Introduction
Diabetes mellitus (DM) is a chronic disease that
exhibits genetic susceptibility (1). DM is caused by decreased levels or an
absolute lack of insulin, resulting from the interaction of various
internal and external factors, and is characterized by high blood
glucose and lipid metabolism of sugar (1). Diabetic patients are disposed to
complications in early and late stages, including nephropathy, eye
disease, foot pathologies and cardiomyopathy; diabetic
cardiomyopathy belongs to the cardiovascular complications of
diabetes and is the predominant cause of mortality in patients with
diabetes (2).
Angiotensin-converting enzyme (ACE)2 is the only
homologue of ACE that has been identified to date; it exhibits 42%
homologous sequences with ACE (3).
ACE2 is an inner membrane carboxypeptidase that can hydrolyze
angiotensin 1 (Ang-1) into Ang-(1–9), and
this may be further hydrolyzed by ACE into Ang-(1–7)
(4). ACE2 can also remove the
terminal tryptophan of Ang-II, thus directly hydrolyzing Ang-II to
Ang-(1–7). Furthermore, in vitro studies
have demonstrated that the enzymatic activity of ACE2 on Ang-II is
400 times of that on Ang-I (4).
The binding of Ang-(1–7) with its specific G-protein coupled
receptor Mas promotes the release of bradykinin, nitric oxide (NO)
and prostaglandins, which serve roles in vessel expansion,
decreasing blood pressure, improving insulin resistance, resisting
inflammation, inhibiting cell proliferation, anticoagulation and
protecting the vascular endothelium (5). ACE2 hydrolyzes vasopressin,
neurotensin and dynorphin A-(1–13),
which are involved in cardiovascular regulation. Cardiovascular
protection is enabled by maintaining homeostasis of the
renin-angiotensin system, by modulating the antagonizing effect of
the ACE2/Ang-(l-7)/Mas receptor axis on the ACE/Ang-1/Ang-II-type 1
(ATI) receptor axis (6).
The vascular endothelial growth factor (VEGF) family
serves a major role in the regulation of the angiogenesis network.
VEGF has direct effects on vascular endothelial cells by promoting
their proliferation and migration; VEGF is also a critical
regulator of angiogenesis in the formation human tumors (7). Furthermore, VEGF may be involved in
regulating the interaction between endothelial cells and between
endothelial cells and the basement membrane, by interacting with
receptors on vascular endothelial cells (8).
Diabetic cardiomyopathy (DCM) is a chronic and
complex pathological process that can alter cell metabolism,
resulting in damage to the endoplasmic reticulum, mitochondria and
other organelles, and ultimately result in myocardial hypertrophy
and increased apoptosis (9).
Furthermore, apoptosis resulting from altered energy metabolism of
the cell serves an additional role in the pathology of DCM; large
scale apoptosis can aggravate myocardial remodeling and cause
further tissue dysfunction, thus creating a destructive pathogenic
cycle (10,11).
The active ingredients of pueraria constitute
isoflavones, including daidzin, daidzein and puerarin (12). Puerarin has wide pharmacological
effects (12); aside from its
application in the treatment of cardiovascular and cerebrovascular
diseases, with broad prospects for development and clinical
application (13). Puerarin
inhibits lipid peroxidation and aldose reductase activity, removes
superoxide ion radicals, and has a protective effect on endothelial
cells (14). Furthermore, puerarin
may significantly slow the glucosamine metabolism of endothelial
cells, reduce endothelin and platelet surface activity, inhibit
platelet aggregation and adhesion, lower blood lipids, cholesterol,
blood viscosity and prevent thrombosis (14). Therefore, puerarin may be useful in
the protection of vascular endothelial cells, the promotion of
angiomalacia and the inhibition of atherosclerosis (15). The present study investigated
whether the myocardial protective effects of puerarin can attenuate
ischemia/reperfusion (I/R)-induced myocardial apoptosis in diabetic
rats, via upregulation of VEGFA/Ang-1 and suppression of apoptosis
in rats, and thus exert cardioprotective effects in diabetics.
Materials and methods
Animals and experimental groups
Male Sprague-Dawley rats (8–10 weeks old, n=40)
weighing 200–220 g were housed at 22–23°C, 55–60% humidity, 12 h
light/dark cycle and access free to food and water, and randomly
assigned into five groups: Sham (n=6); ischemia/reperfusion (I/R;
n=10); I/R+L (lower dosage, n=8, 25 mg/kg puerarin); I/R+M (medium
dosage, n=8, 50 mg/kg puerarin), I/R+H (higher dosage, n=8, 100
mg/kg puerarin). Rats were injected intraperitoneally with
streptozotocin (30 mg/kg) twice, with a rest day between each
injection. The following week, the myocardial I/R model was induced
with 35 mg/kg pentobarbital sodium; the heart was exteriorized with
a left thoracic incision, and a slipknot made using 4-0 silk was
placed around the left anterior descending coronary artery.
Ischemia was performed for 30 min, the slipknot was released and
reperfusion was allowed to occur for 3 h. Rats of the sham group
were anesthetized with 35 mg/kg pentobarbital sodium and underwent
a sham surgery in which the heart was exteriorized without
myocardial I/R. The following day, myocardial I/R rats were gavaged
with 25, 50 or 100 mg/kg/puerarin (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) every 2 days over 4 weeks. The present study
was approved by the ethics committee of Tianjin First Central
Hospital (Tianjin, China).
Assessments of myocardial
function
After treatment with puerarin for 4 weeks, A
hemodynamic analyzing system (Chengdu Xinjin Shifeng Medical
Apparatus & Instrument Co., Ltd., Chengdu, China) was employed
to analyze the left ventricular developed pressure (LVDP), the left
ventricular end-systolic interior dimension (LVIDs) and the left
ventricular end diastolic interior dimension (LVIDd) as described
in a recent study (16). Under
anesthesia (35 mg/kg of pentobarbital sodium), the coronary artery
was ligated; after 4 h, the left ventricle was stored at −80°C for
30 min. The left ventricle was subsequently sliced into 4-mm thick
sections to assess the size of the infarct. The heart area assessed
was the ischemic heart muscles. The infarct size area was assessed
by volume and weight as a percentage of the left ventricle.
Determination of malondialdehyde
(MDA), superoxide dismutase (SOD), tumor necrosis factor-α (TNF-α)
and interleukin (IL)-6 levels, NO production and caspase-3
activity
Rats were anesthetized and blood was collected from
the eye socket of each rat. Plasma was centrifuged at 8,000 × g for
10 min at 4°C. ELISA kits obtained from Wuhan Elabscience
Biotechnology Co., Ltd., (Wuhan, China) were employed to analyze
the levels of MDA (E-EL-0060c), SOD (E-EL-R1424c), TNF-α
(E-EL-R0019c) and IL-6 (E-EL-R0015c). Total NO production was
measured using an NO analyzer (Nanjing Jiancheng Bioengineering
Institute, Nanjing, China). Caspase-3 activity was measured using a
Caspase 3 Activity Assay kit (Beyotime Institute of Biotechnology,
Haimen, China). Absorbance values were measured using a Synergy HT
microplate reader (BioTek Instruments, Inc., Winooski, VT,
USA).
