Introduction
Diabetes is a major public health problem affecting
415 million people worldwide and diabetic retinopathy (DR) is a
major diabetic complication that can cause significant visual
impairment and blindness (1). The
early stages of DR can be inhibited by improvement of glycemic
control suggesting hyperglycemia to initiate the pathology of the
pathology of DR (2,3). Emerging evidence showed that human
retinal endothelial cells (HRECs) dysfunction is the initial event
of microvascular disorder in the development of DR. HRECs damage is
associated with thickening of the capillary endothelial basement
membrane (4). Moreover, current
studies demonstrated that except sterile-inflammation, high glucose
(HG) induced oxidative stress in the retina also plays a critical
role in the pathogenesis of retinopathy (5). Administration of antioxidants or
genetic overexpression of superoxide dismutase has been
demonstrated to inhibit the diabetes-induced degeneration of
retinal capillaries (6–8). Accordingly, strategies against
intracellular reactive oxygen species (ROS) production induced cell
apoptosis help to reduce HRECs injury (9).
Gastrodin, a major active ingredient of Chinese
herbal medicine called Gastrodia elata Blume, has been reported to
exert anti-inflammatory and anti-apoptotic effects in various
diseases (10,11). Importantly, Peng et al
(12) found gastrodin treatment
reduced reactive oxygen species production in macrophages and
protected macrophages against oxidative stress-induced apoptosis.
And a recent study identified that gastrodin could stimulate M2
macrophage polarization and rescue macrophages from oxidative
stress-induced apoptosis and death (13). To date, no literatures involved in
the role of gastrodin on HG-induced HRECs injury.
In the present study, we sought to examine the
potential protective effects of gastrodin on HG-induced HRECs
damage and to investigate the relationship between its effect and
the modulation of TLR4/NF-κBp65 signaling pathway. And our work
suggested that gastrodin may have the potential to be a novel
pharmaceutical approach for the treatment of DR.
Materials and methods
Cell culture
Human retinal endothelial cells (HRECs) were
obtained from Cell Systems (Kirkland, WA, USA). The culture medium
was EGM-2 Bulletkit medium (Lonza, Basel Switzerland) with 100 U/ml
penicillin/100 µg/ml streptomycin (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), containing 10% fetal bovine
serum (Gibco; Thermo Fisher Scientific, Inc.). Cells were
maintained at 37°C and 5% CO2. HRECs of passages 6–10
were used for experiments.
Cell treatment
After an initial 24 h of culture in serum-free
medium, HRECs were treatment with various concentration of
gastrodin (0.1, 1, 10, 100 µM) or 100 nM gastrodin in normal medium
for various time (6, 12, 24 and 48 h) to detect the optimal
concentration and time of intervention. Cells were subjected to
medium containing 5 mM glucose (control group) or high glucose
medium containing 30 mM glucose (HG group). HRECs cultured in
6-well plates were transfected with recombinant plasmid pcDNA3.1 or
pcDNA3.1-SIRT1 (Invitrogen; Thermo Fisher Scientific, Inc.) to
overexpress SIRT1 using Lipofectamine 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.) in accordance with the manufacturer's
protocol.
Cell viability assay
The Cell Counting Kit-8 (CCK-8, Biosharp, Hefei,
China) was performed to determine cell viability of HRECs. In
brief, 5×103 of cells were cultured in a 96-well plate
at 37°C and allowed to attach for 24 h. After starvation for 12 h,
cells were stimulated with different concentrations of gastrodin
for different time in normal median. Then, 10 µl CCK-8 solution was
added to each well, plates were incubated at 37°C for another 2 h.
The absorbance of cells was then measured at 450 nm.
Intracellular reactive oxygen species
(ROS) analysis
The intracellular ROS level was measured by
dihydroethidium (DHE; Beyotime Institute of Biotechnology, Haimen,
China) staining. After various treatment, HRECs were incubated with
5 µM DHE at 37°C for 30 min. Following incubation, cells were fed
with normal growth medium without DHE for 1 h, and rinsed with PBS.
Fluorescence images were observed using a fluorescence microscope
(Carl Zeiss AG, Oberkochen, Germany). The fluorescence intensity
was measured using ImageJ software (NIH, Bethesda, MD, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RT-qPCR analyses were carried out as described
previously (14). Total RNA was
extracted from cells in groups using TRIzol reagent (Invitrogen;
Thermo Fisher Scientific, Inc.) and quantified by measuring the
absorbance at 260 nm. Subsequently, 2 µl of total RNA was used for
the preparation of cDNA by reverse transcription using the
PrimeScript RT reagent kit (Takara Bio, Inc., Otsu, Japan)
according to the manufacturer's instructions. The expression of
HO-1, NQO1, NRF2 and GCLM mRNA were determined on the Applied
Biosystems StepOne Real-Time PCR system (Applied Biosystems; Thermo
Fisher Scientific, Inc.). The reaction condition was 95°C at 10 min
for a hot start, then 40 cycles at 95°C for 15 sec, 60°C for 60 sec
and 72°C for 60 sec. The primer sequences used in this study were
listed in Table I. The gene
expression level was calculated by 2−ΔΔCq methods and
normalized to 18S RNA. All experiments were repeated in three
times.
 | Table I.Forward and reverse primers used for
reverse transcription-quantitative polymerase chain reaction. |
Table I.
