Introduction
Currently, cardiovascular diseases remain a leading
cause of mortality worldwide. Among cardiovascular diseases,
coronary heart diseases (CHDs) are frequently occurring
cardiovascular diseases (1). It is
estimated that 110 million people succumbed to CHD annually in 2015
(2). Approximately half of
patients with CHD suffer from acute myocardial infarction and
sudden cardiac mortality, of which, the complications bring
economic burden to families and society (3,4).
Myocardial ischemia-reperfusion (MI/R) injury is a common clinical
pathophysiological phenomenon in CHD, which fails to restore normal
cardiac function, and aggravates the dysfunction and structural
impairments of the heart (5). The
clinical manifestations of MI/R are arrhythmia, no reflow
phenomenon, myocardial stunning and necrocytosis (6). Currently, there is no effective
therapy to prevent MI/R. Thus, the search of novel therapeutic
agents and therapeutic strategies for MI/R is important.
RAC-α serine/threonine-protein kinase (Akt), also
termed protein kinase B, serves as a critical tumor factor and a
downstream effector of phosphatidylinositol 3′-kinase (PI3K)
(7). Previous studies have
reported that MI/R induces the apoptosis of cardiomyocytes
(8,9), and that the upregulation of PI3K/Akt
pathway may suppress myocardial apoptosis (10). It was additionally demonstrated
that the PI3K/Akt pathway inhibited myocardial apoptosis to protect
the heart through numerous mechanisms, including by suppressing
caspase-3 activation and DNA damage (11), affecting the metabolism of glucose
(12), enhancing the effects of
the Bcl-2 family (13) and
inhibiting the expression of apoptosis-regulating genes (14). However, to the best of our
knowledge, limited research regarding the involvement of the
PI3K/Akt pathway in myocardial apoptosis induced by MI/R injury
exists.
Apigenin is an important flavonoid that exhibits a
variety of physiological and pharmacological activities, and is
associated with the prevention of cardiovascular diseases (15). Previous studies have demonstrated
that apigenin possess multiple pharmacological activities,
including anti-oxidant (16),
anti-mutagenesis (17),
anti-inflammatory (18) and
antitumor (19). However, the
pharmacological activity and effect of apigenin on myocardial
apoptosis induced by MI/R is unclear.
The present study aimed to investigate whether
apigenin acts as a novel therapeutic agent that affects myocardial
apoptosis induced by MI/R. Furthermore, the exact roles and
mechanisms of apigenin, together with the PI3K/Akt pathway in MI/R
injury were investigated.
Materials and methods
Establishment of H9C2 cells subjected
to oxygen-glucose deprivation/reoxygenation (OGD/R) injury as a
model of MI/R
H9C2 rat cardiomyocytes were obtained from The Cell
Bank of Type Culture Collection of Chinese Academy of Sciences
(Shanghai, China). The H9C2 cells were treated as described in
previous literature to establish an in vitro model of MI/R
(20,21), and the protocols were further
developed in the present study. Medium with no serum or glucose
served as the ischemic buffer. The ischemic buffer was incubated in
an atmosphere with a gas mixture of 95% N2 and 5%
CO2 for 2 h. The cells were subsequently cultured in
glucose-free Dulbecco's modified Eagle's medium (DMEM) containing
10% fetal bovine serum (both from Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA), in an anoxic environment (95%
N2 and 5% CO2) at 37°C for 2 h. The H9C2
cells were transferred to fresh medium (DMEM) at 37°C and the gas
mixture of 95% N2 and 5% CO2 was replaced
with air at a flow rate of velocity of 2 l/min (95% O2
and 5% CO2). Following incubation for 1 h, the MI/R cell
model was harvested and cells were subsequently observed under an
inverted microscope.
Grouping
A total of five treatment groups were prepared in
the present study: The control group (H9C2 cells with no
treatment); the MI/R group (H9C2 cells treated with OGD/R injury);
the 1 µM apigenin + MI/R group (H9C2 cells treated with OGD/R
injury, and subsequently treated with 1 µM apigenin); the 6 µM
apigenin + MI/R group (H9C2 cells treated with OGD/R injury, and
subsequently treated with 6 µM apigenin); and the 25 µM apigenin +
MI/R group (H9C2 cells treated with OGD/R injury, and subsequently
treated with 25 µM apigenin). Inhibition of PI3K was performed via
incubation with LY294002 (25 µM) for 2 h as previously described
(22).
