Introduction
Osteosarcoma (OS) is the most aggressive and
widespread bone tumor that typically occurs in children and
adolescents (1). OS causes
numerous cancer-related deaths in adolescents and children every
year because of rapid progression, high metastasis ability, and
poor clinical outcomes (2,3). At present, OS is mainly treated with
surgical resection combined with standard chemotherapy and
radiation (4). Although
considerable efforts have been devoted to developing novel
effective methods for OS treatment, minor advances have been
achieved, and the 5-year overall survival rate of patients with OS
remains low (5). Therefore, the
pathogenesis of OS must be determined.
MicroRNAs (miRNAs) are a class of noncoding RNAs
that measure a length of 18–25 nucleotides and reportedly
participate in nearly all kinds of biological processes (6–9).
miRNAs play essential roles in human cancers, such as head and neck
carcinomas (10), hepatocellular
carcinoma (11), colon cancer
(12), lung cancer (13), and OS (14), by regulating cellular
proliferation, migration, invasion, and apoptosis. miRNAs can
target the 3′-UTR of specific mRNAs and regulate gene expression.
The dysregulation of miRNA expression results in the development or
progression of cancers (15). For
example, miRNA-140 suppresses OS tumor growth by enhancing the
anti-tumor immune response (16).
miRNA-665 suppresses the invasion and metastasis of OS by directly
inhibiting RAB23 (17). miR-188 is
implicated in the regulation of tumor occurrence. For instance,
Zhang et al (18) reported
that miR-188-5p inhibits tumor growth and metastasis in prostate
cancer by repressing LAPTM4B expression. However, the role of
miR-188 in OS remains largely unknown.
In the present study, we demonstrated that miR-188
was downregulated in OS tissues compared with that in adjacent
normal tissues. miR-188 overexpression inhibited the proliferation,
migration, and invasion of OS cells by directly targeting SOX4. Our
findings indicated that miR-188 acted as a tumor suppressor and
might be a promising target for OS treatment.
Materials and methods
Clinical specimens and cell lines
Seventy-four pediatric patients with OS (age range:
5–21 years) were recruited from the People's Hospital of Rizhao,
and adjacent normal tissues were obtained from the same specimens.
Tumor biopsies were collected prior to neoadjuvant therapy,
immediately frozen, stored at −80°C, and histologically
characterized by a pathologist. This study was approved by the
Ethics Committee of the People's Hospital of Rizhao and all
patients gave written informed consent for the usage of their
tissues within the present study.
The OS cell lines U2OS, SAOS2, and MG63 and the
fetal osteoblastic cell line hFOB1.19 were purchased from the
American Type Culture Collection (Manassas, VA, USA) and cultured
in DMEM at 37°C in a humidified incubator with 5%
CO2.
Oligonucleotide and transfection
miR-188 mimics and miR-control were chemically
synthesized by GenePharma (Shanghai, China). The cells were
transfected with miR-188 mimics or miR control by using
Lipofectamine reagent (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). The cells were harvested for further experiments
24 h after transfection.
Cell proliferation assay
Cell viability was monitored by Cell Counting Kit
(CCK)-8 (Dojindo Molecular Technologies, Inc., Kumamoto, Japan).
5×103 OS cells were seeded onto 96-well plates and
incubated for 1, 2 and 3 days. Subsequently, 10 µl of CCK8 reagents
were added to the 96-well plates, after 2 h incubation at 37°C, the
absorbance at 450 nm was measured to evaluate the number of viable
cells by SUNRISE microplate reader (Tecan Group, Ltd., Mannedorf,
Switzerland).
In vitro migration and invasion
assays
The transwell assay was conducted in 24-well BD
Matrigel invasion chambers (BD Biosciences, Franklin Lakes, NJ,
USA) according to the manufacturer's instructions. Briefly,
5×104 OS cells were seeded in the upper well of the
migration chamber in DMEM without serum, and 500 µl DMEM
supplemented with 10% FBS were added to the lower chamber well.
After 24 h incubation, the cells on the top of the well were
removed with a cotton swab, and the bottom cells were fixed with 4%
paraformaldehyde, subsequently stained with 0.1% crystal violet for
30 min. Images were captured in 5 independent fields. For invasion
assay, the membranes were coated with Matrigel (BD
Biosciences).
Reverse transcription-quantitative
polymerase chain reaction
Total RNA was extracted from cultured cells using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol and cDNA was synthesized
from total RNA by a PrimerScript RT Reagent kit (Takara Bio, Inc.,
Otsu, Japan). MiRNA from total RNA was reverse transcribed using
the Prime-Script miRNA cDNA Synthesis kit (Takara Bio, Inc.).
Real-time PCR (RT-PCR) was performed with the SYBR-Green Premix Ex
Taq II (Takara Bio, Inc.) on Applied Biosystems Step One Plus
Real-Time PCR System (Applied Biosystems; Thermo Fisher Scientific,
Inc.). GAPDH was used as the endogenous control for detection of
mRNA expression level, while U6 was used as endogenous control for
miRNA expression analysis. Relative gene expression level was
calculated by 2−ΔΔCT method (19).
Tumor xenograft model
Eight female BALB/c nude mice aged 4–6 weeks were
used for the tumor growth assay. U2OS cells (3×106)
transfected with miR-188 or control were subcutaneously injected
into the dorsal flank of the nude mice. Tumor volume was measured
at indicated time points. The mice were sacrificed on the 4th week
to evaluate the tumor growth. All animal experiments were performed
with the approval of the People's Hospital of Rizhao.