Forward and reverse primers used for
reverse transcription-quantitative polymerase chain reaction.
| Gene name | Direction | Sequence
(5′-3′) |
|---|
| HO-1 | Forward |
GGCAGAGGGTGATAGAAGAGG |
|
| Reverse |
AGCTCCTGCAACTCCTCAAA |
| NQO1 | Forward |
TCCAGAAACGACATC |
|
| Reverse |
GCACCCCAAACCAATACAAT |
| NRF2 | Forward |
AAGAATAAAGTCGCCGCCCA |
|
| Reverse |
AGATACAAGGTGCTGAGCCG |
| GCLM | Forward |
AGTCTCCATGGAAGAACGGCC |
|
| Reverse |
CGATTACGGCTTCACTTGCCT |
| 18S | Forward |
GAGGGGAGAGCGGGTAAGA |
|
| Reverse |
TCGGGGTCCGACAAAACCC |
Cell apoptosis assay
HRECs cell apoptosis rates were measured by flow
cytometric analysis using Annexin V-FITC-PI Apoptosis Detection kit
(Vazyme Biotech, Nanjing, China). Briefly, cells were trypsinized
after treatment and rinsed with PBS to achieve the final
concentration of 5×103/ml. Then, 195 µl cell suspension
and 5 µl Annexin V-FITC were mixed under dark for 15 min followed
by stained with 10 µl of propidium iodide (PI) for another 5 min.
The apoptosis rate was assayed by flow cytometry (FACSCalibur;
Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Western blot analysis
After treatment, HRECs lysates were collected in
RIPA lysis buffer supplemented with protease/phosphatase inhibitor
cocktail (Merck KGaA, Darmstadt, Germany) and total protein
concentrations were measured using the BCA assay (Beyotime
Institute of Biotechnology). Protein samples were loaded on 6–12%
SDS-PAGE, transferred to polyvinylidene fluoride membranes
(Bio-Rad) and were blocked with 5% skim milk for 1 h at room
temperature. The membranes were incubated with the corresponding
primary antibodies against SIRT1 (1:400; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA), Bcl-2 (1:1,000), Bax (1:1,000), cleaved
caspase 3 (1:400), cytochrome C (1:1,000), TLR4 (1:500), NF-κBp65
(1:1,000), p-NF-κBp65 (1:1,000), and GAPDH (1:1,000; all from Cell
Signaling Technology, Inc., Danvers, MA, USA) at 4°C overnight.
Subsequently, the blots were washed with PBST and incubated with a
peroxidase conjugated immunoglobulin G secondary antibody (Santa
