Introduction
Over the last few decades, there has been increasing
interest in the use of natural compounds, often from traditional
medicine, as adjuvants and even as replacement of allopathic
treatment (1). While prescription
medicines have well-defined chemical composition and are supported
by evidence-based published studies, in terms of their efficacy and
toxicity, this has not been the case with traditional medicines.
Indeed, the popular misconception that all ‘natural’ products are
safe has tended to discourage investigation into their potential
toxicity (2). Such problems arise
even in the case of certain therapeutic teas which have even
special monographs in Pharmacopoeias (3).
Pyrrolizidine alkaloids (PAs) are heterocyclic
organic compounds, which are found in more than 6,000 plant species
(approximately 3% of world flora) as secondary metabolites
(4). Approximately 95% of PAs are
found in plants from Senecioneae and Eupatorieae tribes
(Asteraceae), in several genera of the Boraginaceae family, in the
genus Crotalaria (Fabaceae), and in some genera of the
Orchidaceae family (5). PAs are
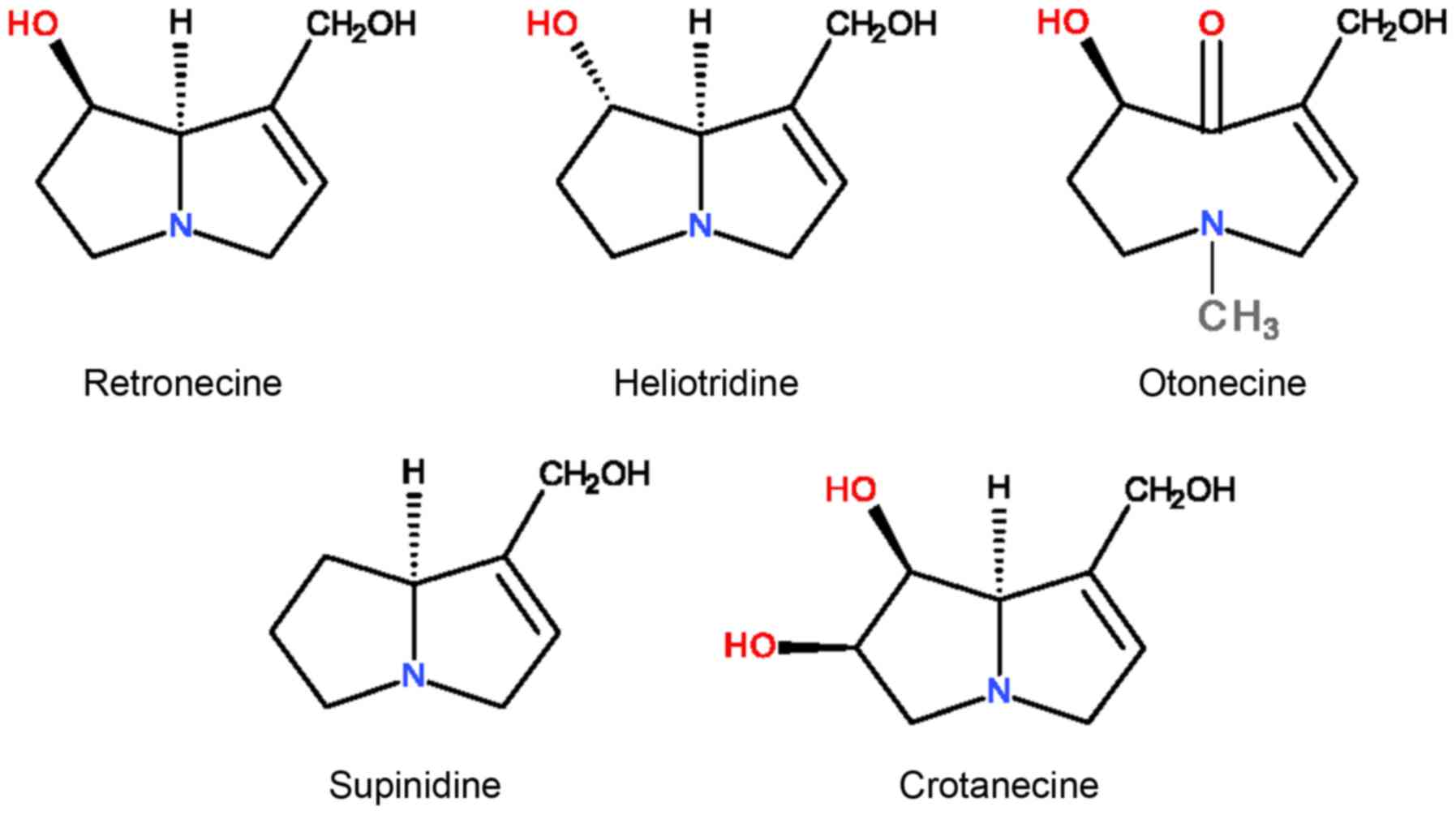
esters of heterocyclic amino alcohols termed necines (Fig. 1), with aliphatic monocarbonic or
dicarbonic acids (necine acids). These alkaloids can be found, in
plants, both as a free base and as the corresponding pyrrolizidine
alkaloids N-oxides (PANOs) (4).