RNA immunoprecipitation (RIP)
The RIP experiment was performed using the Magna
RIP™ RNA-Binding protein immunoprecipitation kit (EMD Millipore,
Billerica, MA, USA) following manufacturer's protocol. U2OS and
SAOS2 cells were lysed using complete RIP lysis bufferand 100 µl of
the whole cell extract was incubated with RIPA buffer containing
magnetic beads conjugated with human anti-Argonaute2 (Ago2)
antibody (EMD Millipore) for 6–8 h at 4°C. Normal mouse IgG (EMD
Millipore) was used as a negative control. Samples were washed with
washing buffer and incubated with proteinase K at 55°C for 30 min
to isolate the RNA-protein complexes from beads. Then
immunoprecipitated RNA was extracted and subjected to qRT-PCR
analysis.
Luciferase reporter assay
MiR-188 mimic, pRL-CMV Renilla luciferase reporter
was co-transfected into the cells using Lipofectamine 2000
(Invitrogen; Thermo Fisher Scientific, Inc.). After 48 h,
luciferase data was measured by using a luciferase assay kit
(Promega Corporation, Madison, WI, USA). Firefly luciferase
activity was normalized against Renilla luciferase activity.
Statistical analysis
All statistical analyses were performed using SPSS
v20.0 (IBM Corp., Armonk, NY, USA) and GraphPad Prism. Student's
t-test and one-way ANOVA followed by Tukey's post hoc test were
used to analyze 2 or multiple groups, respectively, for statistical
significance. Pearson correlation coefficient analysis was used to
determine the correlations. P<0.05 was considered to indicate a
statistically significant difference.
Results
miR-188 was overexpressed in OS
tissues and cell lines
To investigate the role of miR-188 in the regulation
of OS progression, we initially analyzed the expression of miR-188
in 74 pairs of OS tissues and adjacent normal tissues through
real-time quantitative polymerase chain reaction (RT-qPCR). The
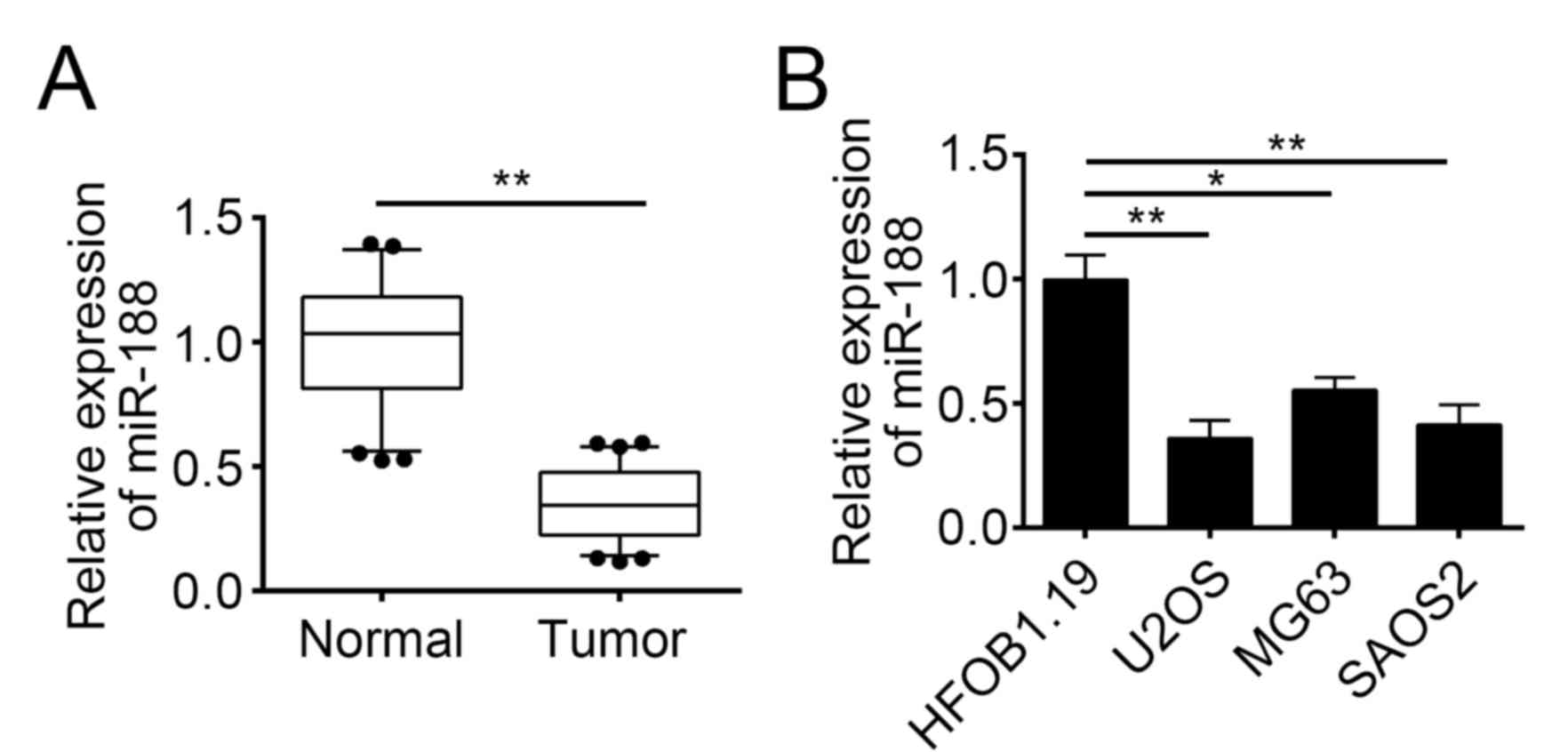
results indicated that the miR-188 expression was downregulated in
tumor tissues compared with that in normal tissues (Fig. 1A). We then determined the
expression patterns of miR-188 in OS cell lines by RT-qPCR. We
found that miR-188 was underexpressed in OS cell lines, including
U2OS, MG63, and SAOS2 cells (Fig.
