Introduction
Lipotoxicity refers to excessive accumulation of
lipid in cells, including hepatocytes, pancreatic cells and
cardiomyocytes, which contributes to a loss of cellular function
and cell death (1–2). A previous study suggested that
excessive lipid levels may result in the death of mouse
myocardiocytes, possibly causing heart failure (3). Pigment epithelial-derived factor
(PEDF), a 50 kDa multifunctional glycoprotein with antiangiogenic,
antitumor, anti-inflammatory, antioxidative and cardioprotective
properties, protects against hypoxia-induced apoptosis and
necroptosis in primary cardiomyocytes and H9c2 cells (4–6).
Autophagy is an evolutionarily conserved and highly regulated
process that is essential for maintaining cellular homeostasis by
preventing the accumulation of damaged proteins and organelles,
whilst sustaining metabolism during hypoxia (7,8).
However, whether PEDF regulates lipid degradation by inducing
autophagy remains to be elucidated.
The autophagy-associated-gene proteins are major
regulators of the autophagy system, mediating several sequential
steps of the autophagic process (7,8).
Autophagy-related 5 (Atg5) is a protein required for the formation
of autophagosomes (7,9) and sequesters cytoplasmic materials
prior to lysosomal delivery. Atg5 serves a major role in autophagic
processes; however, further investigation is required to understand
whether PEDF promotes autophagy and whether this effect contributes
to cytoprotection. Whether Atg5 is involved in PEDF-promoted
autophagy is yet to be investigated.
Previous studies have revealed that adipose
triglyceride lipase (ATGL) serves as a receptor for PEDF in retinal
epithelial cells and cardiomyocytes (10,11).
PEDF-receptor (PEDF-R), an 80 kDa receptor protein, was used in the
present study. PEDF-R has been reported to exhibit biological
effects by interacting with PEDF on cell-surface (12). PEDF-R is the key enzyme of lipid
catabolism and catalyzes the lipid lipolysis cascade (13); a lack of PEDF-R in cardiac muscle
causes a lipolytic defect, which leads to lipid accumulation,
severe cardiac dysfunction and premature death (14). However, whether PEDF-R is
associated with autophagy and whether PEDF-R-mediated autophagy is
associated with lipid degradation requires further
investigation.
The aim of the present study was to examine whether
PEDF-promoted autophagy may protect H9c2 cells under hypoxic
conditions, and whether PEDF stimulates lipid degradation by
promoting autophagy. It was also investigated whether PEDF-promoted
autophagy is regulated by Atg5 in H9c2 cells.
Materials and methods
Antibodies and reagents
Anti-microtubule-associated protein-light chain 3
(LC3)B (cat. no. 2775) and anti-cleaved caspase-3 (cat. no. 9662)
antibodies were purchased from Cell Signaling Technology, Inc.
(Danvers, MA, USA). Alexa Fluor 488 (cat. no. 705-546-147) was
purchased from Jackson ImmunoResearch Laboratories, Inc. (West
Grove, PA, USA). Cell counting Kit-8 (CCK-8; cat. no. WO97/38985)
was purchased from Dojindo Molecular Technologies, Inc. (Kumamoto,
Japan). The LDH Cytotoxicity Assay kit was purchased from Nanjing
KeyGen Biotech Co., Ltd (Nanjing, China). A triglyceride (TG) assay
kit (GPO-POD; cat. no. E1003) was purchased from (Applygen
Technologies Inc., Beijing, China). The in situ cell death
detection kit (cat. no. 11684795910) was purchased from
Sigma-Aldrich; (Merck KGaA, Darmstadt, Germany). Anti-Atg5 antibody
(cat. no. ab227132) was purchased from Abcam (Cambridge, UK).
Recombinant PEDF was synthesized by Cusabio Biotech Co., Ltd.
(Hubei, China). The special inhibitor of autophagy 3-methyladenine
(3-MA; cat. no. KGATGR006; Nanjing KeyGen Biotech Co., Ltd.) was
donated from Dr Xiaofang Yang (Laboratory of Clinical and
Experimental Pathology, Xuzhou Medical University, Jiangsu,
China).
Recombinant lentivirus constructs and
viral production
Recombinant lentivirus (LV) was prepared as
previously described (15). The
Lentivirus expressing PEDF or PEDF-R_shRNA were purchased from
GENECHEM, Inc. (Daejeon, Korea). The concentrated titer of virus
suspension was 2×1012 Tu/l. Transient transfection of
H9c2 cells with short interfering RNA (siRNA) targeting the PEDF-R
genes were performed using Lipofectamine 3000 according to the
manufacturer's instructions (Invitrogen; Thermo Fisher Scientific,
Inc., Waltham, MA, USA).
Preparations of PEDF protein
Recombinant rat PEDF (GenBank™ accession
no. NM_177927) was synthesized by Cusabio Biotech, Co., Ltd.
(Wuhan, China).
Cell culture and hypoxia
The H9c2 cells embryonic rat heart-derived cell line
was obtained from American Type Culture Collection (ATCC; Manassas,
VA, USA) and maintained in Dulbecco's modified Eagle's medium
supplemented with 10% fetal bovine serum (both from Gibco; Thermo
Fisher Scientific, Inc.) and 100 mg/ml penicillin/streptomycin at
37°C in a humidified atmosphere containing 5% CO2.
