Introduction
Lung cancer, including small cell lung carcinoma and
non-small cell lung carcinoma (NSCLC), is the primary cause of
cancer death globally (1). NSCLC
consists of lung squamous cell carcinoma (LUSC), adenocarcinoma and
large cell carcinoma (2), which
are responsible for 80% of lung cancer mortality (3). LUSC is associated with tobacco
smoking, and more frequently occurs in men (4). It has the characteristics of poor
therapeutic response, high relapse rate and poor prognosis
(5), inducing ~400,000
mortalities/year worldwide (6).
Therefore, revealing the genes involved in the prognosis of LUSC is
of great importance.
Long non-coding RNAs (lncRNAs) are a class of
non-protein-coding RNAs that mediate gene expression, which play
important roles in multiple biological processes and diverse
carcinomas (7). Previous studies
have identified a number of lncRNAs to be associated with prognosis
of LUSC. For example, Zhang et al (8) reported that lncRNA 1133
(LINC01133) is overexpressed in patients with LUSC and may
shorten their survival time, thus LINC01133 is a promising
biomarker for LUSC. The lncRNA metastasis associated lung
adenocarcinoma transcript 1 is reported to have tumor-promoting
functions and is associated with the survival time of patients with
NSCLC (9,10). The lncRNA HOX transcript antisense
intergenic RNA (HOTAIR) mediates the cell invasion and
metastasis of NSCLC by downregulating homeobox A5, indicating that
HOTAIR may serve as a prognostic marker and therapeutic
target in patients with NSCLC (11,12).
By inhibiting the expression of Kruppel like factor 2 (KLF2)
and p21, lncRNA antisense non-coding RNA in the INK4 locus
promotes cell proliferation and suppresses cellular apoptosis NSCLC
(13,14). Furthermore, lncRNA associated with
microvascular invasion in HCC regulates NSCLC cell proliferation
and invasion, thus its overexpression may be used as a prognostic
biomarker for NSCLC (15).
However, the lncRNAs associated with the prognosis of LUSC have not
been completely elucidated.
The present study further examined the key lncRNAs
associated with the prognosis of LUSC through a series of
bioinformatics methods. The lncRNA expression profiles of LUSC were
downloaded from The Cancer Genome Atlas, and the differentially
expressed lncRNAs (DELs) between LUSC and adjacent samples were
identified. Following prognosis-associated DEL screening, a
prognostic risk model was established and evaluated. Finally, the
co-expression genes of important lncRNAs were obtained and their
functions were predicted. The present study may contribute to
predicting the prognosis of LUSC and revealing novel molecules
associated with the disease.
Materials and methods
Data source
The lncRNA expression profiles of LUSC and the
relevant clinical data were downloaded from The Cancer Genome Atlas
(TCGA; cancergenome.nih.gov) database
(downloaded on April 18, 2017). Samples without clinical data were
removed and the data of 501 patients were obtained (Table I). In addition, the data of 49
tumor-adjacent normal lung tissues were obtained.
 | Table I.Clinical characteristics of patients
(n=501) with lung squamous cell carcinoma in the present study. |
Table I.
Clinical characteristics of patients
(n=501) with lung squamous cell carcinoma in the present study.
| Variable | No. patients |
|---|
| Age, years |
|
|
≤65 | 190 |
|
>65 | 311 |
| Sex |
|
|
Female | 139 |
|
Male | 362 |
| Pathological
stage |
|
| I | 244 |
| II | 162 |
|
III | 84 |
| IV | 7 |
| NA | 4 |
| T stage |
|
| T1 | 114 |
| T2 | 293 |
| T3 | 71 |
| T4 | 23 |
| N stage |
|
| N0 | 319 |
| N1 | 131 |
|
N2-N3 | 45 |
| NA | 6 |
| M stage |
|
| M0 | 411 |
| M1 | 7 |
| NA | 83 |
| Radiotherapy |
|
|
Yes | 53 |
| No | 384 |
| NA | 64 |
| Targeted molecular
therapy |
|
|
Yes | 133 |
| No | 306 |
| NA | 62 |
| Residual tumor |
|
| R0 | 398 |
|
R1+R2 | 16 |
| NA | 87 |
| Neoplasm
recurrence |
|
|
Yes | 135 |
| No | 285 |
| NA | 81 |
| Vital status |
|
|
Alive | 289 |
|
Deceased | 212 |
| NA | 6 |
DEL screening
Subsequent to obtaining the RNA-sequencing data of
LUSC from TCGA, lncRNA annotation was performed using the GENCODE
database (www.gencodegenes.org) (16). The lncRNAs with average expression
values (counts/million) >0.1 were considered as sample
expressing lncRNAs. The two independent R (version 3.1.0) (17) packages edgeR (version 3.8.5;
www.bioconductor.org/packages/release/bioc/html/edgeR.html)
(18) and DEseq (version 1.16.0;
www.bioconductor.org/packages/release/bioc/html/DESeq.html)
(19) were used for screening DELs
between LUSC samples and adjacent samples. The adjusted P-value
<0.05 and |log fold change (FC)| >1 were set as thresholds.
The intersecting DELs predicted by the edgeR and DEseq packages
were regarded as significant DELs.
Establishment of prognostic risk
model
The DELs expressed in <10% of LUSC samples and
patients with a survival time of <30 days were eliminated. The
patients were randomly divided into a test set and validation set.
For the test set, Cox univariate regression analysis was used to
analyze the correlation between DELs and overall survival (OS). The
lncRNAs with a P-value <0.05 was screened as
prognosis-associated DELs. Subsequently, these prognosis-associated
DELs were analyzed using Cox multivariate regression analysis to
establish the prognostic risk model, with P-value <0.05 as the
threshold. The prognosis risk score was calculated using the
following formula (20):
Prognosis risk score=expression value of gene 1 ×
risk coefficient of gene 1 + expression value of gene 2 × risk
coefficient of gene 2 + expression value of gene 3 × risk
coefficient of gene 3.
In the test set, the expression levels of the
prognosis-associated DELs in cancerous samples and adjacent samples
were compared using the non-parametric Wilcoxon signed-rank
test.
Detection of the classification effect
of the prognostic risk model
The test set was divided into a high risk group and
a low risk group, according to the median prognostic risk score. To
assess the effect of prognostic risk score in determining the
prognosis of patients, the difference between the survival curves
of the high and low risk groups was analyzed by drawing receiver
operating characteristic (ROC) curves using survivalROC package
(version 1.0.3; https://cran.r-project.org/web/packages/survivalROC/index.html).
Determination of the correlation
between prognostic risk score and prognosis
Using Cox univariate regression analysis, the
correlation between prognostic risk score and clinical
characteristics, including the OS of patients, were further
analyzed. Subsequently, Cox multivariate regression analysis was
applied to determinate whether the prognostic risk score was an
independent prognostic factor. A P-value <0.05 was set as the
threshold value. The hazard ratio and its 95% confidence intervals
were used for evaluation. In order to assess the prediction
accuracy of the prognostic risk model for time-dependent disease
consequences, the survival ROC package (cran.r-project.org/web/packages/survivalROC/index.html)
(21) in R was applied for drawing
ROC curve. Furthermore, the OS differences among the patients in
the high and low risk groups were evaluated by a log rank test
using Kaplan Meier (KM) survival analysis (22). Based on a χ2 test
(23), the correlations between
DELs and clinical characteristics were detected. In addition, ROC
curves were used to assess the prediction significance of the
prognostic risk score following treatment, with the two-sided
P-value <0.05 as the threshold.