Cruz Biotechnology, Inc.) for 1 h at room temperature. Signals were
visualized using an enhanced chemiluminescence kit (GE Healthcare,
Chicago, IL, USA).
Statistical analysis
All data are expressed as the mean ± standard
deviation, and analyzed with GraphPad Prism 5 (GraphPad Software,
Inc., La Jolla, CA, USA). Statistical significance was tested using
one-way analysis of variance (ANOVA) with Tukey's post hoc test,
Kruskal-Wallis test with Dunn's post hoc test and two-way ANOVA.
<P 0.05 was considered to indicate a statistically significant
difference. All experiments were performed at least three
times.
Results
Gastrodin ameliorated HG induced
inhibition of HRECs viability
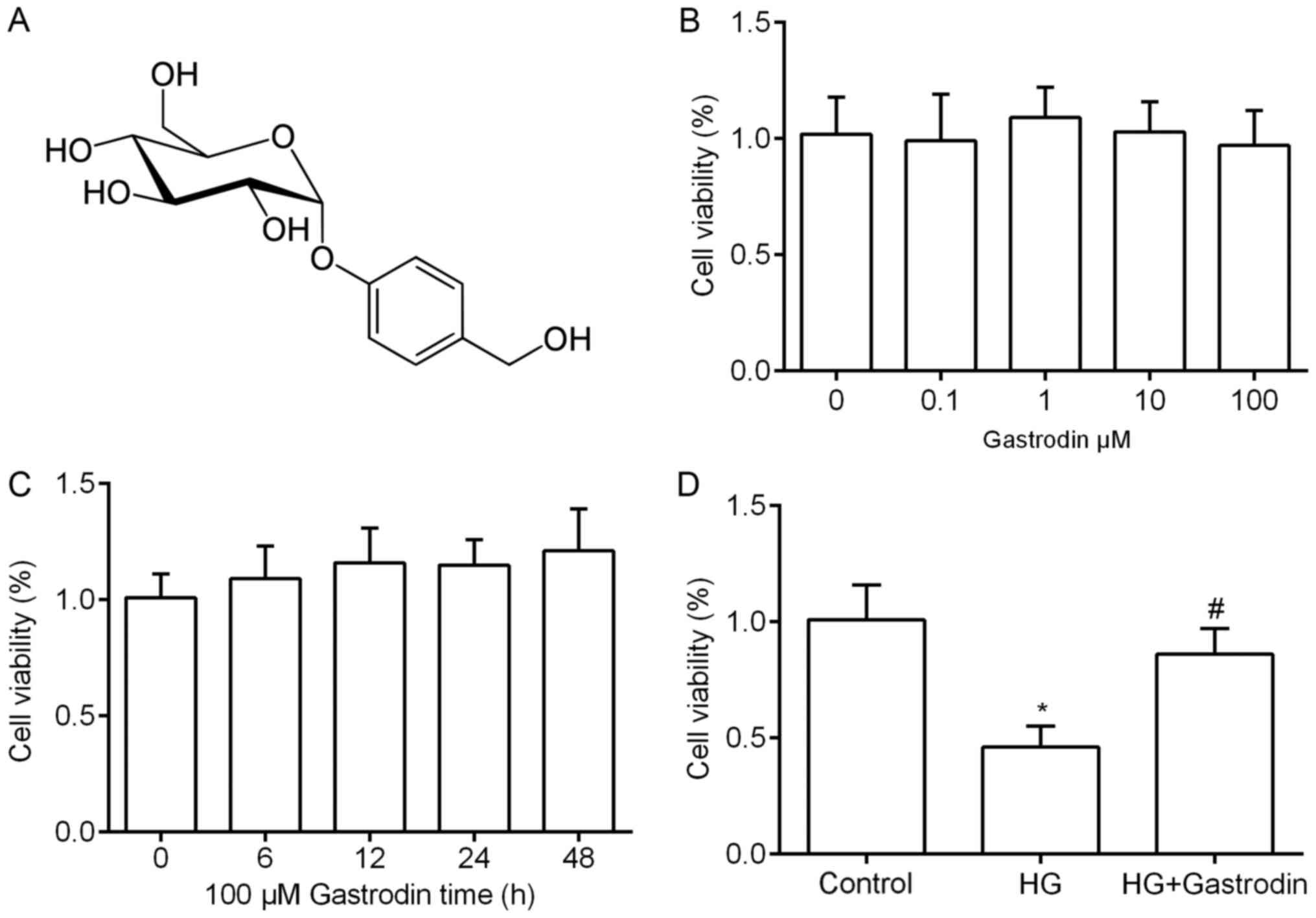
Firstly, to determine whether gastrodin (Fig. 1A) itself affects the cell viability
of HRECs in normal median, cells were treated with various
concentration of gastrodin. The results revealed that gastrodin had
no effects on cell viability even with high concentration at 100 µM
(P>0.05; Fig. 1B). In addition,
100 µM gastrodin had no significant effect on cell viability within
48 h (P>0.05; Fig. 1C). Then,
we found HRECs treated with HG for 24 h showed significant decrease
in cell viability compared to the control group, but 100 µM
gastrodin markedly ameliorated the inhibitory effect caused by HG
(Fig. 1D).
Gastrodin inhibited HG-induced HRECs
apoptosis
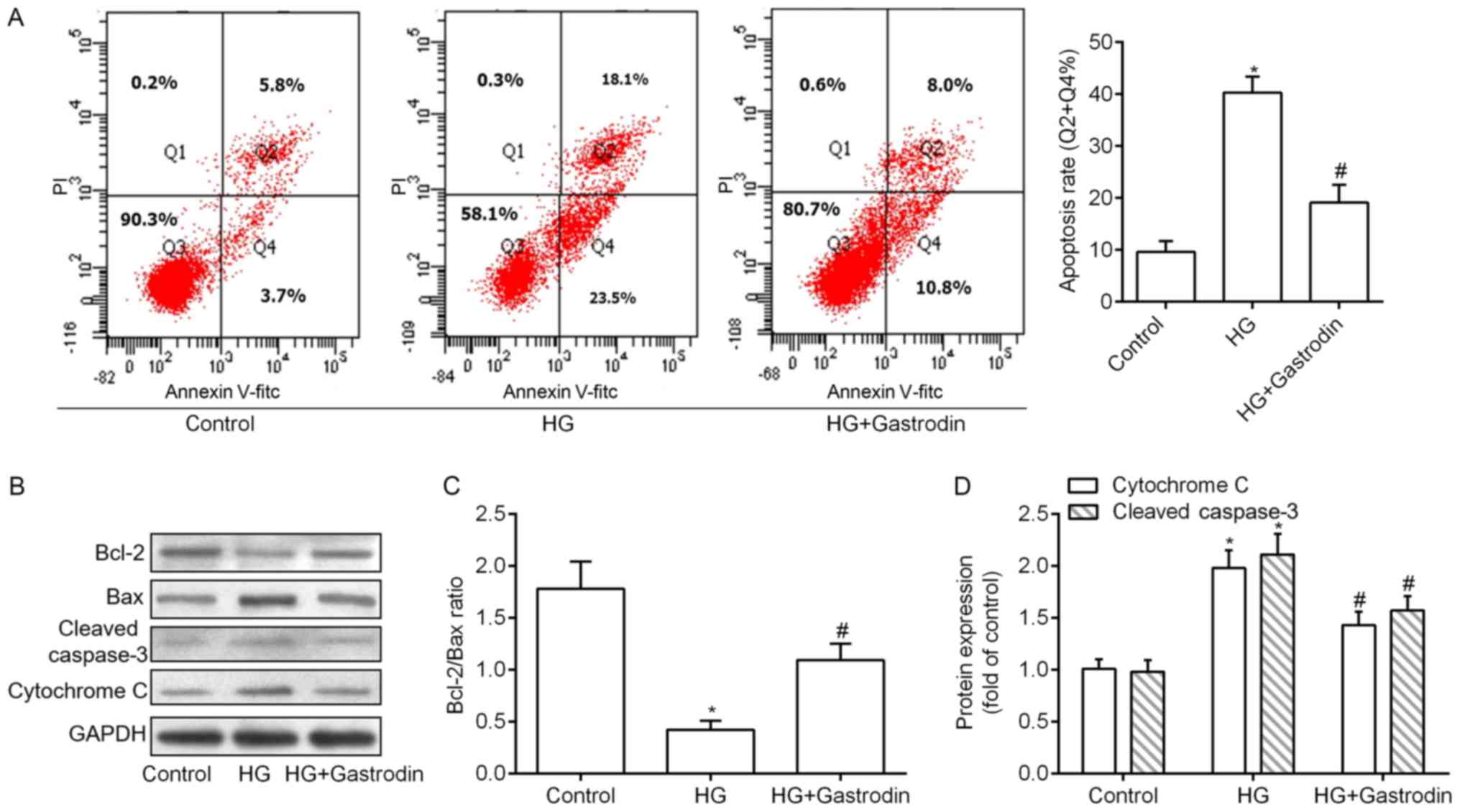
HRECs apoptosis rates was measured and Fig. 2A demonstrated that, comparised with
the control group, treating of HRECs with HG for 24 h significantly
increased apoptosis, which could be reversed by addition of 100 µM
gastrodin (Fig. 2A). Additionally,
we examined the expression levels of mitochondrial apoptotic
markers by Western blot analysis. Expectedly, the ratio of
Bcl-2/Bax was decreased, whereas the expressions of cytochrome C
and cleaved caspase 3 were increased with the treatment of HG. When
the cells were treated with both HG and 100 µM gastrodin, above
changes were at least partly abolished (Fig. 2B-D).