1B). We also analyzed the correlation of miR-188 expression
with the clinical characteristics of OS tissues and found that the
miR-188 expression level was negatively correlated with the tumor
size, metastasis, and TNM stage of OS (Table I).
 | Table I.Correlation between
clinicopathological features and the expression of miR-188 in
pediatric OS tissues. |
Table I.
Correlation between
clinicopathological features and the expression of miR-188 in
pediatric OS tissues.
|
| miR-188 |
|
|---|
|
|
|
|
|---|
| Feature | Low | High | P-value |
|---|
| All cases | 40 | 34 |
|
| Tumor size (cm) |
|
| 0.035 |
|
<5 | 14 | 21 |
|
| ≥5 | 26 | 13 |
|
| Metastases |
|
| 0.034 |
| No | 13 | 20 |
|
|
Yes | 27 | 14 |
|
| Clinical stage |
|
| 0.012 |
|
I/II | 15 | 23 |
|
|
III | 25 | 11 |
|
Upregulation of miR-188 inhibited OS
cell proliferation but induced apoptosis
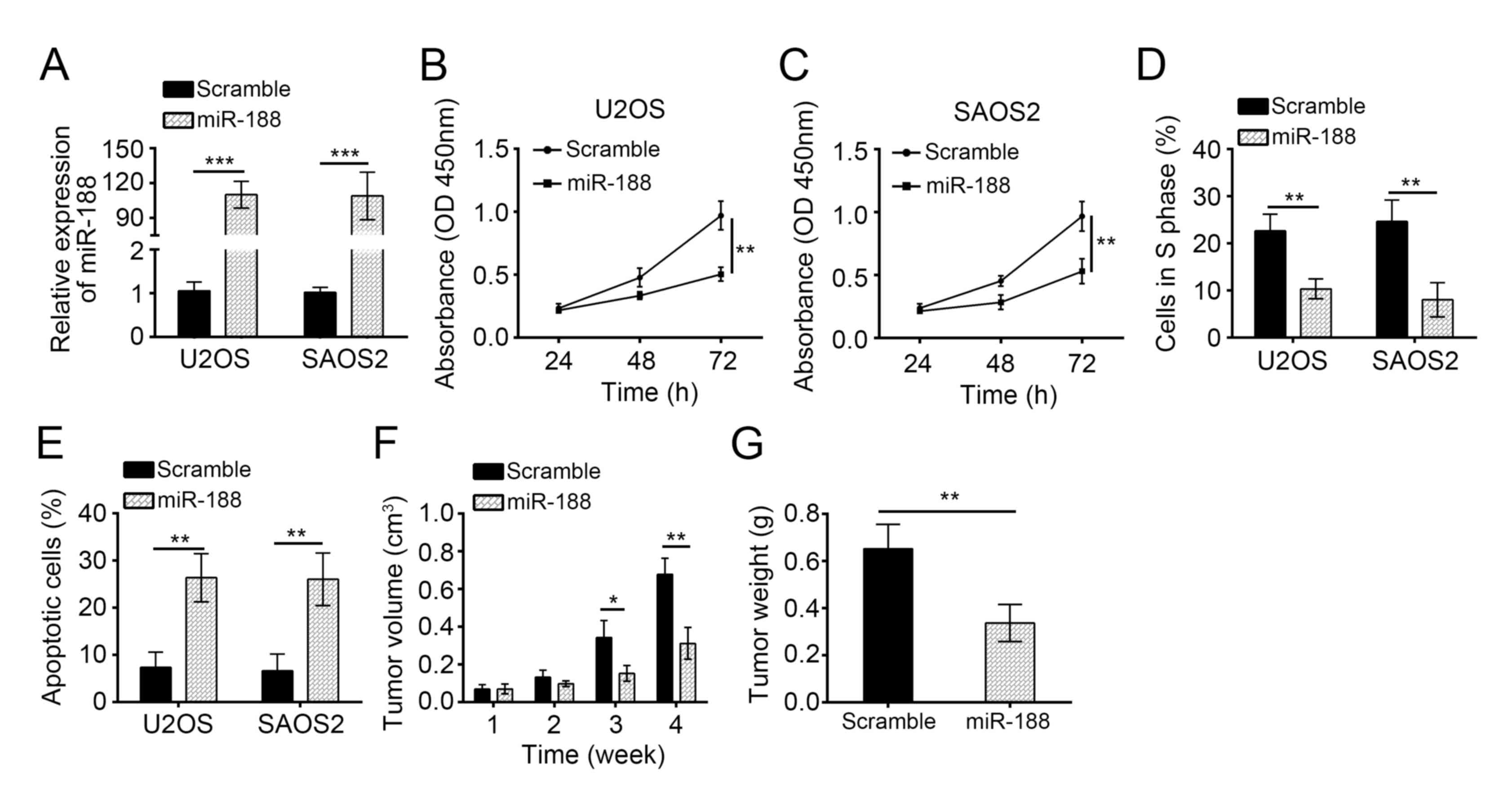
To investigate the function of miR-188 in OS cells,
we overexpressed miR-188 in U2OS and SAOS2 cells by transfecting
them with miR-188 mimics. RT-qPCR revealed that the miR-188
expression was significantly upregulated in U2OS and SAOS2 cells
(Fig. 2A). We then performed CCK8
assays to assess the effects of miR-188 on OS cell proliferation.