Functional analysis of important
lncRNAs
Associated genes (co-expression genes) of lncRNAs
were obtained through the Human RNAseq expression data platform in
the Multi-Experiment Matrix (MEM; biit.cs.ut.ee/mem/index.cgi) online tool (24). Using the STRING database
(string-db.org) (25), a protein-protein interaction (PPI)
network was established, with an associated score >0.4 and a
number of associated nodes >3 set as the thresholds.
Subsequently, the PPI network of lncRNAs and their associated genes
was visualized using Cytoscape software 3.5.1 (www.cytoscape.org) (26). Additionally, Gene Ontology (GO;
www.geneontology.org) (27) and Kyoto Encyclopedia of Genes and
Genomes (KEGG; www.genome.ad.jp/kegg) (28) pathway enrichment analyses were
performed based on the Database for Annotation, Visualization and
Integrated Discovery (DAVID 6.7; david.ncifcrf.gov) (29) bioinformatics tool, with a P-value
<0.05 set as the threshold.
Results
DEL screening
A total of 5,515 DELs were identified between LUSC
samples and adjacent samples, including 2,537 DELs from the edgeR
package and 2,048 DELs from the DEseq package. Finally, a total of
2,041 significant DELs were obtained by selecting the intersecting
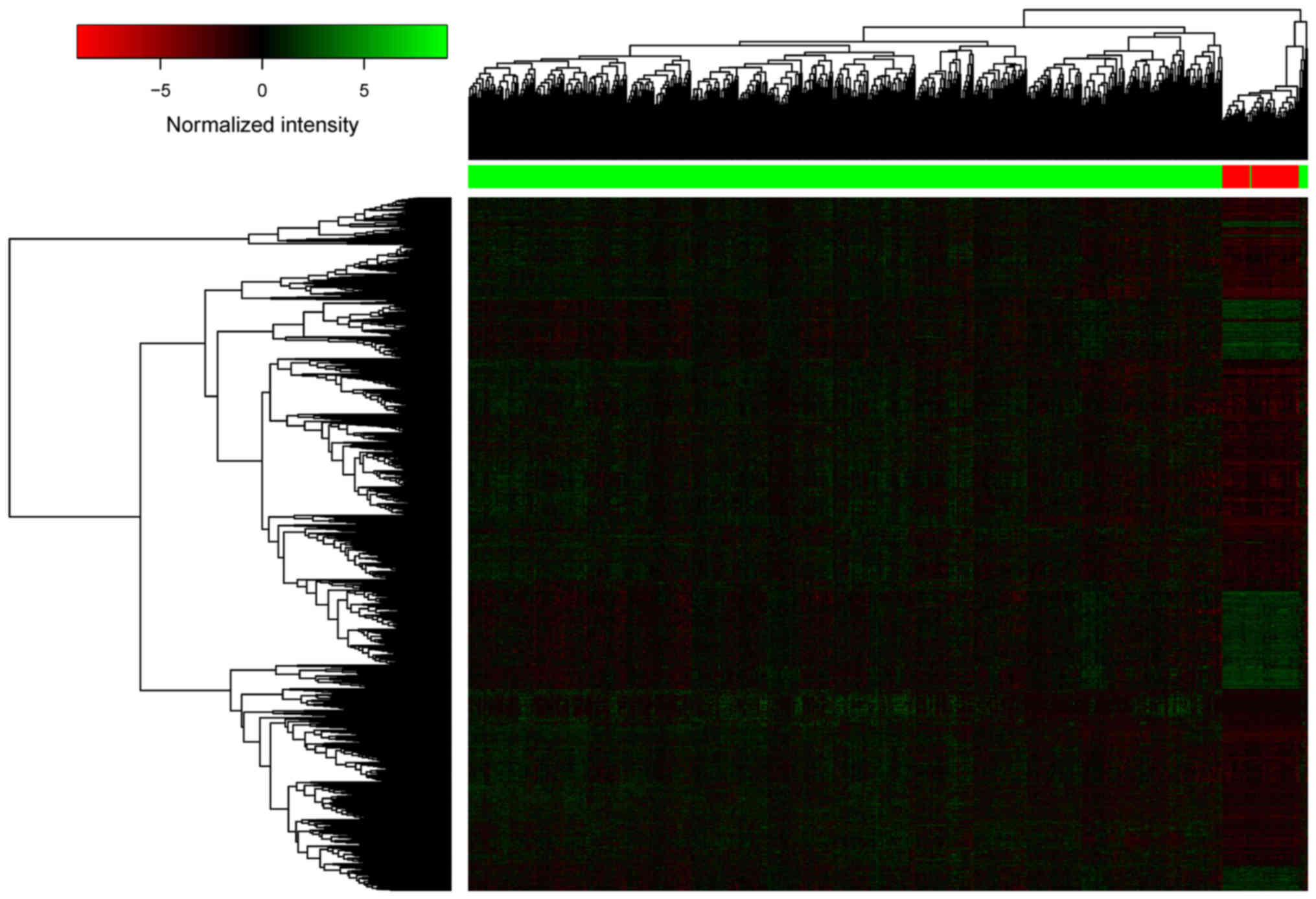
DELs predicted by the edgeR and DEseq packages. The heatmap of the
2,041 DELs is presented in Fig. 1,
indicating that the lncRNA expression profiles of LUSC and adjacent
samples were different.
Establishment of the prognostic risk
model
A total of 489 samples and 1,468 DELs were included
in the survival analysis. The patients were randomly divided into
test (n=245) and validation (n=244) sets. Cox univariate regression
analysis demonstrated that there were 68 prognosis-associated DELs
in the test set. Subsequently, Cox multivariate regression analysis
indicated that 3 prognosis-associated DELs (including RP5-821D11.7,
APCDD1L-AS1 and RP11-277P12.9) had important
prognostic value. The prognostic risk score was calculated using
the following formula: Prognostic risk score=expression value of
RP5-821D11.7 × (−0.392) + expression value of
APCDD1L-AS1 × (0.101) + expression value of
RP11-277P12.9 × (−0.114).