Gastrodin alleviated HG-induced
activation of oxidative stress
Sincere the key role of oxidative stress in
HG-triggered cell apoptosis, we tested the levels of ROS production
in HRECs. Intriguing, HG treatment caused increase in ROS
generation compared to the control and 100 µM gastrodin alleviated
ROS generation significantly (Fig.
3A). Subsequently, we tested the expression levels of oxidative
stress related genes including hemeoxygenase-1 (HO-1), NAD(P)H
dehydrogenase quinone 1 (NQO1), NF-E2-related factor 2 (NRF2), and
γ-glutamate-cysteine ligase modifier (GCLM) via RT-qPCR. HG
dramatically increased the mRNA levels of above four genes compared
to the control group. However, co-treatment with HG and gastrodin
resulted in a decrease in the expression of oxidative stress
related genes (Fig. 3B-E).
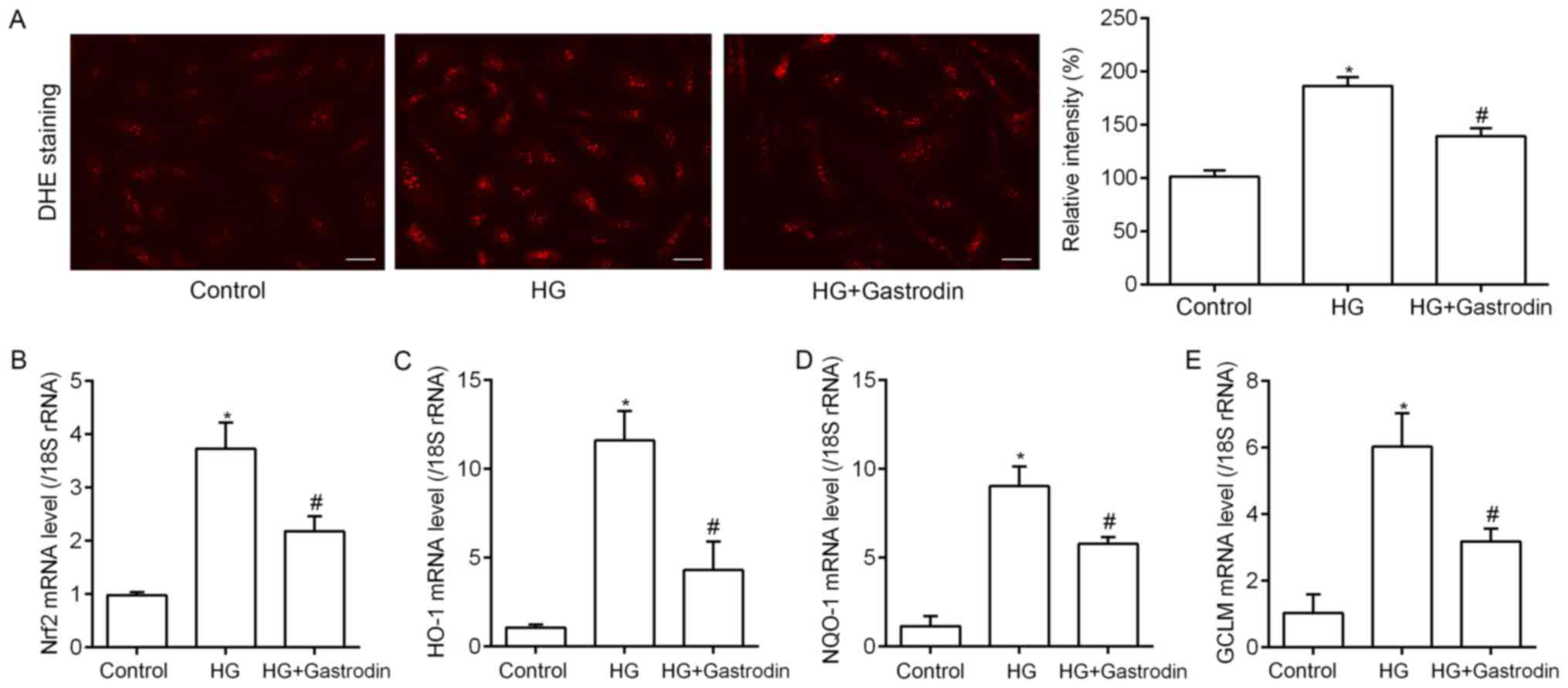 | Figure 3.Gastrodin suppresses HG-induced HREC
oxidative stress. (A) ROS production was detected by DHE staining
and the results revealed that 100 µM gastrodin significantly
inhibited the increased ROS levels in HRECs induced by HG (scale
bars, 50 µm). Changes in the transcription levels of the oxidative
stress associated genes (B) NRF2, (C) HO-1, (D) NQO1 and (E) GCLM
were detected through reverse transcription-quantitative polymerase
chain reaction. The results demonstrated that HG induced
significant gene upregulation, which gastrodin treatment
attenuated. *P<0.05 vs. control; #P<0.05 vs. HG.