The miR-188 overexpression significantly inhibited the
proliferation of U2OS and SAOS2 cells (Fig. 2B and C). We analyzed the cell cycle
by FACS and observed that miR-188 overexpression markedly inhibited
the cells in the S phase (Fig.
2D), indicating that miR-188 inhibited cell cycle progression.
Moreover, AnnexinV/PI staining showed that miR-188 overexpression
significantly increased the apoptotic U2OS and SAOS2 cells
(Fig. 2E). To further evaluate the
effects of miR-188 on tumor growth, we conducted a xenograft
experiment and measured the tumor volumes at the indicated time
points. We found that miR-188 overexpression significantly delayed
the tumor growth in vivo (Fig.
2F). We evaluated the tumor weights at the end of the
experiment and demonstrated that miR-188 overexpression
significantly reduced the tumor size (Fig. 2G). Overall, miR-188 overexpression
prevented OS cell proliferation but induced apoptosis.
MiR-188 overexpression inhibited OS
cell migration and invasion
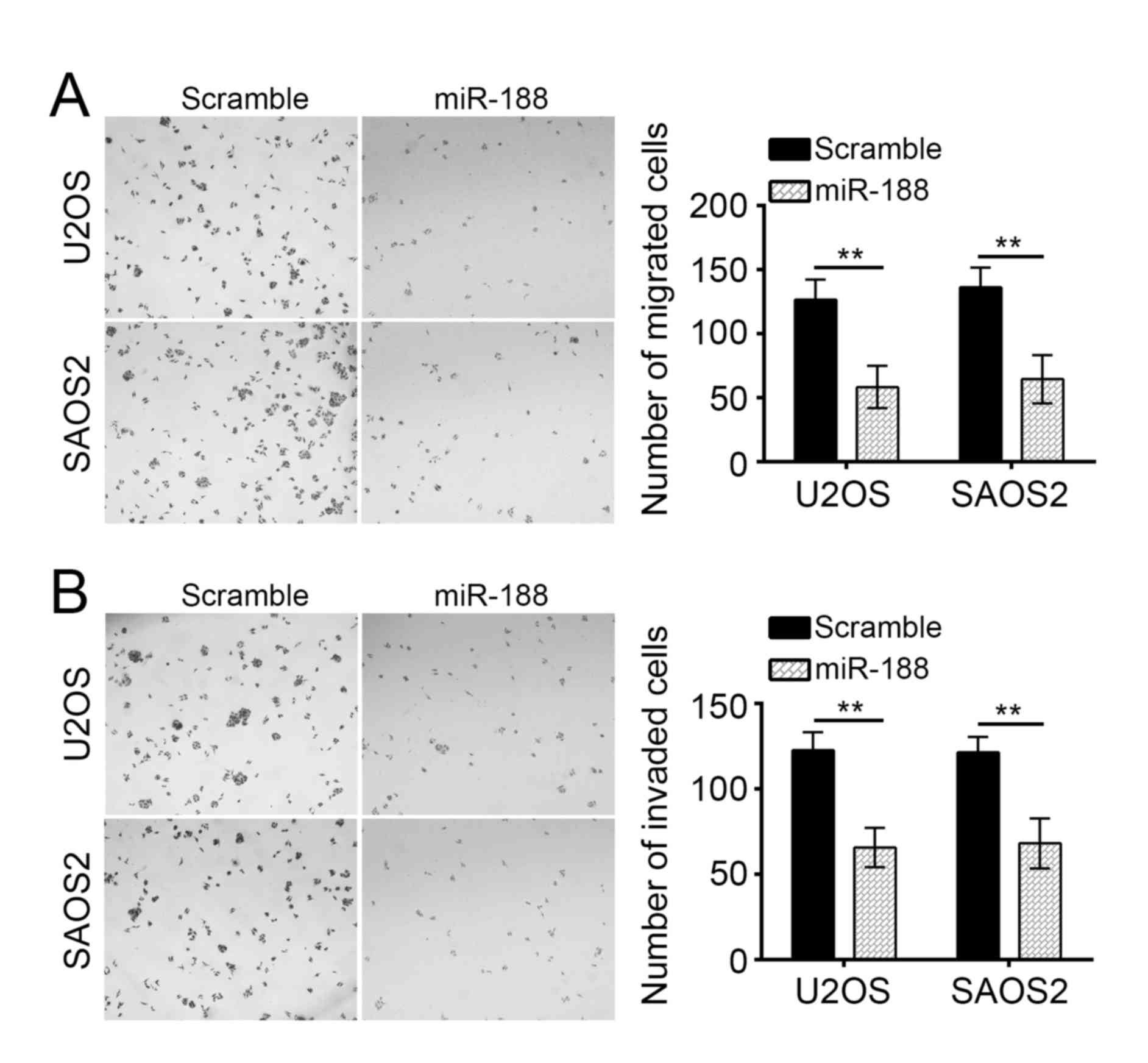
Tumor metastasis substantially contributed to the
poor outcomes of patients with OS. To assess the influence of
miR-188 on tumor metastasis, we conducted Transwell assays. miR-188
overexpression significantly inhibited the migration and invasion
of U2OS and SAOS2 cells in vitro (Fig. 3A and B).