Hypoxia was achieved by culturing the cells in D-Hank's liquid with
glucose deprivation in a tri-gas incubator (Heal Force, Shanghai,
China) saturated with 5% CO2/1% O2 at 37°C
for 4 h.
TUNEL analysis
Cells were seeded in 48-well plates (Corning Inc.,
Corning, NY, USA). Following hypoxic incubation, cells were treated
with 4% paraformaldehyde for 10 min at room temperature, and then
washed three times in PBS, (pH 7.4). Next cells were incubated with
1% goat serum diluted in PBS for 1 h at room temperature. Terminal
dexynucleotidyl transferase (TdT)-mediated dUTP nick end labeling
(TUNEL) staining was then performed using an in situ cell
death detection kit (Roche Molecular Diagnostics, Pleasanton, CA,
USA) according to the manufacturer's instructions. The cells were
incubated with reaction buffer containing enzyme solution and label
solution with an enzyme-to-label ratio of 159 at 37°C for 1 h 33
min. Cells were counterstained using Hoechst stain (Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) for 15 min at room temperature. The
cells were observed under a fluorescence microscope (Olympus,
Tokyo, Japan). A total of 30 fields of view were used.
Magnification, ×20.
Western blotting
Western blotting was performed following standard
procedures (6). The cells were
lysed in radioimmunoprecipitation assay lysis buffer: 100 mmol/l
Tris-HCl, 4% SDS, 20% glycerine, 200 mmol/l dithithreitol and
protease inhibitors (pH 6.8). Total cellular protein was denatured
by boiling for 10 min with an equal volume of 2 3 Tris-glycine SDS
buffer. Protein concentration was determined using a bicinchonic
acid assay. An equal amount of protein (50 ng) for each sample was
resolved via 8–15% SDS-PAGE and transferred onto a nitrocellulose
membrane (EMD Millipore, Billerica, MA, USA). The membrane was
blocked with 5% nonfat milk/PBS-Tween-20 for 2 h at room
temperature and subsequently incubated with
anti-microtubule-associated protein-light chain 3B (LC3B) (cat. no.
2775; 1:1,000) and anti-cleaved caspase-3 (cat. no. 9662; 1:1,000)
antibodies overnight at 4°C, respectively. Subsequently,
fluorescent-labeled secondary antibodies were added at room
temperature for 2 h and then scanned by the Odyssey Infrared
Imaging System with Odyssey 3.0 software (both from LI-COR
Biosciences, Lincoln, NE, USA).
Immunofluorescence
H9c2 cells were cultured in 48-well plates at a
density of 1×104 cells/ml. Following hypoxic treatment
for 4 h (6), H9c2 cells were
washed twice with PBS and fixed with freshly prepared 4%
paraformaldehyde at room temperature for 15 min (6). Antigen accessibility was increased by
treatment with 2% Triton X-100 at room temperature for 10 min. H9c2
cells were subsequently blocked with 3% bovine serum albumin (cat.
no. VIC018; Vicmed Co., Ltd., Xuzhou, China) at room temperature
for 30 min. Following incubation with anti-microtubule-associated
protein-light chain 3B (LC3B) (cat. no. 2775, 1:1,000) and
anti-cleaved caspase-3 (cat. no. 9662; 1:1,000) primary antibodies
at 4°C overnight, respectively. Then cells were washed and
incubated with anti-mouse secondary antibody (cat. no. A21207;
1:200; Thermo Fisher Scientific, Inc.) under light-protected
conditions for 1 h at room temperature. H9c2 cells were then washed
three times with PBS for 5 min, captured and analyzed using TCS SP8
STED 3X (Leica Microsystems GmbH, Wetzlar, Germany).
Transmission electron microscopy
(TEM)
H9c2 cells were fixed with 4% paraformaldehyde and
2.5% glutaraldehyde at room temperature overnight. Subsequently,
samples were incubated while protected from light with 1% osmium
tetroxide at room temperature for 1 h. Following washing in
distilled water, the samples were dehydrated in graded ethanol
concentrations and then embedded in molds with fresh resin.
Ultrathin sections (70 nm) were obtained with an EM UC7 (Leica
Microsystems GmbH), the samples ultrathin sections were then
incubated in 2% uranyl acetate for 0.5 h and stained with lead
citrate for 10 min at room temperature. Finally, the samples were
observed with a Tecnai G2 T12 at room temperature (FEI; Thermo
Fisher Scientific, Inc.).
H9c2 cell viability test
H9c2 cells were seeded in 96-well plates at a
density of 1×104 cells/ml. Following hypoxic treatments
(6), H9c2 cells viability was
analyzed using the CCK-8 kit according to the manufacturer's
protocol. Absorbance was measured at 450 nm with a microplate
reader (BioTek Synergy 2; BioTek Instruments, Inc., Winooski, VT,
USA).
Lactate dehydrogenase (LDH) release
assay
H9c2 cells were seeded in 96-well plates
(1×104 cells/ml). Following hypoxic treatments the
activity of LDH within H9c2 cells released into the medium was
assessed as previously described (6) by a microplate reader (BioTek Synergy
2) analysis at 440 nm, using an LDH Cytotoxicity Assay kit
according to the manufacturer's instructions.
