Introduction
Opioids are used clinically for pain management;
they exert an anti-nociceptive effect by activating µ-opioid
receptors located in the dorsal root ganglia, spinal cord and
brain. However, their continuous administration, particularly
morphine, is associated with the development of analgesic
tolerance. Investigation into the mechanisms of morphine tolerance
has been a focus of interest for numerous years (1–5).
Certain studies have suggested that the spinal glucocorticoid
receptors (GRs) may serve an important role in the mechanisms of
morphine tolerance (6,7); however, the molecular and cellular
mechanisms underlying morphine tolerance remain undetermined.
During the past two decades, a number of associated
intracellular signaling cascades, including the mitogen-activated
protein kinase (MAPK) signaling cascades have been described. MAPKs
may be activated by morphine via opioid receptors, and their
activation has been observed in synaptic plasticity and addiction
(8–14). In vivo, a role of MAPKs in
opioid analgesia and sedation has additionally been proposed
(15). The extracellular
signal-regulated kinase (ERK) pathway is among numerous signal
transduction pathways that may alter gene expression in distinct
brain regions in response to repeated opioid exposure (15,16).
Neuronal GRs have been located within a number of
central regions and implicated in neuronal plastic alterations
(17,18). The regulatory role of GR may be
critical to the cellular mechanisms of morphine tolerance, as the
development of morphine tolerance was attenuated by the GR
antagonist RU38486 (6,7). Furthermore, the GR-mediated effect on
morphine tolerance was abolished in adrenalectomized rats,
indicating that endogenous corticosteroids serve an important role
in GR function following chronic morphine exposure (19). Clark and Lasa (20) summarized the association between
glucocorticoids and the MAPK signaling pathways.
The GR and ERK pathways are involved in neuropathic
pain, which shares the same mechanism as morphine tolerance
(21–23). In a rat model of morphine
tolerance, the hypothesis that spinal GRs may serve an important
role in the development of tolerance to the antinociceptive effect
of morphine through ERK was examined in the present study.
Materials and methods
Animals
A total of 50 male Sprague-Dawley rats (10–12 weeks
old, weighing 250–350 g) were used in the present study. The
animals were provided by the Peking Union Medical College Animal
Center (Beijing, China) and were housed in plastic cages, with free
access to water and food available ad libitum. The rats were
housed under 12-h light/dark conditions in a room with controlled
temperature (22–26°C) and relative humidity (60–80%). The animals
adapted to this environment for 7 days prior to the start of the
experiment, and every effort was made to minimize the number of
animals used and their suffering. All animal procedures in the
present study were approved by the Animal Care and Use Committee of
Tianjin Medical University and in accordance with the National
Institutes of Health Guide for the Care and Use of Laboratory
Animals.
Implantation of intrathecal (IT)
catheters and administration of drugs
Under anesthesia with chloral hydrate [300 mg/kg by
intraperitoneal (IP) injection], a PE10 catheter was implanted in
each rat, according to a previously published method (24), inserting to the level of the lumbar
enlargement (7.5 cm from the incision site). A total of five
animals that exhibited neurological defects, including paralysis,
following the IT catheter implantation were excluded from the
experiments (25). The rats were
housed individually following surgery and recovered for 3 days
prior to the following test. An IT treatment regimen of 10 µg
morphine was given twice daily for 6 consecutive days to induce
chronic morphine tolerance. The following drugs were purchased from
Sigma-Aldrich; Merck KGaA (Darmstadt, Germany): Mifepristone
(RU38486; batch no. M8046); dexamethasone (Dex; batch no. D1756);
and PD98059 (batch no. P215). Morphine was dissolved in normal
saline and PD98059 was dissolved in a 0.4% dimethyl sulfoxide
solution. The other drugs were dissolved in a 10% ethanol solution
(Table I).
 | Table I.Experimental groups. |
Table I.
Experimental groups.
| A, Experiment
1 |
|---|
|
|---|
| Group | Saline, µl | Morphine, µg | RU38486, µg | Dex, µg | 0.4% DMSO, µl | PD98059, µg |
|---|
| C | 1 | – | – | – | 10 | – |
| M | – | 10 | – | – | – | – |
| M/D | – | 10 | – | 4 | – | 10 |
| M/R | – | 10 | 2 | – | – | – |
|
| B, Experiment
2 |
|
| Group | Saline,
µl | Morphine,
µg | RU38486,
µg | Dex, µg | 0.4% DMSO,
µl | PD98059,
µg |
|
| C | 1 | – | – | – | 10 | – |
| M | – | 10 | – | – | – | – |
| P | – | 10 | – | – | – | 10 |
| P+D | – | 10 | – | 4 | – | 10 |
Behavioral tests
The tail flick latency (TFL) was used as an index to
evaluate antinociceptive responses to thermal pain. During the TFL
testing, a rat was placed in a hard plastic fixator, and the rat
tail was immersed in hot water (52±0.5°C) 3 cm from the distal end
of the tail (26). The routine
tail-flick test was used with baseline latencies of 3–4 sec and a
cutoff time of 10 sec. The TFL was measured in each rat 30 min
following injection every morning. Each measurement was repeated
three times to reduce aberration, with a measurement interval of 1
min. The baseline value was determined on the first day prior to
the first injection. In order to make the animal accustomed and
remain quiet in the tail flick test situation, ~one week prior to
the start of the test, the rats were allowed to acclimate in the
test environment for 2 h per day, and were intermittently confined
to the tubular rat fixator to adapt to the pre-experimental state.