The toxicity of PAs is structure-dependent as the
presence of a double bond in the necine base, often referred to as
1,2-unsaturated PAs or dehydroPAs, has been associated with greater
toxicity in comparison with the saturated necine bases (6). PAs are essentially stored in the
plant as protoxins, in the benign N-oxide form, whereas in the
gastrointestinal tract of animals they are reduced to the
corresponding amine becoming toxic (7).
Several cases of poisoning, some fatal, due to the
use of medicinal plants containing PAs have been reported. In
addition, the consumption of cereals and bakery products
contaminated with seeds of species containing PAs has been involved
in mass poisonings in rural areas of Afghanistan, India, South
Africa and the former USSR (8).
Poisoning can manifest as acute or subacute veno-occlusive disease
with specific symptoms such as persistent hepatomegaly, which in
most cases, progresses to cirrhosis (9). Some PAs have been shown to have
genotoxic, mutagenic, teratogenic and carcinogenic effects
(10). Thus, research into the
presence, identification and quantification of PAs as well as their
toxicity is important regarding human consumption of food from
plant origin in general and medicinal plants particularly (11). It is thus important that
commercially available beverages (infusions) of plants should be
tested for their qualitative and quantitative levels of PAs.
Petasites hybridus (butterbur), Tussilago
farfara (coltsfoot) and Symphytum officinale (comfrey)
are species traditionally used in phytotherapy and are commonly
found in specialized shops for tea beverages. Petasites
hybridus root has been used in the treatment of migraine,
dysmenorrhea, asthma and allergic rhinitis (12). Tussilago farfara leaves are
mainly used to relieve dry cough and other respiratory disease
symptoms (13). Findings have
shown that a methanolic extract obtained from the leaves and stems
of this species could be used in anti-cancer therapy as a
TNF-related apoptosis-inducing ligand sensitizer (14). Symphytum officinale root is
used in cases of gastro-intestinal and respiratory tract diseases
(4), whilst Senecio
vernalis (spring groundsel) is not as commonly used in
phytotherapy. However, the aerial parts of Senecio species,
under the generic name of Senecionis herba, have been used
for their antidiarrheal, diuretic, emmenagogue, galactagogue and
expectorant properties (15).
Daphnia magna (Straus) is a freshwater
zooplankton of the order Cladocera that has been routinely used as
a standard test species in ecotoxicology (16) due to their ease of handling in the
laboratory, and also to its relatively high sensitivity to a large
number of toxicants. Daphnia magna are sexually
parthenogenic and their clonal reproduction offers the supreme
advantage of genetic uniformity (17). Artemia salina is a species
of brine shrimp from the Anostraca order, extensively used in
ecotoxicology, due to the reliability, feasibility and
cost-effectiveness of the tests (18).
Most toxicity studies of natural products containing
PAs have been carried out on laboratory animals using isolated
alkaloids including lasiocarpine, senkirkine, retrorsine,
seneciphylline, and riddelliine (19). The toxicity of different plant
extracts obtained from the four studied plant species was
investigated on cell cultures, bacteria or animals (20–23).
To the best of our knowledge, the effect of these species on
aquatic species has not been studied so far.
The present study focuses on the PAs content in some
extracts obtained from the aforementioned plants. To test their
toxicity upon the two in vivo invertebrate models,
Artemia salina and Daphnia magna, and the PAs content
was correlated with the toxic effect.
Materials and methods
Dry plant extracts
Coltsfoot leaves, common butterbur roots teas
(produced by Stef Mar, Ltd., Ramnicu Valcea, Romania) and comfrey
roots tea (produced by Fares, Orastie, Romania) were purchased from
retail stores. Senecio vernalis aerial part was harvested in
May 2013, from Craiova Botanical Garden (Craiova, Romania),
naturally dried and conserved in laboratory conditions. The
morphological characters of the vegetal material were compared with
the ones quoted by literature. A voucher specimen is available in
the collection of the Department of Botany and Cell Biology, ‘Carol
Davila’ University of Medicine and Pharmacy (Bucharest, Romania),
and at ‘Dimitrie Brandza’ Botanical Garden (Bucharest, Romania; no.
405789).
The dry extracts were obtained as previously
described (24). Briefly, the
dried plants were ground (Tyler mesh 48), and 20 g of each plant
material were refluxed twice for 2 h with 1,000 ml of 50% methanol
acidified with citric acid to pH 2.0–3.0. The combined extracts
were evaporated, under reduced pressure with a rotary evaporator
system (Buchi, Flawil, Switzerland) to about 300 ml and atomized
with a Mini Spray Dryer B-290 (Buchi). The extracts were coded as
follows: SEN (Senecio vernalis), SYM (Symphytum
officinale), PET (Petasites hybridus) and TUSS
(Tussilago farfara).
