,
CDK1, CCND1, CCNB2, RRM2, IGFBP3 |
| hsa05219 | Bladder cancer | 6 | 9.83E-04 | CCND1, FGFR3,
VEGFA, CDH1, MMP1, DAPK1 |
| hsa04114 | Oocyte meiosis | 9 | 1.03E-03 | CCNE1, CDK1,
PPP1CA, MAD2L1, PLK1, AURKA, CDC20, ESPL1, PTTG1 |
|
| B, The top 5
enriched pathways of the downregulated DEGs |
|
| Pathway ID | Name | Count | P-value | Genes |
|
| hsa04610 | Complement and
coagulation cascades | 19 | 1.96E-09 | C7, A2M, C5AR1, C3,
F13A1, F8, C1R, SERPING1, BDKRB2, C1S, PLAUR, CD55, F3, SERPINE1,
CFH, TFPI, CFI, CFD, PROS1 |
| hsa04510 | Focal adhesion | 31 | 3.28E-08 | CAV2, CAV1,
PPP1R12B, TNC, MYL9, VCL, LAMB2, COL6A3, ILK, PPP1R12A, COL6A2,
PDGFC, ZYX, PDGFD, THBS1, ITGA1, IGF1, ACTN1, FLNC, COL5A1, COL4A6,
FLNA, LAMA2, VEGFC, ITGA5, FYN, JUN, ITGA8, ITGA7, MYLK, PARVA |
| hsa05410 | Hypertrophic
cardiomyopathy | 15 | 1.55E-05 | ACTC1, IL6,
CACNA2D1, ITGA1, IGF1, TPM2, TPM1, DES, SGCG, ITGA5, DMD, ITGA8,
ITGA7, SGCA, SGCB |
| hsa05205 | Proteoglycans in
cancer | 25 | 2.53E-05 | FGFR1, CAV2, CAV1,
LUM, PPP1R12B, DCN, TIMP3, SDC2, ANK2, PPP1R12A, RRAS, FAS, THBS1,
FGF2, IGF1, FLNC, FZD7, FLNA, ITPR1, WNT2B, PLAUR, FZD10, ITGA5,
HSPB2, HBEGF |
| hsa05414 | Dilated
cardiomyopathy | 15 | 3.70E-05 | CACNA2D1, ACTC1,
ITGA1, IGF1, TPM2, TPM1, DES, SGCG, ITGA5, DMD, PLN, ITGA8, ITGA7,
SGCA, SGCB |
PPI network construction and module
selection
STRING was applied to construct the PPI network of
the DEGs. This PPI network consisted of 959 nodes interacting via
6,400 edges. Seven hub genes appeared in the top 10 genes list in
terms of degree, betweenness and closeness, simultaneously. Among
these genes, Jun proto-oncogene (JUN) showed the highest node
degree, which was 144. The others included cyclin-dependent kinase
1 (CDK1, degree=125), Fos proto-oncogene (fos, degree=122),
proliferating cell nuclear antigen (PCNA, degree=101),
topoisomerase (DNA) II alpha (TOP2A, degree=100), cyclin D1 (CCND1,
degree=98) and cadherin 1 (CDH1, degree=98).
