Introduction
Articular cartilage damage is one of the most common
diseases seen in the clinic, and has been a challenge in orthopedic
medicine due to the poor self-healing ability of cartilage tissue
(1). Severe cartilage damage,
initially caused by degeneration or trauma, may lead to
osteoarthritis (OA) and subsequently cause joint pain and
disability, creating a significant disease burden worldwide.
Although various conservative interventions have been developed for
the treatment of early OA, including oral nonsteroidal
anti-inflammatory drugs and intra-articular hyaluronic acid
injections, they only temporarily alleviate pain. For severe
degenerative joint diseases, surgical arthroplasty is an effective
treatment; however, it has significant postoperative complications.
Due to the potential of chondrogenic differentiation and extensive
proliferation of bone marrow mesenchymal stem cells (BMSCs),
cartilage tissue engineering is currently considered to be one of
the most promising techniques for the treatment of OA (1). To induce chondrogenic differentiation
of BMSCs, numerous growth factors are applied, which have
previously been demonstrated to enhance chondrogenesis and promote
formation of cartilage-like tissue (2–4).
However, growth factors inevitably upregulate the expression levels
of hypertrophic differentiation markers, including collagen x,
matrix metalloproteinase 13 and alkaline phosphatase (ALP), and
functionally contribute to calcification (2–5).
Additional side effects of growth factor treatment limit their
clinical use, including expense, rapid degradation and loss of
activity (6–8). One strategy for addressing these
issues is to investigate safe and low-cost drugs that may
substitute or cooperate with growth factors to promote and maintain
stable chondrogenic differentiation without hypertrophy (6,7).
Herb Epimedium (HEP) is a traditional Chinese herb
and is widely used to treat osteoporosis and OA in China, Japan and
Korea (8). Icariin
(C33H40O15; molecular weight,
676.65) is the primary pharmacologically active compound of HEP.
When chondrocytes were used as seed cells, icariin increased
cartilage extracellular matrix (ECM) synthesis, suppressed ECM
degradation, enhanced cartilage-specific gene expression including
collagen II, aggrecan and SRY-type high mobility group box 9 (SOX9)
in vitro, and additionally improved the repair of cartilage
defects and prevented cartilage degradation in vivo
(6,7,9,10).
The application of chondrocytes in cartilage tissue engineering is
prevalent, yet it faces numerous challenges, including chondrocyte
dedifferentiation, donor site morbidity and limited sources for
harvesting cartilage tissue (1).
In an attempt to overcome these challenges, our recent study
cultivated BMSCs with chondrogenic medium containing icariin in
monolayer culture for 14 days, which is a traditional
two-dimensional (2D) cell culture, and demonstrated that icariin
promoted directed chondrogenesis of BMSCs and had no effect on
hypertrophic differentiation (11). However, chondrogenic potential of
stem cells or chondrocytes cultured in a three-dimensional (3D)
microenvironment is different from 2D culture (12,13).
Thus, icariin may be a potential accelerator for cartilage tissue
engineering; however, its effects on BMSCs in a 3D microenvironment
require further investigation to elucidate its clinical
application.
To culture BMSCs in a 3D microenvironment, the
scaffold is a critical component and should mimic the structural
and functional properties of the native ECM to accommodate cells,
and additionally facilitate cell migration, proliferation and
differentiation. Self-assembling peptides are a relatively novel
class of molecules that have the ability to form stable nanofiber
hydrogels upon exposure to physiological pH and ionic strength. The
self-assembling peptide nanofiber hydrogel scaffold exhibits
excellent biocompatibility, supports the chondrocyte phenotype
(14), promotes BMSCs
proliferation and chondrogenic differentiation in vitro
(15–18), and stimulates cartilage
regeneration or improves clinical symptoms in vivo (19,20).
Therefore, a self-assembling peptide hydrogel scaffold was
considered as an ideal scaffold for 3D cell culture and cartilage
tissue engineering (21).
The present study extended our previous
investigations to observe the effect of icariin on chondrogenic
differentiation of BMSCs in a self-assembling peptide nanofiber
hydrogel scaffold. To the best of our knowledge, this is the first
report of its kind. These findings demonstrated that icariin
promotes BMSCs chondrogenesis; however, has no significant effect
on hypertrophic differentiation in a 3D microenvironment.
Materials and methods
Cell culture of rat BMSCs
OriCell™ Sprague-Dawley rat BMSCs (catalog no.
RASMX-01001) were purchased from Cyagen Biosciences Inc.
(Guangzhou, China). Cell viability, sterility, purity,
proliferation and differentiation ability were tested by the
company, which revealed that cells were highly positive for the
specific mesenchymal markers CD29 (83.99%), CD44 (99.69%) and CD90
(95.05%), and negative for the hematopoietic cell-surface markers
CD34 (0.62%), CD45 (0.28%), and CD11b (4.25%). To verify
pluripotency, BMSCs were able to differentiate into osteoblasts,
chondrocytes and adipocytes. Cells were thawed at 37°C in a water
bath and resuspended in low glucose Dulbecco's modified Eagle's
medium (LG-DMEM) supplemented with 10% fetal bovine serum, 10 U/ml
penicillin G and 10 mg/ml streptomycin, all purchased from Hyclone
(GE Healthcare Life Sciences, Logan, UT, USA). The cell suspension
was subsequently plated into T25 flasks and incubated in a 5%
carbon dioxide humidified incubator at 37°C. Upon achieving 80–90%
confluence, cells were treated with 0.25% trypsin/1 mM
ethylenediaminetetraacetic acid (Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) for 3–5 min. In order to harvest an
adequate number of cells, cells at passage 5 to 7 were used in this
study.
Hydrogel encapsulation and 3D
culture
A BeaverNano™ 3D hydrogel scaffold (catalog no.