Western blot analysis
Heart tissue samples were lysed in
radioimmunoprecipitation assay buffer (Beyotime Institute of
Biotechnology) on ice for 30 min, and the supernatant was
centrifuged at 8,000 × g for 10 min at 4°C. Following
quantification of the protein concentration using a Bicinchoninic
Acid Protein Assay kit (Thermo Fisher Scientific, Inc., Waltham,
MA, USA), proteins (50 µg) were separated by 10% SDS-PAGE and
transferred onto Immobilon-FL transfer membranes (EMD Millipore,
Billerica, MA, USA). Membranes were blocked with 5% non-fat milk
for 1 h at room temperature and incubated overnight at 4°C with
anti-nuclear factor (NF)-κB (sc-71675, 1:1,000), anti-VEGFA
(sc-7269, 1:1,000), anti-Ang-1 (cat. no. sc-6320, 1:1,000),
anti-phosphorylated (p)-endothelial nitric oxide synthase (eNOS;
sc-293032, 1:1,000; all from Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA) and anti-GAPDH (BM3876, 1:5,000; Wuhan Boster
Biological Technology, Ltd., Wuhan, China). Blots were subsequently
washed 3 times with TBS with 0.1% Tween-20 (TBST), and incubated
with goat anti-mouse immunoglobulin G-horseradish peroxidase
secondary antibodies (sc-2005, 1:5,000; Santa Cruz Biotechnology)
in TBST solution for 1 h at 37°C. Bands were visualized using
BeyoECL Star (P0018A; Beyotime Institute of Biotechnology)
quantified using the Quantity image analyzer 3.0 (Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
Data are expressed as the mean ± standard error
(n=3) using SPSS 17.0 (SPSS, Inc. Chicago, IL, USA). Between-group
differences were determined using one-way analysis of variance
followed by Tukey's post-hoc analysis. P<0.05 was considered to
indicate a statistically significant difference.
Results
Puerarin reduces the myocardial
infarct area in diabetic myocardial I/R rats
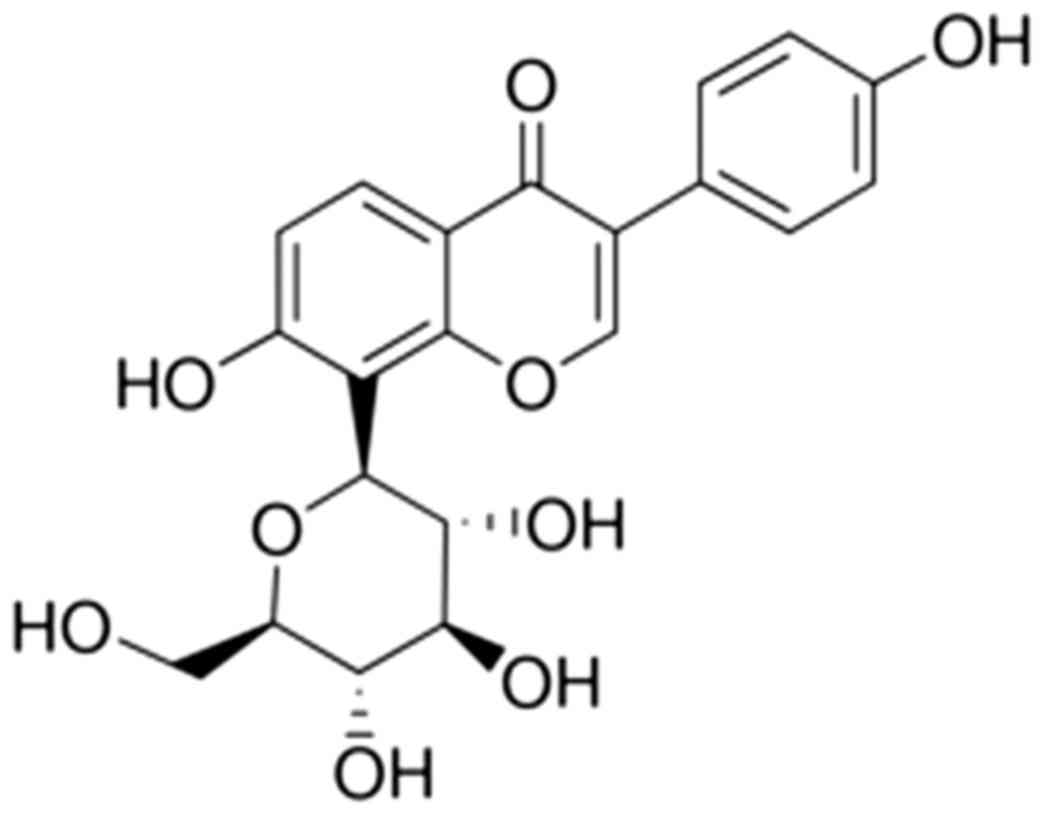
The chemical structure of puerarin is presented in
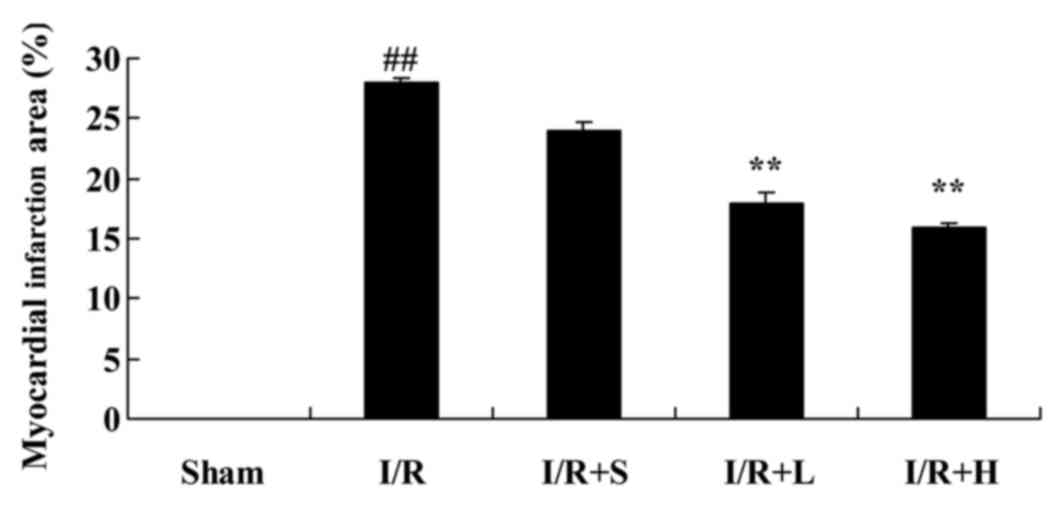
Fig. 1. The myocardial infarct
area was markedly increased in the diabetic I/R model rats,
compared with the sham control group. Treatment with puerarin for 4
weeks markedly reduced the myocardial infarct area, compared with
untreated I/R diabetic rats (Fig.