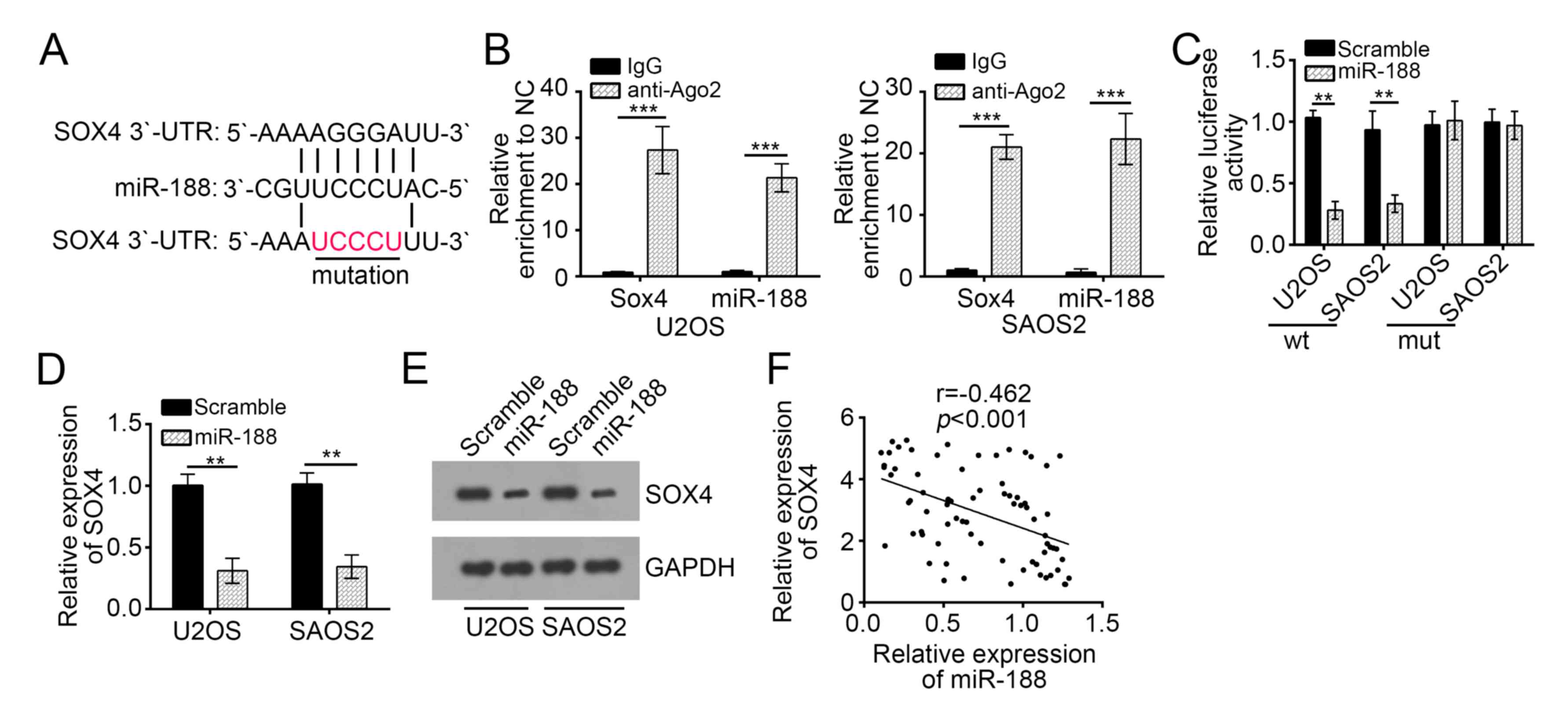
SOX4 is a direct target of miR-188 in
OS cells
miRNAs regulate the expression of target genes. In
our study, we investigated the miR-188-mediated molecular mechanism
of OS. We predicted the target gene of miR-188 by using the
TargetScan and PicTar algorithm software and found that SOX4 is a
potential target of miR-188. SOX4 is a key oncogene in OS
development (20,21). Therefore, we chose SOX4 for further
investigation. As shown in Fig.
4A, an associating site of miR-188 possibly existed in the
3′-UTR of the mRNA of SOX4. It is well documented that miRNAs are
presented in the cytoplasm in the form of miRNA ribonucleoprotein
complexes (miRNPs) containing Ago2, a key component of RNA-induced
silencing complex (RISC). Therefore, RIP assay was conducted in
U2OS and SAOS2 cells using antibody Ago2 to verify whether SOX4 and
miR-188 were in the same RISC complex. The RIP assay results
demonstrated that SOX4 and miR-188 were both significantly enriched
in Ago2-containing miRNPs relative to control group (Fig. 4B), suggesting that SOX4 and miR-188
were in the same RISC complex. To further explore whether SOX4
could directly interact with miR-188, luciferase reporter plasmids
containing the wild-type or mutated miR-188 binding sites in SOX4
3′-UTR were constructed, as presented in Fig. 4A, and cotransfected with
miR-control or miR-188 into U2OS and SAOS2 cells. Luciferase
reporter assay showed that ectopic expression of miR-188
significantly reduced the luciferase activity of SOX4 3′-UTR-WT but
not that of SOX4 3′-UTR-Mut (Fig.