P0030105; Cyagen Biosciences Inc.), which was a self-assembling
peptide nanofiber scaffold with pore sizes between 50 and 200 nm,
was selected because it had 3D nanofiber structures similar to
natural cartilage ECM. The molecular mode
(Ac-RADARADARADARADA-CONH2), microstructure (visualized
by scanning electron microscopy and atomic force microscopy) and
preparation of the hydrogel scaffold are detailed by the
manufacturer (Beaver Nano-Technologies Co., Ltd., Suzhou, China),
and a 0.25% concentration was applied in the present study.
Briefly, 1.0% (w/v) hydrogel stock solution was mixed with an equal
volume of 20.0% (w/v) sucrose solution. Following trypsinization,
BMSCs were centrifuged at 400 × g for 5 min at 4°C,
resuspended in 10.0% (w/v) sucrose solution, and quickly
encapsulated in an equal volume of 0.50% (w/v) concentration
peptide scaffolds to make a final concentration of
1.0×107 cells/ml. The cell density was selected to match
a previous study (15). The
cell/hydrogel mixture (300 µl) was immediately dropped onto the
bottom of each cell culture well (200 mm2/hole; EMD
Millipore, Billerica, MA, USA) and allowed to form a layer ~1.50-mm
thick. LG-DMEM medium (500 µl) was added to the top of the scaffold
to induce self-assembly. The cell/hydrogel scaffolds were incubated
in a humidified atmosphere with 5% CO2 at 37°C.
Chondrogenic differentiation
Following self-assembly, the cell/hydrogel scaffolds
were cultured in control medium without chondrogenic supplements,
or chondrogenic medium (catalog no. GUXMX-90041, Cyagen Biosciences
Inc.) containing 10 ng/ml transforming growth factor (TGF)-β3 in
the presence or absence of 1×10−6 M icariin (labeled
control, TGF-β3 + icariin or TGF-β3 groups, respectively). Icariin
(purity: 99%) was purchased from National Institute for the Control
of Pharmaceutical and Biological Products (Beijing, China).
OriCell™ MSC chondrogenic differentiation medium
contained 0.1 µM dexamethasone, 50 µg/ml ascorbate, 1%
insulin-transferrin-selenium cell culture supplement, 100 µg/ml
sodium pyruvate, 40 µg/ml proline and 10 ng/ml TGF-β3. In the
TGF-β3 + icariin group, icariin was dissolved in dimethyl sulfoxide
and subsequently added to chondrogenic differentiation medium at
concentrations of 1×10−6 M, according to our preliminary
experiments (11). The culture
medium was replaced every other day, and the morphology of BMSCs
was observed under a CKX41 inverted microscope (Olympus
Corporation, Tokyo, Japan). At each time point (days 7, 14 and 21),
the scaffold, cell lysis and culture medium were harvested for
further experiments. All experiments were performed at least three
times.
Histology and
immunohistochemistry
Cell/hydrogel scaffolds were fixed in 4%
paraformaldehyde and permeabilized in PBS containing 0.1% Triton
X-100 at room temperature for 30 min. Following blocking with 5%
bovine serum albumin (GE Healthcare Life Sciences) for 2 h, samples
were treated with the following primary antibodies: Rabbit
anti-collagen II (catalog no. GTX20300; 1:100; GeneTex, Inc.,
Irvine, CA, USA), rabbit anti-SOX9 (catalog no. ab71762; 1:200;
Abcam, Cambridge, UK) or mouse anti-collagen × (catalog no.
ab49945; 1:200; Abcam) at 4°C overnight, washed three times with
PBS, and subsequently incubated with cy3-labeled goat anti-rabbit
fluorescent secondary antibody (catalog no. A0516; 1:1,000;
Beyotime Institute of Biotechnology, Shanghai, China) or
cy3-labeled goat anti-mouse fluorescent secondary antibody (catalog
no. A0521; 1:1,000; Beyotime Institute of Biotechnology) for 2 h at
room temperature in the dark. Cell nuclei were stained with 4,
6-diamidion-2-phenylindole (catalog no. C1006; 1:1,000; Beyotime
Institute of Biotechnology) for 3 min. Subsequently, stained
samples were imaged under a Leica DM4000 B fluorescence microscope
(Leica Microsystems GmbH, Wetzlar, Germany). Additional samples
were washed three times with PBS and stained for sulfated
proteoglycans with toluidine blue dye solution (0.50%; Abcam) for 5
min, and subsequently observed under an inverted microscope
(Olympus Corporation, Tokyo, Japan).
Cell abstraction from hydrogel
scaffold and reverse transcription-quantitative polymerase chain
reaction (RT-qPCR)
At days 7, 14 and 21, BMSCs from hydrogel scaffolds
were harvested by mechanical disruption with a micropipette until
single cells were obtained. Cells were subsequently centrifuged at
400 × g for 5 min at 4°C, and the supernatant was removed,
rinsed with LG-DMEM and centrifuged again under the same
conditions. Total RNA was extracted from culture cells using an
RNAiso Plus reagent (Takara Biotechnology, Co., Ltd., Dalian,
China) following the manufacturer's protocol, and absorbance was
measured at a wavelength of 260 nm using a spectrophotometer
(DU-70; Beckman, Fullerton, CA, USA) to determine the diluted RNA
concentration. Each sample (1 µg) was reverse transcribed using a
PrimeScript™ RT reagent kit with gDNA Eraser (Takara
Biotechnology Co., Ltd.). qPCR reactions were performed using an
Applied Biosystems 7500 Fast Real-Time PCR system (Applied
Biosystems; Thermo Fisher Scientific, Inc.) and SYBR®
Premix Ex Taq™ II reagent (Takara Biotechnology Co., Ltd.). Equal
quantities of cDNA and specific primers were added to the mix, and
the following cycle parameters of PCR was used: Initial
denaturation at 94°C for 30 sec, followed by 40 cycles of
denaturation at 94°C for 5 sec, annealing at 60°C for 15 sec and
extension at 72°C for 10 sec. The sequences of forward and reverse
primers used for collagen type II α 1 (col2α1; collagen II gene),
SOX9, collagen type I α 1 (col1α1; collagen I gene) and collagen
type X α 1 (col10α1; collagen × gene) are listed in Table I. As described previously (11), mRNA expression levels were analyzed
by the 2−ΔΔCq method using the housekeeping gene
glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as an internal
control.