2).
Puerarin increases the LVDP in
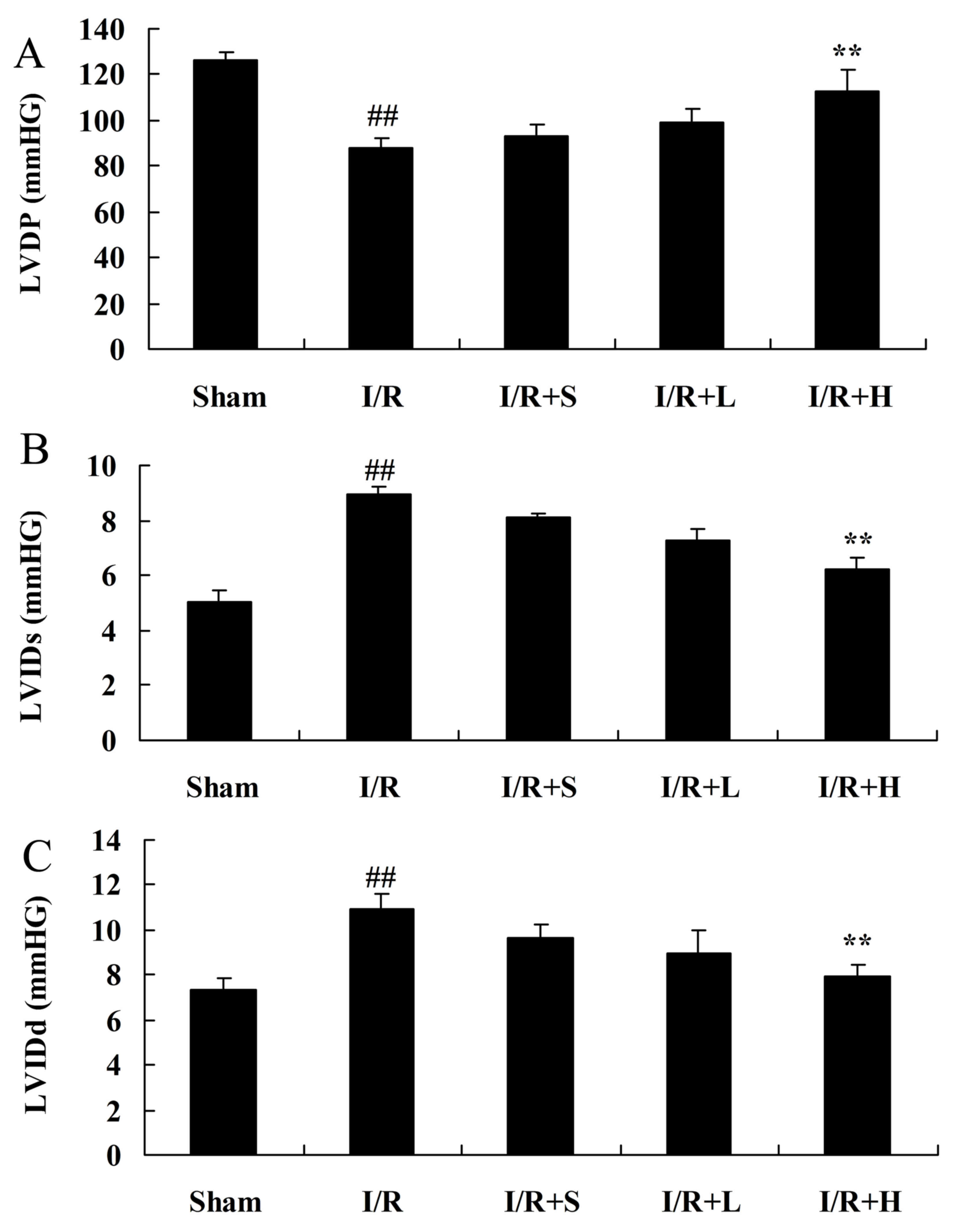
diabetic myocardial I/R rats
A significantly attenuated LVDP in the diabetic I/R
rat model group was observed, compared with the sham control group.
Puerarin treatment increased the LVDP in diabetic myocardial I/R
rats, compared with the diabetic I/R rat model group (Fig. 3A). Notably, a significant increase
was observed in LVIDs and LVIDd in the diabetic I/R rat model
group, compared with the control group (Fig. 3B and C). However, puerarin
treatment markedly decreased LVIDs and LVIDd in diabetic I/R rats,
compared with rats in the diabetic I/R model group.