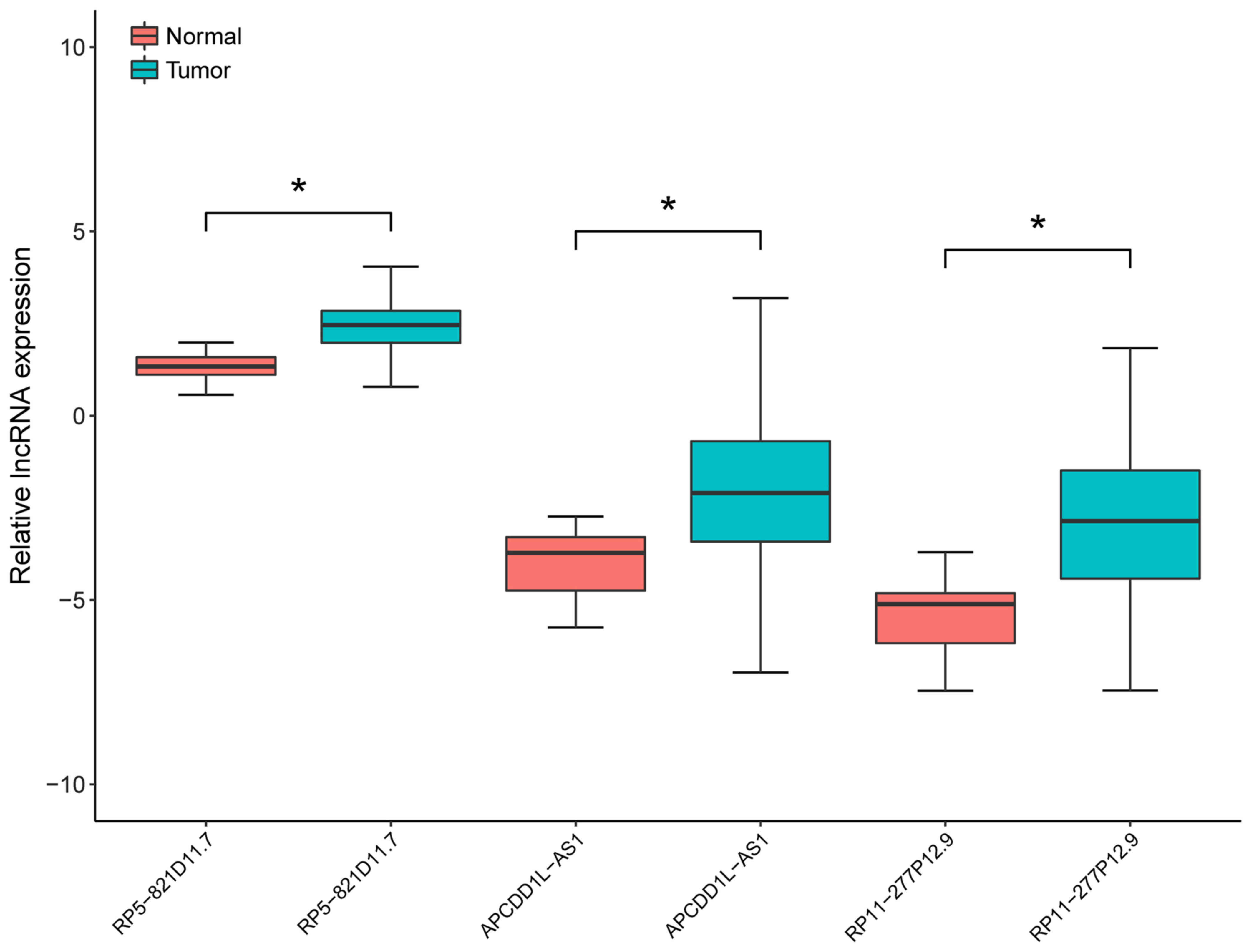
In the test set, the expression levels of the three
prognosis-associated DELs in LUSC samples and adjacent samples were
compared by non-parametric Wilcoxon signed-rank test. As presented
in Fig. 2, the expression levels
were significantly increased in LUSC samples compared with adjacent
samples (P<0.05; Fig. 2). In
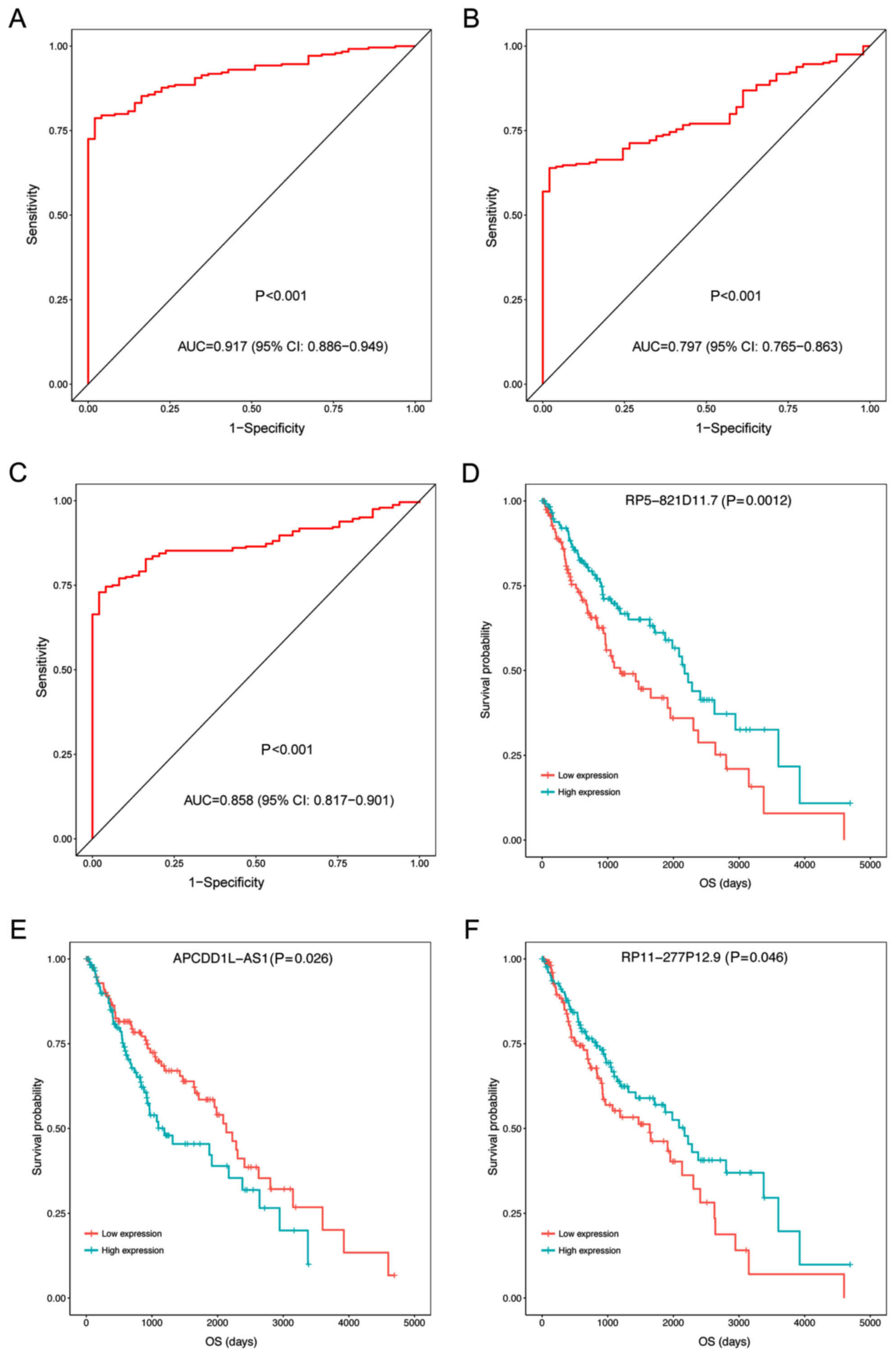
addition, the expression levels of the three prognosis-associated
DELs were able to be used to separate LUSC samples from the
adjacent samples with an area under the ROC curve (AUC) >0.8
(Fig. 3A-C). Furthermore, the KM
survival analysis demonstrated that the samples with high
expression levels of the three prognosis-associated DELs had
significantly lower OS compared with those with low expression
levels (P<0.05; Fig. 3D-F).