TG assay
H9c2 cells were seeded in 96-well plates at a
concentration of 1×104 cells/ml. The levels of
intracellular TG were assessed using a TG assay kit according to
the manufacturer's instructions.
Statistical analysis
Data are expressed as the mean ± standard deviation.
Statistical analysis was performed using repeated-measures or
one-way analysis of variance followed by the Tukey's test for
multiple comparisons. P<0.05 was considered to indicate a
statistically significant difference.
Results
PEDF induces autophagy via PEDF-R
within hypoxic H9c2 cells
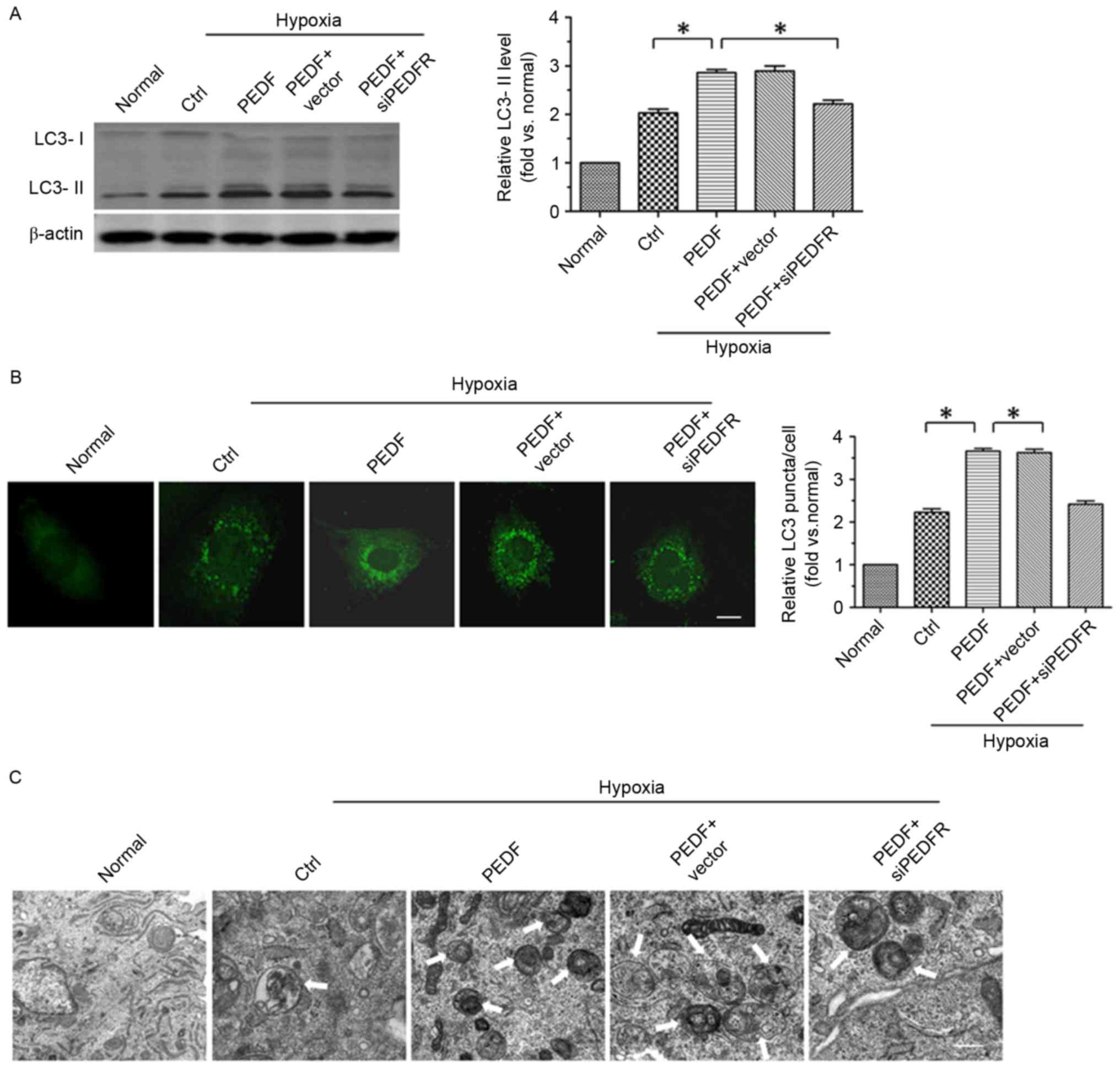
To investigate whether PEDF promotes H9c2 cell
autophagy via PEDF-R under hypoxic conditions, the level of
autophagy marker protein LC3 in H9c2 cells was detected under
normoxic and hypoxic conditions. A total of ≤4 h of hypoxic
conditions resulted in a significantly increase in the expression
of LC3-II in H9c2 (P<0.05). Furthermore, overexpression of PEDF
significantly increased LC3-II expression (P<0.05; Fig. 1A). In addition, LC3 staining
(green) was used to further test PEDF-induced autophagy. As
presented in Fig. 1B, a
significant increase in LC3 puncta was detected in PEDF-treated
H9c2 cells compared with cells under hypoxic conditions only
(P<0.05). To further confirm that PEDF treatment induced H9c2
cell autophagy under hypoxic conditions, TEM analyses were
performed. H9c2 cells treated with PEDF exhibited a marked increase
in the number of autophagosomes compared with cells under hypoxic
conditions only (P<0.05; Fig.