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Gene | Forward (5′-3′) | Reverse (5′-3′) |
|---|
| Col2α1a |
CGCCACGGTCCTACAATGTC |
GTCACCTCTGGGTCCTTGTTCAC |
| SOX9a |
GCAGAGACTGAAGACCCTACACAGA |
GAGGCAACTTCACGCTGCAA |
| Col1α1b |
GCCTCCCAGAACATCACCTA |
GCAGGGACTTCTTGAGGTTG |
| Col10α1b |
GCCAGGACCTCCAGGACTATCA |
CCCAATGTCTCCTTTCGGTCCA |
| GAPDHc |
TATGACTCTACCCACGGCAA |
|
ALP activity
ALP is one of the most common indicators of
hypertrophic differentiation, and was measured in culture
supernatants as described previously (11). At 7, 14 and 21 days, the medium was
replaced with phenol red-free DMEM supplemented with 10% fetal
bovine serum, 10 U/ml penicillin G and 10 mg/ml streptomycin (GE
Healthcare Life Sciences). Supernatants was collected after 1 day
and centrifuged at 1,400 × g for 10 min at 4°C to remove
particles. ALP activity was immediately assayed using a commercial
ALP kit (Nanjing Jiancheng Bioengineering Institute, Nanjing,
China) according to the manufacturer's protocol.
Statistical analysis
All data are expressed as mean ± standard deviation.
The differences between multiple-group comparisons were analyzed by
one-way analysis of variance followed by Tukey's post hoc test.
P<0.05 was considered to indicate a statistically significant
difference. Statistical tests were performed using SPSS software
version 16.0 (SPSS, Inc., Chicago, IL, USA).
Results
Effect of icariin on the biosynthesis
of ECM
To evaluate the effect of icariin on BMSC
chondrogenesis in self-assembling peptide nanofiber hydrogel
scaffolds, immunofluorescence was performed after 7, 14 and 21 days
of culture. Collagen II is a primary component of cartilage ECM
produced by chondrocytes. At each time point, TGF-β3 and TGF-β3 +
icariin groups were positive for collagen II staining; however, the
control medium was negative. Analysis of multiple sections of
hydrogels revealed that icariin treatment led to more intense and
uniformly distributed staining throughout the hydrogel scaffold at
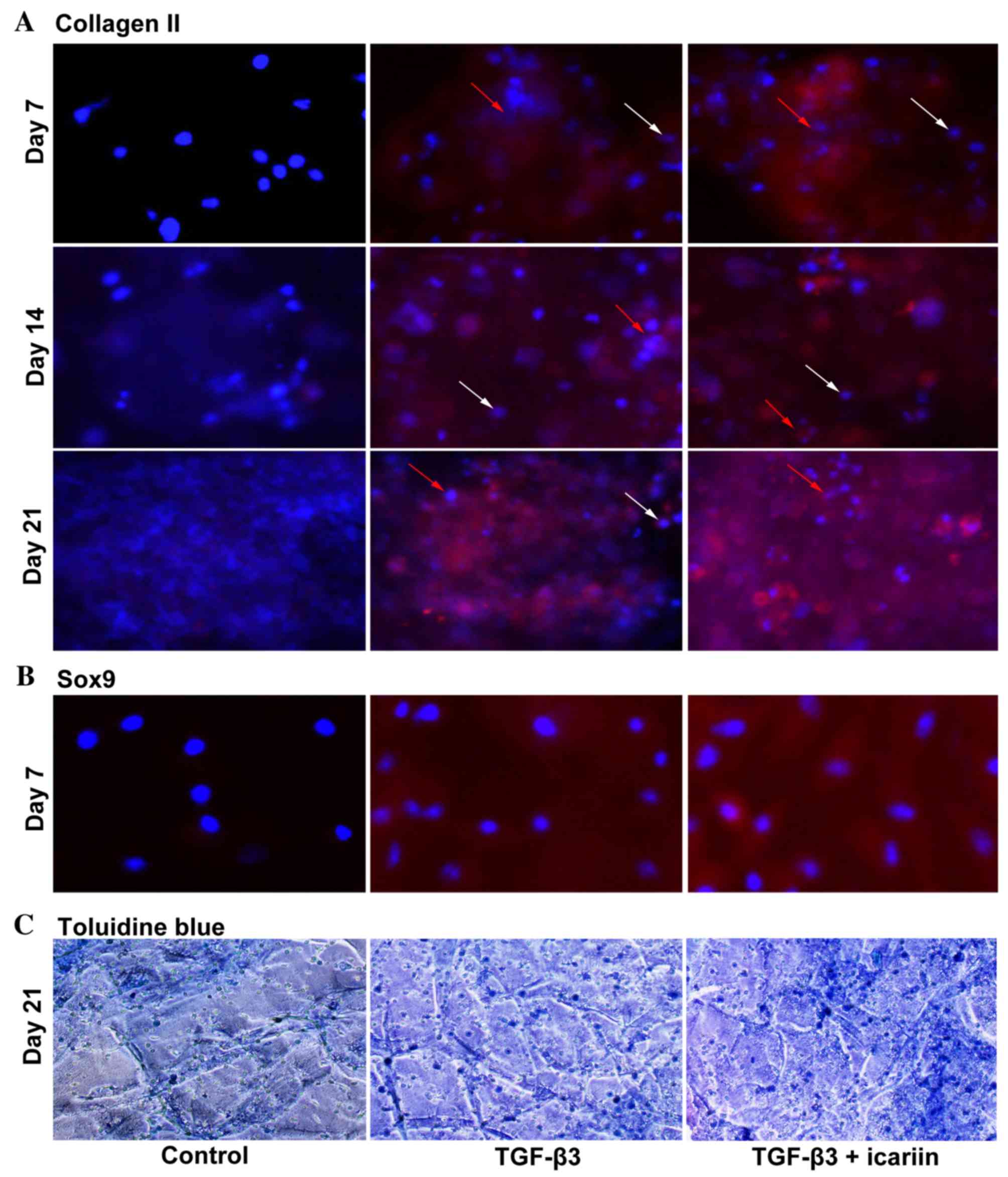
day 7 to 21 compared with the TGF-β3 group (Fig. 1A). Notably, staining was
concentrated to the cell clusters (red arrows), while little
staining was detected in single cells (white arrows).