During each test session, the animals were tested with 20 min
intervals during 2 h and were free to exercise without any
stimulation
Immunofluorescent staining
Under anesthesia with chloral hydrate (300 mg/kg
IP), the lumbar spinal cord segments 3–5 (L3–5) were
dissected, and subsequently mounted in optimum cutting temperature
compound and frozen on dry ice. A total of five spinal cord
sections (5-µm) per animal were fixed with cold pure acetone
(0.7845 g/ml) for 20 min at 4°C, and incubated with 0.3%
H2O2 solution for 10 min. The sections were
blocked with 1% bovine serum (Tianjin Jiaguan Biotechnology
Development Center, Tianjin, China; numbered shs j-4) in 0.3%
Triton X-100 for 30 min at 37°C and subsequently incubated
overnight at 4°C with a primary antibody against GRs (1:50, rabbit
polyclonal; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) and
phosphorylated (p)-ERK1/2 (1:300, rabbit monoclonal; cat. no.
ab201015; Abcam, Cambridge, UK). The sections were incubated for 1
h at 37°C with a corresponding tetramethylrhodamine- or fluorescein
isothiocyanate-conjugated secondary antibody (1:50; Santa Cruz
Biotechnology, Inc.). For double staining, a second primary
antibody was added following the incubation with the first primary
antibody, following the same procedure as described above. A total
of four to six nonadjacent spinal sections were randomly selected,
analyzed using a fluorescence microscope (Olympus Corporation,
Tokyo, Japan) at a magnification, ×400 and processed using Adobe
Photoshop CS3 10.0.1 (Adobe Systems, Inc., San Jose, CA, USA).
Western blot analysis
The rats were rapidly (<1 min) sacrificed by
decapitation on day 7, subsequent to being anesthetized with an IP
injection of 300 mg/kg chloral hydrate. L3–5 were
removed and immediately frozen in liquid nitrogen and stored at
−80°C until the protein extraction was conducted. Tissues were
homogenized in 50 mM radioimmunoprecipitation buffer (20 µl/mg)
containing protease and phosphatase inhibitors. The homogenates
were incubated on ice for 30 min and centrifuged at 12,000 × g for
15 min at 4°C and the supernatant was removed and stored at −20°C.
The protein was lysed by PRO-PREP Protein Extraction Solution
(iNtRON Biotechnology, Sungnam, Korea), and total protein
concentrations were determined spectrophotometrically using the
bicinchoninic acid method. The protein samples were mixed with
equal volumes of electrophoresis loading buffer, boiled for 15 min
and centrifuged at 800 × g at 12,000 × g for 10 min, at 4°C. The
samples (20 µg) were subsequently separated using 15% SDS-PAGE and
transferred electrophoretically to a nitrocellulose membrane. The
membranes were blocked in 5% skim milk in TBST buffer [10 mM Tris
(pH 7.5), 150 mM NaCl, 0.1% Tween-20] for 1 h at room temperature
and incubated with primary antibody, including mouse monoclonal
anti-GR (1:1,000; cat. no. ab9568; Abcam), rabbit monoclonal
anti-phospho-ERK1/2 (1:10,000; cat. no. ab201015; Abcam), and mouse
monoclonal anti-ERK1/2 (1:10,000; cat. no. ab54230; Abcam), and
were gently agitated overnight at 4°C. The blots were rinsed three
times for 30 min in TBST and incubated for 2 h at room temperature
with the appropriate anti-rabbit (cat. no. 7074) and anti-mouse
(cat. no. 7076) secondary horseradish peroxidase-conjugated
antibodies (1:2,000; Cell Signaling Technology, Inc.). Reactive
protein was detected using an enhanced chemiluminescence system
(Amersham ECL™; GE Healthcare, Chicago, IL, USA). β-actin was the
loading control for all the experiments. The ratio of GR/β-actin,
p-ERK/β-actin and p-ERK/total ERK were plotted and analyzed by
ImageJ Software (version 1.49; National Institutes of Health,
Bethesda, MD, USA). All the western blot analyses were performed at
least three times and consistent results were obtained.
Statistical analysis
All results presented are expressed as the mean ±
standard error of the mean as indicated. All experimental data were
repeated three times. The significance of differences was
calculated using repeated measure one-way analysis of variance
followed with post-hoc Newman-Keuls tests. Statistical analysis was
performed with SPSS 19.0 software (IBM Corp., Armonk, NY, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
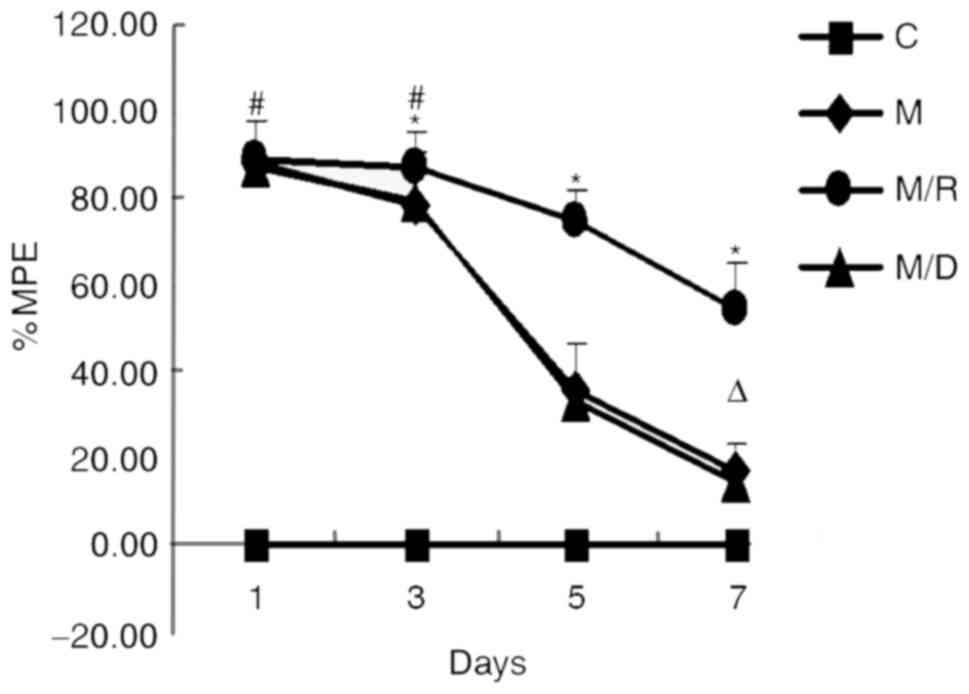
Development of morphine tolerance and
the effect of RU38486
The baseline TFL (3.25±0.52 sec; n=20) was
determined on the first day prior to injection of all the rats. The
IT administration of morphine for 6 days led to the development of
tolerance to morphine-induced analgesia (Fig. 1). As the figure demonstrates, the
injection of morphine produced a significant analgesia to thermal
stimuli on day 1 [% maximal possible effect (MPE)=87.82±10.40%]
compared with the control group; however, the effect of morphine
gradually declined during the following days (between day 3 and day
7; P<0.05), demonstrating that the rats had developed morphine
tolerance. However, cotreatment of the GR antagonist RU38486 with
morphine for 6 consecutive days inhibited the morphine
antinociceptive tolerance; the %MPE was 54.07±11.32 on day 7,
significantly higher compared with the morphine group (16.88±11.88;
P<0.05; Fig. 1). These findings
suggested that the GR antagonist may inhibit the development of
morphine tolerance.