1C). Collectively, the findings of the present study indicate
that PEDF is able to promote H9c2 cell autophagy via PEDF-R under
hypoxic conditions.
PEDF-induced autophagy contributes to
lipid degradation
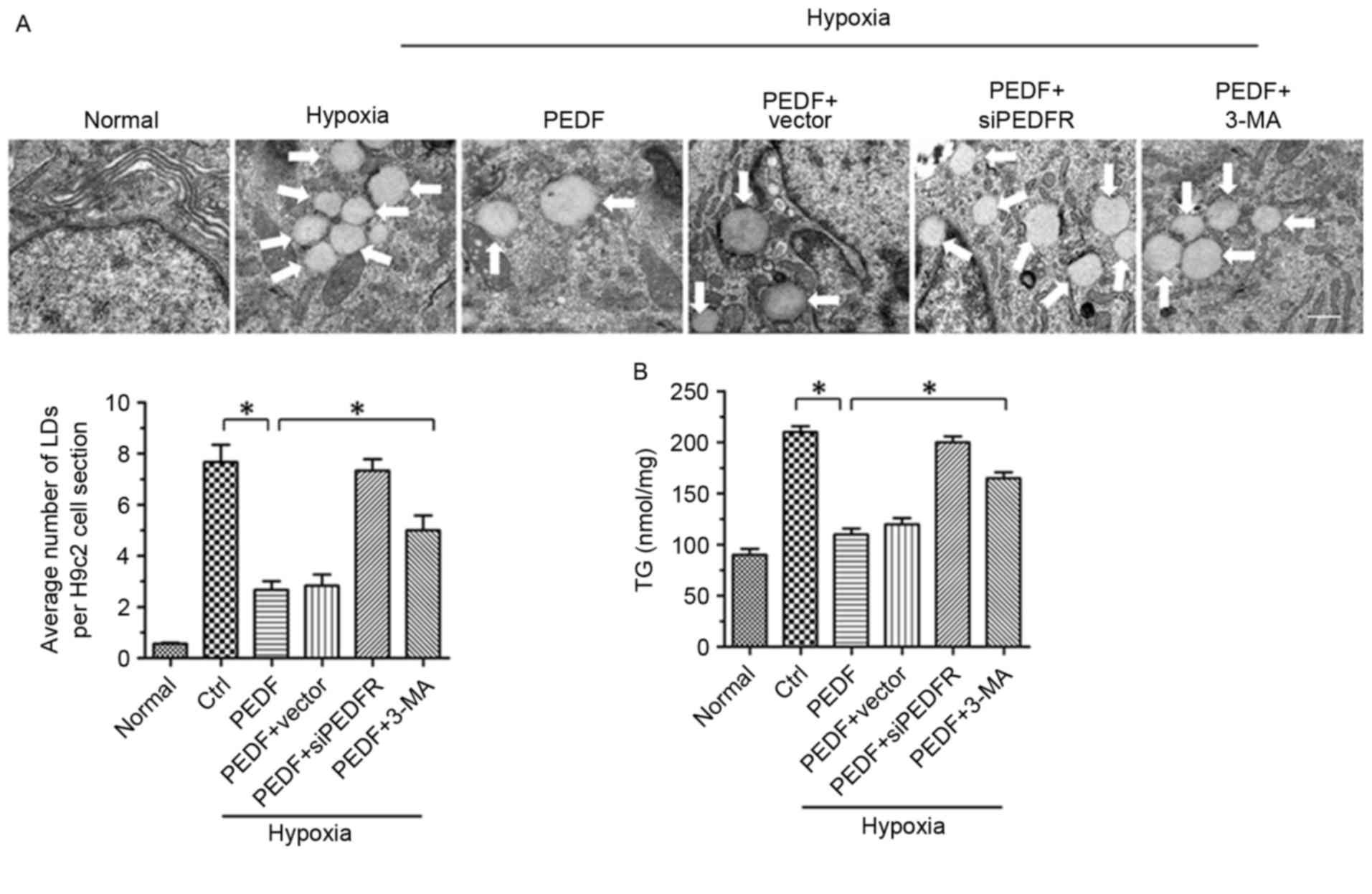
It has previously been reported that PEDF stimulates
cardiac triglyceride degradation via ATGL (11). In addition, it has been
demonstrated that autophagy may regulate lipid metabolism (16). In the present study, the
association between PEDF-promoted autophagy and lipid metabolism
was investigated. As presented in Figs. 2A and 4H of hypoxia led to an increase in lipid
droplets (LDs), whereas PEDF-treatment resulted in decreased LDs
(P<0.05). To investigate the effects of PEDF-induced autophagy
on LDs, H9c2 cells were treated with 3-methyladenine (3-MA). The
addition of 3-MA resulted in a marked increase in LDs (P<0.05),
which suggests that PEDF may induce lipid degradation via
autophagy. In addition, the TG content was significantly increased
within H9c2 cells under hypoxic conditions (P<0.05). Treating
H9c2 cells with PEDF significantly decreased TG levels, whereas the
addition of 3-MA resulted in increased TG content (P<0.05;
Fig. 2B). Collectively, these
findings suggest that PEDF stimulates H9c2 cell lipid degradation
by promoting autophagy.