Cell viability analysis
A Cell Counting Kit-8 (CCK-8; Beyotime Institute of
Biotechnology, Haimen, China) was used to determine the cell
viability of the H9C2 cells. H9C2 cells (6×104 cells/ml)
cultured in the logarithmic phase were seeded in 96-well plates,
and subsequently maintained in a 5% CO2 atmosphere at
37°C for 12 h. Apigenin at different concentrations (1, 3, 6, 12,
25 and 50 µM) was added to the cells. The H9C2 cells were
maintained for 12, 24 and 48 h. Subsequently, 10 µl CCK-8 reagent
was added to the wells of the 96-well plates and the H9C2 cells
were maintained for a further 3 h. A microplate reader (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) was used to record the
absorbance at 450 nm. Cell viability was evaluated as the
percentage of cell survival compared with the control.
Enzyme activity detection
H9C2 cells were seeded into 96-well plates
(6×103 cells/well) and treated according to the
aforementioned protocol. Cells were subsequently collected and the
content/activity of lactate dehydrogenase (LDH), malondialdehyde
(MDA), catalase (CAT), Na+K+-ATPase and
Ca2+-ATPase were determined using a LDH Assay Kit (cat.
no. ab102526), MDA Assay Kit (cat. no. ab118970) (both from Abcam,
Cambridge, UK), CAT Assay Kit (cat. no. BC0205),
Na+K+-ATPase assay kit (cat. no. BC0065) and
Ca2+-ATPase assay kit (cat. no. BC0960) (all from
Beijing Solarbio Science & Technology Co., Ltd.), respectively,
in accordance with the manufacturers' protocol.
Apoptosis assay
Annexin V is a phospholipid binding protein, which
has a high affinity for phosphatidylserine. Annexin V is a
sensitive indicator for detecting early apoptosis of cells.
Propidium iodide (PI) is a type of nucleic acid dye that is not
able to penetrate an intact cell membrane. PI penetrates the cell
membrane as cell membrane permeability increases in the late stage
of apoptosis. Therefore, Annexin V and PI may be used to
distinguish cells in different apoptotic periods. Flow cytometry
(FCM) was conducted to assess the apoptosis of H9C2 cells.
Following washing with PBS, cultured H9C2 cells were trypsinized
with 0.25% trypsin (Beyotime Institute of Biotechnology, Haimen,
China). The supernatant was collected and the H9C2 cells were
prepared for assessment by suspension in an incubation buffer at a
density of 1×106 cells/ml. H9C2 cells were subsequently
incubated with Annexin V-fluorescein isothiocyanate and PI (XiLong
Scientific Co., Ltd., Shantou, China) in the dark at room
temperature for 15 min. A flow cytometer (FACSCalibur; BD
Biosciences, Franklin Lakes, CA, USA) with CellQuest software
version 3.3 was used to measure cellular apoptosis.
Evaluation of mitochondrial membrane
potential (MMP) and reactive oxygen species (ROS)
PBS was added to the cultured H9C2 cells until the
cell density reached 1×106/ml. FL1-H [Multisciences
(Lianke) Biotech Co., Ltd., Hangzhou, China] was added to the H9C2
cells. H9C2 cells were incubated in the dark at room temperature
for 10 min. The supernatant was collected and 100 µl PBS was added
to the H9C2 cells. ROS have the ability to transform
dichloro-dihydro-fluorescein diacetate (DCFH-DA) into
2′,7′-dichlorofluorescein (DCF). The fluorescence of DCF indicates
the ROS level. The DCFH-DA dye (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) was used to detect ROS generation, according to
previous literature (23,24). The fluorescence dye Rhodamine-123
(Rho-123) is able to penetrate the cell membrane. As described in
previous studies (25,26), Rho-123 (Sigma Aldrich; Merck KGaA)
was used to detect the MMP in the present study. FCM was performed
with an excitation wavelength of 488 nm to assess the MMP and ROS
levels in H9C2 cells. Cells (1×104) were collected from
each sample, and the data were analyzed using BD
CellQuest™ Pro software (version 3.3; BD
Biosciences).