Effects of pretreatment with PD98059
in morphine-tolerant rats
Coadministration of morphine (10 µg) and Dex (4 µg)
given twice daily for 7 days significantly accelerated the
development of morphine tolerance (%MPE=14.93±8.29%; Fig. 2). On day 5, the %MPE was
20.40±17.07% in the morphine group; pretreatment with the MEK
inhibitor PD98059 prior to 30 min administration prolonged the TFL
(%MPE=44.00±16.95; P<0.05; Fig.
2), demonstrating that ERKs serve an important role in morphine
tolerance.
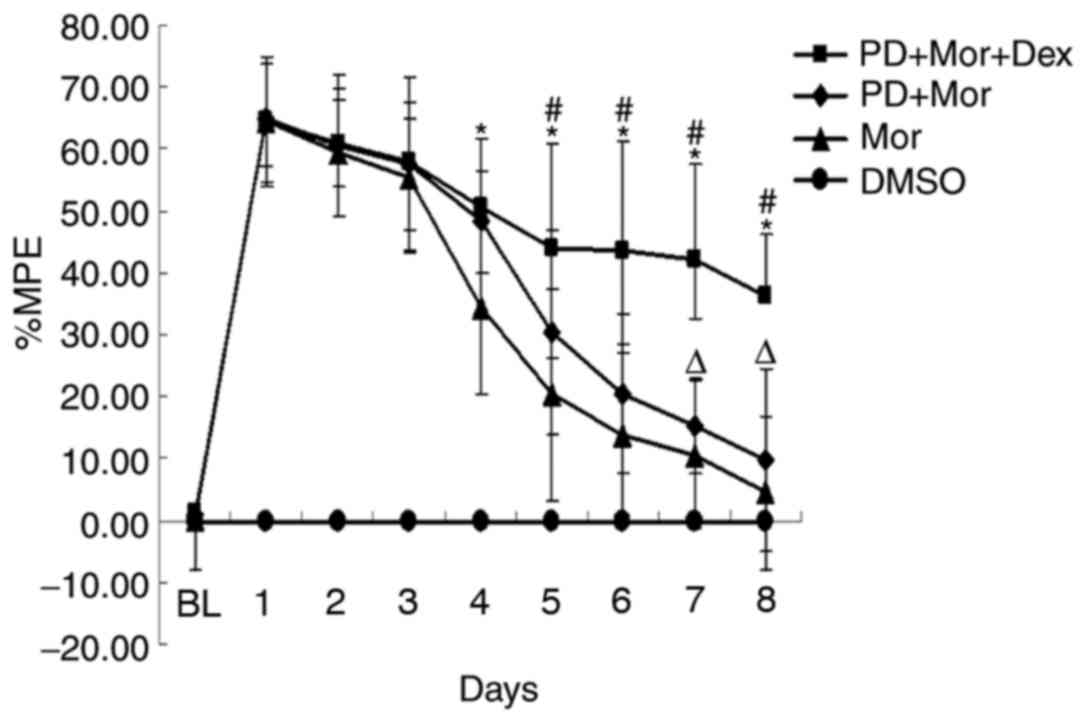 | Figure 2.Effects of pretreatment with PD98059
in the tail-flick test. *P<0.05, PD+Mor+Dex vs. Mor;
#P<0.05, PD+Mor+Dex vs. PD+Mor,
ΔP>0.05, PD+Mor vs. Mor. n=5 for each group. DMSO,
treatment with dimethyl sulfoxide, RU38486 and Dex treatment for 7
days (10 µl twice daily); Mor, treatment with morphine for 7 days
(10 µg twice daily); PD+Mor+Dex, cotreatment of PD98059 (10 µg),
morphine (10 µg) and Dex (2 µg) for 7 days twice daily; PD+Mor,
cotreatment of morphine (10 µg) and PD98059 (10 µg) for 7 days
twice daily; MPE, maximal possible effect; RU38486, mifepristone;
Dex, dexamethasone. |
Colocalization of GR and p-ERK1/2
Double staining immunofluorescence was conducted to
determine the colocalization of GR and p-ERK1/2 in the
morphine-tolerant rats (Fig. 3;
white arrow positioning area). The distribution of GR
immunoreactivity (IR) was primarily within the spinal cord
superficial laminae (I–II) compared with the deeper laminae
(III–IV) (Fig. 3A-D). Following
repeated morphine administration or combined with Dex, the content
of GR increased in the spinal cord dorsal horn (Fig. 3B and D). In addition, p-ERK1/2 was
primarily located in the superficial layer of the spinal cord
(I–II). Subsequent to GR antagonist RU38486 application, the
expression of p-ERK1/2 was increased (Fig. 3G); whereas, the expression of
p-ERK1/2 decreased following the application of Dex (Fig. 3H). The combination of GR and
p-ERK1/2 immunostaining demonstrated that approximately all the
GR-IR sites in the I–II layer expressed p-ERK1/2, indicating that
GR may regulate the role of morphine through ERK (Fig. 3I-K).