PEDF protects H9c2 cells against
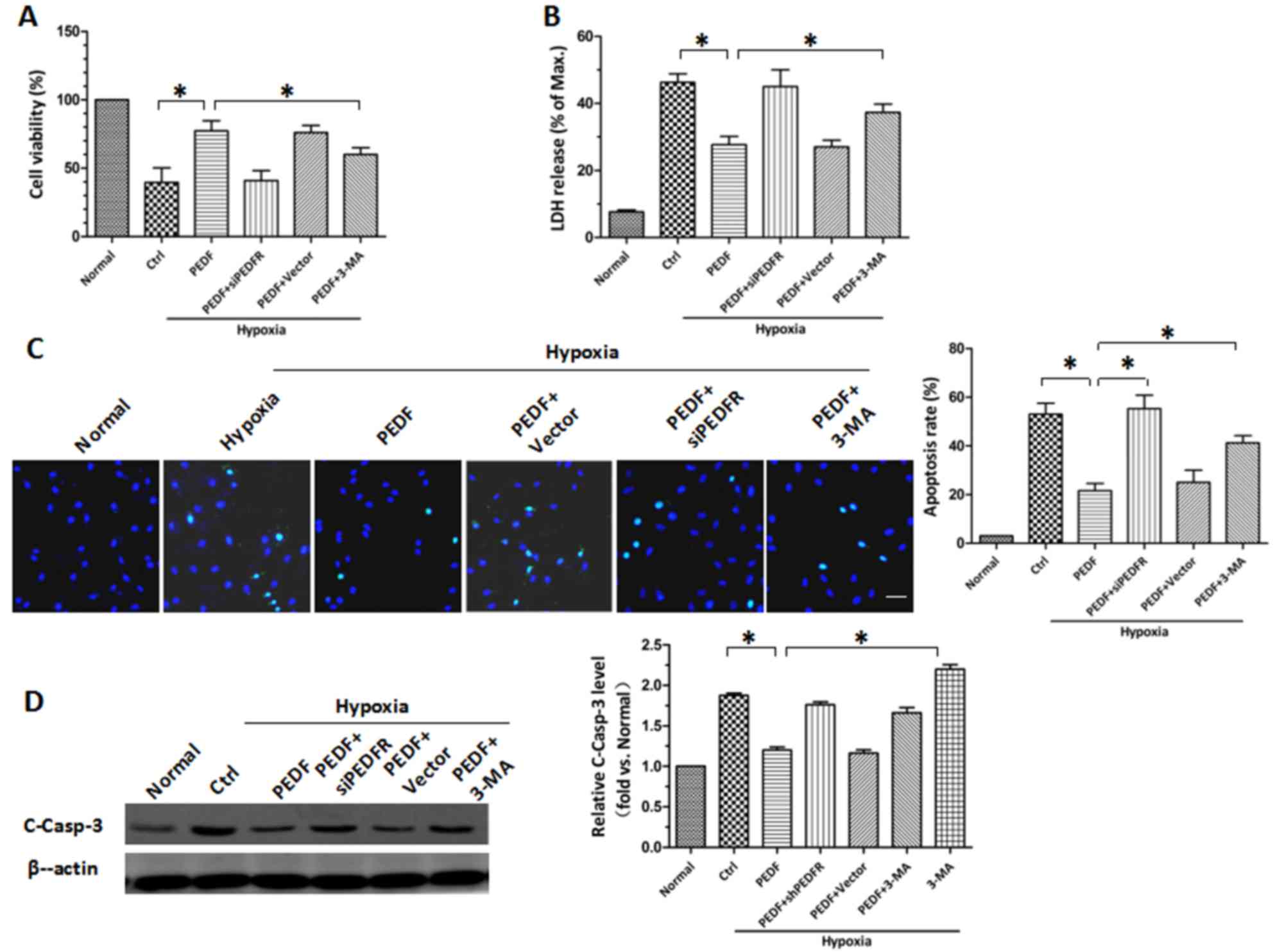
hypoxia-induced apoptosis by inducing autophagy
Following the establishment of PEDF-induced
autophagy, the protective role of PEDF-induced autophagy in hypoxic
H9c2 cells was investigated. CCK-8 and LDH release assays revealed
similar results (P<0.05; Fig. 3A
and B). Under hypoxic conditions, the viability of H9c2 cells
was decreased. PEDF overexpression attenuated the hypoxia-mediated
reduction in cell viability, whereas the autophagy inhibitor 3-MA
attenuated this effect. The protective effects of PEDF may be
achieved via reducing H9c2 cell apoptosis. TUNEL staining was used
to assess H9c2 cell apoptosis and it was demonstrated that PEDF
significantly reduced H9c2 cell apoptosis compared with in hypoxia
only (P<0.05; Fig. 3C).