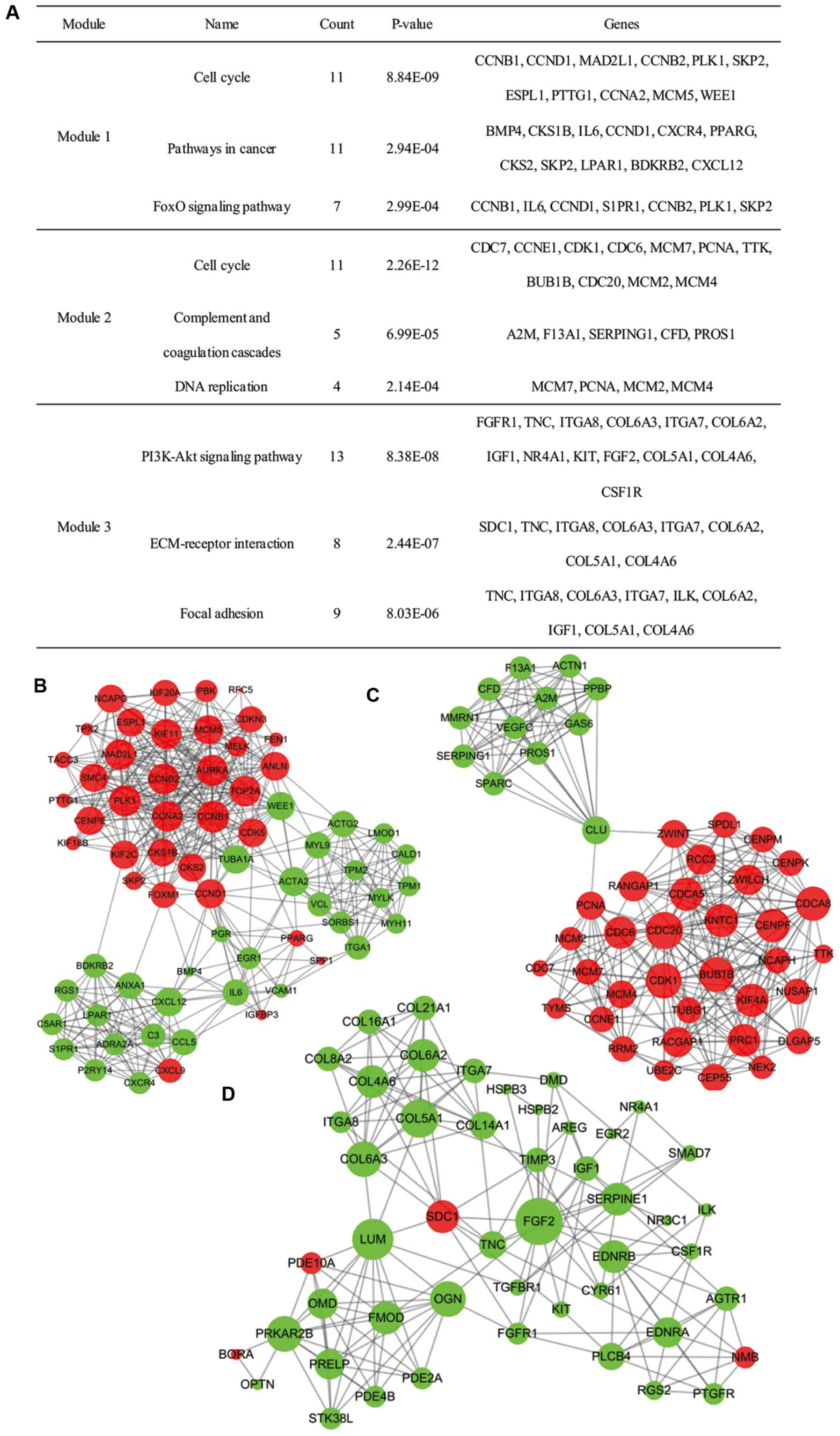
Moreover, 27 functional clusters were selected from
the PPI network using MCODE. The top 3 significant modules were
selected (Fig. 4), and the pathway
enrichment annotation of the genes involved in the modules was
analyzed using KEGG pathway analysis, which revealed that the genes
in modules 1–3 were mainly associated with the ‘cell cycle’
signaling pathway (both appearing in module 1 and module 2), and
‘PI3K-Akt signaling pathway’.
Discussion
BC is one of the most common types of malignant
cancer in China and has a high mortality rate (18). BC is the most common form of
urinary tract malignant tumor. Approximately 95% of bladder tumors
are urothelial, and their treatment mainly centers around surgery;
however, relapse and metastasis after surgery are common (4). The key genes and pathways associated
with BC were identified in the present study using bioinformatics
methods.
In the present study, R was used to extract the
genetic information from GSE7476, and a total of 1,173 genes were
identified to be differentially expressed between BC and HC
samples, among which 314 were upregulated and 859 were
downregulated in BC. The upregulated DEGs were mainly enriched in
‘cell division’, ‘nucleoplasm’ and ‘protein binding’, while the
downregulated DEGs were mainly involved in ‘extracellular matrix
organization’, ‘proteinaceous extracellular matrix’ and ‘heparin
binding’. Moreover, the KEGG pathway enrichment analysis results
showed that the upregulated DEGs were associated with the ‘cell
cycle’, ‘DNA replication’ and the ‘p53 signaling pathway’, whereas
the downregulated DEGs were mainly enriched in the ‘complement and
coagulation cascades’, ‘focal adhesion’ and ‘HCM’.
