Introduction
Many experiments have confirmed that transplantation
of bone marrow mesenchymal stem cells (BMSCs) can improve damaged
cardiac function after acute myocardial infarction (AMI) (1). However due to local inflammation,
ischemia, hypoxia, and other factors, the homing and survival rates
of BMSCs after transplantation are still very low (2). Therefore, in order for stem cell
therapy to be effective, it is very important to increase the
homing and survival rates of BMSCs (3).
Tenascin-C (TN-C) is an extracellular matrix
glycoprotein, which is closely associated with inflammation and
tissue injury (4). TN-C is
detected at low levels in the healthy adult heart, but is more
highly expressed under various pathological conditions, such as
AMI, myocardial hibernation, myocarditis, and dilated
cardiomyopathy. TN-C is secreted by the interstitial cells
surrounding the infarcted myocardium in response to adverse
conditions, such as ischemia, hypoxia, and other factors, and is
involved in injury repair and formation of myocardial fibrosis.
TN-C can thus be used as an independent predictor of left
ventricular remodeling and long-term prognosis (5–7).
Apoptosis is a form of cell death that is generally
triggered by normal, healthy processes in the body. Many factors,
such as inflammation and injury, can increase the apoptotic rate of
cells, which causes harm to the repair of tissue damage. As a
result, excessive apoptosis should be inhibited (8–10).
To date, 11 human and 13 mouse Toll-like receptors
(TLRs) have been identified. Recent studies indicate that BMSCs
express functional TLRs, including TLR4 (11). When activated, TLRs affect many
downstream signaling pathways, such as the mitogen-activated
protein kinase (MAPK), AKT, and Wnt signaling pathways. These
signaling pathways play important roles in the apoptosis,
proliferation, and differentiation of many cells (12–17).
However, it is yet unclear whether TN-C binds to TLR4 on the
surface of BMSCs and activates some downstream signaling pathways,
resulting in its biological effects.
We hypothesized that in the simulated AMI
microenvironment, TN-C promotes the migration, proliferation, and
differentiation of BMSCs via TLR4-mediated signaling pathways, such
as MAPK, AKT, and Wnt. This study was designed to investigate the
effects of TN-C on BMSCs and elucidate its underlying mechanisms
in vitro.
Materials and methods
Animals and preparation of BMSCs
C57BL/6 mice were originally purchased from the
Jackson Laboratory (Bar Harbor, ME, USA). Colonies were
subsequently established by in-house breeding at the Laboratory
Animal Center of Dalian Medical University. The mice (the
experimental mice as well as the breeding pairs) were all housed in
a specific pathogen-free animal facility. Adult C57BL/6 male mice
(weighing 20–30 g) were obtained from the Laboratory Animal Center
of Dalian Medical University. Primary cells from C57BL/6 mice were
isolated, cultured, and passaged according to a previously
described method, with some modifications (18,19).
Cells at passage F3-F4 were harvested at densities of 1 to
2×106 cells/ml and used for subsequent experiments.
The study was reviewed and approved by the
Institutional Ethics Committee on Animal Resources of Dalian
Medical University, and conformed to the guiding principles of the
‘Guide for the Care and Use of Laboratory Animals’ (NIH publication
no. 83-23, revised 1996).
Flow cytometry
After digestion with 2.5 g/l trypsin, the cells were
washed, resuspended (1×106 cells/ml), and incubated for
30 min at 37°C with monoclonal antibodies against CD29, CD44, CD34,
and CD45. The cells were then centrifuged at 1,000 × g for 10 min,
washed three times with phosphate buffered saline (PBS), and
incubated for 30 min with the corresponding FITC-labeled secondary
antibody (1 mg/ml). Homologous IgG and PBS were used as negative
controls. Expression levels of the cell surface markers were
analyzed by flow cytometry.
Cell Counting Kit-8 (CCK-8) assay
BMSCs were seeded at 5×104 cells/ml (100
µl/well) into 96-well plates and treated with different
concentrations of TN-C (0, 0.1, 1, 10, 50, 100, and 150 µg/ml) for
48 h. The cell number was measured using a CCK-8 proliferation
assay kit (Dojindo Molecular Technologies, Inc., Kumamoto, Japan)
following the manufacturer's instructions. The absorbance (optical
density, OD) at 450 nm, representing the survival/proliferation of
BMSCs, was determined using a microplate reader.
BMSCs were seeded at 5×104 cells/ml (100
µl/well) into 96-well plates and treated with different
concentrations of H2O2 (60 or 90 µmol/ml) and
TN-C (0, 1, 10, 50, 100, or 150 µg/ml) for 48 h. Altogether, there
were 13 experimental groups that were treated with different
concentrations of H2O2 and TN-C:
H2O2 0 µmol/ml, TN-C 0 µg/ml;
H2O2 60 µmol/ml, TN-C 0 µg/ml;
H2O2 60 µmol/ml, TN-C 1 µg/ml;
H2O2 60 µmol/ml, TN-C 10 µg/ml;
H2O2 60 µmol/ml, TN-C 50 µg/ml;
H2O2 60 µmol/ml, TN-C 100 µg/ml;
H2O2 60 µmol/ml, TN-C 150 µg/ml;
H2O2 90 µmol/ml, TN-C 0 µg/ml;
H2O2 90 µmol/ml, TN-C 1 µg/ml;
H2O2 90 µmol/ml, TN-C 10 µg/ml;
H2O2 90 µmol/ml, TN-C 50 µg/ml;
H2O2 90 µmol/ml, TN-C 100 µg/ml; and
H2O2 90 µmol/ml, TN-C 150 µg/ml. The cell
number was analyzed as described above. BMSCs pretreated with 1 µM
TAK-242 (inhibitor of TLR4) were cultured with 60–90 µmol/ml
H2O2 and 100–150 µg/ml TN-C for 48 h in a
cell culture incubator (20). The
cell number was analyzed as described above.
Transwell® method
To investigate the effects of TN-C alone on BMSC
migration, Transwell® chambers with 8 µm pores were
obtained from Corning Incorporated, (Corning, NY, USA). Pelleted
BMSCs were resuspended in Dulbecco's modified Eagle's medium (DMEM)
at a concentration of 3×105 cells/ml, and then seeded
into the upper chambers of the 24-well plate. The lower chambers
were filled with 500 µl DMEM at different final concentrations of
TN-C (0, 0.1, 1, 10, 50, 100, and 150 µg/ml). Cells were then
incubated for 12 h. At the end of the experiment, cells that
migrated to the reverse side of the Transwell® membrane
were fixed with 4% paraformaldehyde, stained with 0.5% crystal
violet solution, and then counted under a light microscope at
magnification, ×100. An average of six visual fields was
examined.