Conversely, 3-MA promoted hypoxia-induced cell death (P<0.05;
Fig. 3C). It was also demonstrated
that PEDF significantly suppressed the levels of cleaved caspase-3,
whereas the addition of 3-MA increased its expression (P<0.05;
Fig. 3D). These findings indicate
that PEDF-induced autophagy may be increase cell survival under
hypoxic conditions by reducing hypoxia-induced apoptosis.
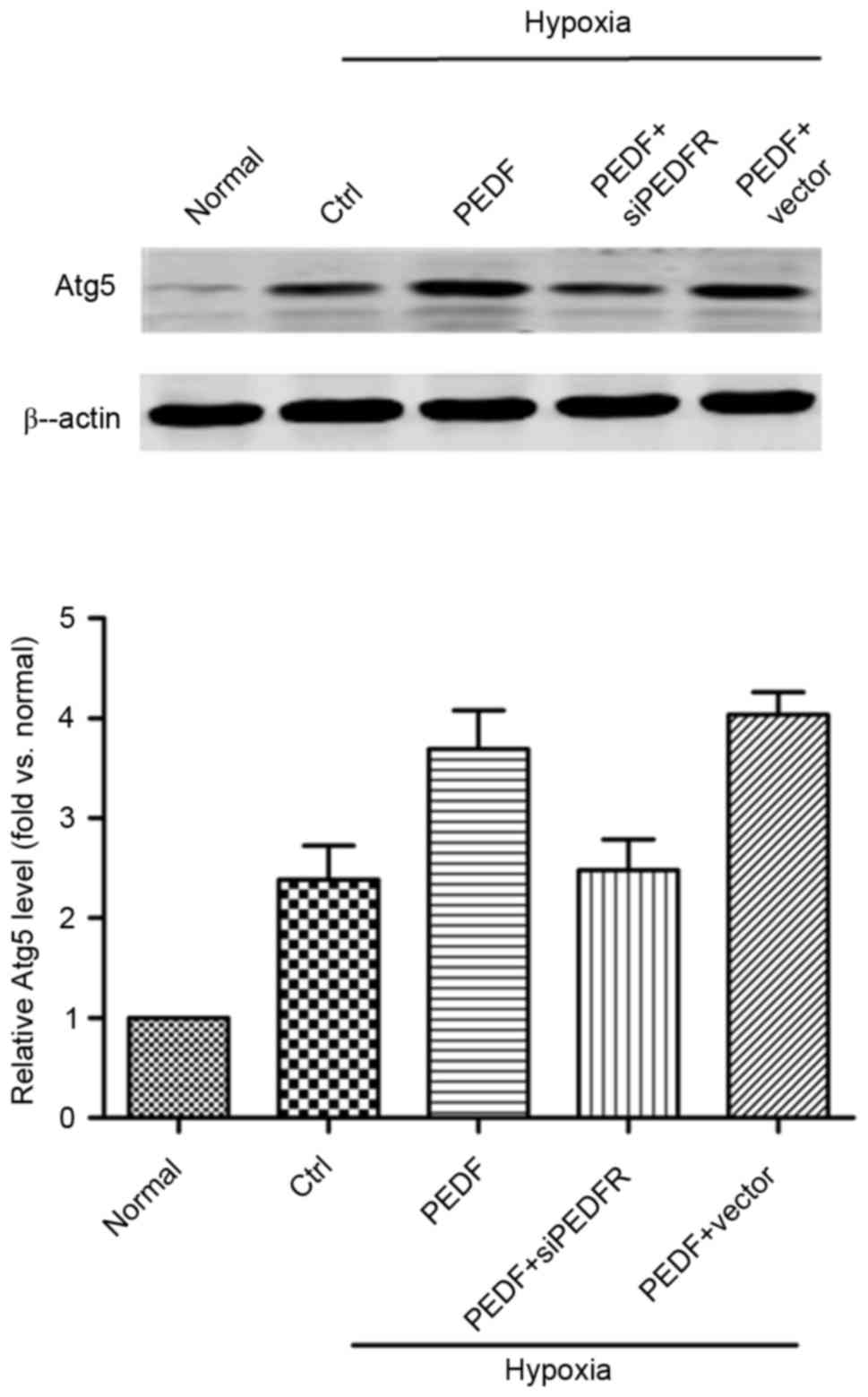
PEDF-induced autophagy is dependent on
the Atg5 pathway
As presented in Fig.
4, the addition of PEDF increased Atg5 expression and the
effect of PEDF treatment was inhibited by PEDF-R siRNA (P<0.05).
These findings suggest that, under hypoxic conditions, PEDF may
promote autophagy via the PEDF-R-induced Atg5 pathway.
Discussion
In the present study, it was confirmed that
PEDF-promoted autophagy stimulates lipid degradation. In addition,
PEDF was demonstrated to protect H9c2 cells against hypoxia-induced
apoptosis by promoting autophagy. The results of the present study
indicate a potential mechanism of PEDF promoted-autophagy that is
dependent on the Atg5 pathway via PEDF-R.
Autophagy is a highly conserved and genetically
programmed process, which maintains metabolic and cellular
homeostasis by recycling intracellular components for use in
macromolecular synthesis and inhibits the accumulation of
aggregated proteins and damaged organelles that may be toxic
(17–20). The results of the present study
revealed that PEDF promotes autophagy via PEDF-R. This mechanism is
dependent on the Atg5 pathway; the Atg5 protein serves a role in
the early stage of autophagosome formation (7,9).