Western blot analysis
Cultured cells were lysed on ice in a
radioimmunoprecipitation assay lysis buffer (Thermo Scientific
Inc.; cat. no. 89900). The cells were fragmented following
treatment with an ultrasonic cell disruptor. Following
centrifugation at 5,000 × g at 4°C, fractionation occurred and the
supernatant was collected. The protein expression level was
measured using a protein assay reagent (Bio-Rad Laboratories, Inc.)
and was performed following the manufacturer's protocol. From each
sample, an equal quantity of protein (50 µg) was separated using 5%
SDS-PAGE. The proteins were obtained and transferred to
nitrocellulose membranes (EMD Millipore, Billerica, MA, USA) for
1.5 h. Nonspecific proteins were blocked by immersing the membranes
into 5% low fat dried milk at room temperature for 2 h following
washing with PBS. The membranes were incubated with primary
antibodies: Anti-poly ADP-ribose polymerase (PARP; 1:5,000; cat.
no. ab32138); anti-cleaved-caspase-3 (1:500; cat. no. ab49822);
tumor necrosis factor receptor superfamily member 6 (Fas; 1:2,000;
cat. no. ab133619); tumor necrosis factor ligand superfamily member
6 (Fasl; 1:8,000; cat. no. ab186671); anti-phospho (p)-PI3K (1:800;
cat. no. ab182651); anti-PI3K (1:1,000; cat. no. ab189403);
anti-p-Akt (1:500; ab38449); anti-Akt (1:6,000; cat. no. ab81283);
anti-apoptosis regulator Bcl-2 (Bcl-2; 1:1,000; cat. no. ab59348);
anti-p-serine/threonine-protein kinase mTOR (mTOR; phospho S2448;
1:1,000; cat. no. ab109268); anti-mTOR (1:1,000; cat. no. ab32028);
and anti-GAPDH (1:2,500; cat. no. ab9485) (all from Abcam)
overnight at 4°C. Horseradish peroxidase-conjugated secondary
antibodies (1:5,000; Abcam; cat. no. ab205718; goat anti-rabbit)
were added and incubated at room temperature for 1 h. Enhanced
chemiluminescent reagents (EMD Millipore) with an enhanced
chemiluminescence system (GE Healthcare Life Sciences, Little
Chalfont, UK) were performed to assess the results. Densitometric
analysis was performed using Quantity One software (version 4.6.9;
Bio-Rad Laboratories, Inc.).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
TRIzol® reagent (Beyotime Institute of
Biotechnology) was used to extract the total RNA from the cultured
H9C2 cells. RNA was reverse transcribed to cDNA using an RT kit
(Beyotime Institute of Biotechnology), according to the
manufacturers' protocol. The temperature protocol used for RT was
as follows: 25°C for 10 min, 42°C for 50 min and 70°C for 5 min.
RT-qPCR analysis was performed using SYBR-Green PCR Master Mix
(Thermo Fisher Scientific, Inc.) on an ABI 7500 Thermocycler
(Applied Biosystems; Thermo Fisher Scientific, Inc.). PCR cycling
conditions were as follows: Pretreatment at 95°C for 10 min; 96°C
for 15 sec; 65°C for 45 sec (45 cycles); 96°C for 15 sec; 65°C for
1 min; 95°C for 15 sec; and a final extension at 75°C for 10 min,
maintained at 4°C. The results were quantified using the
2−ΔΔCq method (27).
The primers were purchased from Invitrogen (Thermo Fisher
Scientific, Inc.): PARP forward, 5′-CTGTTAATGCTAATCGTGAT-3′ and
reverse, 5′-AACTGACTCCTACATATTAG-3′ (product, 223 bp); Fas forward,
5′-AGTACAGCCGGGAAGACAAT-3′ and reverse, 5′-TTTCTGGGCCATGCTTCTCT-3′
(product, 269 bp); Fasl forward, 5′-CAGAAAGCATGATCCGCGAC-3′ and
reverse, 5′-GGTCTGGGCCATAGAACTGA-3′ (product, 215 bp); GAPDH
forward, 5′-TCTGAACTCCAACGATGCCT-3′ and reverse,
5′-TCTTGTCCTTAAGCCTGGGG-3′ (product, 225 bp). GAPDH was used as the
control to normalize the input RNA level.
Statistical analysis
The results are presented as the mean ± standard
deviation. The data was evaluated using one-way analysis of
variance followed by Tukey's multiple comparisons. Statistical
analyses were performed using GraphPad Prism software (version 6.0;
GraphPad Software, Inc., La Jolla, CA, USA). P<0.05 was
considered to indicate a statistically significant difference. All
experiments were performed in triplicate.