To investigate the effects of TN-C and
H2O2 on BMSC migration, pelleted BMSCs were
resuspended in DMEM as described above, and then seeded into the
upper chambers of a 24-well plate. The lower chambers were filled
with 500 µl DMEM supplemented with different final concentrations
of H2O2 and TN-C (H2O2
0 µmol/ml, TN-C 0 µg/ml; H2O2 60 µmol/ml,
TN-C 0 µg/ml; H2O2 60 µmol/ml, TN-C 10 µg/ml;
H2O2 60 µmol/ml, TN-C 50 µg/ml;
H2O2 60 µmol/ml, TN-C 100 µg/ml;
H2O2 90 µmol/ml, TN-C 0 µg/ml;
H2O2 90 µmol/ml, TN-C 10 µg/ml;
H2O2 90 µmol/ml, TN-C 50 µg/ml; and
H2O2 90 µmol/ml, TN-C 100 µg/ml) and
incubated for 12 h. At the end of the experiment, cells that
migrated to the reverse side of the Transwell® membrane
were fixed with 4% paraformaldehyde, stained with 0.5% crystal
violet solution, and then counted under a light microscope at
magnification, ×100. An average of six visual fields was examined.
BMSCs pretreated with 1 µM TAK-242 for 2 h were treated with 90
µmol/ml H2O2 and 10–100 µg/ml TN-C for 12 h,
and then treated as described above. All the above experiments were
repeated at least three times independently.
ICC/IF staining
Pelleted BMSCs were resuspended in DMEM at a
concentration of 1×105 cells/ml. Ten pieces of 10 mm
cell sheets were disinfected with 75% alcohol, air-dried, and
placed into a sterile 12-well plate. Then, 100 µl of the above BMSC
suspension was directly seeded onto each piece of cell sheet, and
cultured for 2 h under standard cell culture conditions. Following
that, 1 ml DMEM was added to each well, and the cells were cultured
for another 24 h. DMEM with different final concentrations of TN-C
(0, 0.1, 1, 10, 50, 100, and 150 µg/ml) was then added to the cell
sheets and they were returned to culture, with the culture medium
replaced every 3 days. After 3 weeks, the cell sheet slices were
fixed with 4% paraformaldehyde, permeabilized with 0.1% Triton
X-100 at room temperature, blocked with goat serum at 37°C for 1 h,
incubated for 2 h with 50 µl 1:100 primary antibody against mouse
α-actin (1 mg/ml) at 37°C, incubated for 1 h with 50 µl 1:200
fluorogenic secondary antibody (FITC-labeled, 1 mg/ml) at 37°C, and
counter-stained for 1 min with 10 µl DAPI at room temperature. Cell
differentiation was observed under a fluorescence microscope. The
nuclei were stained blue, whereas the α-actin was green.
As described above, the BMSC suspension was directly
seeded onto each piece of cell scaffold, and then returned to the
incubator for 2 h. Following that, 1 ml DMEM was added to each well
and they were cultured for 24 h. Next day, DMEM with different
final concentrations of H2O2 and TN-C, as
described in section 2.4.2, was added to the cell scaffolds, and
they were cultured and processed as described above. Finally, cell
differentiation was observed under a fluorescence microscope.
Western blotting
BMSCs were treated with different factors (60
µmol/ml H2O2; H2O2 60
µmol/l, TN-C 100 µg/ml; H2O2 60 µmol/ml, TN-C
100 µg/ml, TAK-242) for 48 h; untreated normal cells were used as
control. At the end of the treatment, the cytoplasmic proteins were
extracted using a cell lysis solution (RIPA: PMSF: Phosphatase
inhibitor=100: 1: 20), and the total protein and phosphorylated
protein levels of p38 MAPK, AKT (Ser473), and β-catenin were then
analyzed by western blot analysis as described previously (21,22).
Statistical analysis
All statistical analyses were performed using
GraphPad Prism v5.0 (GraphPad Software Inc., La Jolla, CA, USA) and
SPSS v13.0 (SPSS Inc., Chicago, IL, USA). All data are presented as
mean ± standard deviation (SD). All OD values, cell numbers, and
protein levels were compared between two groups using one-way
analysis of variance (ANOVA) with LSD analysis. P<0.05 was
considered to indicate a statistically significant difference.
Results
Identification of BMSCs
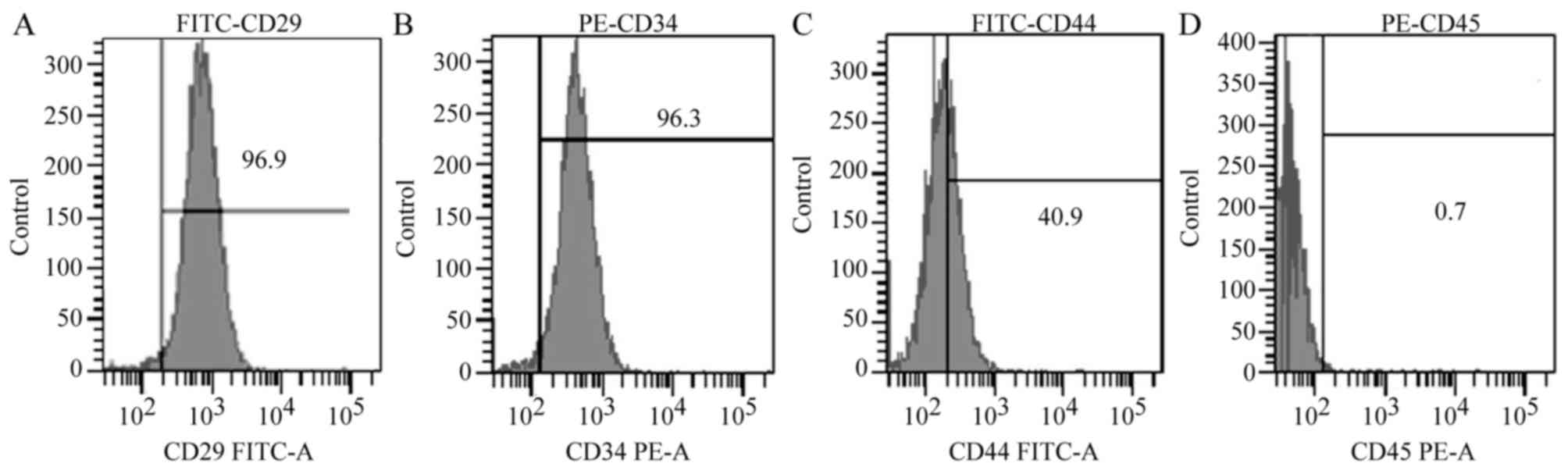
The cells obtained from the mouse bone marrow were
CD29+ (96.9%), CD34+ (96.3%),
CD44+ (40.9%), and CD45− (0.7%), which
confirmed that these cells were BMSCs (Fig. 1).