GC-MS analysis
For the PAs content assay, 2 g of SEN and SYM and 4
g of PET and TUSS were dissolved in 30 ml of methanol acidified
with 50% citric acid to pH 2.0–3.0. After complete dissolution,
zinc powder (Merck KGaA, Darmstadt, Germany) was added in excess
and the solutions were stirred for 3 h using a magnetic stirrer (HI
190M; Hanna Instruments, Woonsocket, RI, USA) to convert PANOs to
PAs. After filtration, the solutions were purified by liquid-liquid
extraction twice with chloroform and twice with diethyl ether. The
aqueous solutions were alkalinized with 25% aqueous ammonia (pH
9–10) and the PAs were extracted three times with 30 ml of
chloroform. The chloroform solutions were dried under nitrogen flow
at room temperature, the residue was dissolved in 2 ml methanol and
filtered through a 0.2 µm syringe filter (Pall Life Science, Port
Washington, NY, USA), and analyzed by GC-MS.
Standard solutions in methanol (HPLC isocratic
grade; Merck KGaA) were prepared from a stock solution of
senecionine (200 mg/ml, GC ≥95%; Carl Roth GmbH & Co. KG,
Karlsruhe, Germany). The GC-MS analysis was carried out on a Focus
gas chromatograph (Thermo Fisher Scientific, Inc., Waltham, MA,
USA) equipped with retention time (TR)-5MS (5% phenyl
polysilphenylene-siloxane) capillary column (30 m × 0.25 mm × 0.25
µm; Thermo Fisher Scientific, Inc.). The capillary column was
directly coupled to a quadrupole mass spectrometer (DSQII; Thermo
Fisher Scientific, Inc.).
Chromatographic conditions were as follows: Samples
(1 µl) were injected in the split mode using helium as carrier gas
(flow rate of 1.0 ml/min); the injector temperature was 225°C and
column temperature program: 100°C for 1 min; ramp to 200°C at
20°C/min; ramp to 300°C at 10°C/min; analysis performed at 300°C,
the ionization by electron impact at 70 eV; detection in full scan
mode within the range m/z 60–650. In order to obtain the Kovats
retention indices (RI), to be further used to compare the retention
times obtained on the TR-5MS with those obtained on the DB-5 type
column (25,26), a mixture of n-alkanes (C21-C40) was
analyzed at 300°C and maintained up to 40 min (26,27).
Identification of the PAs was carried out by means
of the Kovats RI and the comparison of their mass spectra with
those found in the NIST 0.2 database when available, and data from
the literature (25–28). Due to the characteristic mass
fragmentation pattern of PAs, a selected ion monitoring (SIM)
method was used for the determination of retronecine/heliotridine
type (m/z 93, 120 and 136) and otonecine type (m/z 110, 151 and
168).
For the quantitative determination, senecionine was
used as a standard for the calibration curve. The area obtained for
each PA was interpolated on this curve and the results were
expressed in milligrams senecionine equivalents per 100 g of dry
material (dw).
The total amount of PAs obtained was compared to the
results obtained for the same extracts with those obtained by a
modified Ehrlich spectrophotometric method (24).
All the measurements were performed in triplicate.
The results are expressed as means ± standard deviation (SD).
Toxicity assessment
Artemia salina assay
Brine shrimp eggs were obtained from a local
aquarium shop (Bucharest, Romania) and hatched for 48 h in breakers
containing artificial sea water (36 g/l salinity) at 25±1°C under
continuous aeration, in a plant growth chamber (Sanyo MLR-351 H;
Sanyo, San Diego, CA, USA), using a 16 h photoperiod per day. The
newly hatched nauplii were separated from the shells and
transferred to fresh artificial sea water with a micropipette. The
assay was performed in the growth chamber, under the conditions
described above, using different concentrations of extracts
(1.0–1,000.0 µg/ml) in artificial sea water with 1% DMSO, on 50
larvae in 500 µl final volume of each dilution of the extracts.
Concentrations varied for each extract and were chosen based on
results obtained from preliminary studies. Artificial sea water
with 1% DMSO was used as the negative control. After 24 h, the
number of surviving nauplii was counted and recorded. Larvae were
considered dead only if they did not move their appendages for 10
sec during observation (29–31).
Daphnia magna assay
The daphnids, maintained parthenogenetically in
‘Carol Davila’ University, Department of Pharmaceutical Botany and
Cell Biology, were selected according to their size and kept in
fresh synthetic water (32,33)
under continuous aeration, 24 h prior to the experiment. The assay
was performed for the extracts in fresh synthetic water with 1%
DMSO (10–1,000 µg/ml) in 15 ml glass tubes, with 10 daphnids/tube.
Concentrations for each extract were settled after several
preliminary tests. Fresh synthetic with 1% DMSO was used as the
control. Lethality was recorded after 24, 72 and 120 h. The
daphnids were considered dead only if they did not move their
appendages for 30 sec during observations (34). During the experiment the daphnids
were kept at 25±1°C under continuous aeration, in a plant growth
chamber (Sanyo MLR-351 H), using a 16 h photoperiod per day
(35).