Results
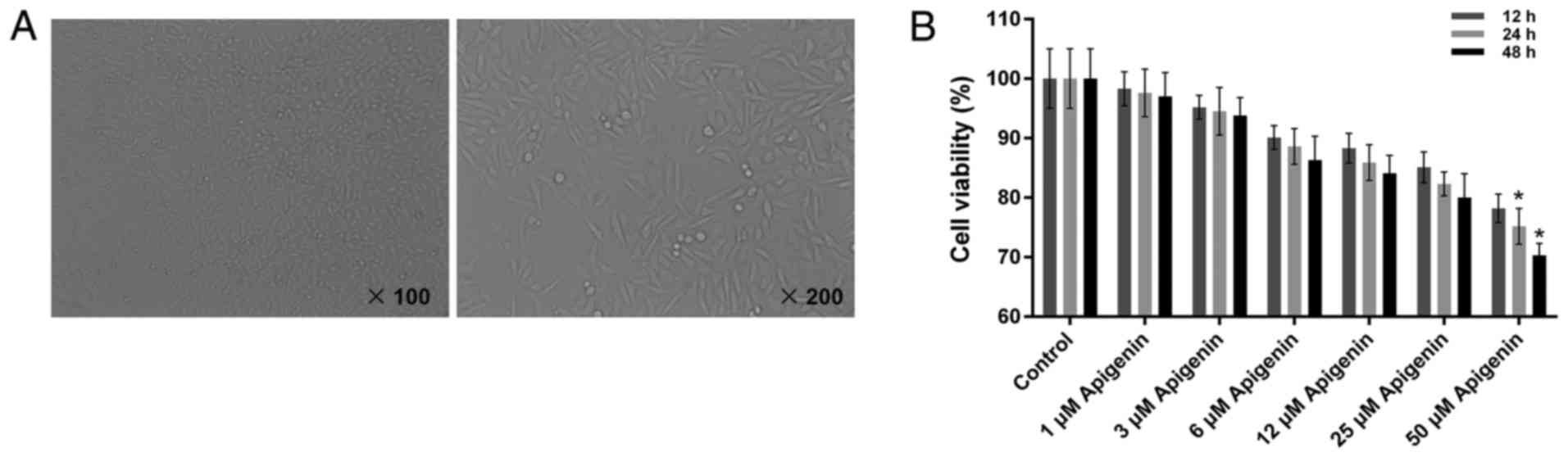
Characterization of the H9C2 rat
myocardial cell line
The cultured cardiomyocytes were observed with an
inverted microscope (Fig. 1A). The
shape of the H9C2 cells was round prior to adhesion. However, after
4–6 h, the majority of the cardiomyocytes began to adhere to the
wall of culture bottle, exhibiting a round or spindle shape.
Following culture for 24 h, the cells were arrayed in long
fusiform, with a few arranged in triangular or irregular polygonal
form. Additionally, cell nuclei were small and the cytoplasm was
dark. After 48 h, cells dispersed gradually at the bottom of
culture bottle, forming a cell monolayer. The cardiomyocytes were
subsequently harvested for further experiments.
Apigenin has limited cytotoxic
activity on the cell viability of H9C2 cells
To evaluate the cytotoxic activity of apigenin on
normal H9C2 cells, a CCK-8 assay was performed to determine the
cell viability of H9C2 cells. The present results demonstrate that
apigenin at concentrations of 1, 3, 6, 12 and 25 µM did not induce
significant cytoxicity in H9C2 cells. The cell viability of H9C2
cells treated with apigenin was >80% with the exception that the
cell viability of H9C2 cells was significantly lower compared with
the control when the concentration of apigenin was 50 µM (Fig. 1B; P<0.05). Thus, we selected 1,
6 and 25 µM to treat the H9C2 cells for 12 h for the subsequent
experiments.