Puerarin inhibits oxidative stress in
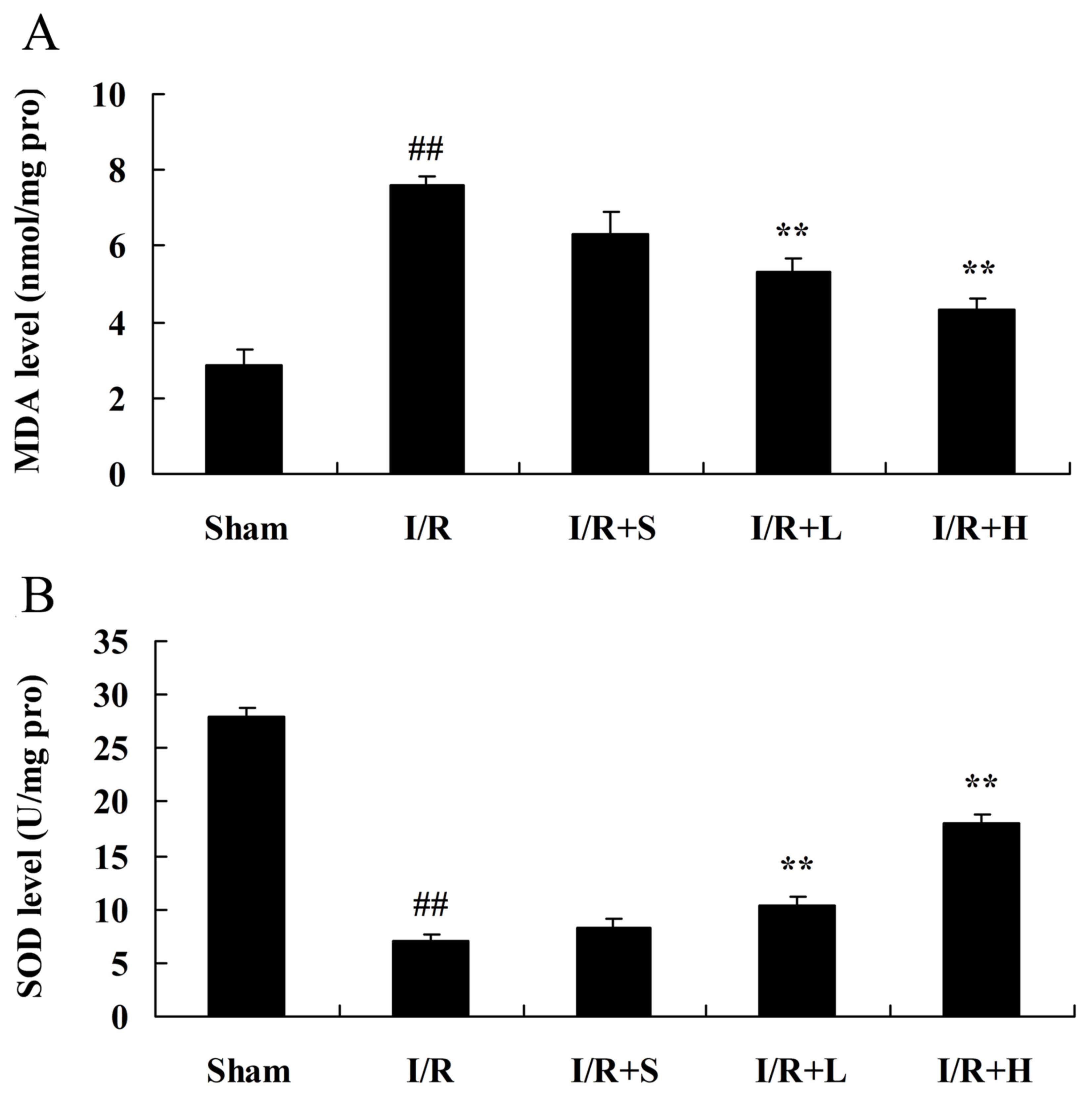
diabetic myocardial I/R rats
MDA and SOD activity were measured to investigate
the protective effect of puerarin on myocardial I/R in diabetic
rats. Increased MDA activity (Fig.
4A) and decreased SOD activity (Fig. 4B) were observed in the diabetic
myocardial I/R rat model, compared with the sham control group.
Puerarin treatment reversed these alterations to MDA and SOD
activity, compared with rats in the diabetic myocardial I/R model
group (Fig. 4).
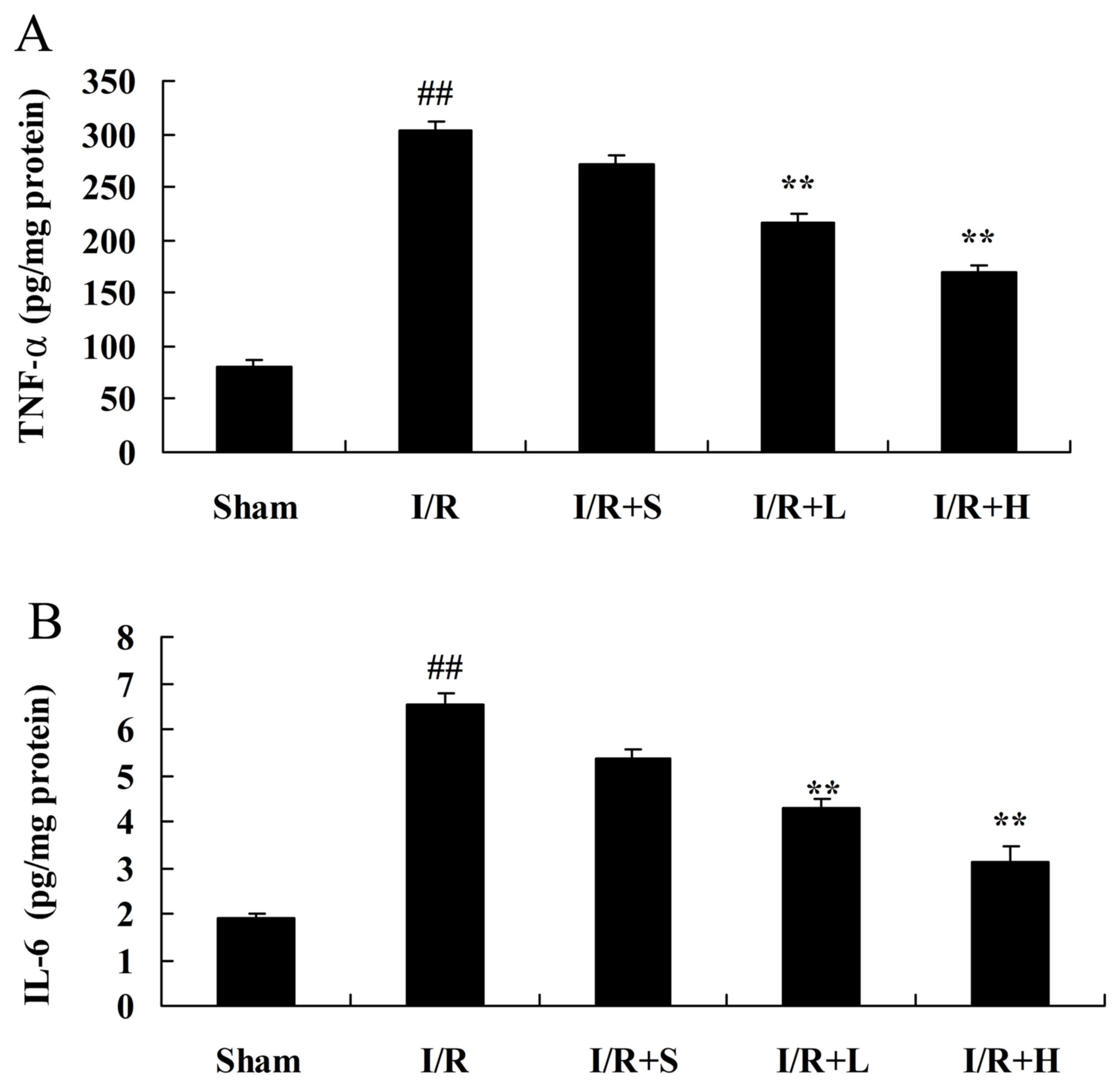
Puerarin inhibits inflammation in
diabetic myocardial I/R rats
The protective effects of puerarin on myocardial I/R
in diabetic rats were further examined by investigating the plasma
levels of TNF-α and IL-6. TNF-α and IL-6 levels were significantly
increased in the plasma of rats in the diabetic myocardial I/R
model, compared with the sham control group (Fig. 5A and B, respectively). However,
treatment with puerarin significantly reduced TNF-α and IL-6
levels, compared with rats in the diabetic myocardial I/R model
group.