PEDF treatment resulted in an increase in Atg5. PEDF-R, a
lipase-linked cell membrane receptor for PEDF (10), binds to PEDF and stimulates
triglyceride degradation (12).
PEDF may therefore stimulate the activity of PEDF-R, generating
intracellular signaling molecules and ultimately upregulating Atg5
under hypoxic conditions. The results of the present study suggest
that PEDF upregulates the expression of Atg5 via PEDF-R to promote
autophagy. However, further investigation is required to confirm
the mechanisms underlying the induction of Atg5 initiated by PEDF-R
in hypoxic H9c2 cells treated with PEDF.
During acute myocardial infarction, TG accumulation
due to reduced oxygen availability results in H9c2 cell toxicity
and serves a crucial role in the progression of cardiomyopathy and
heart failure (2,21). Various diseases associated with
excess lipid accumulation, including obesity and diabetes, have
been reported to feature lipotoxicity (1,22).
Therefore, inhibiting TG accumulation may contribute to the
treatment of these diseases. In the present study, the number of
LDs was significantly increased under hypoxic conditions, whereas
H9c2 cells overexpressing PEDF had a markedly lower number of LDs.
Conversely, the addition of 3-MA resulted in an increase in LDs. TG
levels in H9c2 cells increased under hypoxic conditions and
decreased in response to PEDF. Treatment with 3-MA resulted in
increased TG levels. In the present study, it was demonstrated that
PEDF-induced autophagy may stimulate TG degradation within hypoxic
H9c2 cells. The results demonstrated a previously unreported
association between PEDF-induced autophagy and lipid metabolism.
However, further investigation is required to fully elucidate the
molecular mechanisms of PEDF-induced autophagy in the regulation of
lipid metabolism in response to hypoxia.
Autophagy is an important cell survival mechanism
that eliminates aggregated proteins and unwanted organelles
(23,24) and is associated with cardiovascular
diseases, inflammation, hypoxia, and oxidized lipoproteins
(25–27). Previous studies have demonstrated
that PEDF serves a protective role in hypoxic H9c2 cells via its
antioxidative effect, as well as inhibiting p53 mitochondrial
translocation (5,6). To the best of the authors' knowledge,
the effect of PEDF on autophagy in hypoxic H9c2 cells have not
previously been investigated. In the present study, treatment with
PEDF markedly increased the H9c2 cell viability compared with
hypoxia alone, whereas the addition of 3-MA significantly weakened
this effect. However, treatment with 3-MA could reverse the
downregulation of PEDF on, the level of cleaved caspase-3. The
results of the present study confirm that the autophagy facilitated
by PEDF serves a protective role in H9c2 cells via inhibiting the
hypoxia-induced apoptosis. The findings of the present study are
generally consistent with an earlier report that autophagy may be a
harmful form of procedural cell death under pathological conditions
(28). Inconsistencies in the
findings may be due to experimental factors, including the cell
type employed, the application of the model in vivo or in
vitro and treatment conditions. The results of the present
study suggest that PEDF may enhance hypoxia-induced autophagy and
has the potential to be a novel therapeutic approach for the
treatment of H9c2 cell injury.
Acknowledgements
The present study was supported by the National
Nature Science Foundation of China (grant nos. 81570242 and
81270173) and the Technology Bureau of Xuzhou of China (grant no.
KC14SH106). We thank Dr. Xiaofang Yang (Laboratory of Clinical and
Experimental Pathology, Xuzhou Medical University, Jiangsu, China)
for providing autophagy inhibitor 3-MA.
References
|
1
|
Brookheart RT, Michel CI and Schaffer JE:
As a matter of fat. Cell Metab. 10:9–12. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Huss JM, Levy FH and Kelly DP: Hypoxia
inhibits the peroxisome proliferator-activated receptor
alpha/retinoid X receptor gene regulatory pathway in cardiac
myocytes: A mechanism for O2-dependent modulation of
mitochondrial fatty acid oxidation. J Biol Chem. 276:27605–27612.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chiu HC, Kovacs A, Ford DA, Hsu FF, Garcia
R, Herrero P, Saffitz JE and Schaffer JE: A novel mouse model of
lipotoxic cardiomyopathy. J Clin Invest. 107:813–822. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kawaguchi T, Yamagishi SI and Sata M:
Structure-function relationships of PEDF. Curr Mol Med. 10:302–311.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gao X, Zhang H, Zhuang W, Yuan G, Sun T,
Jiang X, Zhou Z, Yuan H, Zhang Z and Dong H: PEDF and PEDF-derived
peptide 44mer protect cardiomyocytes against hypoxia-induced
apoptosis and necroptosis via anti-oxidative effect. Sci Rep.
4:56372014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang X, Zhang Y, Lu P, Zhang H, Li Y, Dong
H and Zhang Z: PEDF attenuates hypoxia-induced apoptosis and
necrosis in H9c2 cells by inhibiting p53 mitochondrial
translocation via PEDF-R. Biochem Biophys Res Commun. 465:394–401.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Klionsky DJ: Autophagy: From phenomenology
to molecular understanding in less than a decade. Nat Rev Mol Cell
Biol. 8:931–937. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
He C and Klionsky DJ: Regulation
mechanisms and signaling pathways of autophagy. Annu Rev Genet.