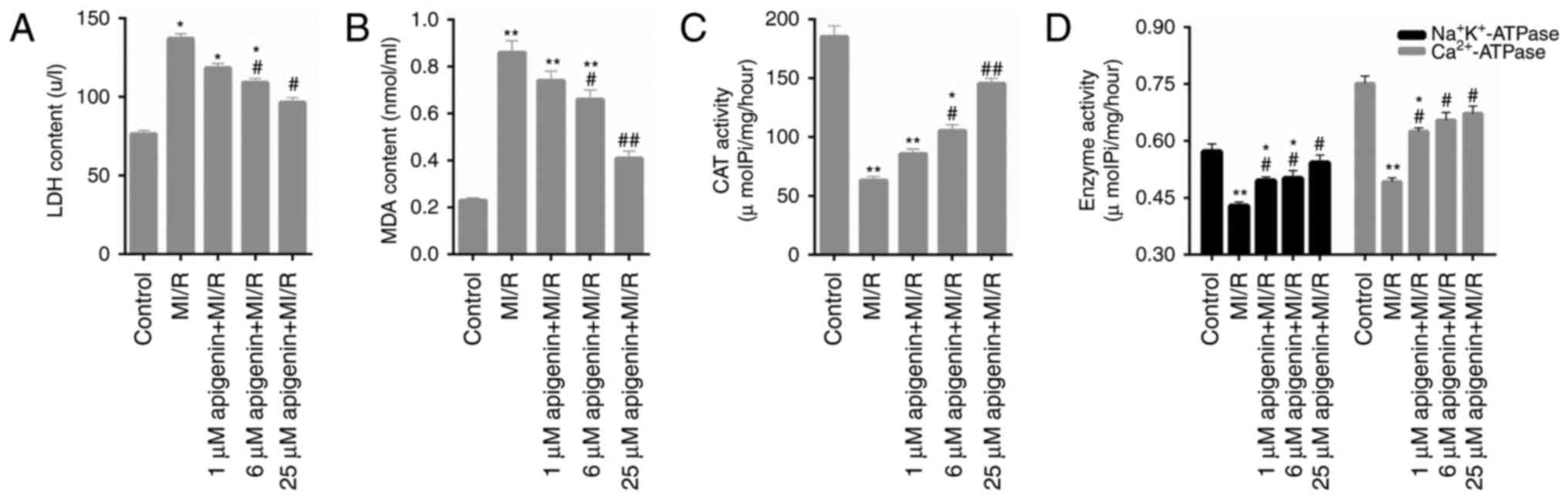
Oxidative stress in MI/R-induced H9C2
cells is decreased by apigenin
Numerous oxidative stress markers in H9C2 cells,
including LDH, MDA, CAT and ATPase activity, were assessed in the
present study. The data indicated that the LDH content was
significantly increased by MI/R injury (Fig. 2A; P<0.05); however, reduced upon
treatment with apigenin. MDA content in H9C2 cells additionally
exhibited similar trends following treatment with MI/R injury and
apigenin (Fig. 2B; P<0.05) at
different concentrations. The CAT activity in H9C2 cell treated
with MI/R injury was significantly decreased compared with the
control group (Fig. 2C;
P<0.01). However, apigenin markedly enhanced the activity of CAT
in MI/R-induced H9C2 cells (Fig.
2C). It was additionally observed that apigenin significantly
increased the activities of Na+K+-ATPase and
Ca2+-ATPase in H9C2 cells induced by MI/R injury
(Fig. 2D; P<0.05).