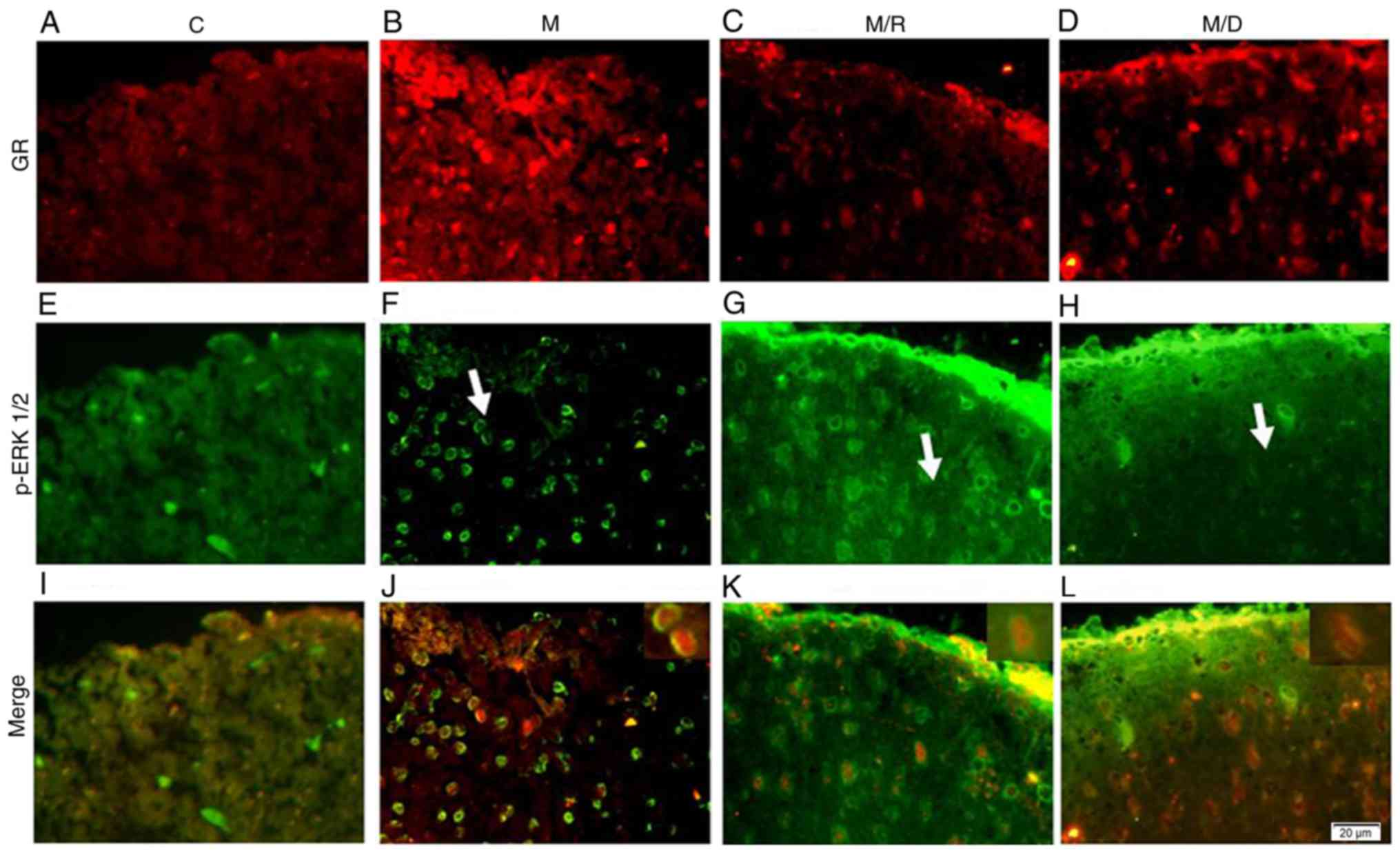 | Figure 3.Colocalization of GR and p-ERK1/2
(indicated by the white arrows). Double immunostaining of GR in (A)
C group, (B) M group, (C) M/R group and (D) M/D group, and of p-ERK
in (E) C group, (F) M group, (G) M/R group and (H) M/D group,
revealed the colocalization in the dorsal horn of the lumbar spinal
cord as indicated in yellow. The images of (I) C group, (J) M
group, (K) M/R group and (L) M/D group are merged (the
magnification of smaller images in K and L is ×1,000). Scale bar
(in F): A-L, 20 µm. n=5 for each group. C, Saline treatment for 6
days (10 µl, twice daily); M, treatment with morphine for 6 days
(10 µg twice daily); M/R, cotreatment of morphine (10 µg) and