Previous studies have demonstrated that tumor
development is associated with the activation of the coagulation
cascade. The exact mechanism through which coagulation proteins
promote tumorigenesis remains unclear; however, it is possibly
associated with hemostatic factor changes and peritumoral
deposition of fibrin (19–21). The cell cycle is the series of
events that occur between cell duplication and division, and is
closely associated with cell growth, anabolism and proliferation
(22). Uncontrolled cell
proliferation and DNA replication comprise one of the hallmarks of
cancer (23). p53 is known to be
mutated in >50% of all human cancers, including bladder
carcinoma (24). Alterations in
p53 expression levels are correlated with tumor recurrence, lower
survival rates (25) and poor
prognosis in BC patients (26).
Therefore, investigating these signaling pathways may aid in
elucidating the carcinogenic mechanism of BC.
In addition, a PPI network was constructed to
identify the key DEGs. We used proteins that corresponded to genes
to construct the PPI network, and found that seven hub genes (JUN,
CDK1, FOS, PCNA, TOP2A, CCND1 and CDH1) appeared in each of the top
10 gene lists in terms of degree, betweenness and closeness. JUN
and FOS both exhibited downregulated expression, and were
identified as main hub genes, with degree values of 144 and 122,
respectively. FOS and JUN are proto-oncogenes belonging to the
family of activator protein 1 (AP1) transcription factors (27,28).
Ye et al (29) reported
that AP-1 plays a vital role in cellular migration, metastasis,
proliferation, transformation, apoptosis and inflammation. C-FOS, a
major member of the FOS family, has been demonstrated to be
involved in the regulation of cell growth, differentiation,
proliferation, transformation and apoptosis (30). Previous studies have shown that the
level of C-FOS in BC tissues is significantly higher than that in
adjacent non-cancer and normal tissues (31,32).
Most of the research on c-Jun (a major member of the JUN family),
indicates that it may contribute to tumor initiation and
invasiveness (33,34). Huhe et al (35) revealed that high c-Jun expression
served a vital role in tumor progression, and may be a diagnostic
and therapeutic biomarker in urothelial carcinoma of the
bladder.
The results of our study also showed that CCND1 and
CDH1 were enriched in several pathways in BC. CCND1, a cell cycle
regulatory factor, promotes the progression of the cell cycle
through the G1/S phase limit points. Overexpression of the CCND1
gene can result in uncontrolled cell proliferation and tumor
occurrence by shortening the G1 phase (36,37).
Many patients with cancer have been found to overexpress CCND1, and
thus to have a poor prognosis (38,39).
Xu et al (40) reported
that the expression of CCND1 is associated with the progression of
BC; therefore, CCND1 may be considered as an auxiliary diagnostic
factor and potential prognostic marker for BC patients. CDH1
encodes a classical cadherin of the cadherin superfamily. A
previous report (41) showed that
CDH1 plays an important role in suppressing the invasive phenotype
of urothelial BC cells. Many studies have shown that the classical
cadherins and related molecular pathways may be attractive
therapeutic targets to restrain tumor progression in patients with
BC (42–46).
Cell cycle progression is controlled by
cyclin-dependent kinases (CDKs) and cyclins. Cell cycle
dysregulation may lead to uncontrolled cell proliferation and the
subsequent development of cancer (47). CDK1 regulates the G1-S transition
in the cell cycle, a process that is important for the development
of centrosome mutation (48). CDK1
is a vital regulator in cell proliferation, and overexpression may
lead to high tumor aggressiveness and poor prognosis (49–51).
Some antibodies, including anti-CDK1, have been used to investigate
cell proliferation (52). One
study revealed that determination of the specific activity of CDK1
may be useful in the prediction of outcomes in breast cancer
patients (53). Therefore, CDK1
may also play an important role in BC tumorigenesis, and further
study is required to identify whether it may serve as a potential
molecular marker associated with BC.