HG, high glucose; HRECs, human retinal endothelial cells; ROS,
reactive oxygen species; DHE, dihydroethidium; NRF2, nuclear
factor-E2-related factor 2; HO-1, hemeoxygenase-1; NQO1,
nicotinamide adenine dinucleotide phosphate dehydrogenase quinone
1; GCLM, γ-glutamate-cysteine ligase modifier. |
Gastrodin induced activation of SIRT1
and subsequent inhibition of TLR4/NF-κBp65 signaling pathway in
HRECs
Activation of NF-κB was shown to promote expression
of various pro-apoptotic regulators in HRECs (15) and TLR-mediated NF-κB activation
were commonly shown in HRECs under HG conditions (16). We thereby investigated whether
TLR4/NF-κBp65 signaling pathway was involved in protective of
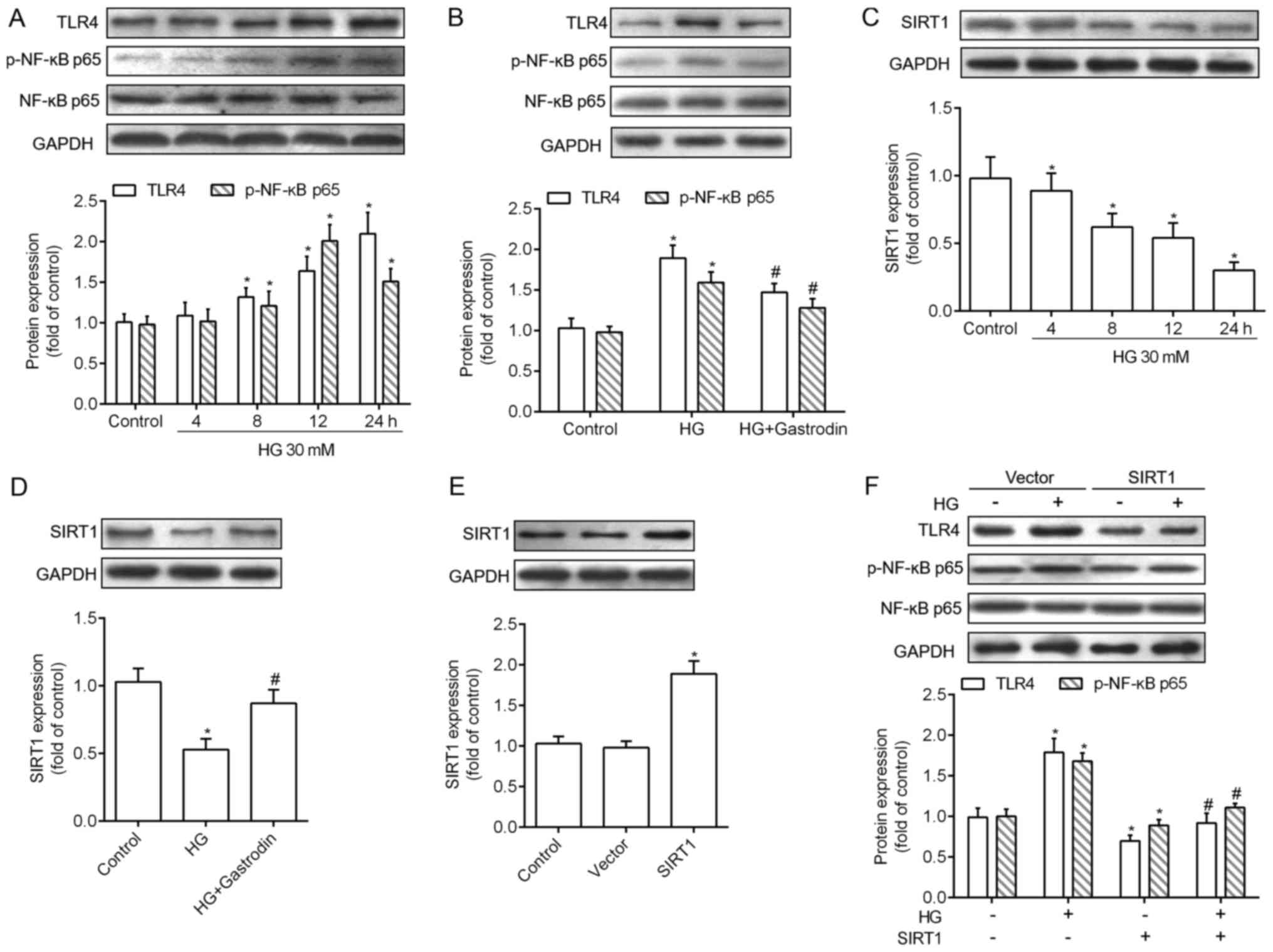
gastrodin on HRECs. As shown in Fig.
4A, HG inhibited the increase in TLR4 protein expression level
and phosphorylation of NF-κB in a concentration dependent manner.