Effect of TN-C on the
survival/proliferation of BMSCs
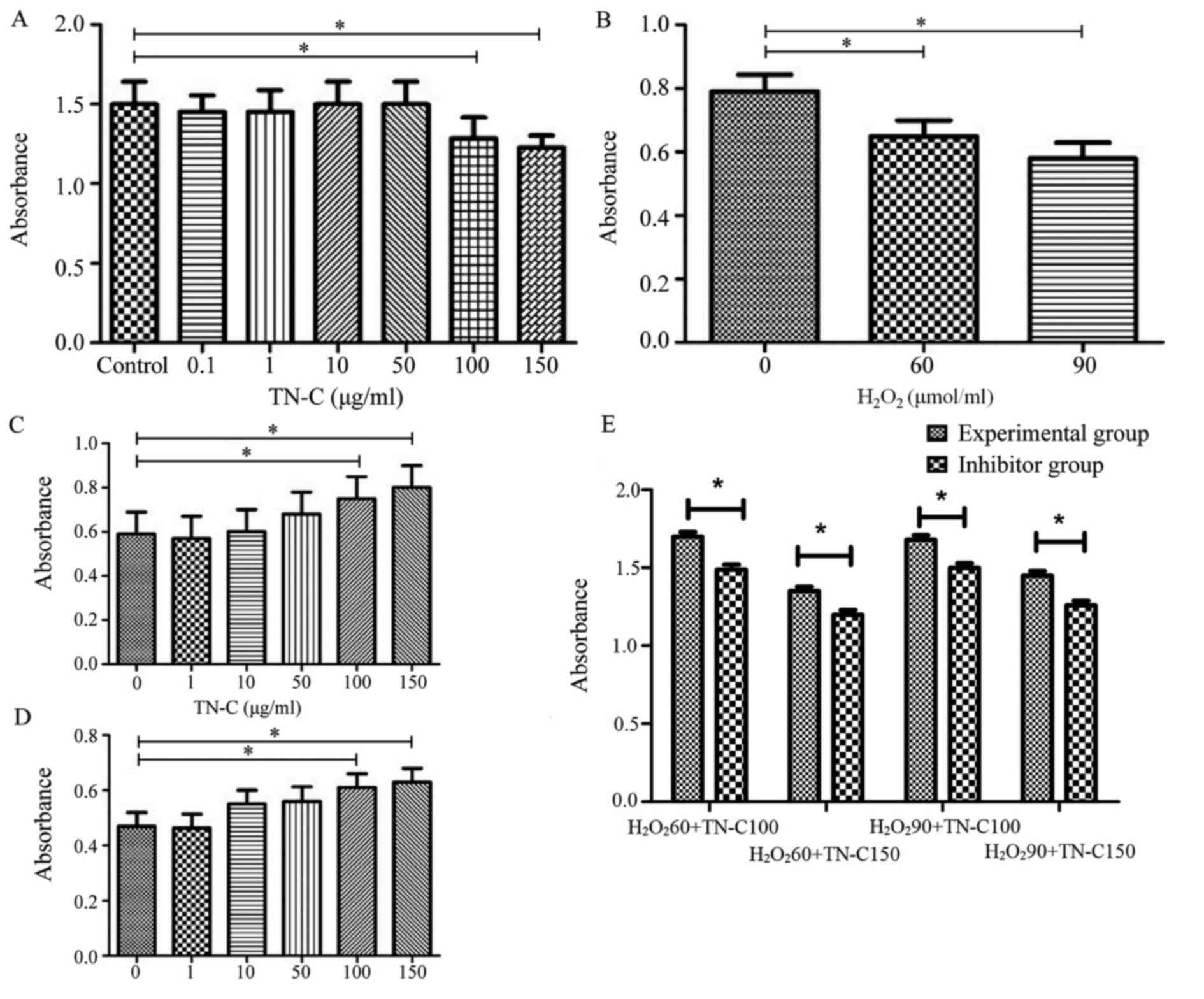
TN-C did not promote the proliferation of BMSCs: OD
values of BMSCs treated with different concentrations of TN-C (0.1,
1, 10, 50, 100, or 150 µg/ml) were no higher than those of control
BMSCs (P>0.05); however, OD values of BMSCs treated with high
concentrations of TN-C (100 or 150 µg/ml) were lower than those of
controls (P<0.05; Fig. 2A).
H2O2 reduced the survival rate
of BMSCs: OD values of BMSCs treated with either 60 or 90 µmol/ml
H2O2 were lower than those of control BMSCs
(P<0.05), which showed that 60–90 µmol/ml
H2O2 could cause BMSC death (Fig. 2B).
TN-C protected BMSCs from cell death caused by
H2O2: OD values of BMSCs treated with 60
µmol/ml H2O2 together with high
concentrations of TN-C (100 or 150 µmol/ml) were higher than those
of BMSCs treated with 60 µmol/ml H2O2 alone
(P<0.05). OD values of BMSCs treated with 90 µmol/ml
H2O2 together with high concentrations of
TN-C (100 or 150 µmol/ml) were also higher than those of BMSCs
treated with 90 µmol/ml H2O2 alone
(P<0.05; Fig. 2C and D).
TAK-242 reduced the protective effect of TN-C: OD
values of BMSCs treated with TAK-242 were less than those of normal
cells treated with the same concentrations of
H2O2 and TN-C (P<0.05; Fig. 2E).