RU38486 (2 µg) for 6 days twice daily; M/D, cotreatment of morphine
(10 µg) and RU38486 (4 µg) for 6 days twice daily; GR,
glucocorticoid receptor; p-ERK, phosphorylated extracellular
signal-regulated kinase; RU38486, mifepristone. |
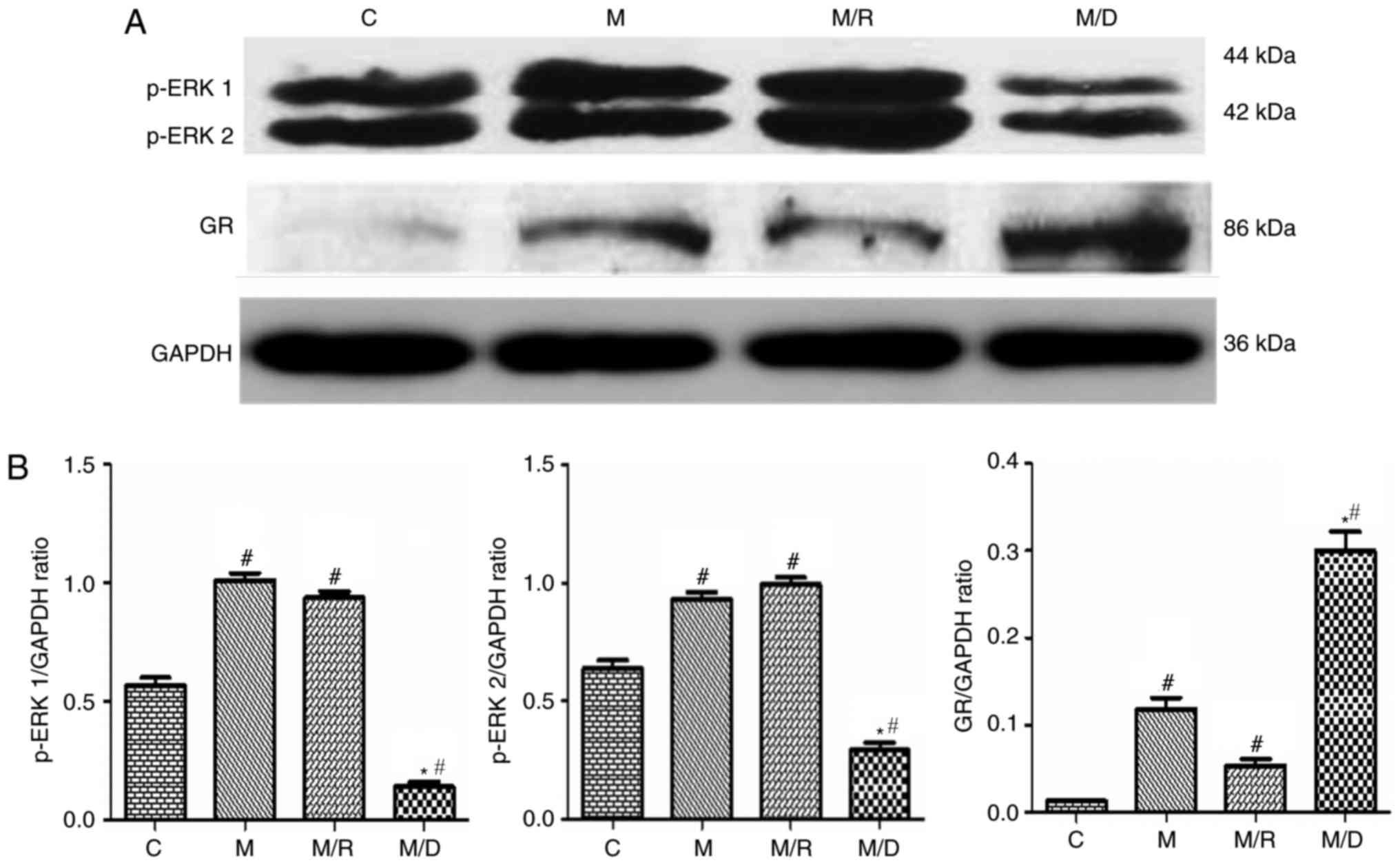
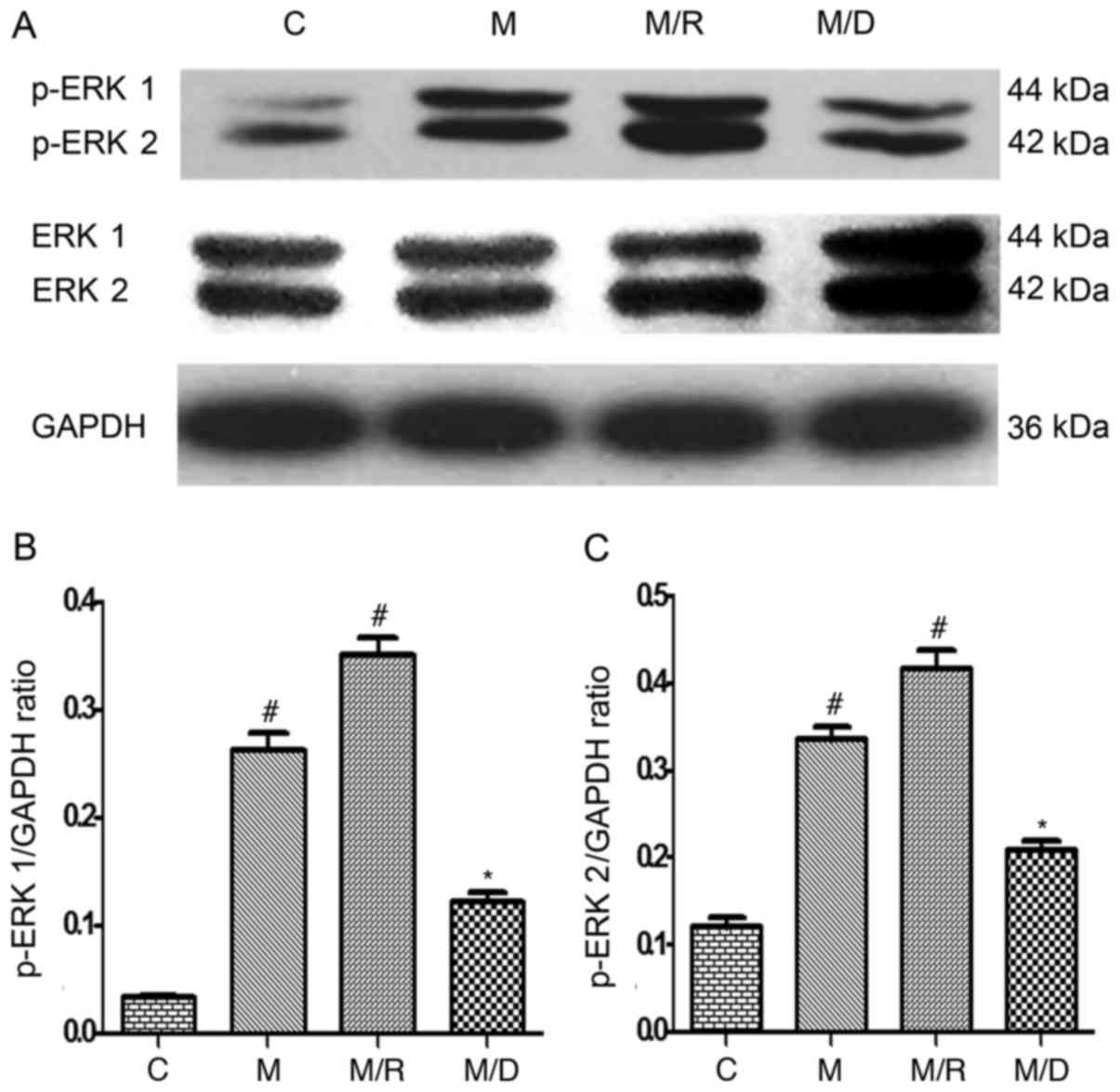
Expression of spinal cord GR and
p-ERK
The results of the western blot analysis
demonstrated that compared with the saline control, there was an
upregulation of GR and p-ERK1/2 within the spinal cord following
chronic treatment with morphine on day 7 (Fig. 4A; P<0.05; n=4–5), which is
consistent with previous studies that demonstrated a time-dependent
increase in spinal GR expression following chronic treatment with
morphine (10 µg twice daily for 6 days) (6,7).