Statistical analysis
The toxicity tests were performed in duplicate. The
lethality percentage was calculated and plotted against the
logarithm of concentrations and the lethality-concentration curves
were calculated using the least squares fit method. The lethal
concentrations (LC50) which produce a lethality value of
50, were determined by interpolation on lethality-concentration
curves. The upper and lower limits of the 95% confidence interval
(95% CI) were calculated. The correlation between the
LC50 values for the two invertebrate models was
determined by the use of Pearson coefficient. All calculations were
performed using GraphPad Prism version 5.01 software (GraphPad
Software, Inc., La Jolla, CA, USA).
Results
GC-MS analysis
Both the qualitative and quantitative analysis of
the extracts were validated. Thus, the RI values were determined on
a TR-5MS column. The consulted literature did not report any RI
values for PAs using this type of column, but there were reports on
a DB-5 type column (25,26). Fletcher et al (27) analyzed the PAs from
Crotalaria sp. by GS-MS using a TR-5MS column and observed
that the same retention order for the compounds, although RI values
were all 1.02-fold higher than those obtained on DB-5 columns.
Similarly, our results showed that for the TR-5MS type column the
elution order of the PAs from the 4 extracts coincided with that
reported in the literature for a DB-5 type column and the RI values
were 1.03-fold higher (Table I).
Therefore, we can assume that the retention times were correctly
assigned.
 | Table I.Pyrrolizidine alkaloid content and
composition of SEN, PET, TUSS and SYM. |
Table I.
Pyrrolizidine alkaloid content and
composition of SEN, PET, TUSS and SYM.
| Extract | PA (type) | TR
(min) |
RIDB-5 |
RITR-5MS | PA
contenta |
|---|
| SEN | Senecivernine
(R) | 13.10 | 2,330 | 2,395 | 3.78 |
|
| Senecionine
(R) | 13.23 | 2,341 | 2,412 | 346.14 |
|
| Seneciphylline
(R) | 13.45 | 2,360 | 2,439 | 40.40 |
|
| Integerrimine
(R) | 13.77 | 2,402 | 2,481 | 26.26 |
|
| Senkirkine (O) | 14.71 | 2,530 | 2,608 | 78.28 |
| SYM | Intermedine
(R) | 11.97 | 2,188 | 2,253 | 7.48 |
|
| Symphytine (R) | 12.45 | 2,244 | 2,312 | 117.92 |
|
| Lasiocarpine
(H) | 12.55 | 2,257 | 2,325 | 31.64 |
|
| Symveridine
(R) | 13.97 | 2,325 | 2,406 | 0.52 |
| TUSS | Senecionine
(R) | 13.23 | 2,341 | 2,412 | 0.74 |
|
| Senkirkine (O) | 14.66 | 2,530 | 2,604 | 2.44 |
| PET | Senecionine
(R) | 13.24 | 2,341 | 2,413 | 1.84 |
|
| Integerrimine
(R) | 13.78 | 2,402 | 2,482 | 0.47 |
|
| Senkirkine (O) | 14.69 | 2,530 | 2,605 | 0.86 |
For the quantitative analysis, the total amount of
PAs obtained by the GC method was compared to the results of a
spectrophotometric quantitative analysis which also quantified PAs,
expressed as mg senecionine/100 g dry weight of each extract.
Results are presented in Table
II.
 | Table II.Comparison of the quantitative
analysis results for the extracts. |
Table II.
Comparison of the quantitative
analysis results for the extracts.
|
| Total PAs (mg
senecionine/100 g dry weight extract) |
|---|
|
|
|
|---|
| Extract | GC-MS |
Spectrophotometrica |
|---|
| SEN | 424.92 | 416.58 |
| SYM | 150.24 | 157.56 |
| PET |
2.11 |
2.31 |
| TUSS |
0.97 |
0.74 |
Toxicity assessment
Preliminary tests showed, in case of Artemia
salina, that a lethality >90% was caused by SEN (over 250
µg/ml), TUSS (over 500 µg/ml), PET (over 400 µg/ml) and SYM (over
750 µg/ml) (Table III).
Lethality vs. the logarithm of concentration regression curves were
afterwards plotted, and the LC50 and 95% CIs (α=0.05)
could be calculated (Table
III).
 | Table III.Acute toxicity of the extracts on
Artemia salina and Daphnia magna. |
Table III.