Effect of TN-C on the migration of
BMSCs
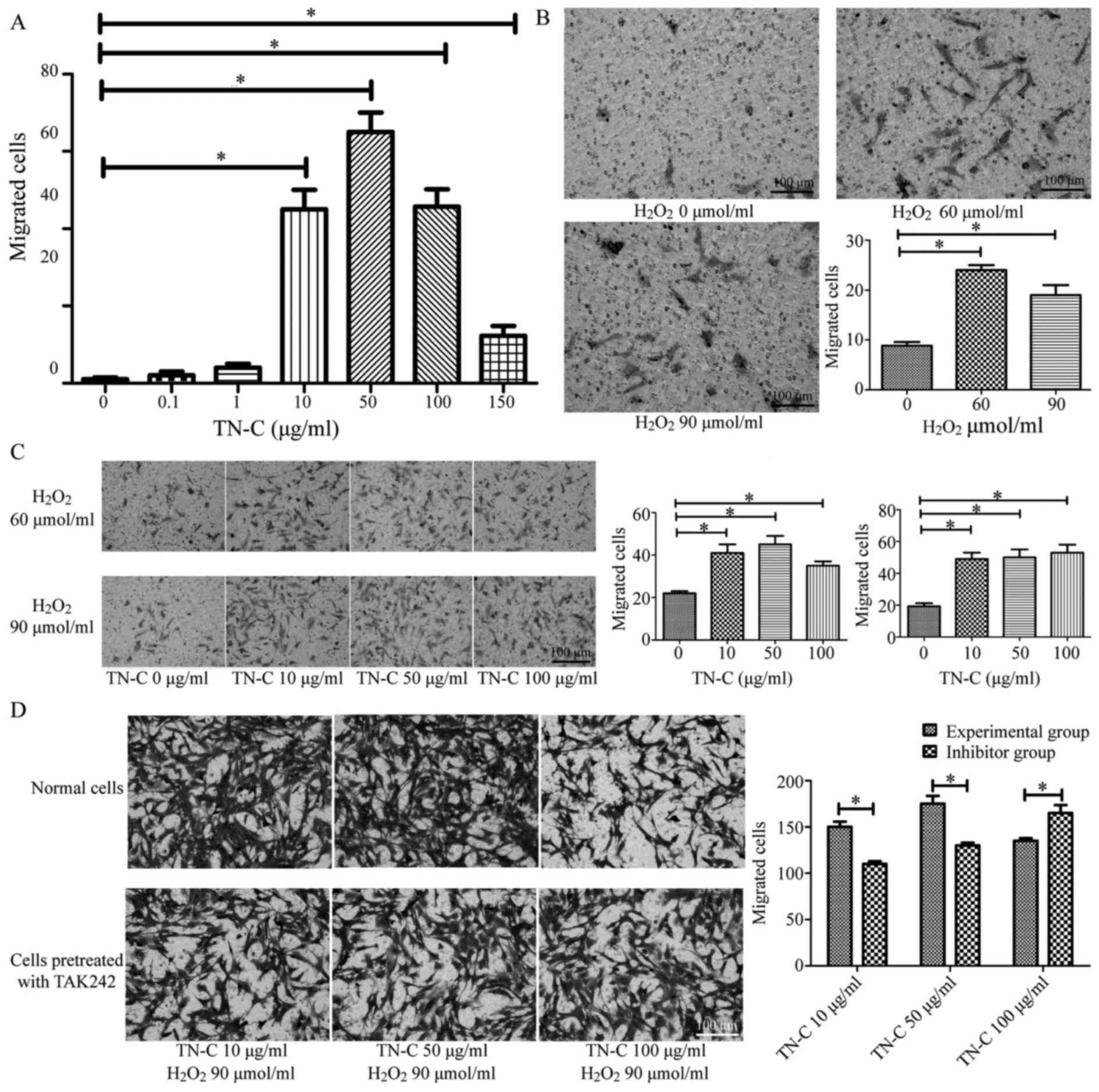
Migrated BMSCs were observed under an inverted
microscope at high magnification, ×200. Long spindle-shaped or
irregularly shaped cells stained purple with crystal violet were
the migrated BMSCs. After treatment with different factors,
including TN-C, H2O2, and TAK-242, the
migrated BMSCs were observed and counted.
High concentrations of TN-C promoted the migration
of BMSCs: High concentrations of TN-C (10–150 µg/ml) promoted the
migration of BMSCs, whereas low concentrations of TN-C (0.1–1
µg/ml) showed no significant effect (Fig. 3A).
H2O2 promoted the migration of
BMSCs: The number of migrated BMSCs in cultures treated with 60 or
90 µmol/ml H2O2 was greater than that in
control untreated cultures (P<0.05), demonstrating that
H2O2 promotes the migration of BMSCs.
However, we found no significant difference between the groups
treated with 60 or 90 µmol/ml H2O2
(P>0.05; Fig. 3B).
Combined treatment with TN-C and
H2O2 further promoted the migration of BMSCs:
When BMSCs were treated with either concentration of
H2O2 (60 or 90 µmol/ml) together with
different concentrations of TN-C (10, 50, or 100 µg/ml), increased
numbers of migrated cells were observed compared to that in
cultures treated with different concentrations of
H2O2 alone (60 or 90 µmol/ml) (P<0.05).
However, there was no significant difference among the groups
treated with different concentrations of TN-C (10, 50, or 100
µg/ml) (P>0.05; Fig. 3C).
TAK-242 reduced the migration-promoting effect of
TN-C: In cultures of BMSCs treated with 90 µmol/ml
H2O2, different concentrations of TN-C (10 or
50 µg/ml), and 1 µM TAK-242, there were fewer number of migrated
cells than in cultures treated with 90 µmol/ml
H2O2 and different concentrations of TN-C (10
or 50 µg/ml) (P<0.05). However, in cultures of BMSCs treated
with 90 µmol/ml H2O2, 100 µg/ml TN-C, and 1
µM TAK-242, the number of migrated cells was higher than that in
cultures treated only with 90 µmol/ml H2O2
and 100 µg/ml TN-C (P<0.05; Fig.
3D).