Whereas, 100 µM gastrodin inhibited the activation of TLR4/NF-κBP65
induced by HG (Fig. 4B). In
addition, emerging evidence suggested that SIRT1 appears to target
numerous cellular factors to regulate oxidative stress and
apoptosis resulting in improving diabetic retinopathy. Fig. 4C revealed that the expression level
of SIRT1 protein was decreased in a HG concentration-dependent
manner. However, when the HRECs were co-treated with gastrodin with
HG, SIRT1 protein expression was significantly increased as
compared with that treated with HG along (Fig. 4D). Moreover, overexpression of
SIRT1 was then conducted to further validate the pathway involved.
As a result, SIRT1 was increased by pcDNA3.1-SIRT1 but no
significant change with HG or pcDNA3.1-vector treatment were
observed (Fig. 4E). Compared with
vector group with no treatment, TLR4/NF-κBP65 pathway was inhibited
after SIRT1 overexpressed. Besides, overexpression of SIRT1 at
least partly reversed TLR4/NF-κBP65 activation induced by HG in
HRECs in the vector group (Fig.
4F).
Discussion
Diabetic retinopathy (DR) is one of the main
microvascular complications of diabetes mellitus and one of the
leading causes of blindness worldwide (17). A large body of evidences showed
that inhibition of HRECs apoptosis may have protective effects
against DR (18,19). In this study, we demonstrated that
interference of gastrodin counteracted HG-induced oxidative
stress-mediated apoptosis of HRECs.
Gastrodin, which is also known a 4-Hydroxybenzyl
alcohol 4-O-beta-D-glucopyranoside, was widely used clinically as
an anticonvulsant, an analgesic, and a sedative that was effective
against vertigo, general paralysis, epilepsy and tetanus (20,21).
It is available in the market in the form of tablets, capsules and
injections. Gastrodin can be absorbed sufficiently and rapidly in
the intestine (22), which was
facilitated by SGLT1 (23). A
study identified that administration of gastrodin effectively
attenuated the allodynia and hyperalgesia-related to the
experimental diabetes by reciprocal regulation of sodium and
potassium currents in small dorsal root ganglion neurons (24). Researchers also indicated that
gastrodin could restrain the hypoxia-induced calcium ion and nitric
oxide increase in cultured rat hippocampal neurons (25). Besides, gastrodin was found to
decrease cell apoptosis and reduce the release of inflammatory
factors (10). Similarly, the
anti-apoptosis effect of gastrodin was observed in our study in
HRECs stimulated by HG. In mitochondria-dependent apoptosis, the
released cytochrome c activates caspase-9 and sequentially
activates the downstream effector caspase-3 (26). Antiapoptotic Bcl-2 protein inhibits
the release of cytochrome c, while proapoptotic Bax enhances the
progression of apoptosis (27).
Similar with previous studies (10,28),
we showed that HG-mediated reduction of the Bcl-2/Bax ratio was
increased by the treatment with gastrodin in HRECs. In addition,
HG-induced activation of the caspase 3 was suppressed by gastrodin.
The underlying molecular mechanisms of gastrodin-mediated
protection of HG-indcued apoptosis were then investigated.
To date, mitochondrial production of ROS in response
to HG may also be a key initiating step in the pathogenesis of DR
(29). Our results showed that HG
stimulation caused significant increase in the production of ROS in
HRECs and the involvement of transcription levels of the oxidative
stress associated genes was confirmed. Due to the anti-oxidant and
anti-inflammation activities of gastrodin (12), we found treatment of HG exposed
HRECs with gastrodin could effectively HG-stimulated oxidative
stress. These clarified that gastrodin protected HRECs from
HG-induced cell apoptosis via inhibition of oxidative stress
related pathway.
It is known that Toll-like receptor 4 (TLR4)
signaling pathway participates in the induction of several
immune-related diseases (30) and
is a major contributor of inflammation including TNF-α and IL-1.
MyD88 is a common signaling molecule for all TLRs, leading to
downstream activation of nuclear factor-kappa beta (NF-κB)
(31). Studies revealed that TLR4
is involved in the activity of late endothelial progenitor cells
(32) and was upregulated in the
retina of DR rats and in HRECs cultured in HG (33). The TLR4/NF-κB signaling pathway was
activated in retinal ganglion cells under high glucose and TLR4
inhibition suppressed HG-induced apoptosis (34). Consist with previous studies, the
activation of TLR4/NF-κB pathway was observed with the treatment of
HG in a dose-dependent manner. Moreover, we identified that SIRT1
may act as a regulator of TLR4/NF-κB pathway in HG-induced HRECs
in vitro. A recent study demonstrated that knockdown of
SIRT1 markedly augmented the protein expression of TLR4 and
p-NF-κBp65 in renal inner medullary collecting duct cells treated
with lipopolysaccharide (35). In
this study, we found SIRT1 protein expression was decreased by HG
in a dose-dependent manner and restored SIRT1 significantly caused
inhibition of TLR4/NF-κB pathway in HRECs under HG. The role of
SIRT1 in diabetic retinopathy had been widely investigated.
Hyperglycemia reduced the expression of SIRT1 and restored SIRT1
leading to decreased apoptosis, inflammation, oxidative stress and
mitochondrial damage and protects against DR (36). Although a study had identified that
SIRT1 could suppress NF-κB to reduce inflammatory responses and
inhibit cell apoptosis (15), the
exact molecular mechanism is still unclear. We firstly determined
the key role of TLR4 in regulating NF-κB signaling under the
inhibition of SIRT1 in HG-treated HRECs. We demonstrated that
SIRT1/TLR4/NF-κB pathway may be involved in the protective effects
of gastrodin on HG-induced. However, the effects and the mechanism
of gastrodin on HG-induced impairment of retinal angiogenesis
should be further explored in vitro and in vivo.