Acute toxicity of the extracts on
Artemia salina and Daphnia magna.
|
|
| AS | DM |
|---|
|
|
|
|
|
|---|
| Extract | Determination time
(h) | LC50
(µg/ml) | 95% CI (µg/ml) | LC50
(µg/ml) | 95% CI (µg/ml) |
|---|
| SEN | 24 | 131.22 | 109.14–148.59 | 95.67 | 95.58–95.75 |
|
| 72 | nt | nt | 83.31 | 74.73–92.51 |
|
| 120 | nt | nt | 5.28 | a |
| SYM | 24 | 707.95 | 698.23–726.11 | b | a |
|
| 72 | nt | nt | 801.0 | 690.42–876.48 |
|
| 120 | nt | nt | 412.3 | 237.18–565.89 |
| PET |
|
|
|
|
|
|
| 24 | 296.48 | 269.15–343.56 | 339.8 | 256.9–449.4 |
|
| 72 | nt | nt | 178.6 | 51.42–620.3 |
|
| 120 | nt | nt | 43.52 | a |
| TUSS | 24 | 222.33 | 181.13–270.40 | 509.04 | a |
|
| 72 | nt | nt | 189.97 | 105.02–459.30 |
|
| 120 | nt | nt | 37.40 | 5.84–314.20 |
In case of Daphnia magna SEN, TUSS and PET
induced lethality of >90% in the concentration range of
500–1,000 µg/ml and SYM at 1,000 µg/ml. After logarithm of the
concentration vs. lethality regression curves were plotted,
LC50 could not be calculated for SYM at 24 h, therefore
it was considered >2,000 µg/ml. In all cases tested, the
viability of the controls was 100%.
Discussion
Even though the identification of PAs by GC-MS was
first carried out in the early 1990s (25), their assay, mainly in plants used
as therapeutic teas, remains of interest due to their potential
toxicity (36–39). This report presents four plant
extracts, which were analyzed for their qualitative and
quantitative content in PAs: Senecio vernalis, Petasites
hybridus, Tussilago farfara and Symphytum officinale.
While the last three are commonly used in phytoterapy, Senecio
vernalis was analyzed mainly for its already known high content
of PAs (40), in an attempt to
emphasize the risk of using PAs in therapy, if any. Moreover, while
the extracts from the last three plants (PET, TUSS and SYM) were
obtained from commercial teas (dry plant material), SEN extract was
obtained from plants harvested and prepared similarly to teas
(aerial parts were dried in the conditions in which common teas are
dried) mainly as it contained a significant amount of
senecionine.
A novel GC-MS method for the qualitative and
quantitative determination of certain PAs from dry vegetable
extracts of the four plants was developed using a TR-5MS capillary
column. In order to compare our results to those already described
in the literature, which used a DB-5 column (25,26),
the Kovats RI was computed for senecivernine, senecionine,
seneciphylline, integerrimine, senkirkine, intermedine, symphytine,
lasiocarpine, and symveridine. Both the order of elution for these
compounds and the ratio between the retention times obtained in
this study and those reported on a TR-5 column, of 1.03, were the
same as in the case of DB-5ms and DB-5 columns (27).
The largest amount of PAs was found in SEN (494.86
mg/100 g dw), the predominant alkaloid being senecionine (346.14
mg/100 g dw). For TUSS and PET the total PAs concentration was
similar, 3.17 mg/100 g dw and 3.18 mg/100 g dw, respectively. The
main PA for PET was senecionine (1.84 mg/100 g dw) and for TUSS the
otonecine - type PA senkirkine (2.44 mg/100 g dw). For SYM the
total PAs concentration was 157.56 mg/100 g dw with symphytine as
the main alkaloid (117.92 mg/100 g dw). There is currently no
international standard for the maximum allowable level of PAs in
foods. However, in the Netherlands, the total PA content (including
PANOs) in herbal preparations or herbal extracts, must not exceed 1
µg/kg (41). These limits are far
exceeded by the tested extracts.
The toxicity of the extracts was determined using
two experimental models of crustaceans - Artemia salina and
Daphnia magna. These models can be predictive for the
toxicity of extracts containing PAs in animals and humans because
numerous studies have demonstrated the presence of cytochrome P450
in these organisms, enzymes needed for the biotransformation of PAs
to the toxic pyrrole derivatives (42–45).
The assays consisted in the exposure of the invertebrates at
increasing concentrations of test substances and determination of
the lethal concentrations 50% (LC50) at different time
intervals (24–120 h). Substances with LC50 <1,000
µg/ml are considered toxic (46).
In case of Artemia salina, the low
LC50 obtained for SEN (131.22 µg/ml) indicates that this
extract is the most toxic of those studied. TUSS and PET are
approximately 1.5-fold and 2.3-fold less toxic and SYM is about
5.4-fold less toxic. The CIs (P<0.05) could be calculated for
all extracts, and they presented very high limits for PET and TUSS,
but were narrow for SEN (approximately 50 µg/ml) and SYM
(approximately 30 µg/ml). It should be noted that all tested
extracts were found to be toxic in the case of this species.
Concerning Daphnia magna, it was observed
that the extracts were generally toxic in the following order: SEN
> PET > TUSS > SYM. Thus, SEN has the highest toxicity,
TUSS and PET exhibit similar toxicity, with LC50 values
being very similar, particularly on the 3rd and 5th day. SYM is
substantially non-toxic at 24 h, has low toxicity after 72 h and
moderate after 120 h of exposure.