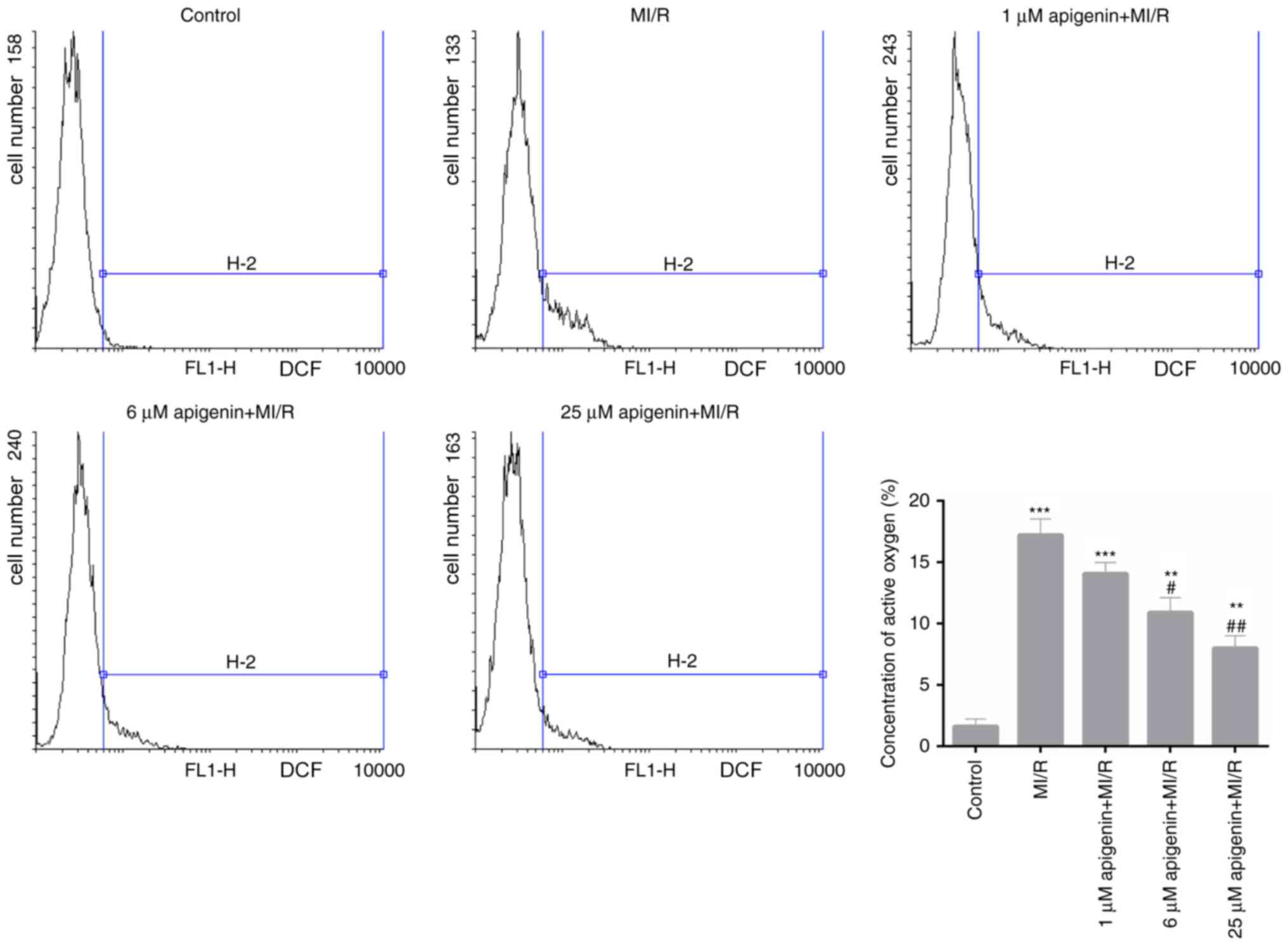
Apigenin reduces ROS production in
MI/R-induced H9C2 cells
The ROS content was measured in H9C2 cells from each
treatment group. According to the FCM data, MI/R injury
significantly increased the ROS production in H9C2 cells (Fig. 3; P<0.001). However, following
treatment with apigenin at different concentration levels, a
dose-dependent decrease in the ROS content in MI/R-induced H9C2
cells was observed, which was significant at 6 and 25 µM compared
with MI/R injury (Fig. 3;
P<0.05).
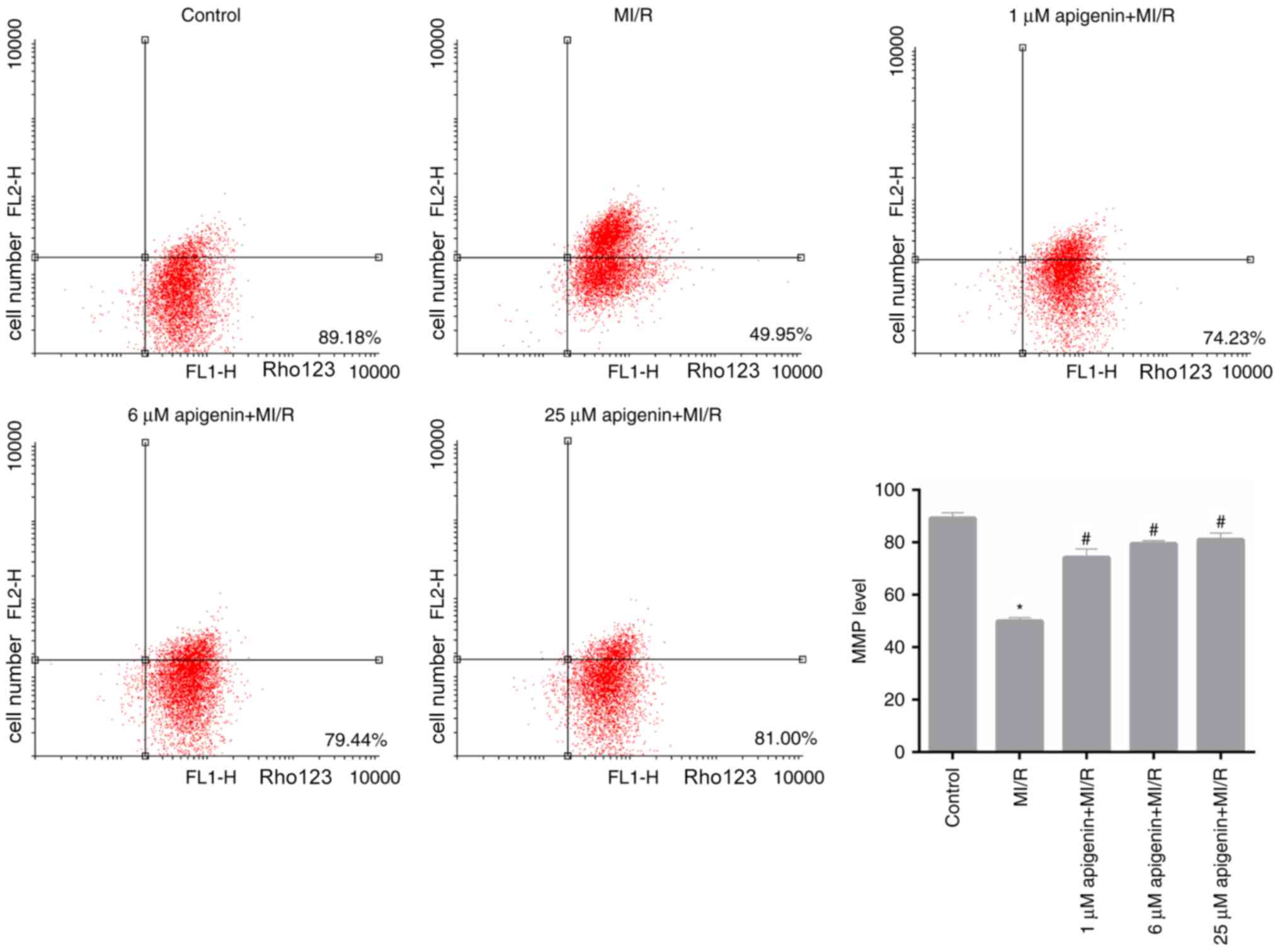
Apigenin modulates the MMP level in
MI/R-induced H9C2 cells
Furthermore, in the present study, MMP level in H9C2
cells, which were treated with MI/R and apigenin at different
concentrations, was measured. Based on the FCM data, it was
observed that MI/R significantly reduced the MMP level in H9C2
cells compared with the control (Fig.
4; P<0.05). Following treatment with apigenin at different