PCNA, which encodes a nuclear protein that functions
as a cofactor of DNA polymerase delta, serves as an important
proliferative marker in carcinogenesis (54). The synthesis rate of PCNA has a
direct impact on the proliferative rate of cells (55). An early study reported that
significant clinical information obtained from immunohistochemical
staining for PCNA may be helpful in the initial selection of
therapies and the evaluation of chemotherapeutic effects (56). Malkas et al (57) reported that polyclonal antibodies
against cancer-associated PCNA (caPCNA), which can serve as a
diagnostic marker of breast cancer, have been developed. Therefore,
further investigation is necessary to clarify the underlying
biological links between PCNA and BC.
TOP2A is an essential nuclear enzyme involved in DNA
replication, and its expression is decreased at the end of mitosis
and increased during the S to G2/M phases in bladder urothelial
carcinoma (58). Many studies have
reported that the expression of TOP2A is increased in skin, breast,
brain, ovarain and small cell lung cancers, and such increased
expression is associated with shortened survival (59–63).
Overexpression of TOP2A has been demonstrated to be related to
recurrence and increased risk of death (64), and with late-stage BC (65). Lindén et al (66) reported that TOP2A could serve as a
vital urinary biomarker candidate for BC. However, further
investigation is required to elucidate the exact mechanism of
action of TOP2A in the development and progression of BC.
The module analysis in the PPI network demonstrated
that the development of BC was associated with the cell cycle
signaling pathway and PI3K-Akt signaling pathway. It is well known
that the cell cycle signaling pathway plays a key role in
controlling the normal progression of the cell cycle. In the entire
cell cycle regulatory network, abnormalities in various types of
molecules can affect cell proliferation and apoptosis, potentially
leading to uncontrolled cell growth and ultimately causing tumors.
Akt plays a central role in the signaling pathways involved in cell
growth, proliferation, angiogenesis, metabolism, apoptosis and
migration (67,68), and has already been found to be
associated with cancer (69). Many
studies have identified PI3K/Akt overexpression and activation in a
variety of tumor tissues, such as ovarian cancer, colorectal
cancer, lymphoma, pancreatic cancer, non-small cell lung cancer,
lymphoma and gastric cancer (70–75).
Therefore, blocking the cell cycle and inhibiting the PI3K-AKT
signaling pathway are promising approaches for therapeutic
intervention in BC patients.
In conclusion, the current study aimed to identify
DEGs involved in the progression of BC via comprehensive
bioinformatics analysis. This study provides several key genes and
pathways for future investigation into the mechanisms and
biomarkers of BC. However, a lack of experimental verification is a
limitation of this study. Further experimental research is
necessary to investigate the pathogenic mechanism of BC.
Acknowledgements
The authors would like to thank Mr. Weipeng Zheng at
the Department of Orthopedics, Guangzhou First People's Hospital,
Guangzhou Medical University (Guangzhou, China) for his assistance
in the use of R programming language.
Funding
The present study was supported by Guangzhou Science
and Technology Project of China (grant no. 201510010272).
Availability of data and materials
The datasets of gene expression profiles for bladder
cancer (GSE7476) are available in the GEO on the NCBI website
(http://www.ncbi.nlm.nih.gov/geo).
Authors' contributions
ZH and FT conceived and coordinated the study. FT
and ZH designed methods, analyzed the data, interpreted the results
and wrote and reviewed the manuscript. HL, YC and ZL co-analyzed
and interpreted the data regarding the functional and pathway
enrichment and PPI network construction. GZ and HW downloaded the
gene expression profile from the GEO and interpreted the primary
data regarding bladder cancer. All authors contributed to, read and
approved the manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interest.
References
|
1
|
Antoni S, Ferlay J, Soerjomataram I, Znaor
A, Jemal A and Bray F: Bladder cancer incidence and mortality: A
global overview and recent trends. Eur Urol. 71:96–108. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pinto IG: Systemic therapy in bladder
cancer. Indian J Urol. 33:118–126. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Burger M, Catto JW, Dalbagni G, Grossman
HB, Herr H, Karakiewicz P, Kassouf W, Kiemeney LA, La Vecchia C,
Shariat S and Lotan Y: Epidemiology and risk factors of urothelial
bladder cancer. Eur Urol. 63:234–241. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Choueiri TK and Raghavan D: Chemotherapy
for muscle-invasive bladder cancer treated with definitive
radiotherapy: Persisting uncertainties. Nat Clin Pract Oncol.