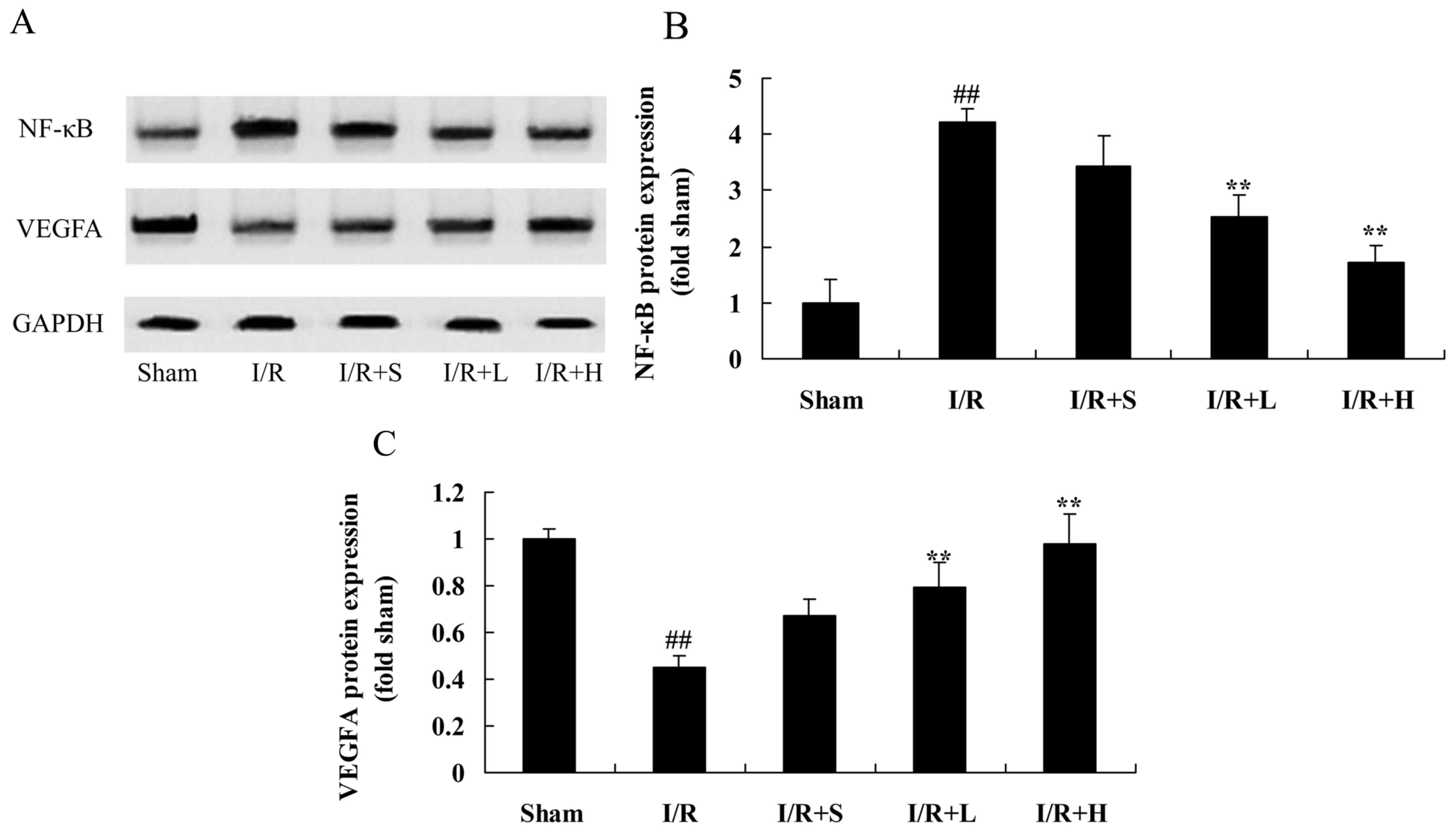
Puerarin inhibits NF-κB and VEGFA
protein expression in diabetic myocardial I/R rats
NF-κB and VEGFA protein expression levels were
measured to examined the anti-inflammatory effect of puerarin on
diabetic myocardial I/R in rats. NF-κB protein expression was
significantly induced and VEGFA protein expression was
significantly suppressed in diabetic myocardial I/R model rats,
compared with the sham control group (Fig. 6). However, puerarin treatment
significantly suppressed NF-κB and elevated VEGFA protein
expression in puerarin-treated diabetic I/R rats, compared with the
untreated diabetic I/R model rats.
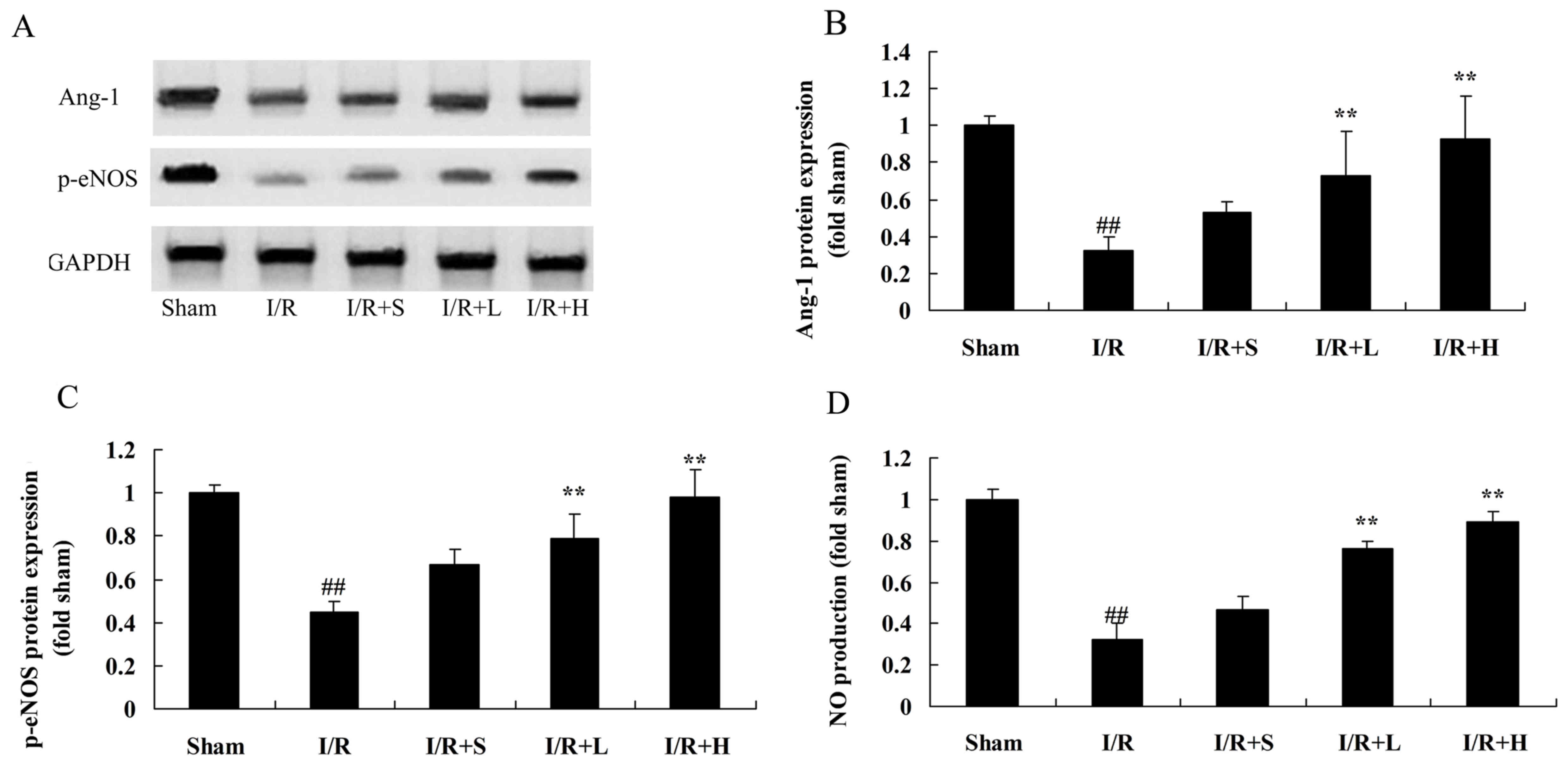
Puerarin increases Ang-1 and p-eNOS
protein expression and NO production in diabetic myocardial I/R
rats
The role of Ang-1 and p-eNOS in puerarin-induced
diabetic myocardial I/R protection was investigated. Inhibition of
Ang-1 and p-eNOS protein expression and NO production was observed
in diabetic myocardial I/R rats, compared with the sham control
group (Fig. 7). Puerarin treatment
significantly alleviated the I/R-induced inhibition of Ang-1 and
p-eNOS protein expression and NO production, compared with the
untreated diabetic myocardial I/R rats.
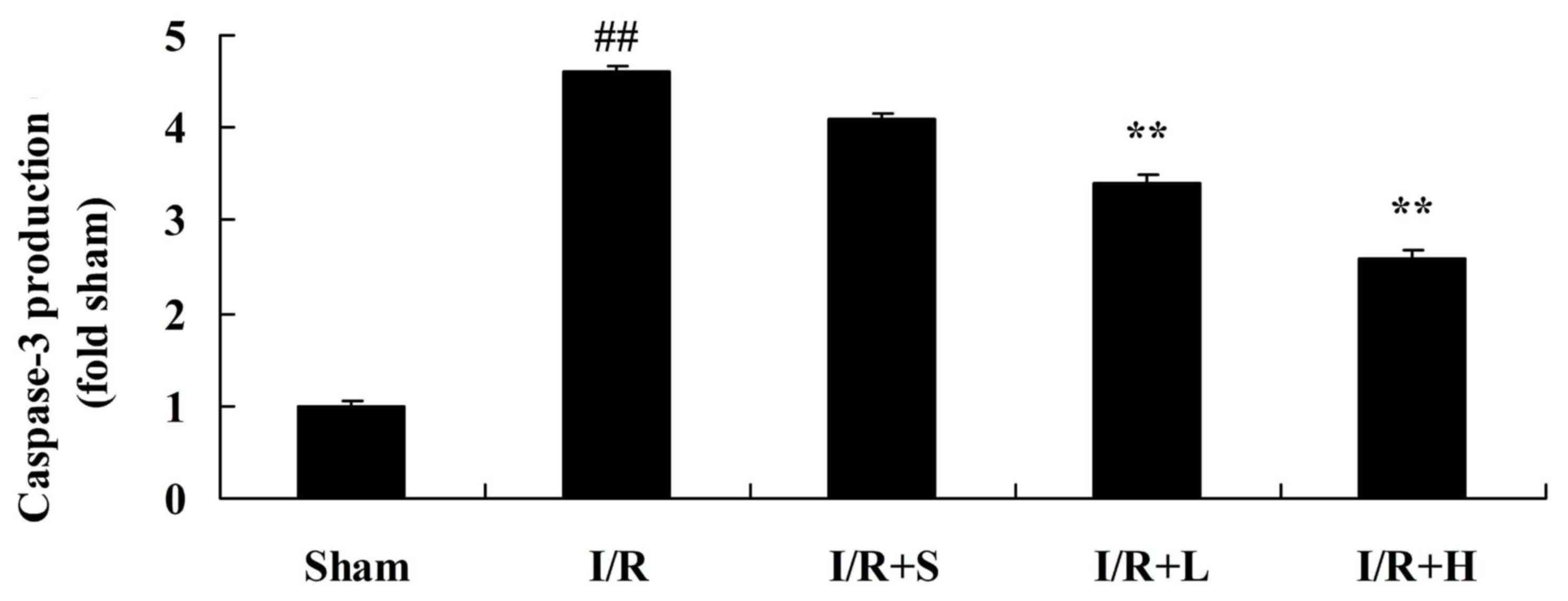
Puerarin decreases caspase-3 activity
in diabetic myocardial I/R rats
To investigate whether puerarin protects against
apoptosis, caspase-3 activity was assessed (Fig. 8). Increased activation of caspase-3