SOX9 is considered to be an early chondrogenic
marker and serves a pivotal role in MSC chondrogenesis. The present
study stained hydrogel scaffolds with a SOX9-specific antibody
after 7 days in culture, and the results demonstrated that cells
cultured with TGF-β3 + icariin medium exhibited markedly increased
positive staining compared with cells in TGF-β3 only medium, and no
staining was present in control medium (Fig. 1B).
Toluidine blue staining of day 21 hydrogels
(Fig. 1C) was consistent with the
collagen II staining (Fig. 1A),
demonstrating that the cells were enclosed in a metachromatic
matrix. The TGF-β3 + icariin group had more proteoglycan deposition
compared with the TGF-β3 group, which was reflected by more intense
staining. In addition, chondrocyte-like rounded cells were observed
in the TGF-β3 + icariin and TGF-β3 groups.
Effect of icariin on expression levels
of chondrogenesis-specific genes
To further assess chondrogenesis of BMSCs in
self-assembling peptide nanofiber hydrogel scaffolds at a genetic
level, mRNA expression levels of the chondrogenesis-specific genes
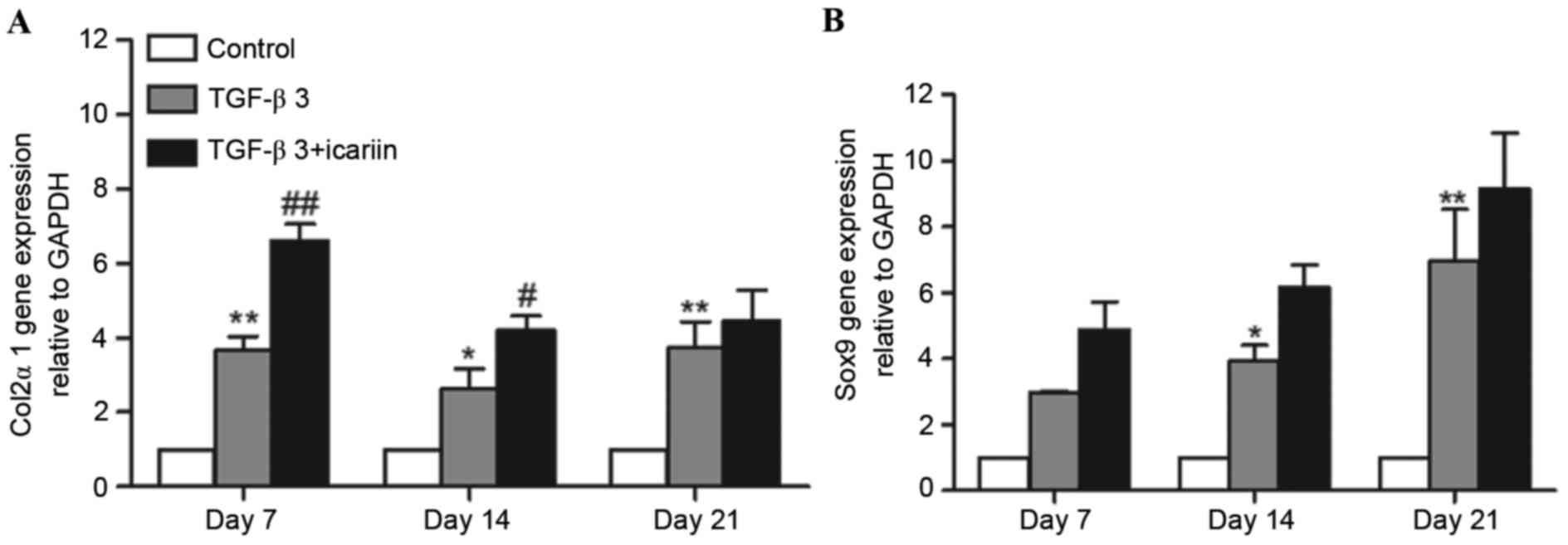
col2α1 and SOX9 were determined using RT-qPCR. Compared with the
control group, the expression levels of col2α1 and SOX9 mRNA
continued to significantly increase from days 7 to 21 in the TGF-β3
and TGF-β3 + icariin groups. Compared with the TGF-β3 group, col2α1
gene expression in the TGF-β3 + icariin group was increased by
1.80-fold (P<0.01), 1.60-fold (P<0.05) and 1.20-fold
(P>0.05) at days 7, 14 and 21, respectively (Fig. 2A). Consistent with the expression
levels of col2α1 mRNA, icariin treatment led to a 1.65-, 1.56- and
1.31-fold increase in SOX9 expression levels at days 7, 14 and 21
in comparison with the TGF-β3 group (Fig. 2B).