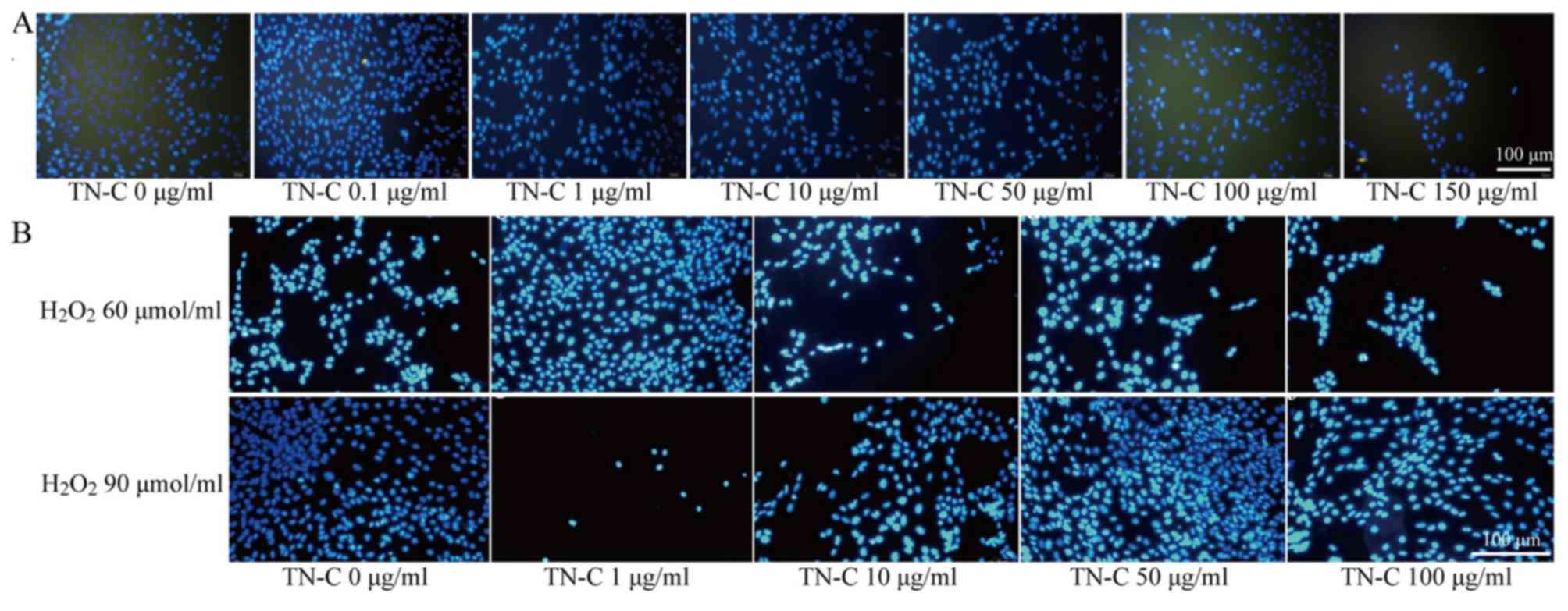
TN-C was unable to promote
differentiation of BMSCs
Staining for α-actin was negative in all culture
groups, indicating that none of the conditions tested induced the
BMSCs to differentiate into cardiomyocytes (Fig. 4A).
When the same experiment was performed in the
presence of H2O2 (60 or 90 µmol/ml) to
simulate oxidative stress in the microenvironment of AMI, TN-C was
still unable to induce the differentiation of BMSCs into
cardiomyocytes (Fig. 4B).
The effect of TN-C on MAPK, AKT, and
Wnt signaling pathways
TN-C decreased the phosphorylation levels of p38
MAPK, which were inhibited byTAK-242: The phosphorylation level of
p38 MAPK in the 60 µmol/ml H2O2 group was
higher than that in the control group (P<0.05), whereas the
phosphorylation level of p38 MAPK in the 100 µg/ml TN-C group was
lower than that in the 60 µmol/ml H2O2 group
(P<0.05). In contrast, the phosphorylation level of p38 MAPK in
the TAK-242 group was higher than that in the 100 µg/ml TN-C group
(P<0.05; Fig. 5A and B).
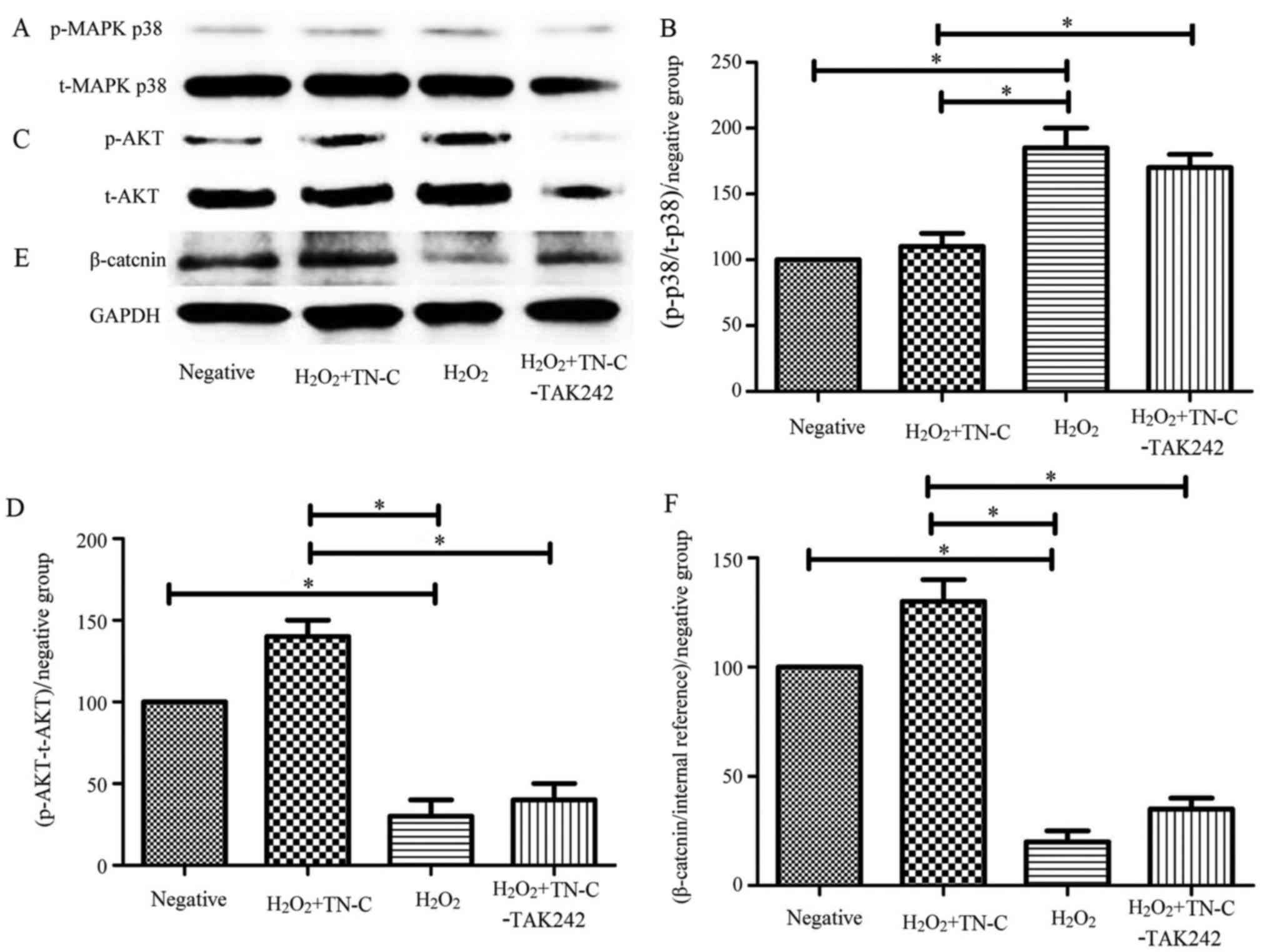 | Figure 5.Effect of TN-C on MAPK, AKT, and Wnt
signaling pathways. (A and B) TN-C reduced the phosphorylation
levels of p38 MAPK, and this effect could be inhibited by TAK-242.