5:444–454. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Forbes SA, Bindal N, Bamford S, Cole C,
Kok CY, Beare D, Jia M, Shepherd R, Leung K, Menzies A, et al:
COSMIC: Mining complete cancer genomes in the catalogue of somatic
mutations in cancer. Nucleic Acids Res. 39:(Database Issue).
D945–D950. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Goebell PJ and Knowles MA: Bladder cancer
or bladder cancers? Genetically distinct malignant conditions of
the urothelium. Urol Oncol. 28:409–428. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cancer Genome Atlas Research Network:
Comprehensive molecular characterization of urothelial bladder
carcinoma. Nature. 507:315–322. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Guo W, Xie L, Zhao L and Zhao Y: mRNA and
microRNA expression profiles of radioresistant NCI-H520 non-small
celllung cancer cells. Mol Med Rep. 12:1857–1867. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mengual L, Burset M, Ars E, Lozano JJ,
Villavicencio H, Ribal MJ and Alcaraz A: DNA microarray expression
profiling of bladder cancer allows identification of noninvasive
diagnostic markers. J Urol. 182:741–748. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Smyth GK: Limma: Linear models for
microarray dataBioinform Comput Biol Sol Using R Bioconduct.
Springer; pp. 397–420. 2005
|
|
12
|
Hardcastle TJ: Generalized empirical
Bayesian methods for discovery of differential data in
high-throughput biology. Bioinformatics. 32:195–202.
2016.PubMed/NCBI
|
|
13
|
Huang da W, Sherman BT and Lempicki RA:
Systematic and integrative analysis of large gene lists using DAVID
bioinformatics resources. Nat Protoc. 4:44–57. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Stelzl U, Worm U, Lalowski M, Haenig C,
Brembeck FH, Goehler H, Stroedicke M, Zenkner M, Schoenherr A,
Koeppen S, et al: A human protein-protein interaction network: A
resource for annotating the proteome. Cell. 122:957–968. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Von Mering C, Huynen M, Jaeggi D, Schmidt
S, Bork P and Snel B: STRING: A database of predicted functional
associations between proteins. Nucleic Acids Res. 31:258–261. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Szklarczyk D, Franceschini A, Kuhn M,
Simonovic M, Roth A, Minguez P, Doerks T, Stark M, Muller J, Bork
P, et al: The STRING database in 2011: Functional interaction
networks of proteins, globally integrated and scored. Nucleic Acids
Res. 39:(Database Issue). D561–D568. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bandettini WP, Kellman P, Mancini C,
Booker OJ, Vasu S, Leung SW, Wilson JR, Shanbhag SM, Chen MY and
Arai AE: MultiContrast delayed enhancement (MCODE) improves
detection of subendocardial myocardial infarction by late
gadolinium enhancement cardiovascular magnetic resonance: A
clinical validation study. J Cardiovasc Magn Reson. 14:832012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dai QS, He HC, Cai C, Chen JH, Han ZD, Qin
GQ, Liang YX and Zhong WD: Multicenter case-control study of the
relationship between smoking and bladder cancer in China. Zhonghua
Yi Xue Za Zhi. 91:2407–2410. 2011.(In Chinese). PubMed/NCBI
|
|
19
|
Boccaccio C and Medico E: Cancer and blood
coagulation. Cell Mol Life Sci. 63:1024–1027. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gay LJ and Felding-Habermann B:
Contribution of platelets to tumour metastasis. Nat Rev Cancer.
11:123–134. 2011.Falanga A, Marchetti M and Vignoli A: Coagulation
and cancer: Biological and clinical aspects. J Thromb Haemost 11:
223–233, 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhao M, Li Z and Qu H: An evidence-based
knowledgebase of metastasis suppressors to identify key pathways
relevant to cancer metastasis. Sci Rep. 5:154782015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
MacLachlan TK, Sang N and Giordano A:
Cyclins, cyclin-dependent kinases and cdk inhibitors: Implications
in cell cycle control and cancer. Crit Rev Eukaryot Gene Expr.
5:127–156. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dalbagni G, Presti J, Reuter V, Fair WR
and Cordon-Cardo C: Genetic alterations in bladder cancer. Lancet.