In order to determine whether there is a correlation
between the LC50 values obtained from Artemia
salina and Daphnia magna (at 24, 72 and 120 h) assays
for each extract, Pearson coefficient was calculated for the pairs
Artemia salina (at 24 h) and Daphnia magna at 24, 48
and 72 h. The values obtained were greater than 0.97, which
indicates a strong positive Pearson correlation. SEN, the extract
with the highest concentration of PAs, exhibits the highest
toxicity and the LC50 obtained for PET and TUSS,
extracts that have similar PAs concentrations, had similar values.
SYM, the extract with the second PAs concentration 157.56 mg/100 g
dw, presented in both cases the lowest toxicity. This result could
be explained by the fact that Symphytum officinale contained
senecionine under the limit of detection, if any. Alternately,
other components present in the extract could reduce the effect of
existing PAs in SYM. Further studies are to focus on both
hypotheses.
The LC50 computed for each extract
corresponded to an LC50 of total PAs of 2.15–1,063 µg/l
for the Artemia salina assay and 0.36–1,203 µg/l for the
Daphnia magna assay. Mulder et al (47) determined the occurrence of PAs in
herbal teas from different European countries. For the teas
containing no PA-producing plants (n=166), in the majority of
samples (91%), one or more PAs were detected, with a mean total
value of 6.13 µg/l. For the tea infusion obtained from PA-producing
plants (n=12), the PAs levels were 2.4–414 µg/l. It can be seen
that PAs levels in these infusions are in the same range as the
LC50 for the two aquatic organisms.
In conclusion, all four extracts containing PAs were
shown to be toxic in the model tests on aquatic organisms. Toxicity
increased with the PAs concentration, except for the extract from
Symphytum officinale. PAs LC50 was in the same
range as PAs levels found in some herbal tea infusion from
Europe.
Acknowledgements
In memoriam: The authors would like to respectfully
dedicate this article to Ms. Mihaela Ilie, who passed away on
January 1, 2018.
Funding
The authors acknowledge the financial support
offered by UEFISCDI (Romania - grant no. 8BM/2016) and NRF (South
Africa), through the Romania - South Africa Joint
Collaboration.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
OCS, OTO, MI and DMM defined the research theme.
OCS, OTO, CMG and GMN designed the methods and experiments and
performed the laboratory experiments. SN, CEZ and CNP analyzed and
interpreted the data. OCS and OTO wrote the manuscript. MI, DAS,
MDC, AMT and DMM critically revised the paper for important
intellectual content. AMT and DMM gave the final approval of the
version to be published. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Demetrios A. Spandidos is the Editor-in-Chief for
the journal, but had no personal involvement in the reviewing
process, or any influence in terms of adjudicating on the final
decision, for this article.
Glossary
Abbreviations
Abbreviations:
|
PAs
|
pyrrolizidine alkaloids
|
|
PANOs
|
pyrrolizidine alkaloids N-oxides
|
|
SEN
|
Senecio vernalis extract
|
|
SYM
|
Symphytum officinale
extract
|
|
PET
|
Petasites hybridus extract
|
|
TUSS
|
Tussilago farfara extract
|
|
RI
|
Kovats retention indices
|
|
TR
|
retention time
|
|
dw
|
dry material
|
|
LC50
|
lethal dose 50%
|
|
CI 95%
|
95% confidence interval
|
References
|
1
|
Han E, Johnson N, DelaMelena T, Glissmeyer
M and Steinbock K: Alternative therapy used as primary treatment
for breast cancer negatively impacts outcomes. Ann Surg Oncol.
18:912–916. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Margină D, Ilie M, Grădinaru D,
Androutsopoulos VP, Kouretas D and Tsatsakis AM: Natural products -
friends or foes? Toxicol Lett. 236:154–167. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shikov AN, Pozharitskaya ON, Makarov VG,
Wagner H, Verpoorte R and Heinrich M: Medicinal plants of the
Russian Pharmacopoeia; their history and applications. J
Ethnopharmacol. 154:481–536. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Roeder E: Medicinal plants in Europe
containing pyrrolizidine alkaloids. Pharmazie. 50:83–98.
1995.PubMed/NCBI
|
|
5
|
Reimann A, Nurhayati N, Backenköhler A and
Ober D: Repeated evolution of the pyrrolizidine alkaloid-mediated
defense system in separate angiosperm lineages. Plant Cell.
16:2772–2784. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hartmann T: Chemical ecology of
pyrrolizidine alkaloids. Planta. 207:483–495. 1999. View Article : Google Scholar
|
|
7
|
Coulombe RA Jr: Pyrrolizidine alkaloids in
foodsAdvances in Food and Nutrition Research. Taylor SL: 45.