4C). Taken together, these data indicated that SOX4 directly
interacted with miR-188. Furthermore, we found that miR-188
overexpression significantly inhibited the mRNA and protein levels
of SOX4 in U2OS and SAOS2 cells (Fig.
4D and E). Finally, the expression of SOX4 was inversely
correlated with that of miR-188 in OS tissues (Fig. 4F). Overall, our findings
demonstrated that SOX4 is a target of miR-188 in OS cells.
Ectopic SOX4 expression alleviated the
miR-188-mediated inhibition of the proliferation, migration, and
invasion of OS cells
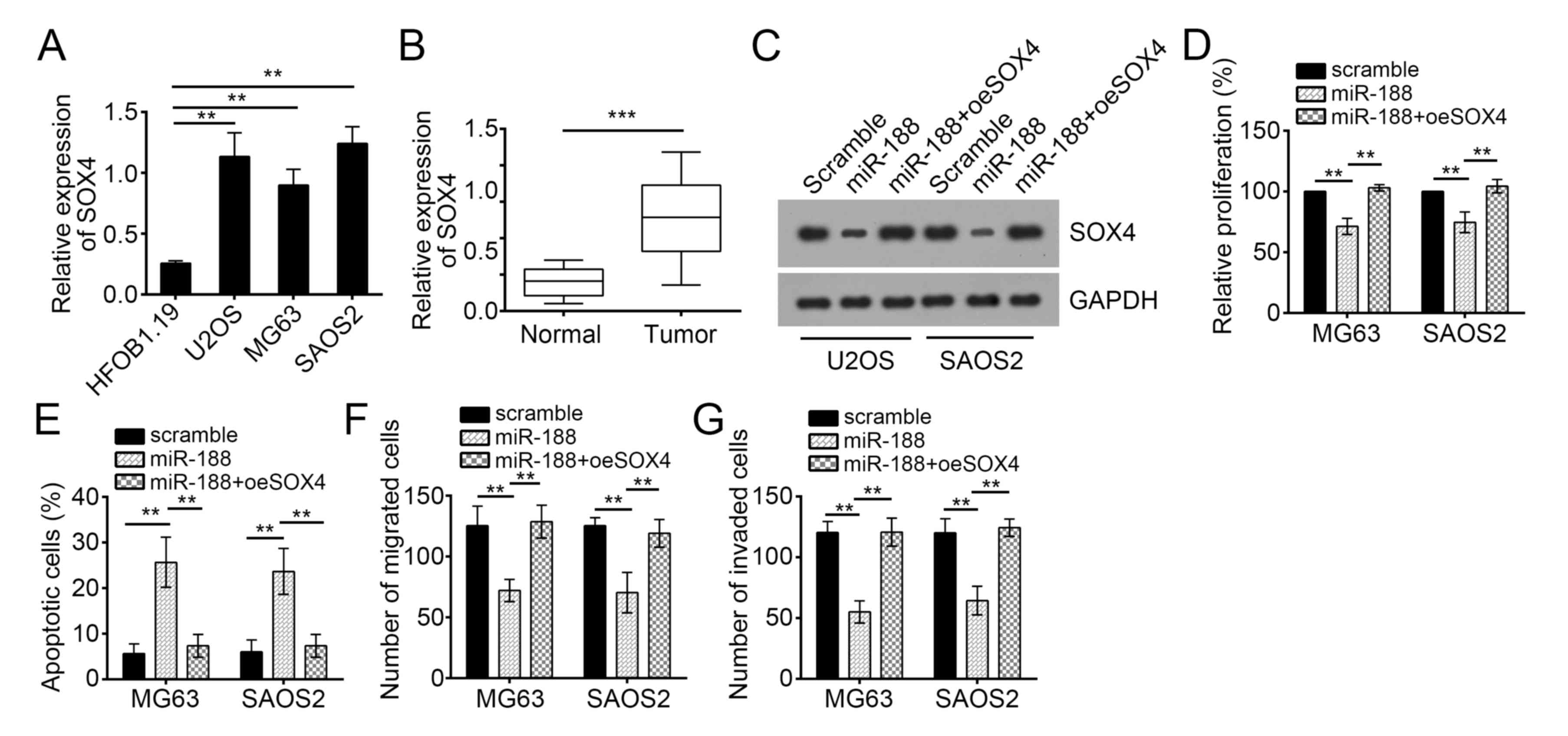
We analyzed the expression patterns of SOX4 in OS
cells through RT-qPCR. SOX4 expression was upregulated in OS cell
lines compared with that in hFOB cells (Fig. 4A). The SOX4 expression was
upregulated in OS tissues compared with that in normal tissues
(Fig. 5B). To determine whether
SOX4 is responsible for the inhibitory function of miR-188 in OS
cells, we restored the SOX4 expression in miR-188-overexpressed
U2OS and SAOS2 cells (Fig. 5C).
Subsequent functional analyses demonstrated that the restoration of
SOX4 rescued the miR-188-mediated reduction of the proliferation,
migration, and invasion of U2OS and SAOS2 cells but inhibited their
apoptosis (Fig. 5D-G). Therefore,
miR-188 prevented OS progression by inhibiting SOX4.