342:469–471. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cote RJ, Dunn MD, Chatterjee SJ, Stein JP,
Shi SR, Tran QC, Hu SX, Xu HJ, Groshen S, Taylor CR, et al:
Elevated and absent pRb expression is associated with bladder
cancer progression and has cooperative effects with p53. Cancer
Res. 58:1090–1094. 1998.PubMed/NCBI
|
|
26
|
Cordon-Cardo C, Wartinger D, Petrylak D,
Dalbagni G, Fair WR, Fuks Z and Reuter VE: Altered expression of
the retinoblastoma gene product: Prognostic indicator in bladder
cancer. J Natl Cancer Inst. 84:1251–1256. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bossis G, Malnou CE, Farras R,
Andermarcher E, Hipskind R, Rodriguez M, Schmidt D, Muller S,
Jariel-Encontre I and Piechaczyk M: Down-regulation of c-Fos/c-Jun
AP-1 dimer activity by sumoylation. Mol Cell Biol. 25:6964–6979.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hess J, Angel P and Schorpp-Kistner M:
AP-1 subunits: Quarrel and harmony among siblings. J Cell Sci.
117:5965–5973. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ye N, Ding Y, Wild C, Shen Q and Zhou J:
Small molecule inhibitors targeting activator protein 1 (AP-1). J
Med Chem. 57:6930–6948. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Durchdewald M, Angel P and Hess J: The
transcription factor Fos: A Janus-type regulator in health and
disease. Histol Histopathol. 24:1451–1461. 2009.PubMed/NCBI
|
|
31
|
Yao HQ, Peng Y, Zhong ZZ, He HX and Li ZH:
Association of the expressions of platelet-derived growth factor
receptor and c-Fos with the biological characteristics of bladder
cancer. Di Yi Jun Yi Da Xue Xue Bao. 24:177–179. 2004.PubMed/NCBI
|
|
32
|
Lan G, Yang L, Xie X, Peng L and Wang Y:
MicroRNA-490-5p is a novel tumor suppressor targeting c-FOS in
human bladder cancer. Arch Med Sci. 11:561–569. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Vleugel MM, Greijer AE, Bos R, van der
Wall E and van Diest PJ: c-Jun activation is associated with
proliferation and angiogenesis in invasive breast cancer. Human
Pathol. 37:668–674. 2006. View Article : Google Scholar
|
|
34
|
Eferl R, Ricci R, Kenner L, Zenz R, David
JP, Rath M and Wagner EF: Liver tumor development. c-Jun
antagonizes the proapoptotic activity of p53. Cell. 112:181–192.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Huhe M, Liu S, Zhang Y, Zhang Z and Chen
Z: Expression levels of transcription factors c-Fos and c-Jun and
transmembrane protein HAb18G/CD147 in urothelial carcinoma of the
bladder. Mol Med Rep. 15:2991–3000. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hunter T and Pines J: Cyclins and cancer.
II: Cyclin D and CDK inhibitors come of age. Cell. 79:573–582.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhong Z, Yeow WS, Zou C, Wassell R, Wang
C, Pestell RG, Quong JN and Quong AA: Cyclin D1/cyclin-dependent
kinase 4 interacts with filamin A and affects the migration and
invasion potential of breast cancer cells. Cancer Res.
70:2105–2114. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Feakins RM, Nickols CD, Bidd H and Walton
SJ: Abnormal expression of pRb, p16, and cyclin D1 in gastric
adenocarcinoma and its lymph node metastases: Relationship with
pathological features and survival. Hum Pathol. 34:1276–1282. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jovanovic IP, Radosavljevic GD,
Simovic-Markovic BJ, Stojanovic SP, Stefanovic SM, Pejnovic NN and
Arsenijevic NN: Clinical significance of Cyclin D1, FGF3 and p21
protein expression in laryngeal squamous cell carcinoma. J BUON.
19:944–952. 2014.PubMed/NCBI
|
|
40
|
Xu S, Gu G, Ni Q, Li N, Yu K, Li X and Liu
C: The expression of AEG-1 and Cyclin D1 in human bladder
urothelial carcinoma and their clinicopathological significance.