Additionally, recent research has demonstrated that
DR is not only a microvascular disease but may be a result of
neurodegenerative processes (37).
As the neuroprotective effect of gastrodin had been widely accepted
and a recent study found it could also exert a neuroprotective
effect on retinal ganglion cells via inhibiting microglia
activation and microglial-mediated neuroinflammation (38), we believe gastrodin might be
developed as potential candidate for the treatment of DR for both
microvascular dysfunction and neurodegeneration.
In summary, this study demonstrated that gastrodin
effectively attenuated HG-induced oxidative stress and associated
apoptosis regulation of SIRT1/TLR4/NF-κBP65 signaling pathway.
These findings identified that gastrodin may be an avenue during
the treatment of DR.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
QJ designed the study; CMH and XG performed the
research on cell viability and apoptotic analysis; LLH performed
the ROS-associated analysis; and THZ and JW performed the western
blot analysis. XG performed statistical analysis, and THZ and JW
prepared the manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
DR
|
diabetic retinopathy
|
|
HG
|
high glucose
|
|
HRECs
|
human retinal endothelial cells
|
|
ROS
|
reactive oxygen species
|
|
TLR4
|
Toll-like receptor 4
|
|
NF-κB
|
nuclear factor-κB
|
References
|
1
|
Sabanayagam C, Yip W, Ting DS, Tan G and
Wong TY: Ten emerging trends in the epidemiology of diabetic
retinopathy. Ophthalmic Epidemiol. 23:209–222. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
ADVANCE Collaborative Group, . Patel A,
MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M,
Cooper M, Glasziou P, et al: Intensive blood glucose control and
vascular outcomes in patients with type 2 diabetes. N Engl J Med.
358:2560–2572. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zoungas S, Arima H, Gerstein HC, Holman
RR, Woodward M, Reaven P, Hayward RA, Craven T, Coleman RL and
Chalmers J: Collaborators on Trials of Lowering Glucose (CONTROL)
group: Effects of intensive glucose control on microvascular
outcomes in patients with type 2 diabetes: A meta-analysis of
individual participant data from randomised controlled trials.
Lancet Diabetes Endocrinol. 5:431–437. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Geraldes P, Hiraoka-Yamamoto J, Matsumoto
M, Clermont A, Leitges M, Marette A, Aiello LP, Kern TS and King
GL: Activation of PKC-delta and SHP-1 by hyperglycemia causes
vascular cell apoptosis and diabetic retinopathy. Nat Med.
15:1298–1306. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Roy S, Kern TS, Song B and Stuebe C:
Mechanistic insights into pathological changes in the diabetic
retina: Implications for targeting diabetic retinopathy. Am J
Pathol. 187:9–19. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kowluru RA, Tang J and Kern TS:
Abnormalities of retinal metabolism in diabetes and experimental
galactosemia. VII. Effect of long-term administration of
antioxidants on the development of retinopathy. Diabetes.
50:1938–1942. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hou B, He S, Gong Y and Li Z: Effect of
obtusifolin administration on retinal capillary cell death and the
development of retinopathy in diabetic rats. Cell Biochem Biophys.
70:1655–1661. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gao J, Zheng Z, Gu Q, Chen X, Liu X and Xu
X: Deacetylation of MnSOD by PARP-regulated SIRT3 protects retinal
capillary endothelial cells from hyperglycemia-induced damage.
Biochem Biophys Res Commun. 472:425–431. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu GD, Xu C, Feng L and Wang F: The
augmentation of O-GlcNAcylation reduces glyoxal-induced cell injury
by attenuating oxidative stress in human retinal microvascular
endothelial cells. Int J Mol Med. 36:1019–1027. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen J, Gu YT, Xie JJ, Wu CC, Xuan J, Guo
WJ, Yan YZ, Chen L, Wu YS, Zhang XL, et al: Gastrodin reduces
IL-1β-induced apoptosis, inflammation and matrix catabolism in
osteoarthritis chondrocytes and attenuates rat cartilage
degeneration in vivo. Biomed Pharmacother. 97:642–651. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang Z, Zhou J, Song D, Sun Y, Liao C and
Jiang X: Gastrodin protects against LPS-induced acute lung injury
by activating Nrf2 signaling pathway. Oncotarget. 8:32147–32156.
2017.PubMed/NCBI
|
|
12
|
Peng Z, Wang S, Chen G, Cai M, Liu R, Deng
J, Liu J, Zhang T, Tan Q and Hai C: Gastrodin alleviates cerebral
ischemic damage in mice by improving anti-oxidant and
anti-inflammation activities and inhibiting apoptosis pathway.
Neurochem Res. 40:661–673. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jia J, Shi X, Jing X, Li J, Gao J, Liu M,
Lin CI, Guo X and Hua Q: BCL6 mediates the effects of Gastrodin on
promoting M2-like macrophage polarization and protecting against
oxidative stress-induced apoptosis and cell death in macrophages.
Biochem Biophys Res Commun. 486:458–464. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li Q, Niu C, Zhang X and Dong M: Gastrodin
and isorhynchophylline synergistically inhibit MPP (+)-induced
oxidative stress in SH-SY5Y cells by targeting ERK1/2 and GSK-3β
pathways: Involvement of Nrf2 nuclear translocation. ACS Chem
Neurosci. 2017.doi: 10.1021/acschemneuro.7b00247.
|
|
15
|
Zhao S, Li T, Li J, Lu Q, Han C, Wang N,
Qiu Q, Cao H, Xu X and Chen H: miR-23b-3p induces the cellular
metabolic memory of high glucose in diabetic retinopathy through a
SIRT1-dependent signalling pathway. Diabetologia. 59:644–654. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang L, Wang J, Fang J, Zhou H, Liu X and
Su SB: High glucose induces and activates Toll-like receptor 4 in
endothelial cells of diabetic retinopathy. Diabetol Metab Syndr.