was observed in diabetic myocardial I/R rats, compared with the
sham control group. Treatment with puerarin significantly decreased
caspase-3 activity in untreated diabetic myocardial I/R rats.
Discussion
DCM is a chronic cardiac complication directly
caused by diabetes, and is independent of coronary heart disease,
hypertension and valvular heart disease (17,18).
Early stage DCM predominantly manifests with left ventricular
hypertrophy and diastolic dysfunction (19). The left ventricular ejection score
at this stage may be normal or even elevated; however, systolic
dysfunction and a decline in the ejection fraction value are
presented as the disease progresses, and these may ultimately
result in heart failure (20). DCM
pathology also includes cardiac hypertrophy and apoptosis, heart
wall thickening, capillary basement membrane thickening, capillary
endothelial lesions and microvascular lesions (10). The pathogenesis of DCM is complex;
high blood sugar has been recognized as a leading risk factor,
accompanied by lipid toxicity (triglycerides in the blood),
oxidative stress, inflammation, autonomic neuropathy, microvascular
disease and activation of renin-angiotensin-aldosterone system
(20). In addition, mitochondrial
dysfunction and epigenetic changes have been demonstrated to
participate in the occurrence of DCM (21). The results of the present study
indicated that puerarin may reduce the myocardial infarct area,
increase LVDP and decrease LVIDs and LVIDd in diabetic myocardial
I/R rats.