Elsevier Science, Ltd.; Oxford, UK: pp. 61–99. 2003, View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Neuman MG, Cohen L, Opris M, Nanau RM and
Hyunjin J: Hepatotoxicity of pyrrolizidine alkaloids. J Pharm Pharm
Sci. 18:825–843. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Prakash AS, Pereira TN, Reilly PEB and
Seawright AA: Pyrrolizidine alkaloids in human diet. Mutat Res.
443:53–67. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen T, Mei N and Fu PP: Genotoxicity of
pyrrolizidine alkaloids. J Appl Toxicol. 30:183–196.
2010.PubMed/NCBI
|
|
11
|
Stahlmann R and Horvath A: Risks, risk
assessment and risk competence in toxicology. Ger Med Sci.
13:Doc092015.(In English, German). PubMed/NCBI
|
|
12
|
Fiebich BL, Grozdeva M, Hess S, Hüll M,
Danesch U, Bodensieck A and Bauer R: Petasites hybridus
extracts in vitro inhibit COX-2 and PGE2 release by direct
interaction with the enzyme and by preventing p42/44 MAP kinase
activation in rat primary microglial cells. Planta Med. 71:12–19.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nedelcheva A, Kostova N and Sidjimov A:
Pyrrolizidine alkaloids in Tussilago farfara from Bulgaria.
Biotechnol Biotechnol Equip. 29 Suppl 1:S1–S7. 2015. View Article : Google Scholar
|
|
14
|
Lee HJ, Cho HS, Jun SY, Lee JJ, Yoon JY,
Lee JH, Song HH, Choi SH, Kim SY, Saloura V, et al: Tussilago
farfara L. augments TRAIL-induced apoptosis through MKK7/JNK
activation by inhibition of MKK7 TIPRL in human hepatocellular
carcinoma cells. Oncol Rep. 32:1117–1123. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mogosanu DG, Pintea A, Bejenaru LE,
Bejenaru C, Rǎu G and Popescu H: HPLC analysis of carotenoids from
Senecio vernalis and S. jacobaea (Asteraceae).
Farmacia. 57:780–786. 2009.
|
|
16
|
Martins J, Teles Oliva L and Vasconcelos
V: Assays with Daphnia magna and Danio rerio as alert
systems in aquatic toxicology. Environ Int. 33:414–425. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Toumi H, Boumaiza M, Millet M, Radetski
CM, Camara BI, Felten V and Ferard J-F: Investigation of
differences in sensitivity between 3 strains of Daphnia
magna (crustacean Cladocera) exposed to malathion
(organophosphorous pesticide). J Environ Sci Health B. 50:34–44.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nunes BS, Carvalho FD, Guilhermino LM and
Van Stappen G: Use of the genus Artemia in ecotoxicity
testing. Environ Pollut. 144:453–462. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fu PP, Xia Q, Lin G and Chou MW:
Pyrrolizidine alkaloids - genotoxicity, metabolism enzymes,
metabolic activation, and mechanisms. Drug Metab Rev. 36:1–55.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Behninger C, Abel G, Röder E, Neuberger V
and Göggelmann W: Studies on the effect of an alkaloid extract of
Symphytum officinale on human lymphocyte cultures. Planta
Med. 55:518–522. 1989.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chou MW and Fu PP: Formation of
DHP-derived DNA adducts in vivo from dietary supplements and
chinese herbal plant extracts containing carcinogenic pyrrolizidine
alkaloids. Toxicol Ind Health. 22:321–327. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Benedek B, Ziegler A and Ottersbach P:
Absence of mutagenic effects of a particular Symphytum
officinale L. liquid extract in the bacterial reverse mutation
assay. Phytother Res. 24:466–468. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gomes MFPL, de Oliveira Massoco C, Xavier
JG and Bonamin LV: Comfrey (Symphytum officinale. l.) and
experimental hepatic carcinogenesis: A short-term carcinogenesis
model study. Evid Based Complement Alternat Med. 7:197–202. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Seremet O, Olaru OT, Bǎlǎlǎu D and Negres
S: The effect of certain plant extracts containing pyrrolizidine
alkaloids on Lactuca sativa radicle growth. Rom J Biophys.
24:1–9. 2014.
|
|
25
|
Witte L, Rubiolo P, Bicchi C and Hartmannt
T: Comparative analysis of pyrrolizidine alkaloids from natural
sources by gas chromatography-mass spectrometry. Phytochemistry.
32:187–196. 1992. View Article : Google Scholar
|
|
26
|
Gardner DR, Thorne MS, Molyneux RJ,
Pfister JA and Seawright AA: Pyrrolizidine alkaloids in Senecio
madagascariensis from Australia and Hawaii and assessment of
possible livestock poisoning. Biochem Syst Ecol. 34:736–744. 2006.