Int J Clin Exp Med. 8:21222–21228. 2015.PubMed/NCBI
|
|
41
|
Mao Q, Li Y, Zheng X, Yang K, Shen H, Qin
J, Bai Y, Kong D, Jia X and Xie L: Up-regulation of E-cadherin by
small activating RNA inhibits cell invasion and migration in 5637
human bladder cancer cells. Biochem Biophys Res Commun.
375:566–570. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cavallaro U, Schaffhauser B and
Christofori G: Cadherins and the tumour progression: Is it all in a
switch? Cancer Lett. 176:123–128. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Mialhe A, Levacher G, Champelovier P,
Martel V, Serres M, Knudsen K and Seigneurin D: Expression of E-,
P-, n-cadherins and catenins in human bladder carcinoma cell lines.
J Urol. 164:826–835. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wheelock MJ, Shintani Y, Maeda M, Fukumoto
Y and Johnson KR: Cadherin switching. J Cell Sci. 121:727–735.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Molinari M: Cell cycle checkpoints and
their inactivation in human cancer. Cell Prolif. 33:261–274. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hochegger H, Takeda S and Hunt T:
Cyclin-dependent kinases and cell-cycle transitions: Does one fit
all? Nat Rev Mol Cell Biol. 9:910–916. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Möröy T and Geisen C: Cyclin E. Int J
Bioch Cell Biol. 36:1424–1439. 2004. View Article : Google Scholar
|
|
48
|
Sutherland RL and Musgrove EA: Cyclins and
breast cancer. J Mammary Gland Biol Neoplasia. 9:95–104. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lee MH and Yang HY: Regulators of G1
cyclin-dependent kinases and cancers. Cancer Metast Rev.
22:435–449. 2003. View Article : Google Scholar
|
|
50
|
Sávio AL, da Silva GN and Salvadori DM:
Inhibition of bladder cancer cell proliferation by allyl
isothiocyanate (mustard essential oil). Mutat Res. 771:29–35. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kim SJ, Nakayama S, Miyoshi Y, Taguchi T,
Tamaki Y, Matsushima T, Torikoshi Y, Tanaka S, Yoshida T, Ishihara
H and Noguchi S: Determination of the specific activity of CDK1 and
CDK2 as a novel prognostic indicator for early breast cancer. Ann
Oncol. 19:68–72. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Leonardi E, Girlando S, Serio G, Mauri FA,
Perrone G, Scampini S, Dalla Palma P and Barbareschi M: PCNA and
Ki67 expression in breast carcinoma: Correlations with clinical and
biological variables. J Clin Pathol. 45:416–419. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Al-Dhaheri WS, Hassouna I, Al-Salam S and
Karam SM: Characterization of breast cancer progression in the rat.
Ann N Y Acad Sci. 1138:121–131. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Bravo R, Frank R, Blundell PA and
Macdonald-Bravo H: Cyclin/PCNA is the auxiliary protein of DNA
polymerase. Nature. 326:515–517. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Nagase Y, Moriyama N, Kurimoto S, Tajima
A, Higashihara E and Aso Y: Histochemical expression of
proliferating cell nuclear antigen (PCNA) for pre and post
chemotherapeutic bladder cancer. Nihon Hinyokika Gakkai Zasshi.
86:985–990. 1995.(In Japanese). PubMed/NCBI
|
|
56
|
Inagaki T, Ebisuno S, Uekado Y, Hirano A,
Hiroi A, Shinka T and Ohkawa T: PCNA and p53 in urinary bladder
cancer: Correlation with histological findings and prognosis. Int J
Urol. 4:172–177. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Malkas LH, Herbert BS, Abdel-Aziz W,
Dobrolecki LE, Liu Y, Agarwal B, Hoelz D, Badve S, Schnaper L,
Arnold RJ, et al: A cancer-associated PCNA expressed in breast
cancer has implications as a potential biomarker. Proc Natl Acad
Sci USA. 103:pp. 19472–19477. 2006; View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Koren R, Kugel V, Dekel Y, Weissman Y,
Livne PM and Gal R: Human DNA topoisomerase-IIalpha expression as a
prognostic factor for transitional cell carcinoma of the urinary
bladder. BJU Int. 91:489–492. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Mu XC, Tran TA, Ross JS and Carlson JA:
Topoisomerase II-alpha expression in melanocytic nevi and malignant
melanoma. J Cutan Pathol. 27:242–248. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Holden JA and Townsend JJ: DNA
topoisomerase II-alpha as a proliferation marker in astrocytic
neoplasms of the central nervous system: Correlation with MIB1
expression and patient survival. Mod Pathol. 12:1094–1100.