(C and D) TN-C increased the phosphorylation levels of Ser473 AKT,
and this effect could be inhibited by TAK-242. (E and F) TN-C
increased the phosphorylation levels of β-catenin, and this effect
could be inhibited by TAK-242. *P<0.05, as indicated. TN-C,
Tenascin-C; BMSCs, bone marrow mesenchymal stem cells; MAPK,
mitogen-activated protein kinase; AKT, protein kinase B; p-,
phosphorylated; t-, total. |
TN-C increased the phosphorylation levels of Ser473
AKT, which were inhibited byTAK-242: The phosphorylation level of
Ser473 AKT in the 60 µmol/ml H2O2 group was
lower than that in the control group (P<0.05), whereas the
phosphorylation level of Ser473 AKT in the 100 µg/ml TN-C group was
higher than that in the 60 µmol/ml H2O2 group
(P<0.05). Furthermore, the phosphorylation level of Ser473 AKT
in the TAK-242 group was lower than that in the 100 µg/ml TN-C
group (P<0.05; Fig. 5C and
D).
TN-C increased the phosphorylation levels of
β-catenin, which were inhibited byTAK-242: The phosphorylation
level of β-catenin in the 60 µmol/ml H2O2
group was lower than that in the control group (P<0.05), whereas
the phosphorylation level of β-catenin in the 100 µg/ml TN-C group
was higher than that in the 60 µmol/ml H2O2
group (P<0.05). Furthermore, the phosphorylation level of
β-catenin in the TAK-242 group was lower than that in the 100 µg/ml
TN-C group (P<0.05; Fig. 5E and
F).
Discussion
Our results showed that TN-C acts in a
dose-dependent manner to promote the migration of BMSCs. When
H2O2 was added to the culture to simulate
oxidative stress in the cardiac microenvironment after AMI, TN-C
promoted the migration of BMSCs and protected them from cell death.
However, TN-C had no effect on promoting the proliferation or
differentiation of BMSCs. Investigation of possible signaling
mechanisms indicated that TN-C bound to TLR4 expressed on the
surface of BMSCs, and then activated the downstream signaling
pathways, including MAPK, AKT, and Wnt.
Many signaling molecules and their ligands are
involved in the migration of BMSCs to areas of damage. Among them,
stromal cell-derived factor-1 (SDF-1) is, so far, the only known
natural chemokine that can bind to and activate the CXC chemokine
receptor type 4 (CXCR4) receptor (23–25).
In rats, this interaction between SDF-1 and CXCR4 has been shown to
play a key role in the homing of BMSCs to the infarct area
(23). Here, we demonstrated that
TN-C promotes the migration of BMSCs in vitro, but it is
unclear whether TN-C still exerts the same effect in
vivo.
Our experiments showed that 60–90 µmol/ml
H2O2 causes apoptosis of BMSCs. Different
concentrations of TN-C were able to promote migration of BMSCs in
the simulated oxidative stress environment of AMI, modeled in
vitro by H2O2. However, it is unclear
whether this effect would be reproduced in vivo, where many
other factors are involved. Hence, further experiments are
needed.
TLR4 is the best-studied immune sensor, which
detects invading microbes. It is broadly distributed on cells
throughout the immune system. It has been revealed that BMSCs also
express functional TLR4 (26–33).
Activation of TLR4 signaling in BMSCs has diverse effects and is
likely to influence their survival, differentiation, proliferation,
migration, and pro-inflammatory cytokine secretion ability
(26,30,31).
After AMI, ischemia leads to the activation of TLR4/MyD88-dependent
and independent downstream pathways. Among these, activation of the
MyD88-dependent signaling pathway is thought to mediate the
response of the innate immune system, promote the rapid release of
cytokines and inflammatory mediators in the heart tissue, and
recruit inflammatory cells to the lesion sites for repair (34–36).
Lipopolysaccharide serves as a specific ligand for TLR4, activating
the TLR-4/MyD88 pathway to promote BMSC proliferation and reduce
BMSC apoptosis, which suggests that the TLR4 pathway is involved in
promoting the survival and proliferation of BMSCs (34–36).
In this study, we showed that TN-C (10 or 50 µg/ml)
promotes BMSC migration, and this effect can be reduced by
treatment with the TLR4 inhibitor, TAK-242. These results suggest
that TN-C binds to TLR4 through which it exerts its effects.