Detection of the classification effect
of the prognostic risk model
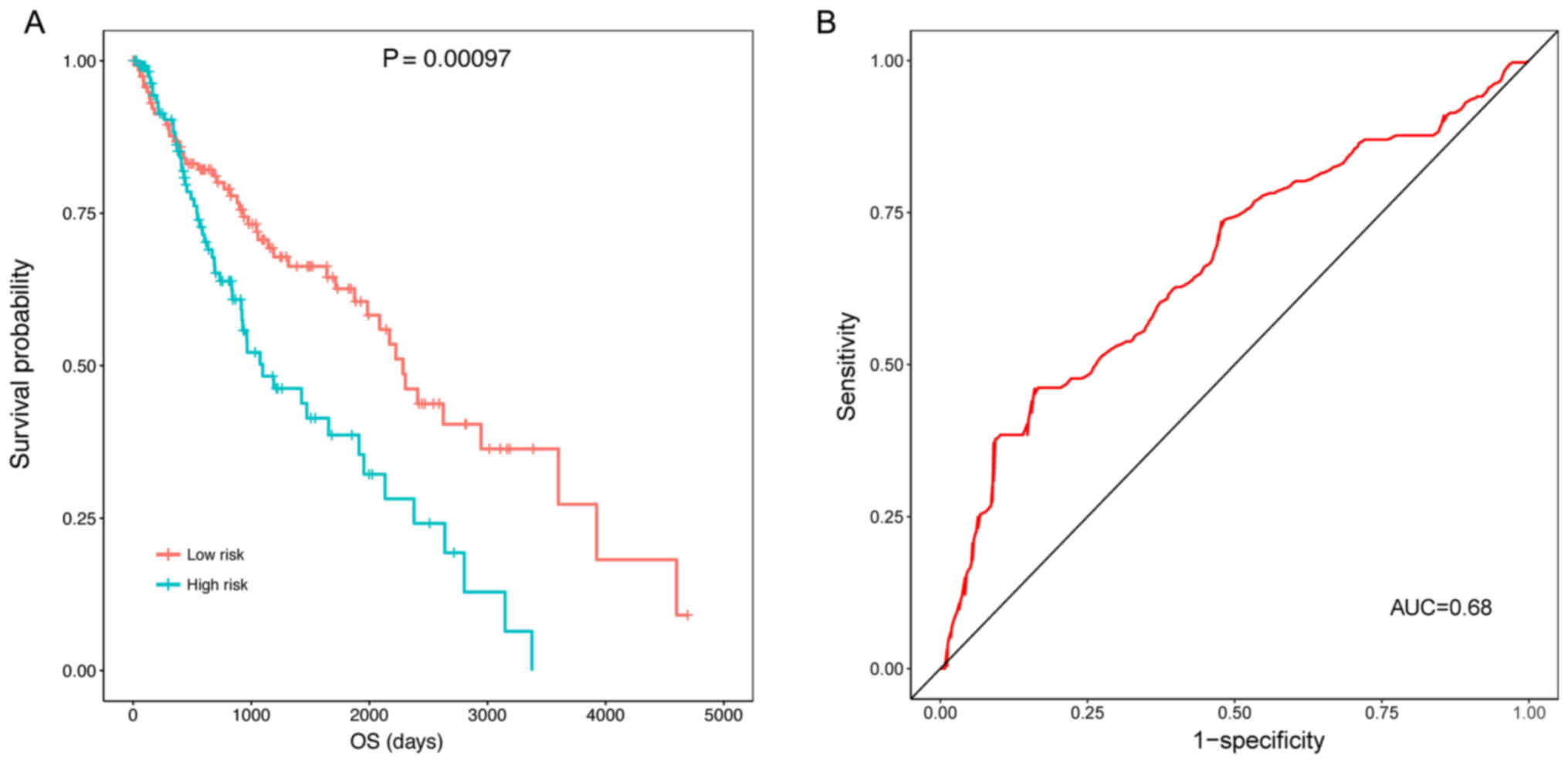
The KM survival analysis indicated that the samples
in the high risk group (OS, 769.1±713.3 days) had a significantly
lower OS compared with those in the low risk group (OS,
1240.0±1030.1 days) (P=0.00097) (Fig.
4A). The ROC curve demonstrated that the prognostic risk scores
were able to predict the 5-year survival of patients to a certain
degree (AUC, 0.68; Fig. 4B).
Evaluation of prognostic risk as an
independent prognostic factor
Cox univariate regression analysis demonstrated that
certain clinical characteristics were associated with the OS of
patients, including risk score (P<0.001), neoplasm recurrence
(P<0.001), tumor-node-metastasis (TNM) stage (P<0.001),
tobacco smoking history (P=0.028), M stage (P=0.030), and T stage
(P=0.038) (Table II). However,
Cox multivariate regression analysis suggested that only risk score
(P<0.001), and tobacco smoking history (P=0.048) had potential
as independent prognostic factors (Table III).
 | Table II.Cox univariate regression analysis
between prognostic risk scores/clinical characteristics and overall
survival of patients. |
Table II.
Cox univariate regression analysis
between prognostic risk scores/clinical characteristics and overall
survival of patients.
| Risk
scores/clinical characteristics | Hazard ratio | Lower.95 | Upper.95 | P-value |
|---|
| Risk score | 2.718 | 1.720 | 4.297 | <0.001 |
| Neoplasm
recurrence | 2.479 | 1.594 | 3.856 | <0.001 |
| TNM stage | 2.254 | 1.402 | 3.625 | <0.001 |
| Tobacco smoking
history | 0.633 | 0.421 | 0.951 | 0.028 |
| M stage | 3.637 | 1.137 | 11.635 | 0.030 |
| T stage | 1.663 | 1.030 | 2.686 | 0.038 |
| Target molecular
therapy | 0.689 | 0.409 | 1.160 | 0.161 |
| Residual tumor | 1.215 | 0.821 | 1.799 | 0.331 |
| Radiotherapy | 1.349 | 0.731 | 2.489 | 0.338 |
| N stage | 1.188 | 0.781 | 1.806 | 0.421 |
| Gender | 1.200 | 0.744 | 1.935 | 0.455 |
| Age | 1.016 | 0.671 | 1.538 | 0.939 |
 | Table III.Cox multivariate regression analysis
between prognostic risk scores/clinical characteristics and overall
survival of patients. |
Table III.
Cox multivariate regression analysis
between prognostic risk scores/clinical characteristics and overall
survival of patients.