1999.PubMed/NCBI
|
|
61
|
Costa MJ, Hansen CL, Holden JA and Guinee
D Jr: Topoisomerase II alpha: Prognostic predictor and cell cycle
marker in surface epithelial neoplasms of the ovary and peritoneum.
Int J Gynecol Pathol. 19:248–257. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Dingemans AM, Witlox MA, Stallaert RA, van
der Valk P, Postmus PE and Giaccone G: Expression of DNA
topoisomerase IIalpha and topoisomerase IIbeta genes predicts
survival and response to chemotherapy in patients with small cell
lung cancer. Clin Cancer Res. 5:2048–2058. 1999.PubMed/NCBI
|
|
63
|
Depowski PL, Rosenthal SI, Brien TP,
Stylos S, Johnson RL and Ross JS: Topoisomerase IIalpha expression
in breast cancer: Correlation with outcome variables. Mod Pathol.
13:542–547. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Koren R, Kugel V, Dekel Y, Weissman Y,
Livne PM and Gal R: Human DNA topoisomerase-IIalpha expression as a
prognostic factor for transitional cell carcinoma of the urinary
bladder. BJU Int. 91:489–492. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Simon R, Atefy R, Wagner U, Forster T,
Fijan A, Bruderer J, Wilber K, Mihatsch MJ, Gasser T and Sauter G:
HER-2 and TOP2A coamplification in urinary bladder cancer. Int J
Cancer. 107:764–772. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Lindén M, Segersten U, Runeson M, Wester
K, Busch C, Pettersson U, Lind SB and Malmström PU: Tumour
expression of bladder cancer-associated urinary proteins. BJU Int.
112:407–415. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Manning BD and Cantley LC: AKT/PKB
signaling: Navigating downstream. Cell. 129:1261–1274. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Song G, Ouyang G and Bao S: The activation
of Akt/PKB signaling pathway and cell survival. J Cell Mol Med.
9:59–71. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Nicholson KM and Anderson NG: The protein
kinase B/Akt signalling pathway in human malignancy. Cell Signal.
14:381–395. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Yousif NG: Fibronectin promotes migration
and invasion of ovarian cancer cells through up-regulation of
FAK-PI3K/Akt pathway. Cell Biol Int. 38:85–91. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Chen Y, Wang Z, Chang P, Xiang L, Pan F,
Li J, Jiang J, Zou L, Yang L, Bian Z and Liang H: The effect of
focal adhesion kinase gene silencing on 5-fluorouracil
chemosensitivity involves an Akt/NF-kappaB signling pathway in
colorectal carcrinomas. Int J Cancer. 127:195–206. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Xu ZZ, Xia ZG, Wang AH, Wang WF, Liu ZY,
Chen LY and Li JM: Activation of the PI3K/AKT/mTOR pathway in
diffuse large B cell lymphoma: Clinical significance and inhibitory
effect of rituximab. Ann Hematol. 92:1351–1358. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Ripka S, Neesse A, Riedel J, Bug E, Aigner
A, Poulsom R, Fulda S, Neoptolemos J, Greenhalf W, Barth P, et al:
CUX1: Target of Akt signaling ang mediator of resisitance to
apoptosis in pancreatic cancer. Gut. 59:1101–1110. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Cumberbatch M, Tang X, Beran G, Eckersley
S, Wang X, Ellston RP, Dearden S, Cosulich S, Smith PD, Behrens C,
et al: Identification of a subset of human non-small cell lung
cancer patients with high PI3Kβ and low PTEN expression, more
prevalent in squamous cell carcinoma. Clin Cancer Res. 11:595–603.
2014. View Article : Google Scholar
|
|
75
|
Xie X, Tang B, Zhou J, Gao Q and Zhang P:
Inhibition of the PI3K/Akt pathway increases the chemosensitivity
of gastric cancer to vincristine. Oncol Rep. 30:773–782. 2013.
View Article : Google Scholar : PubMed/NCBI
|