43:67–93. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hammond EM, Brunet CL, Johnson GD,
Parkhill J, Milner AE, Brady G, Gregory CD and Grand RJ: Homology
between a human apoptosis specific protein and the product of APG5,
a gene involved in autophagy in yeast. FEBS Lett. 425:391–395.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Notari L, Baladron V, Aroca-Aguilar JD,
Balko N, Heredia R, Meyer C, Notario PM, Saravanamuthu S, Nueda ML,
Sanchez-Sanchez F, et al: Identification of a lipase-linked cell
membrane receptor for pigment epithelium-derived factor. J Biol
Chem. 281:38022–38037. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang H, Sun T, Jiang X, Yu H, Wang M, Wei
T, Cui H, Zhuang W, Liu Z, Zhang Z and Dong H: PEDF and
PEDF-derived peptide 44mer stimulate cardiac triglyceride
degradation via ATGL. J Transl Med. 13:682015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Subramanian P, Locatelli-Hoops S, Kenealey
J, DesJardin J, Notari L and Becerra SP: Pigment epithelium-derived
factor (PEDF) prevents retinal cell death via PEDF Receptor
(PEDF-R): Identification of a functional ligand binding site. J
Biol Chem. 288:23928–23942. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zimmermann R, Strauss JG, Haemmerle G,
Schoiswohl G, Birner-Gruenberger R, Riederer M, Lass A, Neuberger
G, Eisenhaber F, Hermetter A and Zechner R: Fat mobilization in
adipose tissue is promoted by adipose triglyceride lipase. Science.
306:1383–1386. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Haemmerle G, Moustafa T, Woelkart G,
Büttner S, Schmidt A, van de Weijer T, Hesselink M, Jaeger D,
Kienesberger PC, Zierler K, et al: ATGL-mediated fat catabolism
regulates cardiac mitochondrial function via PPAR-α and PGC-1. Nat
Med. 17:1076–1085. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang H, Wang Z, Feng SJ, Xu L, Shi HX,
Chen LL, Yuan GD, Yan W, Zhuang W, Zhang YQ, et al: PEDF improves
cardiac function in rats with acute myocardial infarction via
inhibiting vascular permeability and cardiomyocyte apoptosis. Int J
Mol Sci. 16:5618–5634. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Singh R, Kaushik S, Wang Y, Xiang Y, Novak
I, Komatsu M, Tanaka K, Cuervo AM and Czaja MJ: Autophagy regulates
lipid metabolism. Nature. 458:1131–1135. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mizushima N, Levine B, Cuervo AM and
Klionsky DJ: Autophagy fights disease through cellular
self-digestion. Nature. 451:1069–1075. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Levine B and Kroemer G: Autophagy in the
pathogenesis of disease. Cell. 132:27–42. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mizushima N, Ohsumi Y and Yoshimori T:
Autophagosome formation in mammalian cells. Cell Struct Funct.
27:421–429. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Viventi J and Blanco JA: Development of
high resolution, multiplexed electrode arrays: Opportunities and
challenges. Conf Proc IEEE Eng Med Biol Soc. 2012:pp. 1394–1396.
2012; PubMed/NCBI
|
|
21
|
van Herpen NA and Schrauwen-Hinderling VB:
Lipid accumulation in non-adipose tissue and lipotoxicity. Physiol
Behav. 94:231–241. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chavez JA and Summers SA: Lipid
oversupply, selective insulin resistance and lipotoxicity:
Molecular mechanisms. Biochim Biophys Acta. 1801:252–265. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tan SH, Shui G, Zhou J, Li JJ, Bay BH,
Wenk MR and Shen HM: Induction of autophagy by palmitic acid via
protein kinase C-mediated signaling pathway independent of mTOR
(mammalian target of rapamycin). J Biol Chem. 287:14364–14376.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kroemer G, Mariño G and Levine B:
Autophagy and the integrated stress response. Mol Cell. 40:280–293.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sutton MG and Sharpe N: Left ventricular
remodeling after myocardial infarction: Pathophysiology and
therapy. Circulation. 101:2981–2988. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xuan H, Xue W, Pan J, Sha J, Dong B and
Huang Y: Downregulation of miR-221, −30d and −15a contributes to
pathogenesis of prostate cancer by targeting Bmi-1. Biochemistry
(Mosc). 80:276–283. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ma X, Liu H, Murphy JT, Foyil SR, Godar
RJ, Abuirqeba H, Weinheimer CJ, Barger PM and Diwan A: Regulation
of the transcription factor EB-PGC1α axis by beclin-1 controls
mitochondrial quality and cardiomyocyte death under stress. Mol
Cell Biol. 35:956–976. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Park M, Sabetski A, Kwan Chan Y, Turdi S
and Sweeney G: Palmitate induces ER stress and autophagy in H9c2
cells: Implications for apoptosis and adiponectin resistance. J
Cell Physiol. 230:630–639. 2015. View Article : Google Scholar : PubMed/NCBI
|