Discussion
In the present study, we investigated the role of
miR-188 in OS cells. miR-188 expression was downregulated in OS
tissues compared with that in adjacent normal tissues. The ectopic
miR-188 expression suppressed the proliferation, migration, and
invasion of OS cells but promoted the apoptosis of these cells. We
also identified SOX4 as a direct target gene of miR-188 in OS
cells. miR-188 and SOX4 expression were negatively correlated in OS
tissues. The restoration of SOX4 could also reverse the
miR-188-mediated effect on OS cells. Therefore, miR-188 served as a
tumor suppressor in the development and progression of OS.
miRNAs are a class of noncoding RNAs that have a
length of 18–25 nucleotides and modulate gene expression via
posttranscriptional regulation (22–24).
miRNAs are important regulators for OS development and progression.
For instance, Tian et al (25) reported that the miR-635 expression
is significantly decreased in OS specimens, and miR-635
overexpression inhibits OS development by enhancing cell apoptosis.
Jia et al (26) showed that
miR-300 decreases the cell viability, inhibits the migration, and
promotes the apoptosis of OS cells by downregulating Twist1. In
addition, miR-302a suppresses the proliferation, migration, and
invasion of OS cells by targeting ADAM9 (27). Previous studies reported that
miR-188 acts as a tumor suppressor in certain cancers. For example,
Wang et al (28) reported
that miRNA-188 is downregulated in oral squamous cell carcinoma,
and it inhibits proliferation and invasion by targeting SIX1.
However, the functions of miR-188 in OS have yet to be defined. In
our study, we confirmed that miR-188 overexpression significantly
inhibited OS growth in vitro and in vivo. By
contrast, the ectopic miR-188 expression significantly enhanced
cellular apoptosis. The miR-188 expression in OS tissues was
positively correlated with tumor size, metastasis, and TNM stage.
Therefore, we demonstrated the tumor suppressive role of miR-188 in
OS.
SOX4 is a member of the Sry-related high-mobility
group box (Sox) family of transcription factors and closely related
to the development and progression of various cancers (29). An abnormal SOX4 overexpression is
often linked to tumorigenicity and cancer stemness (30). SOX4 is upregulated in various
cancers, such as ovarian cancer (29), renal cell carcinoma (31), lung adenocarcinoma (32), gastric carcinoma (33), and OS (34). Previous studies showed that SOX4 is
regulated by miRNAs in OS and involved in the promotion of OS
progression. For example, Liu et al (35) reported that miRNA-132 inhibits cell
growth and metastasis in OS cell lines possibly by targeting SOX4.
In line with previous findings, our results revealed that SOX4 was
upregulated in OS tissues and cell lines, and SOX4 served as a
direct target gene of miR-188. miR-188 expression was inversely
correlated with SOX4 expression in OS tissues. Furthermore, the
restoration of SOX4 reversed the effect of miR-188 on OS cells.
Therefore, our findings demonstrated that miR-188 suppressed OS
cell proliferation and invasion by targeting SOX4.
In conclusion, we revealed that miR-188 acted as a
tumor suppressor by targeting SOX4 in OS. Our study provided novel
insights into the design of therapeutic targets for OS
intervention.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
LP conceived and designed the study, interpreted the
results and wrote the manuscript. LM, FL and LC performed the
experiments. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
the People's Hospital of Rizhao and all enrolled patients provided
written informed consent. All procedures involving animals
conformed to the national guidelines of and were approved by the
Animal Care Ethics Committee of People's Hospital of Rizhao.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ottaviani G and Jaffe N: The epidemiology
of osteosarcoma. Cancer Treat Res. 152:3–13. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mirabello L, Troisi RJ and Savage SA:
Osteosarcoma incidence and survival rates from 1973 to 2004: Data
from the surveillance, epidemiology and end results program.
Cancer. 115:1531–1543. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shen L, Wang P, Yang J and Li X:
MicroRNA-217 regulates WASF3 expression and suppresses tumor growth
and metastasis in osteosarcoma. PLoS One. 9:e1091382014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ando K, Heymann MF, Stresing V, Mori K,
Rédini F and Heymann D: Current therapeutic strategies and novel
approaches in osteosarcoma. Cancers (Basel). 5:591–616. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Leary SE, Wozniak AW, Billups CA, Wu J,
McPherson V, Neel MD, Rao BN and Daw NC: Survival of pediatric
patients after relapsed osteosarcoma: The St. Jude Children's
Research Hospital experience. Cancer. 119:2645–2653. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nana-Sinkam SP and Croce CM: Clinical
applications for microRNAs in cancer. Clin Pharmacol Ther.
93:98–104. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shen J, Stass SA and Jiang F: MicroRNAs as
potential biomarkers in human solid tumors. Cancer Lett.
329:125–136. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Agrawal L, Sahu S, Ghosh S, Shiga T,
Fujita D and Bandyopadhyay A: Inventing atomic resolution scanning
dielectric microscopy to see a single protein complex operation
live at resonance in a neuron without touching or adulterating the
cell. J Integra Neurosci. 15:435–462. 2016. View Article : Google Scholar
|
|
9
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tan H, Zhu G, She L, Wei M, Wang Y, Pi L,
Chen C, Zhang D, Tan P, Chen J, et al: MiR-98 inhibits malignant
progression via targeting MTDH in squamous cell carcinoma of the
head and neck. Am J Cancer Res. 7:2554–2565. 2017.PubMed/NCBI
|
|
11
|
Xu FF, Xie WF, Zha GQ, Chen HW and Deng L:
MiR-520f promotes cell aggressiveness by regulating fibroblast
growth factor 16 in hepatocellular carcinoma. Oncotarget.