| Risk
scores/clinical characteristics | Hazard ratio | Lower.95 | Upper.95 | P-value |
|---|
| Risk score | 3.747 | 1.765 | 7.957 | <0.001 |
| Tobacco smoking
history | 0.585 | 0.344 | 0.995 | 0.048 |
| Neoplasm
recurrence | 1.486 | 0.871 | 2.535 | 0.146 |
| M stage | 3.026 | 0.550 | 16.640 | 0.203 |
| TNM stage | 1.711 | 0.658 | 4.451 | 0.271 |
| T stage | 0.838 | 0.325 | 2.161 | 0.714 |
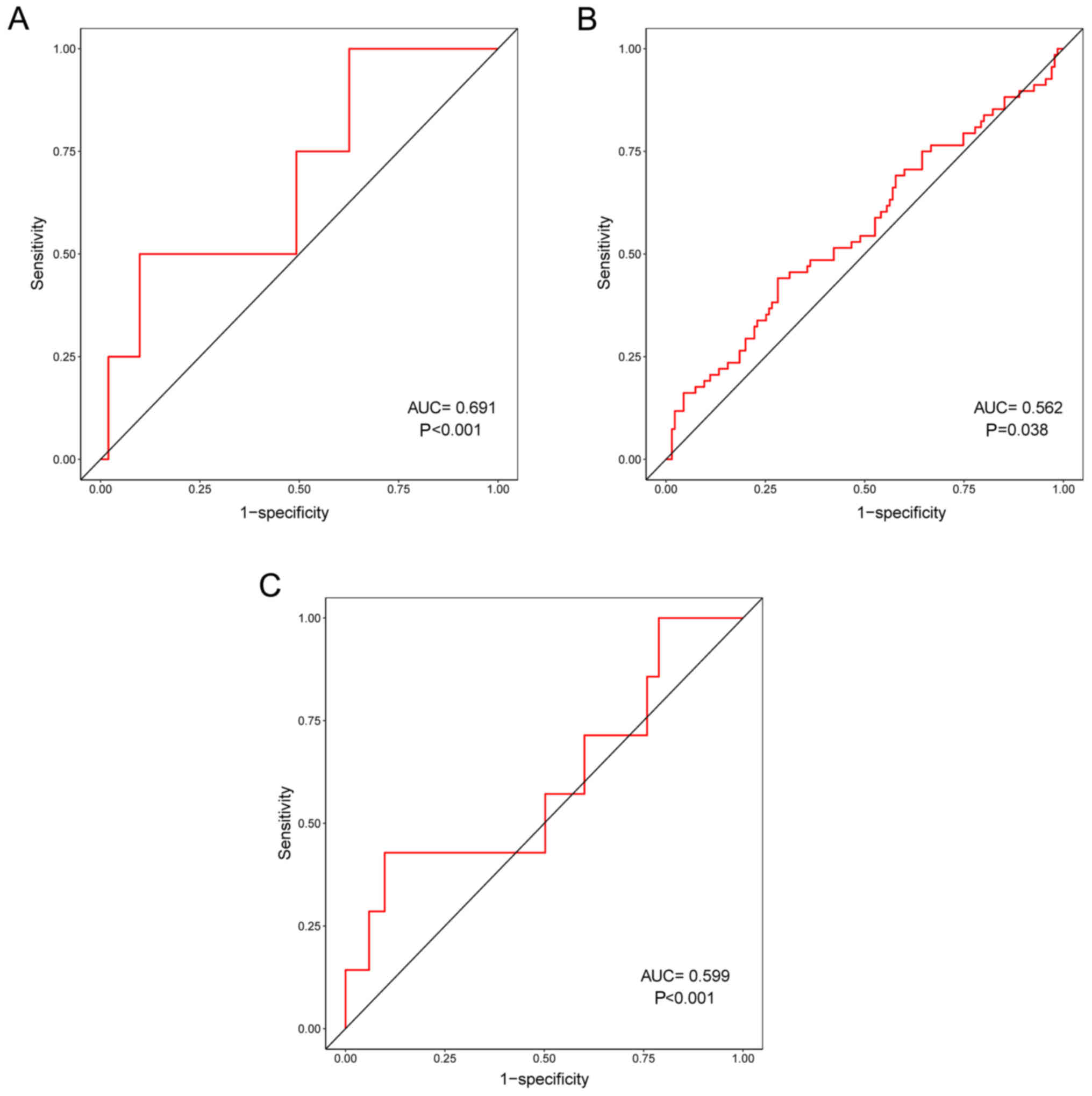
In addition, the correlation between prognostic risk
scores and other clinical characteristics were evaluated. ROC
curves demonstrated that prognostic risk scores were correlated
with distant metastasis (pM) (P<0.001; Fig. 5A), tumor recurrence (P=0.038;
Fig. 5B) and residual tumor
(P=0.001; Fig. 5C). In particular,
pM had the highest correlation with prognostic risk scores,
indicating that the three prognosis-associated DELs may serve
important roles in the distant metastasis of LUSC.
Assessment of the prognostic values of
prognostic risk scores in the validation and universal sets
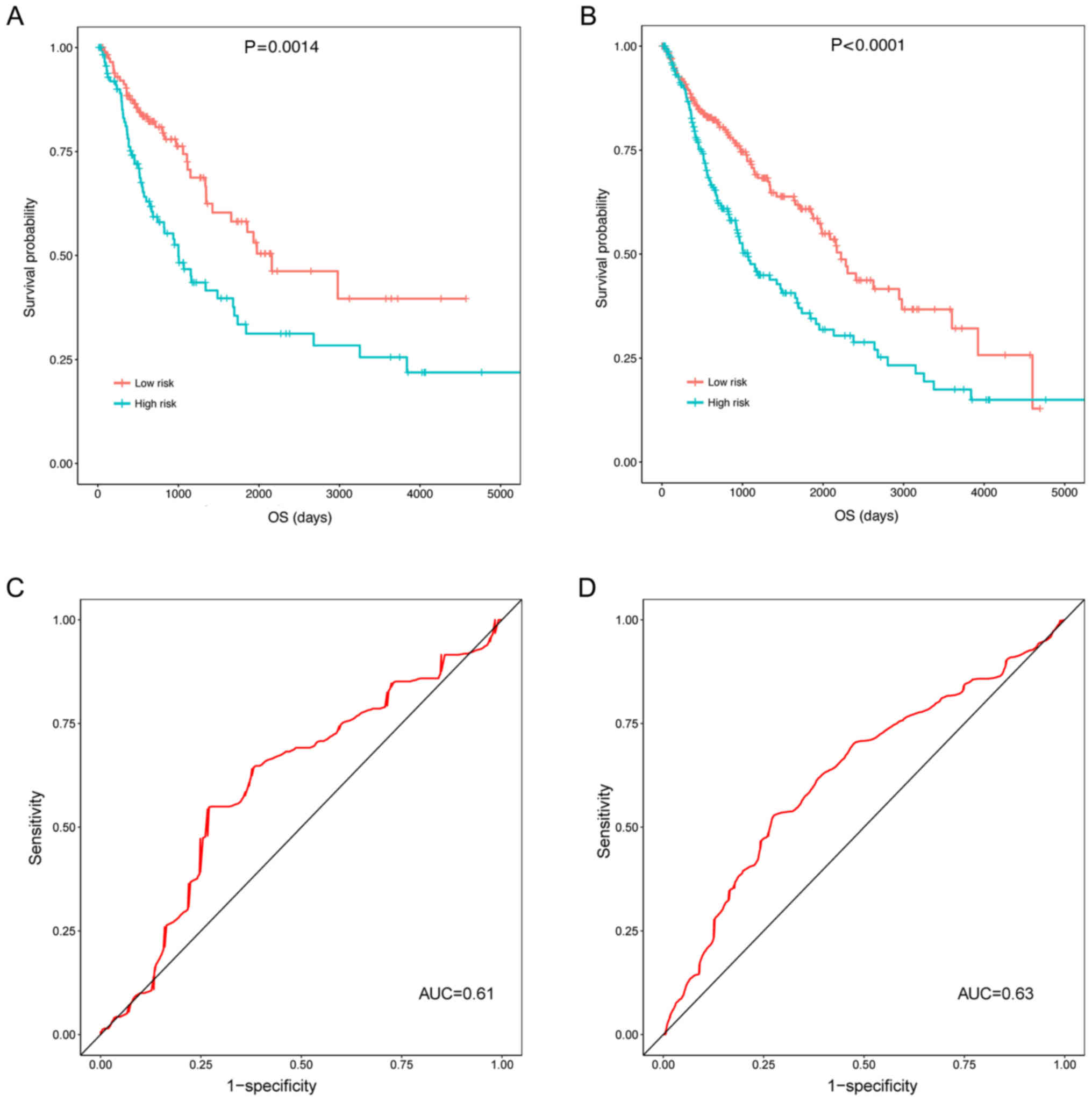
The three prognosis-associated DELs were validated
in the validation and universal sets (including the test set and
the validation set). In the validation set (P=0.0014; Fig. 6A) and the universal set
(P<0.0001; Fig. 6B), the OS of
patients in the high risk group was significantly decreased
compared with the low risk group. Additionally, the ROC curves
illustrated that the prognostic risk scores of the three
prognosis-associated DELs were able to predict the 5-year survival
of patients in the validation set (AUC, 0.61; Fig. 6C) and the universal set (AUC, 0.63;
Fig. 6D). Though the AUCs of the
ROCs predicting 5-year survival were small (0.61 and 0.63; Fig. 6C and D), the KM curves did
illustrate an association between the three DEL-signature and
survival (P=0.0014 and P<0.0001; Fig. 6A and B).