Effect of icariin on cell morphology
during chondrogenesis
Due to the transparency of the self-assembled
peptide nanofiber scaffold, the morphology of BMSCs encapsulated in
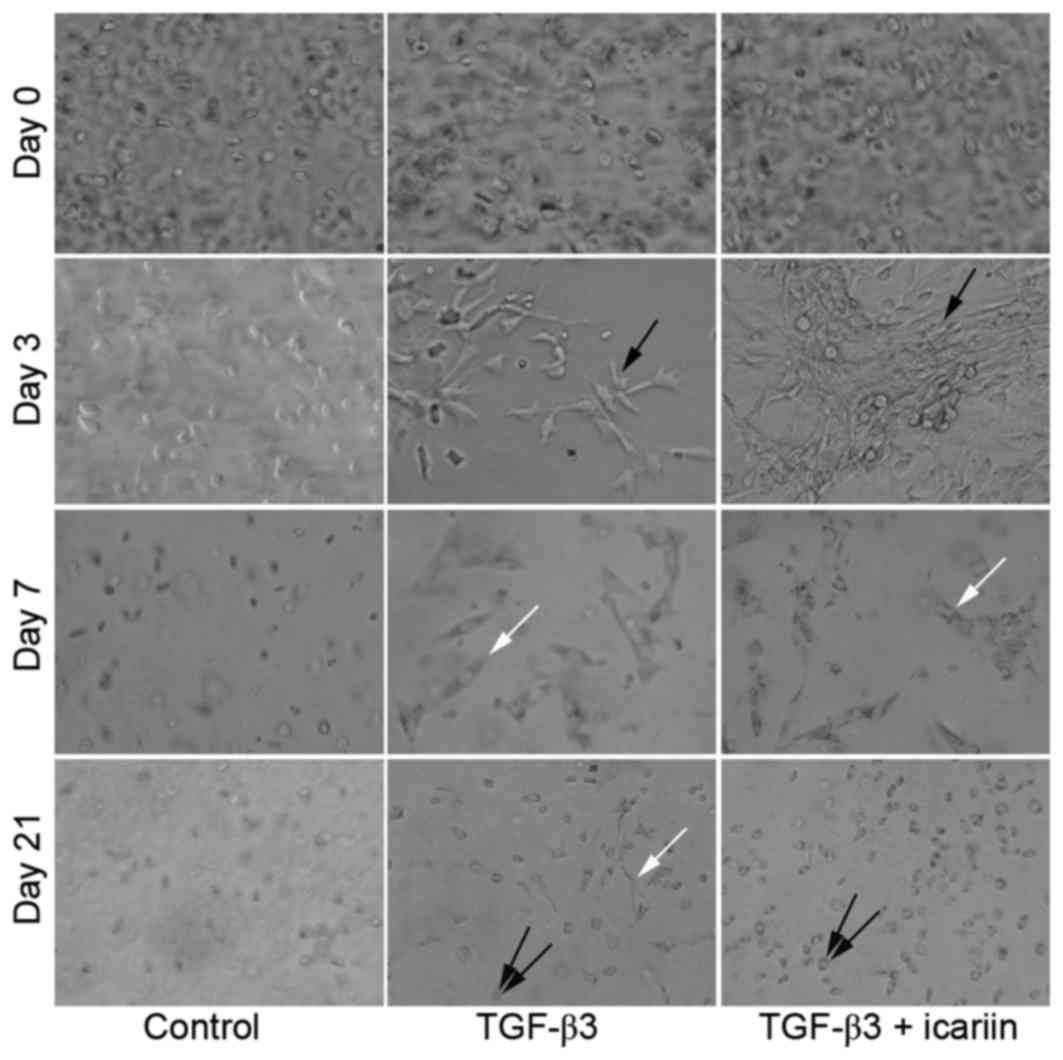
the scaffold during chondrogenesis was easily observed. As
presented in Fig. 3, spherical,
isolated and uniformly seeded cells were observed prior to
chondrogenic induction at day 0. In the TGF-β3 and TGF-β3 + icariin
groups, BMSCs became elongated with long processes and cell-cell
contacts with a clustered morphology at day 3, and the shape of
BMSCs further altered from clusters to fibroblasts, and exhibited a
spread morphology at day 7. In the control medium, cells maintained
the same spherical, isolated morphology as at day 0. Despite these
early differences, cells cultured in TGF-β3 + icariin medium had a
more chondrocyte-like rounded morphology compared with the TGF-β3
only group at day 21, whereas a fibroblastic morphology was visible
in the TGF-β3 group.