Treatment with Dex induced an upregulation of spinal GR expression
compared with the morphine group and the saline control (Fig. 4B; P<0.05; n=4–5), indicating
that coadministration of RU38486 decreased the expression of spinal
GR induced by morphine. In this experiment, compared with the
morphine group, the expression level of spinal p-ERK1/2 increased
following coadministration of morphine with RU38486 for 6 days
(Figs. 4 and 5; P<0.05; n=4–5).
Discussion
The mechanisms underlying opioid analgesic tolerance
are complex and involve adaptations in opioid receptor activity at
the cellular and molecular levels, and at the level of spinal or
superspinal neuronal network signal nociception (27–29).
The spinal cord dorsal horn is the first transit point of the
nociceptive pathway; from Aδ and C fibers, afferent nociceptive
information ascending through the dorsal horn projection neurons is
passed to the upper senior center. The spinal cord receives
nociceptive information and processes and integrates this noxious
information (30). It is an
important pain modulation center. DeLander et al (31) indicated that the spinal cord is a
key site of opioid tolerance, and drugs that inhibit µ opioid
receptor expression can significantly antagonize opioid
tolerance.
There are numerous methods of inducing chronic
morphine tolerance; the present study used the method of IT
catheters to deliver drugs directly to the spinal cord lumbar
enlargement. In the assessment of opioid tolerance, the majority of
behavioral studies examine the TFL, a pain response indicator that
is a simple spinal reflex without the involvement of the
superspinal cord nervous system; it coordinates information on
noxious stimulation of the spinal cord itself (32). In the first experiment, following 6
days of consecutive injection, morphine resulted in a significant
decrease in TFL compared with the control group rats.
Coadministration of morphine and the GR antagonist RU38486
prevented the successive declining of TFL between days 5 and 7, and
the effect of Dex was the opposite.
Double immunofluorescence staining was conducted to
determine the colocalization of GRs and p-ERKs. Studies have
demonstrated that GRs are located within the spinal cord dorsal
horn, and activation of neuronal GRs contributes to neural
plasticity associated with neuronal injury (17,33,34).
Furthermore, activation of GRs has been demonstrated to modulate
morphine-induced antinociception and morphine tolerance (6,35).
ERK as a member of the MAPK family is downstream of numerous
kinases and is activated in primary sensory neurons, dorsal horn
neurons and spinal glial cells when exposed to a number of factors,
including nociceptive stimuli, growth factors and inflammatory
mediators. The activation of ERK consequently contributes to the
induction and maintenance of sensitization via transcriptional,
translational and posttranslational regulation (15). Data from in vitro and in
vivo experiments suggest that the phosphorylation of MAPK
serves a role in the chronic morphine-induced increase in
calcitonin gene-associated peptide and substance P levels in dorsal
root ganglion neurons, indicating MAPK involvement in
morphine-induced antinociception (35). As a part of a critical signaling
pathway, the association between GR and ERK was first tested. It
was revealed that certain neurons contained IR sites for GR and
p-ERK in morphine-tolerant animals and other groups, indicating an
association between GR and ERK in response to chronic morphine
exposure.
The expression of spinal GR and p-ERK1/2 was
assessed by western blotting; compared with the saline control,
upregulation of GR and p-ERK1/2 was observed within the spinal cord
following chronic treatment with morphine on day 7, similar to a
previous study, which demonstrated that an increase in p-ERK1/2
level in the spinal cord was detected following IT injection of
morphine (15 µg/day) for 7 days in rats (36). Rats treated with morphine and
RU38486 demonstrated that the expression of spinal GR decreased
compared with treatment with morphine, suggesting that RU38486 may
prevent the development of morphine tolerance, and the behavior
test was in accordance with this. However, the expression of spinal
p-ERK1/2 was decreased subsequent to treatment with Dex and
morphine on day 7 compared with the morphine group. The tail flick
test demonstrated that the treatment with Dex and morphine for 6
days was unable prevent the development of morphine tolerance. In
the second experiment, IT administration of the MEK inhibitor
PD98059 (10 µg/10 µl) prior to IT injection of morphine and Dex
delayed the development of tolerance in the rats. The possible
mechanism is that Dex functions independently, as demonstrated by
Croxtall et al (37). This
previous study demonstrated that Dex reduces the level of p-ERK1/2
in a GR-dependent manner; however, a transcription-independent
mechanism was observed in A549 cells. A study examining
ERK-deficient mice may be helpful to further elucidate the role of
ERK in tolerance and dependence (38). It is additionally important to note
that the MEK inhibitor PD98059 requires sufficient pretreatment
time (>20 min) to obtain optimal membrane permeability (36).
The addition of a second drug to a morphine infusion
has been demonstrated to be an effective strategy for attenuating
morphine tolerance and maintaining the antinociceptive efficacy of
morphine in chronic morphine-infused rats (39–41).
The present results suggested that a GR inhibitor, including
RU38486 may be useful in preventing the development of opioid
tolerance, an issue of substantial clinical relevance. There is a
clear association between spinal GR and the expression of p-ERK1/2,
contributory to the development of morphine tolerance. The present
study is different from the previous study published by the present
research group (42). The
double-staining immunofluorescence technique detected the
expression of GR and p-ERK1/2, and demonstrated the association
between the two. In combination with the results of the western
blot analyses, it was quantitatively demonstrated that ERK is
involved in the development of chronic morphine tolerance.