However, when the concentration of TN-C was 100 µg/ml, the
migration of BMSCs was not reduced, but was promoted. The possible
mechanism was that TN-C (100 µg/ml) could activate other receptors
on the surface of BMSCs when TLR4 was inhibited by TAK-242, which
promoted the migration of BMSCs. Further analysis of the downstream
signaling pathways showed that TN-C reduced the phosphorylation
levels of p38 MAPK, but increased the phosphorylation of both
Ser473 AKT and β-catenin, and all of these effects could be
inhibited by TAK-242. Taken together, these results demonstrate the
possible mechanism of action of TN-C, wherein TN-C binds to TLR4
expressed on the surface of BMSCs, and activates the MAPK, AKT, and
Wnt signaling pathways to exert its biological effects. This result
is consistent with that reported in a previous study (28–33).
There are many signaling pathways and proteins involved in the
action of TN-C, but in this study, only the major signaling
pathways and proteins were investigated. To further elucidate
whether TN-C exerts its effects through the TLR4-mediated signal
transduction pathways, more research is required.
There is a bottleneck in stem cell therapy due to
the low homing and survival rates of stem cells after
transplantation. Our results showed that TN-C promoted the
migration of BMSCs as well as protected them from cell death. This
study provides a new theoretical basis for improving the homing and
survival rates of transplanted cells, which is very important for
effective stem cell therapy.
When AMI occurs, a series of complex changes take
place in the microenvironment of the infarct area, and hence the
effect of H2O2 alone cannot reflect the real
situation in the body following AMI. As a result, in vivo
experiments are crucial.
When AMI occurs, a series of complex changes take
place in the microenvironment of the infarct area. Consequently,
the effect of H2O2 alone cannot reflect the
real situation in the body, and as a result, in vivo
experiments are necessary. However, addition of extracorporeal
H2O2 to simulate oxidative stress in the
microenvironment following AMI showed that TN-C reduces BMSC
apoptosis and promotes the migration of BMSCs under these
conditions. This finding provides a new theoretical basis for
animal experiments.
In summary, TN-C acts in a dose-dependent manner to
promote the migration of BMSCs in vitro. In the simulated
AMI microenvironment, TN-C promoted the migration of BMSCs and
protected them from cell death, but did not promote BMSC
proliferation or differentiation. The possible mechanism suggested
was that TN-C binds to TLR4 expressed on the surface of BMSCs, and
then activates the downstream signaling pathways, such as MAPK,
AKT, and Wnt. This study provides a new theoretical basis for
improving the homing and survival rates of transplanted cells,
which is very important for effective stem cell therapy.
Acknowledgements
The authors would like to thank Dr. Ming Tian, Dr.
Zhishuai Ye and Dr. Shengnan Zhu in the Department of Cardiology of
The First Affiliated Hospital of Dalian Medical University
(Liaoning, P.R. China) for cell isolation and culture.
Funding
The present study was supported by National Natural
Science Foundation of China (grant nos. 81100220 and 81670324) and
Liaoning Provincial Natural Science Foundation of China (grant no.
2015020295).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
Conception: HD, MJ and RH. Data curation: HD, MJ,
DL, SW, JZ and RH. Experiments: HD, MJ, DL and RH. Analysis: HD,
DL, XS and RH. Validation: HD, MJ, SW, XS and RH. Funding: RH.
Project administration: HD, MJ, DL and RH. Original draft of
manuscript: HD and MJ. Reviewing and editing of manuscript: HD and
RH.
Ethics approval and consent to
participate
The present study was reviewed and approved by the
Institutional Ethics Committee on Animal Resources of Dalian
Medical University (Liaoning, China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Tomita S, Li RK, Weisel RD, Mickle DA, Kim
EJ, Sakai T and Jia ZQ: Autologous transplantation of bone marrow
cells improves damaged heart function. Circulation. 100 19
Suppl:II247–II256. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Geng YJ: Molecular mechanisms for
cardiovascular stem cell apoptosis and growth in the hearts with
atherosclerotic coronary disease and ischemic heart failure. Ann N
Y Acad Sci. 1010:687–697. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Przybyt E and Harmsen MC: Mesenchymal stem
cells: Promising for myocardial regeneration? Curr Stem Cell Res
Ther. 8:270–277. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sato A, Aonuma K, Imanaka-Yoshida K,
Yoshida T, Isobe M, Kawase D, Kinoshita N, Yazaki Y and Hiroe M:
Serum tenascin-C might be a novel predictor of left ventricular
remodeling and prognosis after acute myocardial infarction. J Am
Coll Cardiol. 47:2319–2325. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jones FS and Jones PL: The tenascin family
of ECM glycoproteins: Structure, function, and regulation during
embryonic development and tissue remodeling. Dev Dyn. 218:235–259.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Niebroj-Dobosz I: Tenascin-C in human
cardiac pathology. Clin Chim Acta. 413:1516–1518. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sato A, Hiroe M, Akiyama D, Hikita H,
Nozato T, Hoshi T, Kimura T, Wang Z, Sakai S, Imanaka-Yoshida K, et
al: Prognostic value of serum tenascin-C levels on long-term
outcome after acute myocardial infarction. J Card Fail. 18:480–486.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Loreto C, Musumeci G and Leonardi R:
Chondrocyte-like apoptosis in temporomandibular joint disc internal
derangement as a repair-limiting mechanism. Histol Histopathol.
24:293–298. 2009.PubMed/NCBI
|
|
9
|
Musumeci G, Castrogiovanni P, Loreto C,
Castorina S, Pichler K and Weinberg AM: Post-traumatic caspase-3
expression in the adjacent areas of growth plate injury site: A
morphological study. Int J Mol Sci. 14:15767–15784. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Puzzo D, Loreto C, Giunta S, Musumeci G,
Frasca G, Podda MV, Arancio O and Palmeri A: Effect of
phosphodiesterase-5 inhibition on apoptosis and beta amyloid load
in aged mice. Neurobiol Aging. 35:520–531. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pevsner-Fischer M, Morad V, Cohen-Sfady M,
Rousso-Noori L, Zanin-Zhorov A, Cohen S, Cohen IR and Zipori D:
Toll-like receptors and their ligands control mesenchymal stem cell
functions. Blood. 109:1422–1432. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gutiérrez-Uzquiza Á, Arechederra M,
Bragado P, Aguirre-Ghiso JA and Porras A: p38α mediates cell
survival in response to oxidative stress via induction of
antioxidant genes: Effect on the p70S6K pathway. J Biol Chem.