Functional analysis of important
lncRNAs
Using the MEM online tool and STRING database,
co-expression genes of RP5-821D11.7 [including proliferating
cell nuclear antigen (PCNA)], APCDD1L-AS1 [including
semaphorin 5A (SEMA5A), semaphorin 6D (SEMA6D),
ADAMTS like 1 (ADAMTSL1), ADAM metallopeptidase with
thrombospondin type 1 motif 6 (ADAMTS6), slit guidance
ligand 3 (SLIT3) and tenascin-C (TNC)] and
RP11-277P12.9 [including Wnt family member 2B
(WNT2B)] were screened. Enrichment analysis demonstrated
that only the co-expression genes of APCDD1L-AS1 were
enriched in certain GO terms and KEGG pathways (Table IV). Notably, ‘positive regulation
of cell migration’ (P=0.048, involving SEMA5A and
SEMA6D) and ‘proteinaceous extracellular matrix’ (P=0.017,
involving ADAMTSL1 and ADAMTS6) were enriched.
 | Table IV.The Gene Ontology terms and pathways
enriched for the co-expression genes of APCDD1L-AS1. |
Table IV.
The Gene Ontology terms and pathways
enriched for the co-expression genes of APCDD1L-AS1.
| Category | Term | P-value | Genes |
|---|
| Pathway | Axon guidance | <0.001 | SEMA5A, SEMA6D,
NTNG1, NFATC4, SLIT3 |
|
| ECM-receptor
interaction | 0.013 | TNC, COL6A3,
ITGA11 |
|
| Olfactory
transduction | 0.043 | OR4C13, OR5J2,
OR1L6, OR8H2 |
| Biological
process | Cell adhesion | <0.001 | NRP2, SEMA5A,
COL7A1, TNC, COL6A3, ITGA11, COL8A1, CDH6 |
|
| Negative
chemotaxis | <0.001 | NRP2, SEMA5A,
SEMA6D, SLIT3 |
|
| Extracellular
matrix organization | <0.001 | COL7A1, TNC,
COL6A3, ITGA11, COL8A1 |
|
| Semaphorin-plexin
signaling pathway | 0.002 | SEMA5A, PLXNA4,
SEMA6D |
|
| Collagen catabolic
process | 0.007 | COL7A1, COL6A3,
COL8A1 |
|
| Proteolysis | 0.014 | CPA4, ADAMTS6,
ADAMTSL1, PAPPA2, HTRA3 |
|
| Facial nerve
structural organization | 0.017 | NRP2,
PLXNA4 |
|
| Axon extension
involved in axon guidance | 0.023 | NRP2,
SLIT3 |
|
| Detection of
chemical stimulus involved in sensory perception of smell | 0.046 | OR4C13, OR5J2,
OR1L6, OR8H2 |
|
| Heart
development | 0.047 | NRP2, FOXL1,
NFATC4 |
|
| Positive regulation
of cell migration | 0.048 | SEMA5A, SEMA6D,
LRRC15 |
|
| Negative regulation
of axon extension involved in axon guidance | 0.048 | SEMA5A,
SEMA6D |
| Cellular
component | Extracellular
matrix | 0.003 | COL7A1, TNC,
COL6A3, COL8A1, SSC5D |
|
| Endoplasmic
reticulum lumen | 0.007 | ADAMTSL1,
COL7A1, COL6A3, COL8A1 |
|
| Extracellular
region | 0.014 | NRP2, PTHLH,
COL7A1, TNC, COL6A3, PAPPA2, HTRA3, COL8A1, SLIT3 |
|
| Proteinaceous
extracellular matrix | 0.017 | ADAMTS6,
ADAMTSL1, COL6A3, SLIT3 |
|
| Semaphorin receptor
complex | 0.022 | NRP2,
PLXNA4 |
| Molecular
function | Metallopeptidase
activity | 0.010 | ADAMTS6,
ADAMTSL1, PAPPA2 |
|
| Syndecan
binding | 0.011 | SEMA5A,
TNC |
|
| Semaphorin receptor
activity | 0.023 | NRP2,
PLXNA4 |
|
| Semaphorin receptor
binding | 0.043 | SEMA5A,
SEMA6D |
|
| Laminin
binding | 0.046 | LRRC15,
SSC5D |
|
| Olfactory receptor
activity | 0.047 | OR4C13, OR5J2,
OR1L6, OR8H2 |
|
| Fibronectin
binding | 0.048 | LRRC15,
SSC5D |
|
| Chemorepellent
activity | 0.050 | SEMA5A,
SEMA6D |
Discussion
In the present study, a total of 2,041 significant
DELs between LUSC and adjacent samples were identified. A
prognostic risk model involving three prognosis-associated DELs
(including RP5-821D11.7, APCDD1L-AS1 and
RP11-277P12.9) was established. The prognostic risk scores
were able to predict the 5-year survival of patients to a certain
degree, indicating that the classification effect of the prognostic
risk model was good. Cox multivariate regression analysis suggested
that only prognostic risk score and tobacco smoking history had the
potential to be used as independent prognostic factors. Besides,
the prognostic risk model was validated in the validation set and
the universal set. In addition, certain co-expression genes of
RP5-821D11.7 (including PCNA), APCDD1L-AS1
(including SEMA5A, SEMA6D, ADAMTSL1, ADAMTS6, SLIT3 and
TNC) and RP11-277P12.9 (including WNT2B) were
screened.
The SLIT/ROBO signaling pathway acts by regulating
tumor cell metastasis, and SLIT3 serves as a promising tumor
suppressor gene in lung adenocarcinoma (LAD) (30,31).
The expression of TNC, S100A10 and S100A11 may
predict survival in patients with LAD, indicating that these
factors may be promising markers for better diagnosis and therapy
in LAD (32,33). TNC expression is markedly
elevated in patients with NSCLC, implying that TNC may serve
as a prognostic marker for NSCLC (34,35).
Semaphorins are a large number of transmembrane,
glycosylphosphatidylinositol-linked and secreted proteins that are
able to suppress and promote tumors (36). Downregulated SEMA5A is
correlated with poor survival in patients with NSCLC, thus
SEMA5A may be a prognostic marker for the disease (37). In the present study, enrichment
analysis suggested that SEMA5A and SEMA6D were
enriched in ‘positive regulation of cell migration’. These results
indicated that APCDD1L-AS1 may affect the prognosis of LUSC
by affecting the expression levels of SEMA5A, SEMA6D, SLIT3
and TNC.