High blood sugar and abnormal glucose metabolism
induce excessive reactive oxygen species (ROS) production by the
mitochondrial electron transportation chain. ROS and oxidative
stress can cause DNA damage, meanwhile activating a DNA repair
enzyme, poly ADP-ribose polymerase (22). Advanced glycation endproducts can
activate nuclear factor kB (NF-κB) via binding to the galectin-3
receptor (23). The NF-κB pathway
can regulate the expression of inflammation-related genes to
increase the synthesis of TNF-α and interleukin, amongst others,
resulting in myocardial damage (23). The present study discovered that
puerarin may reduce oxidative stress and the expression of
inflammatory cytokines and NF-κB, in diabetic myocardial I/R rats.
Furthermore, Li et al (12)
demonstrated that puerarin can reduce diabetic aorta injury via the
suppression of NADPH oxidase-induced oxidative stress and NF-κB p65
in diabetic rats.
Ang-(1–7) predominantly associates with the Mas
receptor; however, a low amount of Ang-(1–7) also
binds with the Ang-II type 2 (AT2) receptor (6). Association of Ang-(1–7) with
the AT2 receptor can antagonize the ATI receptor; activation of
eNOS promotes the release of NO, prostacyclin and other
vasodilators; this increases the activity of bradykinin and thus
antagonizes the effect of Ang-II (5). Furthermore, association of
Ang-(1–7) with the Mas receptor can counteract
the induction of vasoconstriction induced by Ang-II binding to the
ATI receptor (24). In addition,
VEGFA is a potent wound healing cytokine; the main functions of
which include inducing angiogenesis, promoting endothelial cell
proliferation and enhancing microvascular permeability, which
results in widespread leakage of plasma proteins (25). These proteins directly or
indirectly alter the extracellular matrix components to form a
temporary new matrix; this matrix supports the migration of
endothelial cells and fibroblasts, which is conducive to wound
repair. The present study demonstrated that puerarin significantly
increases VEGFA, Ang-1 and p-eNOS protein expression, and activates
NO production in diabetic myocardial I/R rats. Ai et al
(15) demonstrated that puerarin
accelerates cardiac angiogenesis and improves cardiac function via
upregulation of VEGFA, Ang-1 and Ang-2.
Proliferation and apoptosis occur in the early
stages of DCM cardiomyocyte hypertrophy; however, normal heart
function can be maintained (26).
As the disease develops, the myocardial intracellular environment
becomes disordered; myocardial cells lose normal regulation and the
rate of apoptosis exceeds the speed of cell proliferation; the
apoptotic area increases, and the resulting loss of large numbers
of cells eventually leads to a significant reduction in cardiac
function (27,28). The regulation of apoptosis involves
a series of complex cascades, the most important of which are the
caspase-related apoptosis signal transduction pathways, which
include the death receptor-mediated apoptosis signaling pathway,
the mitochondrial/cytochrome c-mediated apoptosis pathway
and the endoplasmic reticulum stress-mediated apoptosis pathway
(29). In the present study,
treatment with puerarin significantly decreased caspase-3 activity
in diabetic rats. Notably, Iribarren et al (25) demonstrated that puerarin may
protect against oxidative stress injury via the downregulation of
caspase-3 in neural cells.
In conclusion, the results of the present study
indicated that puerarin markedly reduces the myocardial infarct
area, increases LVDP and decreases LVIDs and LVIDd in diabetic
myocardial I/R rats. Furthermore, puerarin may reduce oxidative
stress and the expression of inflammatory cytokines via the
inhibition of NF-κB, upregulation of the VEGFA/Ang-1 signaling
pathway and the suppression of apoptosis in diabetic rats. The
results of the present study indicated that puerarin may be useful
as a myocardial protective treatment to reduce cardiomyopathy in
diabetic patients.
Acknowledgements
The present study was supported by the Health Bureau
of Science and Technology fund of Tianjin (grant no.
2015KZ092).
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lingvay I, Pérez Manghi F,
García-Hernández P, Norwood P, Lehmann L, Tarp-Johansen MJ and Buse
JB: DUAL V Investigators: Effect of insulin glargine up-titration
vs insulin degludec/liraglutide on glycated hemoglobin levels in
patients with uncontrolled type 2 diabetes: The DUAL V randomized
clinical trial. JAMA. 315:898–907. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pfeffer MA, Claggett B, Diaz R, Dickstein
K, Gerstein HC, Køber LV, Lawson FC, Ping L, Wei X, Lewis EF, et
al: Lixisenatide in patients with type 2 diabetes and acute
coronary syndrome. N Engl J Med. 373:2247–2257. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bader M: ACE2, angiotensin-(1–7), and mas:
The other side of the coin. Pflugers Arch. 465:79–85. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hamming I, Cooper ME, Haagmans BL, Hooper
NM, Korstanje R, Osterhaus AD, Timens W, Turner AJ, Navis G and van
Goor H: The emerging role of ACE2 in physiology and disease. J
Pathol. 212:1–11. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang LH, Pang XF, Bai F, Wang NP, Shah
AI, McKallip RJ, Li XW, Wang X and Zhao ZQ: Preservation of
glucagon-like peptide-1 level attenuates angiotensin II-induced
tissue fibrosis by altering AT1/AT 2 receptor expression and
angiotensin-converting enzyme 2 activity in rat heart. Cardiovasc
Drugs Ther. 29:243–255. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tikellis C, Pickering R, Tsorotes D, Du
XJ, Kiriazis H, Nguyen-Huu TP, Head GA, Cooper ME and Thomas M:
Interaction of diabetes and ACE2 in the pathogenesis of
cardiovascular disease in experimental diabetes. Clin Sci (Lond).
123:519–529. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zygalaki E, Kaklamanis L, Nikolaou NI,
Kyrzopoulos S, Houri M, Kyriakides Z, Lianidou ES and Kremastinos
DT: Expression profile of total VEGF, VEGF splice variants and VEGF
receptors in the myocardium and arterial vasculature of diabetic
and non-diabetic patients with coronary artery disease. Clin
Biochem. 41:82–87. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jesmin S, Zaedi S, Shimojo N, Iemitsu M,
Masuzawa K, Yamaguchi N, Mowa CN, Maeda S, Hattori Y and Miyauchi
T: Endothelin antagonism normalizes VEGF signaling and cardiac
function in STZ-induced diabetic rat hearts. Am J Physiol
Endocrinol Metab. 292:E1030–E1040. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chitose T, Sugiyama S, Sakamoto K,
Shimomura H, Yamashita T, Hokamaki J, Tsunoda R, Shiraishi S,
Yamashita Y and Ogawa H: Effect of a hydrophilic and a hydrophobic
statin on cardiac salvage after ST-elevated acute myocardial
infarction-a pilot study. Atherosclerosis. 237:251–258. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Muhlestein JB, Lappé DL, Lima JA, Rosen
BD, May HT, Knight S, Bluemke DA, Towner SR, Le V, Bair TL, et al:
Effect of screening for coronary artery disease using CT
angiography on mortality and cardiac events in high-risk patients
with diabetes: The FACTOR-64 randomized clinical trial. JAMA.