7:892015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang SY, Andrews CA, Herman WH, Gardner TW
and Stein JD: Incidence and risk factors for developing diabetic
retinopathy among youths with type 1 or Type 2 diabetes throughout
the united states. Ophthalmology. 124:424–430. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Luo DW, Zheng Z, Wang H, Fan Y, Chen F,
Sun Y, Wang WJ, Sun T and Xu X: UPP mediated diabetic retinopathy
via ROS/PARP and NF-κB inflammatory factor pathways. Curr Mol Med.
15:790–799. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shao J, Yin Y, Yin X, Ji L, Xin Y, Zou J
and Yao Y: Transthyretin exerts pro-apoptotic effects in human
retinal microvascular endothelial cells through a GRP78-dependent
pathway in diabetic retinopathy. Cell Physiol Biochem. 43:788–800.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ojemann LM, Nelson WL, Shin DS, Rowe AO
and Buchanan RA: Tian ma, an ancient Chinese herb, offers new
options for the treatment of epilepsy and other conditions.
Epilepsy Behav. 8:376–383. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xu J and Guo S: Retrospect on the research
of the cultivation of Gastrodia elata Bl, a rare traditional
Chinese medicine. Chin Med J. 113:686–692. 2000.PubMed/NCBI
|
|
22
|
Hua W, Zhu Y and Zhang Q: Determination of
gastrodin in human plasma by LC-MS/MS and its application in
pharmacokinetic study. Chin J Mod Appl Phar. 2010.
|
|
23
|
Cai Z, Huang J, Luo H, Lei X, Yang Z, Mai
Y and Liu Z: Role of glucose transporters in the intestinal
absorption of gastrodin, a highly water-soluble drug with good oral
bioavailability. J Drug Target. 21:574–580. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sun W, Miao B, Wang XC, Duan JH, Ye X, Han
WJ, Wang WT, Luo C and Hu SJ: Gastrodin inhibits allodynia and
hyperalgesia in painful diabetic neuropathy rats by decreasing
excitability of nociceptive primary sensory neurons. PLoS One.
7:e396472012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zeng X, Zhang S, Zhang L, Zhang K and
Zheng X: A study of the neuroprotective effect of the phenolic
glucoside gastrodin during cerebral ischemia in vivo and in vitro.
Planta Med. 72:1359–1365. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Luna-Vargas MP and Chipuk JE:
Physiological and pharmacological control of BAK, BAX and beyond.
Trends Cell Biol. 26:906–917. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vander Heiden MG and Thompson CB: Bcl-2
proteins: regulators of apoptosis or of mitochondrial homeostasis?
Nat Cell Biol. 1:E209–E216. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li M and Qian S: Gastrodin protects neural
progenitor cells against amyloid β (1–42)-induced neurotoxicity and
improves hippocampal neurogenesis in amyloid β (1–42)-injected
mice. J Mol Neurosci. 60:21–32. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li J, Yu S, Ying J, Shi T and Wang P:
Resveratrol prevents ROS-induced apoptosis in high glucose-treated
retinal capillary endothelial cells via the activation of
AMPK/Sirt1/PGC-1α pathway. Oxid Med Cell Longev. 2017:75846912017.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Babazada H, Yamashita F and Hashida M:
Suppression of experimental arthritis with self-assembling
glycol-split heparin nanoparticles via inhibition of TLR4-NF-κB
signaling. J Control Release. 194:295–300. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Takeda K and Akira S: TLR signaling
pathways. Semin Immunol. 16:3–9. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yu M, Wang C, Zeng G, Zeng G, Zhou L, Chen
T, Tan X and Wang Y: Tolllike receptor 4 is expressed and
functional in late endothelial progenitor cells. Mol Med Rep.
16:5549–5554. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Berger EA, Carion TW, Jiang Y, Liu L,
Chahine A, Walker RJ and Steinle JJ: β-adrenergic receptor agonist,
compound 49b, inhibits TLR4 signaling pathway in diabetic retina.
Immunol Cell Biol. 94:656–661. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hu L, Yang H, Ai M and Jiang S: Inhibition
of TLR4 alleviates the inflammation and apoptosis of retinal
ganglion cells in high glucose. Graefes Arch Clin Exp Ophthalmol.
255:2199–2210. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lin QQ, Geng YW, Jiang ZW and Tian ZJ:
SIRT1 regulates lipopolysaccharide-induced CD40 expression in renal
medullary collecting duct cells by suppressing the TLR4-NF-κB
signaling pathway. Life Sci. 170:100–107. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Karbasforooshan H and Karimi G: The role
of SIRT1 in diabetic retinopathy. Biomed Pharmacother. 97:190–194.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zorrilla-Zubilete MA, Yeste A, Quintana
FJ, Toiber D, Mostoslavsky R and Silberman DM: Epigenetic control
of early neurodegenerative events in diabetic retinopathy by the
histone deacetylase SIRT6. J Neurochem. 144:128–138. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang JW, Liu YM, Zhao XF and Zhang H:
Gastrodin protects retinal ganglion cells through inhibiting
microglial-mediated neuroinflammation in an acute ocular
hypertension model. Int J Ophthalmol. 10:1483–1489. 2017.PubMed/NCBI
|