287:2632–2642. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Peti W and Page R: Molecular basis of MAP
kinase regulation. Protein Sci. 22:1698–1710. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wee KB and Aguda BD: Akt versus p53 in a
network of oncogenes and tumor suppressor genes regulating cell
survival and death. Biophys J. 91:857–865. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pillai VB, Sundaresan NR and Gupta MP:
Regulation of Akt signaling by sirtuins: Its implication in cardiac
hypertrophy and aging. Circ Res. 114:368–378. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ling L, Nurcombe V and Cool SM: Wnt
signaling controls the fate of mesenchymal stem cells. Gene.
433:1–7. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim W, Kim M and Jho EH: Wnt/β-catenin
signalling: From plasma membrane to nucleus. Biochem J. 450:9–21.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wakitani S, Saito T and Caplan AI:
Myogenic cells derived from rat bone marrow mesenchymal stem cells
exposed to 5-azacytidine. Muscle Nerve. 18:1417–1426. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Deng W, Bivalacqua TJ, Chattergoon NN,
Jeter JR Jr and Kadowitz PJ: Engineering ex vivo-expanded marrow
stromal cells to secrete calcitonin gene-related peptide using
adenoviral vector. Stem Cells. 22:1279–1291. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hussey SE, Liang H, Costford SR, Klip A,
DeFronzo RA, Sanchez-Avila A, Ely B and Musi N: TAK-242, a
small-molecule inhibitor of Toll-like receptor 4 signalling,
unveils similarities and differences in lipopolysaccharide- and
lipid-induced inflammation and insulin resistance in muscle cells.
Biosci Rep. 33:37–47. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
He PP, Ouyang XP, Tang YY, Liao L, Wang
ZB, Lv YC, Tian GP, Zhao GJ, Huang L, Yao F, et al: MicroRNA-590
attenuates lipid accumulation and pro-inflammatory cytokine
secretion by targeting lipoprotein lipase gene in human THP-1
macrophages. Biochimie. 106:81–90. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen T, Li Z, Tu J, Zhu W, Ge J, Zheng X,
Yang L, Pan X, Yan H and Zhu J: MicroRNA-29a regulates
pro-inflammatory cytokine secretion and scavenger receptor
expression by targeting LPL in oxLDL-stimulated dendritic cells.
FEBS Lett. 585:657–663. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Abbott JD, Huang Y, Liu D, Hickey R,
Krause DS and Giordano FJ: Stromal cell-derived factor-1alpha plays
a critical role in stem cell recruitment to the heart after
myocardial infarction but is not sufficient to induce homing in the
absence of injury. Circulation. 110:3300–3305. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sterlacci W, Saker S, Huber B, Fiegl M and
Tzankov A: Expression of the CXCR4 ligand SDF-1/CXCL12 is
prognostically important for adenocarcinoma and large cell
carcinoma of the lung. Virchows Arch. 468:463–471. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bi J, Li P, Li C, He J, Wang Y, Zhang H,
Fan X, Jia R and Ge S: The SDF-1/CXCR4 chemokine axis in uveal
melanoma cell proliferation and migration. Tumour Biol.
37:4175–4182. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Raicevic G, Rouas R, Najar M, Stordeur P,
Boufker HI, Bron D, Martiat P, Goldman M, Nevessignsky MT and
Lagneaux L: Inflammation modifies the pattern and the function of
Toll-like receptors expressed by human mesenchymal stromal cells.
Hum Immunol. 71:235–244. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liotta F, Angeli R, Cosmi L, Filì L,
Manuelli C, Frosali F, Mazzinghi B, Maggi L, Pasini A, Lisi V, et
al: Toll-like receptors 3 and 4 are expressed by human bone
marrow-derived mesenchymal stem cells and can inhibit their T-cell
modulatory activity by impairing Notch signaling. Stem Cells.
26:279–289. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang ZJ, Zhang FM, Wang LS, Yao YW, Zhao Q
and Gao X: Lipopolysaccharides can protect mesenchymal stem cells
(MSCs) from oxidative stress-induced apoptosis and enhance
proliferation of MSCs via Toll-like receptor (TLR)-4 and PI3K/Akt.
Cell Biol Int. 33:665–674. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Brewster BD, Rouch JD, Wang M and Meldrum
DR: Toll-like receptor 4 ablation improves stem cell survival after
hypoxic injury. J Surg Res. 177:330–333. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Raicevic G, Najar M, Pieters K, De Bruyn
C, Meuleman N, Bron D, Toungouz M and Lagneaux L: Inflammation and
Toll-like receptor ligation differentially affect the osteogenic
potential of human mesenchymal stromal cells depending on their
tissue origin. Tissue Eng Part A. 18:1410–1418. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fiedler T, Salamon A, Adam S, Herzmann N,
Taubenheim J and Peters K: Impact of bacteria and bacterial
components on osteogenic and adipogenic differentiation of
adipose-derived mesenchymal stem cells. Exp Cell Res.
319:2883–2892. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Alvarado AG, Thiagarajan PS,
Mulkearns-Hubert EE, Silver DJ, Hale JS, Alban TJ, Turaga SM,
Jarrar A, Reizes O, Longworth MS, et al: Glioblastoma cancer stem
cells evade innate immune suppression of self-Renewal through
reduced TLR4 expression. Cell Stem Cell. 20:450–461.e4. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Goloviznina NA, Verghese SC, Yoon YM,
Taratula O, Marks DL and Kurre P: Mesenchymal stromal cell-derived
extracellular vesicles promote myeloid-biased multipotent
hematopoietic progenitor expansion via toll-like receptor
engagement. J Biol Chem. 291:24607–24617. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yao Y, Zhang F, Wang L, Zhang G, Wang Z,
Chen J and Gao X: Lipopolysaccharide preconditioning enhances the
efficacy of mesenchymal stem cells transplantation in a rat model
of acute myocardial infarction. J Biomed Sci. 16:742009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Huang C, Pan L, Lin F, Dai H and Fu R:
Monoclonal antibody against Toll-like receptor 4 attenuates
ventilator-induced lung injury in rats by inhibiting MyD88- and
NF-κB-dependent signaling. Int J Mol Med. 39:693–700. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sindhu S, Al-Roub A, Koshy M, Thomas R and
Ahmad R: Palmitate-induced MMP-9 expression in the human monocytic
cells is mediated through the TLR4-MyD88 dependent mechanism. Cell
Physiol Biochem. 39:889–900. 2016. View Article : Google Scholar : PubMed/NCBI
|