Secondly, the introduction of a MAPK inhibitor in the present study
further confirmed that GR may increase the duration of morphine
tolerance by signaling via the MAPK/ERK pathway, which suggests a
more logical conclusion: GR may be involved in chronic morphine
tolerance through ERK1/2. However, it must be emphasized that these
findings do not exclude other interactions between the spinal GR
and morphine tolerance. For example, the spinal cord
N-methyl-D-aspartate (NMDA) receptor and protein kinase Cγ, which
are regulated through GRs, influence the development of morphine
tolerance, and the NMDA receptor and the MAPK pathway are involved
in synaptic plasticity (43–46).
In conclusion, the present study demonstrates that
cotreatment of a GR inhibitor with morphine may attenuate the
development of morphine tolerance, and there may be an association
between spinal GRs and the MAPK signaling pathway.
Acknowledgements
The authors thank Mr. Xie KL for his editorial
support.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
MLZ and YC made contributions to experimental
design, data collection and manuscript writing. CL and JBW
contributed to data collection, data analysis and interpretation.
YHY is responsible for the overall design of the experiment, the
revision of the contents and the publication of the paper.
Ethics approval and consent to
participate
All animal procedures in the present study were
approved by the Animal Care and Use Committee of Tianjin Medical
University and in accordance with the National Institutes of Health
Guide for the Care and Use of Laboratory Animals.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bailey CP, Llorente J, Gabra BH, Smith FL,
Dewey WL, Kelly E and Henderson G: Role of protein kinase C and
mu-opioid receptor (MOPr) desensitization in tolerance to morphine
in rat locus coeruleus neurons. Eur J Neurosci. 29:307–318. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chu J, Zheng H, Loh HH and Law PY:
Morphine-induced mu-opioid receptor rapid desensitization is
independent of receptor phosphorylation and beta-arrestins. Cell
Signal. 20:1616–1624. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sim-Selley LJ, Scoggins KL, Cassidy MP,
Smith LA, Dewey WL, Smith FL and Selley DE: Region-dependent
attenuation of mu opioid receptor-mediated G-protein activation in
mouse CNS as a function of morphine tolerance. Br J Pharmacol.
151:1324–1333. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bull FA, Baptista-Hon DT, Sneddon C,
Wright L, Walwyn W and Hales TG: Src kinase inhibition attenuates
morphine tolerance without affecting reinforcement or psychomotor
stimulation. Anesthesiology. 127:878–889. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wilson-Poe AR, Jeong HJ and Vaughan CW:
Chronic morphine reduces the readily releasable pool of GABA, a
presynaptic mechanism of opioid tolerance. J Physiol.
595:6541–6555. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lim G, Wang S, Zeng Q, Sung B and Mao J:
Spinal glucocorticoid receptors contribute to the development of
morphine tolerance in rats. Anesthesiology. 102:832–837. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lim G, Wang S, Zeng Q, Sung B and Mao J:
Evidence for a long-term influence on morphine tolerance after
previous morphine exposure: Role of neuronal glucocorticoid
receptors. Pain. 114:81–92. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Narita M, Ioka M, Suzuki M, Narita M and
Suzuki T: Effect of repeated administration of morphine on the
activity of extracellular signal regulated kinase in the mouse
brain. Neurosci Lett. 324:97–100. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Robbins TW and Everitt BJ: Drug addiction:
Bad habits add up. Nature. 398:567–570. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Williams JT, Christie MJ and Manzoni O:
Cellular and synaptic adaptations mediating opioid dependence.
Physiol Rev. 81:299–343. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Deng XT, Han Y, Liu WT and Song XJ: B
vitamins potentiate acute morphine antinociception and attenuate
the development of tolerance to chronic morphine in mice. Pain Med.
18:1961–1974. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pan Y, Sun X, Jiang L, Hu L, Kong H, Han
Y, Qian C, Song C, Qian Y and Liu W: Metformin reduces morphine
tolerance by inhibiting microglial-mediated neuroinflammation. J
Neuroinflammation. 13:2942016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bao Y, Gao Y, Yang L, Kong X, Yu J, Hou W
and Hua B: The mechanism of mu-opioid receptor (MOR)-TRPV1
crosstalk in TRPV1 activation involves morphine anti-nociception,
tolerance and dependence. Channels (Austin). 9:235–243. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jiang C, Xu L, Chen L, Han Y, Tang J, Yang
Y, Zhang G and Liu W: Selective suppression of microglial
activation by paeoniflorin attenuates morphine tolerance. Eur J
Pain. 19:908–919. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gutstein HB, Rubie EA, Mansour A, Akil H
and Woodgett JR: Opioid effects on mitogen-activated protein kinase
signaling cascades. Anesthesiology. 87:1118–1126. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fukuda K, Kato S, Morikawa H, Shoda T and
Mori K: Functional coupling of the delta-, mu-, and kappa-opioid
receptors to mitogen-activated protein kinase and arachidonate
release in Chinese hamster ovary cells. J Neurochem. 67:1309–1316.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cameron SA and Dutia MB: Lesion-induced
plasticity in rat vestibular nucleus neurones dependent on
glucocorticoid receptor activation. J Physiol. 518:151–158. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Garabedian MJ, Harris CA and Jeanneteau F:
Glucocorticoid receptor action in metabolic and neuronal function.