Functional enrichment analysis demonstrated that
ADAMTSL1 and ADAMTS6 were enriched in ‘proteinaceous
extracellular matrix’. The ADAMTS enzymes are zinc
metalloendopeptidases that affect the structure and function of the
extracellular matrix (ECM) (38).
ADAMTSL-1 belongs to the family of ADAMTS-like proteins and
may serve important roles in the ECM (39). The ECM is important in mesenchymal
cancer cells; in particular, its compositional and structural
alterations may affect metastasis of mesenchymal lung cancer cells
(40). Dysregulation of certain
ADAMTS proteinases may be directly implicated in tumor development
and metastasis (41). Therefore,
APCDD1L-AS1 may be associated with the prognosis of LUSC
through ADAMTSL1 and ADAMTS6.
A previous study reported that PCNA is
specifically targeted by miR-363-3p, and may inhibit tumor
growth in LAD (42). PCNA
may be inhibited by the small molecule AOH1160, which suppresses
the growth of SCLC cells without inducing any unacceptable
side-effects and may be used as a potential anticancer therapy
(43). Dysregulated Wnt signaling
functions in the progression and metastasis of lung cancer, and
inhibitors of WNT signaling are promising for the treatment of the
disease (44,45). WNT2 may promote NSCLC cell
growth by activating the Wnt/β-catenin signaling pathway,
suggesting that WNT2 may be a marker for the diagnosis and
prognosis of NSCLC (46). Thus,
RP5-821D11.7 (co-expressed with PCNA) and
RP11-277P12.9 (co-expressed with WNT2B) may be used
for predicting the prognosis of LUSC.
There are certain limitations to the present study.
First, these findings resulted from bioinformatics analysis and
require further validation. Since the sample and experimental
conditions are insufficient, it is not possible to perform
verification experiments at present. Furthermore, it was identified
from the Cox univariate regression analysis that only neoplasm
recurrence, risk score and T stage were associated with the overall
survival of patients. Therefore, only these three indicators were
included in the Cox multivariate regression analysis, and only
neoplasm recurrence and risk score were associated with the overall
survival of patients. It was hypothesized that this may result from
the relatively small sample size and its unbalanced distribution.
For example, the number of patients with T3 and T4 stages was
significantly lower compared with T1 and T2 stages. Similarly, the
number of patients with N2-N3 stages was significantly lower
compared with N0 and N1 stages. In addition, only seven patients
were M1, while 411 patients were M0. However, the present prognosis
risk model may be a valuable tool for further research.
In conclusion, a total of 2,041 significant DELs
were identified in LUSC samples. RP5-821D11.7 (co-expressed
with PCNA), APCDD1L-AS1 (co-expressed with SEMA5A,
SEMA6D, ADAMTSL1, ADAMTS6, SLIT3 and TNC) and
RP11-277P12.9 (co-expressed with WNT2B) may be
important lncRNAs associated with the prognosis of LUSC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZW conceived and designed the study. YL and ZX
designed and performed data analyses. XZ collected the data and
wrote the manuscript. PZ provided critical suggestion and organized
the literature. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Alberg AJ, Brock MV, Ford JG, Samet JM and
Spivack SD: Epidemiology of lung cancer: Diagnosis and management
of lung cancer, 3rd ed: American College of Chest Physicians
evidence-based clinical practice guidelines. Chest. 143 5
Suppl:e1S–e29S. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ettinger DS, Akerley W, Borghaei H, Chang
AC, Cheney RT, Chirieac LR, D'Amico TA, Demmy TL, Ganti AK,
Govindan R, et al: Non-small cell lung cancer. J Natl Compr Canc
Netw. 10:1236–1271. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Raponi M, Dossey L, Jatkoe T, Wu X, Chen
G, Fan H and Beer DG: MicroRNA classifiers for predicting prognosis
of squamous cell lung cancer. Cancer Res. 69:5776–5783. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kenfield SA, Wei EK, Stampfer MJ, Rosner
BA and Colditz GA: Comparison of aspects of smoking among the four
histological types of lung cancer. Tob Control. 17:198–204. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Antal CE, Hudson AM, Kang E, Zanca C,
Wirth C, Stephenson NL, Trotter EW, Gallegos LL, Miller CJ, Furnari
FB, et al: Cancer-associated protein kinase C mutations reveal
kinase's role as tumor suppressor. Cell. 160:489–502. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cancer Genome Atlas Research Network:
Comprehensive genomic characterization of squamous cell lung
cancers. Nature. 489:519–525. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang G, Lu X and Yuan L: LncRNA: A link
between RNA and cancer. Biochim Biophys Acta. 1839:1097–1109. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang J, Zhu N and Chen X: A novel long
noncoding RNA LINC01133 is upregulated in lung squamous cell cancer
and predicts survival. Tumor Biol. 36:7465–7471. 2015. View Article : Google Scholar
|
|
9
|
Schmidt LH, Spieker T, Koschmieder S,
Humberg J, Jungen D, Bulk E, Hascher A, Wittmer D, Marra A,
Hillejan L, et al: The long noncoding MALAT-1 RNA indicates a poor
prognosis in non-small cell lung cancer and induces migration and
tumor growth. J Thorac Oncol Off Pub Int Assoc Study Lung Cancer.
6:1984–1992. 2011.
|
|
10
|
Weber DG, Johnen G, Casjens S, Bryk O,
Pesch B, Jöckel KH, Kollmeier J and Brüning T: Evaluation of long
noncoding RNA MALAT1 as a candidate blood-based biomarker for the
diagnosis of non-small cell lung cancer. BMC Res Notes. 6:5182013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu XH, Liu ZL, Sun M, Liu J, Wang ZX and
Wei D: The long non-coding RNA HOTAIR indicates a poor prognosis
and promotes metastasis in non-small cell lung cancer. BMC Cancer.
13:4642013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nakagawa T, Endo H, Yokoyama M, Abe J,
Tamai K, Tanaka N, Sato I, Takahashi S, Kondo T and Satoh K: Large
noncoding RNA HOTAIR enhances aggressive biological behavior and is
associated with short disease-free survival in human non-small cell
lung cancer. Biochem Biophys Res Commun. 436:319–324. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nie FQ, Sun M, Yang JS, Xie M, Xu TP, Xia
R, Liu YW, Liu XH, Zhang EB, Lu KH and Shu YQ: Long noncoding RNA
ANRIL promotes non-small cell lung cancer cell proliferation and
inhibits apoptosis by silencing KLF2 and P21 expression. Mol Cancer
Ther. 14:268–277. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Naemura M, Murasaki C, Inoue Y, Okamoto H
and Kotake Y: Long noncoding RNA ANRIL regulates proliferation of
non-small cell lung cancer and cervical cancer cells. Anticancer
Res. 35:5377–5382. 2015.PubMed/NCBI
|
|
15
|
Nie FQ, Zhu Q, Xu TP, Zou YF, Xie M, Sun
M, Xia R and Lu KH: Long non-coding RNA MVIH indicates a poor
prognosis for non-small cell lung cancer and promotes cell
proliferation and invasion. Tumour Biol. 35:7587–7594. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wright JC, Mudge J, Weisser H, Barzine MP,
Gonzalez JM, Brazma A, Choudhary JS and Harrow J: Improving GENCODE
reference gene annotation using a high-stringency proteogenomics
workflow. Nat Commun. 7:117782016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
R Core Team: R: A language and environment
for statistical computingR Foundation for Statistical Computing.