8:109546–109558. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang YN, Chen ZH and Chen WC: Novel
circulating microRNAs expression profile in colon cancer: A pilot
study. Eur J Med Res. 22:512017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fan Q, Hu X, Zhang H, Wang S, Zhang H, You
C, Zhang CY, Liang H, Chen X and Ba Y: MiR-193a-3p is an important
tumour suppressor in lung cancer and directly targets KRAS. Cell
Physiol Biochem. 44:1311–1324. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu SY, Deng SY, He YB and Ni GX: miR-451
inhibits cell growth, migration and angiogenesis in human
osteosarcoma via down-regulating IL 6R. Biochem Biophys Res Commun.
482:987–993. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen CZ: MicroRNAs as oncogenes and tumor
suppressors. New Engl J Med. 353:1768–1771. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ji X, Wang E and Tian F: MicroRNA-140
suppresses osteosarcoma tumor growth by enhancing anti-tumor immune
response and blocking mTOR signaling. Biochem Biophys Res Commun.
495:1342–1348. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dong C, Du Q, Wang Z, Wang Y, Wu S and
Wang A: MicroRNA-665 suppressed the invasion and metastasis of
osteosarcoma by directly inhibiting RAB23. Am J Transl Res.
8:4975–4981. 2016.PubMed/NCBI
|
|
18
|
Zhang H, Qi S, Zhang T, Wang A, Liu R, Guo
J, Wang Y and Xu Y: miR-188-5p inhibits tumour growth and
metastasis in prostate cancer by repressing LAPTM4B expression.
Oncotarget. 6:6092–6104. 2015.PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wu X, Zhou H, Yue B, Li M, Liu F, Qiu C,
Chen B and Ma X: Upregulation of microRNA-25-3p inhibits
proliferation, migration and invasion of osteosarcoma cells in
vitro by directly targeting SOX4. Mol Med Rep. 16:4293–4300. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bao ZQ, Zhang CC, Xiao YZ, Zhou JS, Tao YS
and Chai DM: Over-expression of Sox4 and β-catenin is associated
with a less favorable prognosis of osteosarcoma. J Huazhong Univ
Sci Technolog Med Sci. 36:193–199. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yu X and Li Z: Epigenetic deregulations in
chordoma. Cell Prolif. 48:497–502. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yu X and Li Z: The role of miRNAs in
cutaneous squamous cell carcinoma. J Cell Mol Med. 20:3–9. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee H, Jee Y, Hong K, Hwang GS and Chun
KH: MicroRNA-494, upregulated by tumor necrosis factor-α,
desensitizes insulin effect in C2C12 muscle cells. PLoS One.
8:e834712013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tian L, Guo Z, Wang H and Liu X:
MicroRNA-635 inhibits the malignancy of osteosarcoma by inducing
apoptosis. Mol Med Rep. 16:4829–4834. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jia JP, Yin P, Han G, Xu M, Wang W and Bi
WZ: MicroRNA-300 decreases cell viability, inhibits migration and
promotes apoptosis of osteosarcoma cells via downregulation of
Twist1. Mol Med Rep. 16:3613–3618. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang X, Cui Y, Yang F, Sun C and Gao X:
MicroRNA-302a suppresses cell proliferation, migration and invasion
in osteosarcoma by targeting ADAM9. Mol Med Rep. 16:3565–3572.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang L and Liu H: microRNA-188 is
downregulated in oral squamous cell carcinoma and inhibits
proliferation and invasion by targeting SIX1. Tumor Biol.
37:4105–4113. 2016. View Article : Google Scholar
|
|
29
|
Xi J, Feng J and Zeng S: Long noncoding
RNA lncBRM facilitates the proliferation, migration and invasion of
ovarian cancer cells via upregulation of Sox4. Am J Cancer Res.
7:2180–2189. 2017.PubMed/NCBI
|
|
30
|
Ye X and Weinberg RA:
Epithelial-mesenchymal plasticity: A central regulator of cancer
progression. Trends Cell Biol. 25:675–686. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tong Z, Meng X, Wang J and Wang L:
MicroRNA-338-3p targets SOX4 and inhibits cell proliferation and
invasion of renal cell carcinoma. Exp Ther Med. 14:5200–5206.
2017.PubMed/NCBI
|
|
32
|
Wang D, Gao ZM, Han LG, Xu F, Liu K and
Shen Y: Long noncoding RNA CASC2 inhibits metastasis and epithelial
to mesenchymal transition of lung adenocarcinoma via suppressing
SOX4. Eur Rev Med Pharmacol Sci. 21:4584–4590. 2017.PubMed/NCBI
|
|
33
|
Zhang M, Huang S and Long D: MiR-381
inhibits migration and invasion in human gastric carcinoma through
downregulatedting SOX4. Oncol Lett. 14:3760–3766. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wu X, Zhou H, Yue B, Li M, Liu F, Qiu C,
Chen B and Ma X: Upregulation of microRNA-25-3p inhibits
proliferation, migration and invasion of osteosarcoma cells in
vitro by directly targeting SOX4. Mol Med Rep. 16:4293–4300. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu Y, Li Y, Liu J, Wu Y and Zhu Q:
MicroRNA-132 inhibits cell growth and metastasis in osteosarcoma
cell lines possibly by targeting Sox4. Int J Oncol. 47:1672–1684.
2015. View Article : Google Scholar : PubMed/NCBI
|