Effect of icariin on hypertrophic
differentiation
The present study evaluated the hypertrophic
differentiation markers, collagen × (col10α1 gene), ALP and
collagen I (col1α1 gene), using immunofluorescence and RT-qPCR
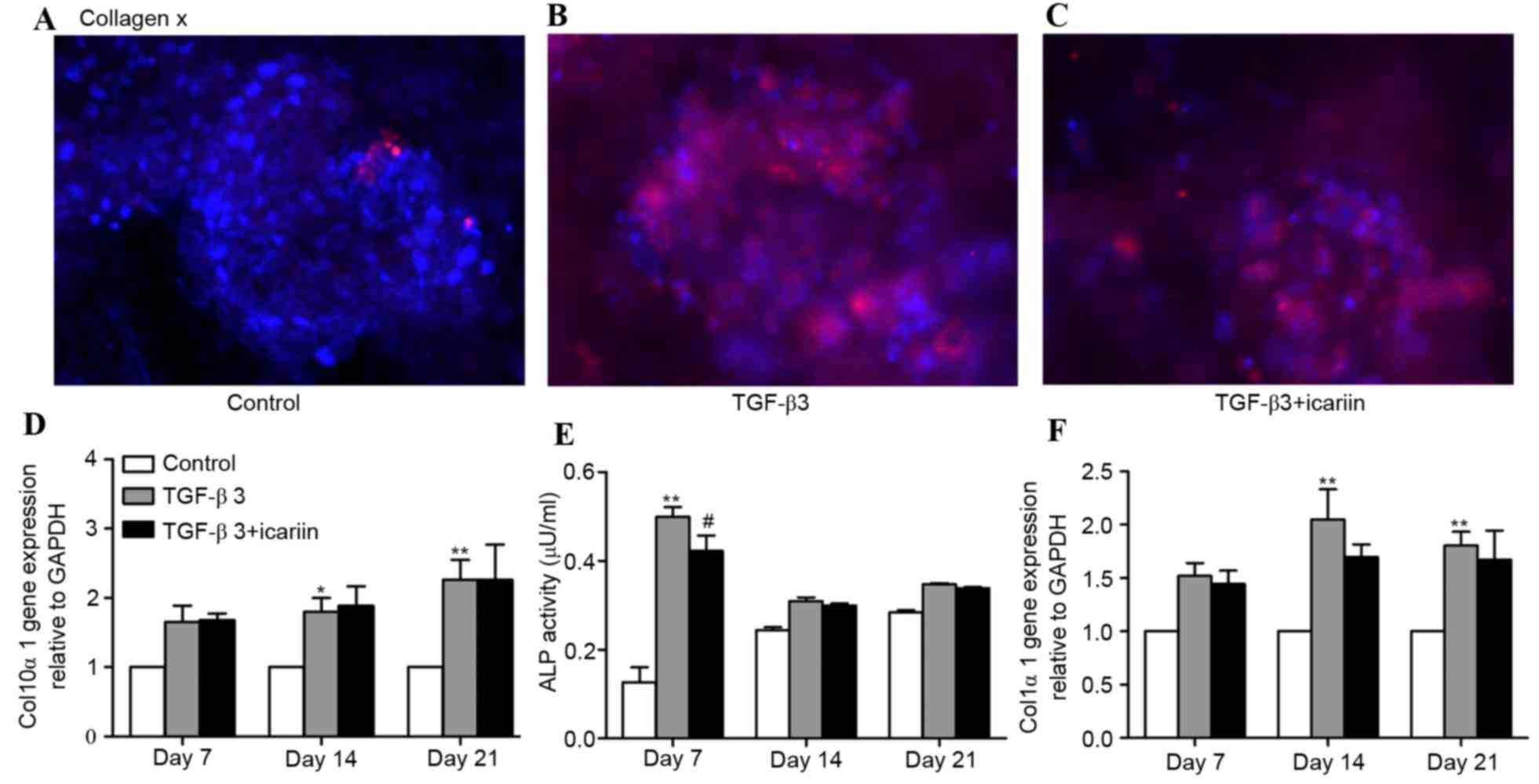
analysis. After 21 days of culture, compared with the control group
(Fig. 4A), which had no collagen ×
staining, the TGF-β3 group revealed strong staining (Fig. 4B). However, treatment with TGF-β3 +
icariin (Fig. 4C) did not lead to
more intense staining compared with the TGF-β3 group, which was
consistent with the results of col10α1 mRNA expression levels
(Fig. 4D). ALP activity was
additionally quantified in supernatants of cell culture. Similar to
the results observed for collagen x, the group treated with TGF-β3
at day 7 exhibited significantly increased ALP activity compared
with the control group, whereas a reduction in ALP activity was
detected in the TGF-β3 + icariin group compared with the TGF-β3
group (Fig. 4E). Collagen I was
considered as a hypertrophic differentiation and dedifferentiation
marker of chondrocytes (22). qPCR
analysis revealed that the expression levels of col1α1 in
the TGF-β3 group were increased significantly at days 14 and 21
compared with the untreated controls; however, the mRNA levels were
decreased in the TGF-β3 + icariin group compared with the TGF-β3
group, although this difference was not significant (Fig. 4F).
Discussion
Based on our previous findings that icariin promoted
chondrogenic differentiation in traditional monolayer 2D culture
(11), the present study
hypothesized that icariin may improve cartilage ECM production and
reduce hypertrophic differentiation in 3D scaffolds seeded with
BMSCs. To maintain a chondrocyte phenotype in monolayer traditional
cultures and promote chondrogenesis, various scaffolds chemically
conjugated with icariin have previously been used for cartilage
tissue engineering, including hyaluronic acid-icariin/collagen
hydrogels (23), hyaluronic
acid-icariin conjugate hydrogels (24) and gelatin/hyaluronic acid-icariin
composite microspheres (25).
Among these biomaterials, self-assembling peptide nanofiber
hydrogels are a relatively new scaffold and have emerged as a
promising cartilage tissue engineering scaffold for simultaneous
cell growth and drug delivery (21). As it is easily loaded with cells,
growth factors and drugs, the present study examined the hypothesis
by adding icariin and TGF-β3 into self-assembling peptide nanofiber
hydrogel scaffolds to induce chondrogenic differentiation, and
subsequently comparing ECM biosynthesis, gene expression levels and
cell morphology. The results of the present study demonstrated that
icariin promotes production of ECM components including collagen II
protein, and additionally enhances chondrogenesis-specific genes
mRNA expression levels, including col2α1 and the early chondrogenic
marker, SOX9.