312:2234–2243. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dombi T, Kwok KK and Sultan MB: A
retrospective, pooled data analysis of the safety of pegaptanib
sodium in the treatment of age-related macular degeneration in
subjects with or without diabetes mellitus. BMC Ophthalmol.
12:372012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li W, Zhao W, Wu Q, Lu Y, Shi J and Chen
X: Puerarin improves diabetic aorta injury by inhibiting NADPH
oxidase-derived oxidative stress in STZ-induced diabetic rats. J
Diabetes Res. 2016:85415202016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shukla R, Pandey N, Banerjee S and
Tripathi YB: Effect of extract of Pueraria tuberosa on expression
of hypoxia inducible factor-1α and vascular endothelial growth
factor in kidney of diabetic rats. Biomed Pharmacother. 93:276–285.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gao Y, Wang X and He C: An
isoflavonoid-enriched extract from Pueraria lobata (kudzu) root
protects human umbilical vein endothelial cells against oxidative
stress induced apoptosis. J Ethnopharmacol. 193:524–530. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ai F, Chen M, Yu B, Yang Y, Xu G, Gui F,
Liu Z, Bai X and Chen Z: Puerarin accelerates cardiac angiogenesis
and improves cardiac function of myocardial infarction by
upregulating VEGFA, Ang-1 and Ang-2 in rats. Int J Clin Exp Med.
8:20821–20828. 2015.PubMed/NCBI
|
|
16
|
Liang J, Yin K, Cao X, Han Z, Huang Q,
Zhang L, Ma W, Ding F, Bi C, Feng D, et al: Attenuation of low
ambient temperature-induced myocardial hypertrophy by atorvastatin
via promoting Bcl-2 expression. Cell Physiol Biochem. 41:286–295.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mingrone G, Panunzi S, De Gaetano A,
Guidone C, Iaconelli A, Nanni G, Castagneto M, Bornstein S and
Rubino F: Bariatric-metabolic surgery versus conventional medical
treatment in obese patients with type 2 diabetes: 5 year follow-up
of an open-label, single-centre, randomised controlled trial.
Lancet. 386:964–973. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Derosa G, Mugellini A, Pesce RM, D'Angelo
A and Maffioli P: Barnidipine compared to lercanidipine in addition
to losartan on endothelial damage and oxidative stress parameters
in patients with hypertension and type 2 diabetes mellitus. BMC
Cardiovasc Disord. 16:662016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Strand E, Pedersen ER, Svingen GF,
Schartum-Hansen H, Rebnord EW, Bjørndal B, Seifert R, Bohov P,
Meyer K, Hiltunen JK, et al: Dietary intake of n-3 long-chain
polyunsaturated fatty acids and risk of myocardial infarction in
coronary artery disease patients with or without diabetes mellitus:
A prospective cohort study. BMC Med. 11:2162013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
McMullan CJ, Lambers Heerspink HJ, Parving
HH, Dwyer JP, Forman JP and de Zeeuw D: Visit-to-visit variability
in blood pressure and kidney and cardiovascular outcomes in
patients with type 2 diabetes and nephropathy: A post hoc analysis
from the RENAAL study and the Irbesartan Diabetic Nephropathy
Trial. Am J Kidney Dis. 64:714–722. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jakljević T, Ruzić A, Bazdarić K,
Zaputović L, Mavrić Z, Champagne S and Teiger E: Detection of
myocardial ischemia in diabetic patients: The limitations of
myocardial perfusion imaging. Coll Antropol. 36:821–826.
2012.PubMed/NCBI
|
|
22
|
Drefs M, Thomas MN, Guba M, Angele MK,
Werner J, Conrad M, Steib CJ, Holdt LM, Andrassy J, Khandoga A and
Rentsch M: Modulation of glutathione hemostasis by inhibition of
12/15-lipoxygenase prevents ROS-mediated cell death after hepatic
ischemia and reperfusion. Oxid Med Cell Longev. 2017:83257542017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Al-Taweel AM, Raish M, Perveen S, Fawzy
GA, Ahmad A, Ansari MA, Mudassar S and Ganaie MA: Nepeta
deflersiana attenuates isoproterenol-induced myocardial injuries in
rats: Possible involvement of oxidative stress, apoptosis,
inflammation through nuclear factor (NF)-κB downregulation.
Phytomedicine. 34:67–75. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Patel VB, Mori J, McLean BA, Basu R, Das
SK, Ramprasath T, Parajuli N, Penninger JM, Grant MB, Lopaschuk GD
and Oudit GY: ACE2 deficiency worsens epicardial adipose tissue
inflammation and cardiac dysfunction in response to diet-induced
obesity. Diabetes. 65:85–95. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Iribarren C, Phelps BH, Darbinian JA,
McCluskey ER, Quesenberry CP, Hytopoulos E, Vogelman JH and
Orentreich N: Circulating angiopoietins-1 and −2, angiopoietin
receptor Tie-2 and vascular endothelial growth factor-A as
biomarkers of acute myocardial infarction: A prospective nested
case-control study. BMC Cardiovasc Disord. 11:312011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shams AS, Mohammed MH, Loka MM and Abdel
Rahman GM: Assessment of the protective role of prenatal zinc
versus insulin supplementation on fetal cardiac damage induced by
maternal diabetes in rat using caspase-3 and KI67
immunohistochemical stains. Cardiol Res Pract. 2016:74695492016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li H, Liu Z, Wang J, Wong GT, Cheung CW,
Zhang L, Chen C, Xia Z and Irwin MG: Susceptibility to myocardial
ischemia reperfusion injury at early stage of type 1 diabetes in
rats. Cardiovasc Diabetol. 12:1332013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yan J, Duan J, Wu X, Guo C, Yin Y, Zhu Y,
Hu T, Wei G, Wen A and Xi M: Total saponins from Aralia taibaiensis
protect against myocardial ischemia/reperfusion injury through AMPK
pathway. Int J Mol Med. 36:1538–1546. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chu LM, Osipov RM, Robich MP, Feng J,
Sheller MR and Sellke FW: Effect of thrombin fragment (TP508) on
myocardial ischemia reperfusion injury in a model of type 1
diabetes mellitus. Circulation. 122 11 Suppl:S162–S169. 2010.
View Article : Google Scholar : PubMed/NCBI
|