F1000Res. 6:12082017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Takahashi M, Sugimachi K and Kaneto H:
Role of adrenal glucocorticoids in the blockade of the development
of analgesic tolerance to morphine by footshock stress exposure in
mice. Jpn J Pharmacol. 51:329–336. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Clark AR and Lasa M: Crosstalk between
glucocorticoids and mitogen-activated protein kinase signalling
pathways. Curr Opin Pharmacol. 3:404–411. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang S, Lim G, Zeng Q, Sung B, Ai Y, Guo
G, Yang L and Mao J: Expression of central glucocorticoid receptors
after peripheral nerve injury contributes to neuropathic pain
behaviors in rats. J Neurosci. 24:8595–8605. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ma W and Quirion R: The ERK/MAPK pathway,
as a target for the treatment of neuropathic pain. Expert Opin Ther
Targets. 9:699–713. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mayer DJ, Mao J, Holt J and Price DD:
Cellular mechanisms of neuropathic pain, morphine tolerance, and
their interactions. Proc Natl Acad Sci USA. 96:7731–7736. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
LoPachin RM, Rudy TA and Yaksh TL: An
improved method for chronic catheterization of the rat spinal
subarachnoid space. Physiol Behav. 27:559–561. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mao J: Opioid-induced abnormal pain
sensitivity: Implications in clinical opioid therapy. Pain.
100:213–217. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cui Y, Chen Y, Zhi JL, Guo RX, Feng JQ and
Chen PX: Activation of p38 mitogen-activated protein kinase in
spinal microglia mediates morphine antinociceptive tolerance. Brain
Res. 1069:235–243. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mao J, Price DD and Mayer DJ: Experimental
mononeuropathy reduces the antinociceptive effects of morphine:
Implications for common intracellular mechanisms involved in
morphine tolerance and neuropathic pain. Pain. 61:353–364. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gardell LR, Wang R, Burgess SE, Ossipov
MH, Vanderah TW, Malan TP Jr, Lai J and Porreca F: Sustained
morphine exposure induces a spinal dynorphin-dependent enhancement
of excitatory transmitter release from primary afferent fibers. J
Neurosci. 22:6747–6755. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Trang T, Quirion R and Jhamandas K: The
spinal basis of opioid tolerance and physical dependence:
Involvement of calcitonin gene-related peptide, substance P, and
arachidonic acid-derived metabolites. Peptides. 26:1346–1355. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Guo D and Hu J: Spinal presynaptic
inhibition in pain control. Neuroscience. 283:95–106. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
DeLander GE, Portoghese PS and Takemori
AE: Role of spinal mu opioid receptors in the development of
morphine tolerance and dependence. J Pharmacol Exp Ther. 231:91–96.
1984.PubMed/NCBI
|
|
32
|
Pitcher GM, Yashpal K, Coderre TJ and
Henry JL: Mechanisms underlying antinociception provoked by
heterosegmental noxious stimulation in the rat tail-flick test.
Neuroscience. 65:273–281. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cintra A, Molander C and Fuxe K:
Colocalization of Fos- and glucocorticoid
receptor-immunoreactivities is present only in a very restricted
population of dorsal horn neurons of the rat spinal cord after
nociceptive stimulation. Brain Res. 632:334–338. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
De Nicola AF, Moses DF, Gonzalez S and
Orti E: Adrenocorticoid action in the spinal cord: Some unique
molecular properties of glucocorticoid receptors. Cell Mol
Neurobiol. 9:179–192. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Capasso A, Di Giannuario A, Loizzo A,
Pieretti S and Sorrentino L: Central interaction of dexamethasone
and RU-38486 on morphine antinociception in mice. Life Sci.
51:PL139–PL143. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang Z, Ma W, Chabot JG and Quirion R:
Cell-type specific activation of p38 and ERK mediates calcitonin
gene-related peptide involvement in tolerance to morphine-induced
analgesia. FASEB J. 23:2576–2586. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Croxtall JD, Choudhury Q and Flower RJ:
Glucocorticoids act within minutes to inhibit recruitment of
signalling factors to activated EGF receptors through a
receptor-dependent, transcription-independent mechanism. Br J
Pharmacol. 130:289–298. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mazzucchelli C, Vantaggiato C, Ciamei A,
Fasano S, Pakhotin P, Krezel W, Welzl H, Wolfer DP, Pages G,
Valverde O, et al: Knockout of ERK1 MAP kinase enhances synaptic
plasticity in the striatum and facilitates striatal-mediated
learning and memory. Neuron. 34:807–820. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tai YH, Wang YH, Wang JJ, Tao PL, Tung CS
and Wong CS: Amitriptyline suppresses neuroinflammation and
up-regulates glutamate transporters in morphine-tolerant rats.
Pain. 124:77–86. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wen ZH, Wu GJ, Chang YC, Wang JJ and Wong
CS: Dexamethasone modulates the development of morphine tolerance
and expression of glutamate transporters in rats. Neuroscience.
133:807–817. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wong CS, Hsu MM, Chou R, Chou YY and Tung
CS: Intrathecal cyclooxygenase inhibitor administration attenuates
morphine antinociceptive tolerance in rats. Br J Anaesth.
85:747–751. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chen Y, Yu YH, Zhai ML, Wang Z and Wang
GL: Participation of glucocorticoid receptors in morphine tolerance
development through the signal pathway of extracellular
signal-regulated kinase. Zhonghua Yi Xue Za Zhi. 91:1272–1275.
2011.(In Chinese). PubMed/NCBI
|
|
43
|
Martel MA, Wyllie DJ and Hardingham GE: In
developing hippocampal neurons, NR2B-containing
N-methyl-D-aspartate receptors (NMDARs) can mediate signaling to
neuronal survival and synaptic potentiation, as well as neuronal
death. Neuroscience. 158:334–343. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Urakubo H, Honda M, Froemke RC and Kuroda
S: Requirement of an allosteric kinetics of NMDA receptors for
spike timing-dependent plasticity. J Neurosci. 28:3310–3323. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ota KT, Pierre VJ, Ploski JE, Queen K and
Schafe GE: The NO-cGMP-PKG signaling pathway regulates synaptic
plasticity and fear memory consolidation in the lateral amygdala
via activation of ERK/MAP kinase. Learn Mem. 15:792–805. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Toyoda H, Zhao MG, Xu H, Wu LJ, Ren M and
Zhuo M: Requirement of extracellular signal-regulated
kinase/mitogen-activated protein kinase for long-term potentiation
in adult mouse anterior cingulate cortex. Mol Pain. 3:362007.
View Article : Google Scholar : PubMed/NCBI
|