Consistent with these results, Li et al
(7) added icariin into
chondrocyte-hydrogel scaffolds and reported that icariin markedly
upregulated cartilage-specific gene expression levels of seeded
chondrocytes, and accelerated the formation of cartilage-like
tissue. They additionally investigated the effect of icariin on the
restoration of osteochondral defects, and observed that icariin
improved the restoration efficiency and enhanced the integration of
new-formed cartilage with subchondral bone. Similarly, when icariin
was chemically conjugated to hyaluronic acid/collagen hydrogel
scaffolds, the fixed icariin was gradually released, effectively
maintaining chondrocyte morphology, promoting cartilage matrix
synthesis and forming improved new cartilage tissue (23). Others have investigated the effects
of icariin on chondrgogenesis for either chondrocytes or BMSCs in
2D culture, and have demonstrated that the promotion effects of
icariin are consistent with those in 3D culture (6,11).
Therefore, these results suggested that icariin promotes the growth
of neocartilage and may be a substitute for the use of certain
growth factors in cartilage tissue engineering.
Self-assembling peptide nanofiber hydrogel scaffolds
are transparent, allowing cells to be observed directly and
clearly. In the present study, BMSC morphology began to alter
clearly at day 3, and cell-cell contacts with a clustered
morphology were observed in the TGF-β3 and TGF-β3 + icariin groups.
At day 7, the shape of BMSCs further altered from clusters to
fibroblasts, exhibiting a spread morphology, and subsequently
altered to a chondrocyte-like rounded morphology at day 21, whereas
cells maintained the same spherical, isolated morphology in the
control medium. The morphological events during chondrogenesis were
consistent with numerous previous reports that BMSCs encapsulate in
self-assembling peptides (15),
pellet culture systems (26) and
collagen gel scaffolds (27).
Particularly, the aggregation phase was commonly observed in
monolayer 2D cultures and scaffold 3D cultures (11,15,27).
Ichinose et al (27)
demonstrated that aggregation of chondroprogenitors was the first
step for cartilage formation. Kopesky et al (15) reported that cell-cell contact
stimulates cell mitotic activity, and subsequently undergo overt
chondrogenesis. The present study revealed that staining was
concentrated to cell clusters, whereas little staining was detected
in single cells. Thus, increased cell-cell contact in the TGF-β3 +
icariin group compared with the TGF-β3 group may be influential in
promoting the chondrogenic effect of icariin.
The present study additionally examined hypertrophic
differentiation markers and demonstrated that TGF-β3 medium
increased the mRNA expression levels of col10α1, and col1α1, and
increased the activity of ALP. Previous reports have demonstrated
that growth factors enhance chondrogenesis, promote formation of
cartilage-like tissue, and inevitably upregulate the expression
levels of hypertrophic differentiation markers (2–5). The
TGF-β3 + icariin group exhibited reduced expression levels of these
hypertrophic differentiation markers, suggesting that icariin had
no promotion effect on hypertrophy. Consistent with these results,
Zhang et al (6) and Li
et al (7) reported that
icariin downregulates col1α1 mRNA expression levels in
chondrocytes.
In conclusion, the present study investigated the
effects of icariin treatment on BMSC chondrogenic specific gene
expression and ECM synthesis, and characterized cell morphology
alterations in self-assembling peptide nanofiber hydrogel
scaffolds. It was demonstrated that icariin treatment enhanced
cartilage ECM synthesis and cartilage-specific genes expression
levels in a 3D microenvironment. However, icariin treatment did not
promote hypertrophic differentiation, suggesting that it may
inhibit growth factor activity, thus preventing further
hypertrophic differentiation. Therefore, these results indicated
that icariin may be a potential compound useful for cartilage
tissue engineering.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81273508 and
81350017).
References
|
1
|
Lubis AM and Lubis VK: Adult bone marrow
stem cells in cartilage therapy. Acta Med Indones. 44:62–68.
2012.PubMed/NCBI
|
|
2
|
Giovannini S, Diaz-Romero J, Aigner T,
Heini P, Mainil-Varlet P and Nesic D: Micromass co-culture of human
articular chondrocytes and human bone marrow mesenchymal stem cells
to investigate stable neocartilage tissue formation in vitro. Eur
Cell Mater. 20:245–259. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Aung A, Gupta G, Majid G and Varghese S:
Osteoarthritic chondrocyte-secreted morphogens induce chondrogenic
differentiation of human mesenchymal stem cells. Arthritis Rheum.
63:148–158. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pelttari K, Winter A, Steck E, Goetzke K,
Hennig T, Ochs BG, Aigner T and Richter W: Premature induction of
hypertrophy during in vitro chondrogenesis of human mesenchymal
stem cells correlates with calcification and vascular invasion
after ectopic transplantation in SCID mice. Arthritis Rheum.
54:3254–3266. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mueller MB, Fischer M, Zellner J, Berner
A, Dienstknecht T, Kujat R, Prantl L, Nerlich M, Tuan RS and Angele
P: Effect of parathyroid hormone-related protein in an in vitro
hypertrophy model for mesenchymal stem cell chondrogenesis. Int
Orthop. 37:945–951. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang L, Zhang X, Li KF, Li DX, Xiao YM,
Fan YJ and Zhang XD: Icariin promotes extracellular matrix
synthesis and gene expression of chondrocytes in vitro. Phytother
Res. 26:1385–1392. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li D, Yuan T and Zhang X, Xiao Y, Wang R,
Fan Y and Zhang X: Icariin: A potential promoting compound for
cartilage tissue engineering. Osteoarthritis Cartilage.