Vienna: 2016
|
|
18
|
Robinson MD, McCarthy DJ and Smyth GK:
edgeR: A Bioconductor package for differential expression analysis
of digital gene expression data. Bioinformatics. 26:139–140. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Anders S and Huber W: Differential
expression of RNA-Seq data at the gene level-the DESeq package.
EMBL. 2013.
|
|
20
|
Zeng JH, Liang L, He RQ, Tang RX, Cai XY,
Chen JQ, Luo DZ and Chen G: Comprehensive investigation of a novel
differentially expressed lncRNA expression profile signature to
assess the survival of patients with colorectal adenocarcinoma.
Oncotarget. 8:16811–16828. 2017.PubMed/NCBI
|
|
21
|
Heagerty PJ, Thomas L and Pepe MS:
Time-dependent ROC curves for censored survival data and a
diagnostic marker. Biometrics. 56:337–344. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Porcher R: CORR Insights(®):
Kaplan-meier survival analysis overestimates the risk of revision
arthroplasty: A meta-analysis. Clin Orthopaed Relat Res.
473:3431–3442. 2015. View Article : Google Scholar
|
|
23
|
McHugh ML: The chi-square test of
independence. Biochem Med (Zagreb). 23:143–149. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Adler P, Kolde R, Kull M, Tkachenko A,
Peterson H, Reimand J and Vilo J: Mining for coexpression across
hundreds of datasets using novel rank aggregation and visualization
methods. Genome Biol. 10:R1392009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Franceschini A, Szklarczyk D, Frankild S,
Kuhn M, Simonovic M, Roth A, Lin J, Minguez P, Bork P, von Mering C
and Jensen LJ: STRING v9. 1: Protein-protein interaction networks,
with increased coverage and integration. Nucleic Acids Res.
41:(Database Issue). D808–D815. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Saito R, Smoot ME, Ono K, Ruscheinski J,
Wang PL, Lotia S, Pico AR, Bader GD and Ideker T: A travel guide to
Cytoscape plugins. Nat Methods. 9:1069–1076. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
The Gene Ontology Consortium: Gene
ontology consortium: Going forward. Nucleic Acids Res.
43:D1049–D1056. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kanehisa M, Sato Y, Kawashima M, Furumichi
M and Tanabe M: KEGG as a reference resource for gene and protein
annotation. Nucleic Acids Res. 44:D457–D462. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dennis G Jr, Sherman BT, Hosack DA, Yang
J, Gao W, Lane HC and Lempicki RA: DAVID: Database for annotation,
visualization, and integrated discovery. Genome Biol. 4:P32003.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang C, Guo H, Li B, Sui C, Zhang Y, Xia
X, Qin Y, Ye L, Xie F, Wang H, et al: Effects of Slit3 silencing on
the invasive ability of lung carcinoma A549 cells. Oncol Rep.
34:952–960. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kong R, Yi F, Wen P, Liu J, Chen X, Ren J,
Li X, Shang Y, Nie Y, Wu K, et al: Myo9b is a key player in
SLIT/ROBO-mediated lung tumor suppression. J Clin Invest.
125:4407–4420. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gocheva V, Naba A, Bhutkar A, Guardia T,
Miller KM, Li CM, Dayton TL, Sanchez-Rivera FJ, Kim-Kiselak C,
Jailkhani N, et al: Quantitative proteomics identify Tenascin-C as
a promoter of lung cancer progression and contributor to a
signature prognostic of patient survival. Proc Natl Acad Sci USA.
114:pp. E5625–E5634. 2017; View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tang YA, Chen CH, Sun HS, Cheng CP, Tseng
VS, Hsu HS, Su WC, Lai WW and Wang YC: Global Oct4 target gene
analysis reveals novel downstream PTEN and TNC genes required for
drug-resistance and metastasis in lung cancer. Nucleic Acids Res.
43:1593–1608. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gebauer F, Gelis S, Zander H, Meyer KF,
Wolters-Eisfeld G, Izbicki JR, Bockhorn M and Tachezy M: Tenascin-C
serum levels and its prognostic power in non-small cell lung
cancer. Oncotarget. 7:20945–20952. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Onion D, Isherwood M, Shridhar N, Mikalena
X, Madeleine LC, Laura JD, Maria A GM, Robert GP, Alex MRS, John
HS, et al: Multicomponent analysis of the tumour microenvironment
reveals low CD8 T cell number, low stromal caveolin-1 and high
tenascin-C and their combination as significant prognostic markers
in non-small cell lung cancer. Oncotarget. 9:1760–1771.
2017.PubMed/NCBI
|
|
36
|
Potiron VA, Roche J and Drabkin HA:
Semaphorins and their receptors in lung cancer. Cancer Lett.
273:1–14. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lu TP, Mong-Hsun T, Jang-Ming L, Hsu CP,
Chen PC, Lin CW, Shih JY, Yang PC, Hsiao CK, Lai LC and Chuang EY:
Identification of a novel biomarker, SEMA5A, for non-small cell
lung carcinoma in nonsmoking women. Cancer Epidemiol Biomark
Prevent. 19:2590–2597. 2010. View Article : Google Scholar
|
|
38
|
Kelwick R, Desanlis I, Wheeler GN and
Edwards DR: The ADAMTS (A disintegrin and metalloproteinase with
thrombospondin motifs) family. Genome Biol. 16:1132015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hirohata S, Wang LW, Miyagi M, Yan L,
Seldin MF, Keene DR, Crabb JW and Apte SS: Punctin, a novel
ADAMTS-like molecule, ADAMTSL-1, in extracellular matrix. J Biol
Chem. 277:12182–12189. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Peng DH, Ungewiss C, Tong P, Byers LA,
Wang J, Canales JR, Villalobos PA, Uraoka N, Mino B, Behrens C, et
al: ZEB1 induces LOXL2-mediated collagen stabilization and
deposition in the extracellular matrix to drive lung cancer
invasion and metastasis. Oncogene. 36:1925–1938. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sun Y, Huang J and Yang Z: The roles of
ADAMTS in angiogenesis and cancer. Tumour Biol. 36:pp4039–4051.
2015. View Article : Google Scholar
|
|
42
|
Wang Y, Chen T, Huang H, Jiang Y, Yang L,
Lin Z, He H, Liu T, Wu B, Chen J, et al: miR-363-3p inhibits tumor
growth by targeting PCNA in lung adenocarcinoma. Oncotarget.
8:20133–20144. 2017.PubMed/NCBI
|
|
43
|
Gu L, Hickey RJ, Reckamp KL and Malkas LH:
Structural analysis identifies an orally active PCNA inhibitor that
inhibits the growth of small cell lung cancer cells without causing
significant toxicity to nonmalignant cells. J Thorac Oncol. 11
Suppl:S22–S23. 2016. View Article : Google Scholar
|
|
44
|
Yang J, Chen J, He J, Li J, Shi J, Cho WC
and Liu X: Wnt signaling as potential therapeutic target in lung
cancer. Expert Opin Ther Targets. 20:999–1015. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Stewart DJ: Wnt signaling pathway in
non-small cell lung cancer. J Natl Cancer Inst. 106:djt3562014.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Huang C, Ma R, Xu Y, Li N, Li Z, Yue J, Li
H, Guo Y and Qi D: Wnt2 promotes non-small cell lung cancer
progression by activating WNT/β-catenin pathway. Am J Cancer Res.
5:1032–1046. 2015.PubMed/NCBI
|