View Article : Google Scholar
|
|
27
|
Fletcher MT, McKenzie RA, Blaney BJ and
Reichmann KG: Pyrrolizidine alkaloids in Crotalaria taxa
from northern Australia: Risk to grazing livestock. J Agric Food
Chem. 57:311–319. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Asres K, Sporer F and Wink M: Occurrence
of pyrrolizidine alkaloids in three Ethiopian Solanecio
species. Biochem Syst Ecol. 36:399–407. 2008. View Article : Google Scholar
|
|
29
|
GuŢu CM, Olaru OT, Purdel NC, Ilie M,
NeamŢu MC, Miulescu Dănciulescu R, Avramescu ET and Margină DM:
Comparative evaluation of short-term toxicity of inorganic arsenic
compounds on Artemia salina. Rom J Morphol Embryol.
56:1091–1096. 2015.PubMed/NCBI
|
|
30
|
Olaru OT, Venables L, VAN DE, Venter M,
Nitulescu GM, Margina D, Spandidos DA and Tsatsakis AM: Anticancer
potential of selected Fallopia Adans species. Oncol Lett.
10:1323–1332. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Socea LI, Socea B, Saramet G, Barbuceanu
SF, Draghici C, Constantin VD and Olaru OT: Synthesis and
cytotoxicity evaluation of new 5H-dibenzo[a,d] [7]annulen-5-yl
acetylhydrazones. Rev Chim. 66:1122–1127. 2015.
|
|
32
|
Nitulescu GM, Draghici C and Olaru OT: New
potential antitumor pyrazole derivatives: Synthesis and cytotoxic
evaluation. Int J Mol Sci. 14:21805–21818. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
OECD guidelines for the testing of
chemicals, section 2: ‘Daphnia sp., acute immobilization
test’. http://www.oecd-ilibrary.org/docserver/download/9720201e.pdfFeb
28–2018
|
|
34
|
Olaru OT, Seremet OC, Petrescu M, Salagean
A, Velescu B and Nitulescu GM: Toxicity evaluation and polyphenols
assessment of some extracts from indigenous Euphorbia species. Rom
J Biophys. 24:43–54. 2014.
|
|
35
|
Fan W, Cui M, Liu H, Wang C, Shi Z, Tan C
and Yang X: Nano-TiO2 enhances the toxicity of copper in
natural water to Daphnia magna. Environ Pollut. 159:729–734.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
IPCS: Environmental health criteria 80:
Pyrrolizidine alkaloids. World Health Organization; Geneva: 1988,
http://www.inchem.org/documents/ehc/ehc/ehc080.htmFeb
28–2018
|
|
37
|
European Medicines Agency: Public
statement on the use of herbal medicinal products containing toxic,
unsaturated pyrrolizidine alkaloids (PAs). http://www.ema.europa.eu/docs/en_GB/document_library/Public_statement/2014/12/WC500179559.pdfFeb
28–2018
|
|
38
|
Seremet O, Olaru OT, Ilie M, Negres S and
Bălălău D: HPTLC evaluation of the pyrollizidine alkaloid
senecionine in certain phytochemical products. Farmacia.
61:756–763. 2013.
|
|
39
|
Şeremet OC, Bărbuceanu F, Ionică FE,
Margină DM, GuŢu CM, Olaru OT, Ilie M, Gonciar V, Negreş S and
ChiriŢă C: Oral toxicity study of certain plant extracts containing
pyrrolizidine alkaloids. Rom J Morphol Embryol. 57:1017–1023.
2016.PubMed/NCBI
|
|
40
|
Aniszewski T: Alkaloids - Secrets of Life:
Aklaloid Chemistry, Biological Significance Applications and
Ecological Role. 1st edition. Elsevier; Amsterdam: pp. 1672007
|
|
41
|
Centre for Food Safety: Chemical hazard
evaluation: Pyrrolizidine alkaloids in food. http://www.cfs.gov.hk/english/programme/programme_rafs/files/Pyrrolizidine_Alkaloids_in_Food_e.pdfMarch
6–2018
|
|
42
|
James MO: Cytochrome P450 monooxygenases
in crustaceans. Xenobiotica. 19:1063–1076. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
James MO and Boyle SM: Cytochromes P450 in
crustacea. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol.
121:157–172. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Rewitz KF, Styrishave B, Løbner-Olsen A
and Andersen O: Marine invertebrate cytochrome P450: Emerging
insights from vertebrate and insects analogies. Comp Biochem
Physiol C Toxicol Pharmacol. 143:363–381. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Solé M and Livingstone DR: Components of
the cytochrome P450-dependent monooxygenase system and
‘NADPH-independent benzo[a]pyrene hydroxylase’ activity in a wide
range of marine invertebrate species. Comp Biochem Physiol C
Toxicol Pharmacol. 141:20–31. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Meyer BN, Ferrigni NR, Putnam JE, Jacobsen
LB, Nichols DE and McLaughlin JL: Brine shrimp: A convenient
general bioassay for active plant constituents. Planta Med.
45:31–34. 1982. View Article : Google Scholar
|
|
47
|
Mulder PPJ, López Sánchez P, These A,
Preiss-Weigert A and Castellari M: Occurrence of pyrrolizidine
alkaloids in food. European Food Safety Authority. 1162015.
|