20:1647–1656. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fan JJ, Cao LG, Wu T, Wang DX, Jin D,
Jiang S, Zhang ZY, Bi L and Pei GX: The dose-effect of icariin on
the proliferation and osteogenic differentiation of human bone
mesenchymal stem cells. Molecules. 16:10123–10133. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu MH, Sun JS, Tsai SW, Sheu SY and Chen
MH: Icariin protects murine chondrocytes from
lipopolysaccharide-induced inflammatory responses and extracellular
matrix degradation. Nutr Res. 30:57–65. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sun P, Liu Y, Deng X, Yu C, Dai N, Yuan X,
Chen L, Yu S, Si W, Wang X, et al: An inhibitor of cathepsin K,
icariin suppresses cartilage and bone degradation in mice of
collagen-induced arthritis. Phytomedicine. 20:975–979. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang ZC, Sun HJ, Li KH, Fu C and Liu MZ:
Icariin promotes directed chondrogenic differentiation of bone
marrow mesenchymal stem cells but not hypertrophy in vitro. Exp
Ther Med. 8:1528–1534. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li J, Mareddy S, Tan DM, Crawford R, Long
X, Miao X and Xiao Y: A minimal common osteochondrocytic
differentiation medium for the osteogenic and chondrogenic
differentiation of bone marrow stromal cells in the construction of
osteochondral graft. Tissue Eng Part A. 15:2481–2490. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Caron MM, Emans PJ, Coolsen MM, Voss L,
Surtel DA, Cremers A, van Rhijn LW and Welting TJ:
Redifferentiation of dedifferentiated human articular chondrocytes:
Comparison of 2D and 3D cultures. Osteoarthritis Cartilage.
20:1170–1178. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kisiday J, Jin M, Kurz B, Hung H, Semino
C, Zhang S and Grodzinsky AJ: Self-assembling peptide hydrogel
fosters chondrocyte extracellular matrix production and cell
division: Implications for cartilage tissue repair. Proc Natl Acad
Sci USA. 99:9996–10001. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kopesky PW, Vanderploeg EJ, Sandy JS, Kurz
B and Grodzinsky AJ: Self-assembling peptide hydrogels modulate in
vitro chondrogenesis of bovine bone marrow stromal cells. Tissue
Eng Part A. 16:465–477. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang B, Sun C, Shao Z, Yang S, Che B, Wu Q
and Liu J: Designer self-assembling Peptide nanofiber scaffolds
containing link protein N-terminal peptide induce chondrogenesis of
rabbit bone marrow stem cells. Biomed Res Int. 2014:4219542014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Erickson IE, Huang AH, Chung C, Li RT,
Burdick JA and Mauck RL: Differential maturation and
structure-function relationships in mesenchymal stem cell- and
chondrocyte-seeded hydrogels. Tissue Eng Part A. 15:1041–1052.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kisiday JD, Kopesky PW, Evans CH,
Grodzinsky AJ, McIlwraith CW and Frisbie DD: Evaluation of adult
equine bone marrow- and adipose-derived progenitor cell
chondrogenesis in hydrogel cultures. J Orthop Res. 26:322–331.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Miller RE, Grodzinsky AJ, Vanderploeg EJ,
Lee C, Ferris DJ, Barrett MF, Kisiday JD and Frisbie DD: Effect of
self-assembling peptide, chondrogenic factors, and bone
marrow-derived stromal cells on osteochondral repair.
Osteoarthritis Cartilage. 18:1608–1619. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Miller RE, Grodzinsky AJ, Barrett MF, Hung
HH, Frank EH, Werpy NM, McIlwraith CW and Frisbie DD: Effects of
the combination of microfracture and self-assembling Peptide
filling on the repair of a clinically relevant trochlear defect in
an equine model. J Bone Joint Surg Am. 96:1601–1609. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
He B, Yuan X, Zhou A, Zhang H and Jiang D:
Designer functionalised self-assembling peptide nanofibre scaffolds
for cartilage tissue engineering. Expert Rev Mol Med. 16:e122014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cooke ME, Allon AA, Cheng T, Kuo AC, Kim
HT, Vail TP, Marcucio RS, Schneider RA, Lotz JC and Alliston T:
Structured three-dimensional co-culture of mesenchymal stem cells
with chondrocytes promotes chondrogenic differentiation without
hypertrophy. Osteoarthritis Cartilage. 19:1210–1218. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yuan T, He L, Yang J, Zhang L, Xiao Y, Fan
Y and Zhang X: Conjugated icariin promotes tissue-engineered
cartilage formation in hyaluronic acid/collagen hydrogel. Process
Biochemistry. 50:2242–2250. 2015. View Article : Google Scholar
|
|
24
|
He L, Yang J, Lu J, Xiao Y, Fan Y and
Zhang X: Preparation and characterization of a novel hyaluronic
acid-icariin conjugate hydrogel. Materials Lett. 136:41–44. 2014.
View Article : Google Scholar
|
|
25
|
Yan H, Zhou Z, Huang T, Peng C, Liu Q,
Zhou H, Zeng W, Liu L, Ou V, He S and Huang H: Controlled release
in vitro of icariin from gelatin/hyaluronic acid composite
microspheres. Polymer Bulletin. 73:1055–1066. 2016. View Article : Google Scholar
|
|
26
|
Ichinose S, Muneta T, Koga H, Segawa Y,
Tagami M, Tsuji K and Sekiya I: Morphological differences during in
vitro chondrogenesis of bone marrow-, synovium-MSCs, and
chondrocytes. Lab Invest. 90:210–221. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ichinose S, Tagami M, Muneta T, Mukohyama
H and Sekiya I: Comparative sequential morphological analyses
during in vitro chondrogenesis and osteogenesis of mesenchymal stem
cells embedded in collagen gels. Med Mol Morphol. 46:24–33. 2013.
View Article : Google Scholar : PubMed/NCBI
|