concentrations, the MMP level in MI/R-induced H9C2 cells
significantly recovered (Fig. 4;
P<0.05).
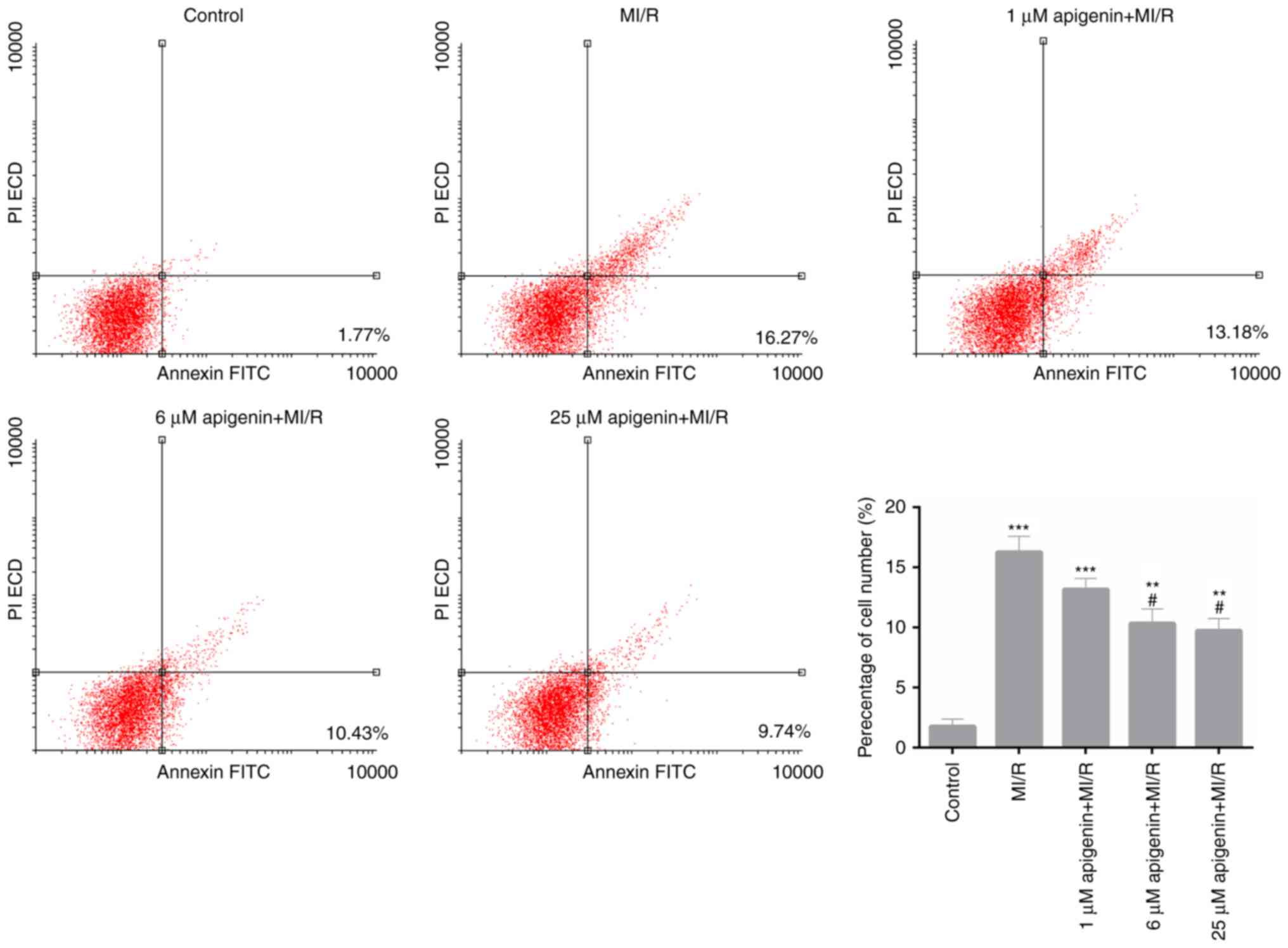
Apigenin suppresses the MI/R-mediated
apoptosis of H9C2 cells
As the aforementioned data suggested that apigenin
may decrease the oxidative stress in MI/R-induced H9C2 cells, the
apoptosis capacity of MI/R-induced H9C2 cells that treated with
apigenin was evaluated. The FCM data demonstrated that the
percentage of apoptotic H9C2 cells in the MI/R group was 16.27%,
which was significantly increased compared with the control
(Fig. 5; 1.77%; P<0.001).
Furthermore, following treatment with apigenin at different
concentrations of 1, 6 and 25 µM, the apoptosis rate of
MI/R-induced H9C2 cells decreased from 16.27 to 13.18, 10.43 and
9.74%, respectively (Fig. 5;
P<0.01). These data indicated that apigenin suppressed the
apoptosis capacity of MI/R-induced H9C2 cells in a dose-dependent
manner.
Expression of apoptosis-associated
proteins is regulated by apigenin
It was demonstrated that apigenin inhibited
apoptosis of H9C2 cells induced by MI/R; therefore,
apoptosis-associated protein expression in H9C2 cells was measured.
According to the RT-qPCR data, PARP, Fas and Fasl expression in
H9C2 cells from the MI/R group was significantly higher than the
control group (Fig. 6A;
P<0.001). The PARP, Fas, and Fasl expression in MI/R-induced
H9C2 cells was markedly decreased upon treatment with apigenin in a
dose-dependent manner (Fig. 6A).
Furthermore, western blot analysis additionally demonstrated that
the expression levels of PARP, cleaved caspase-3, Fas and Fasl in
H9C2 cells were significantly upregulated upon MI/R injury compared
with the control (Fig. 6B;
P<0.0001); whereas, the expression of these molecules was
markedly decreased with the addition of apigenin at different
concentrations (Fig. 6B).
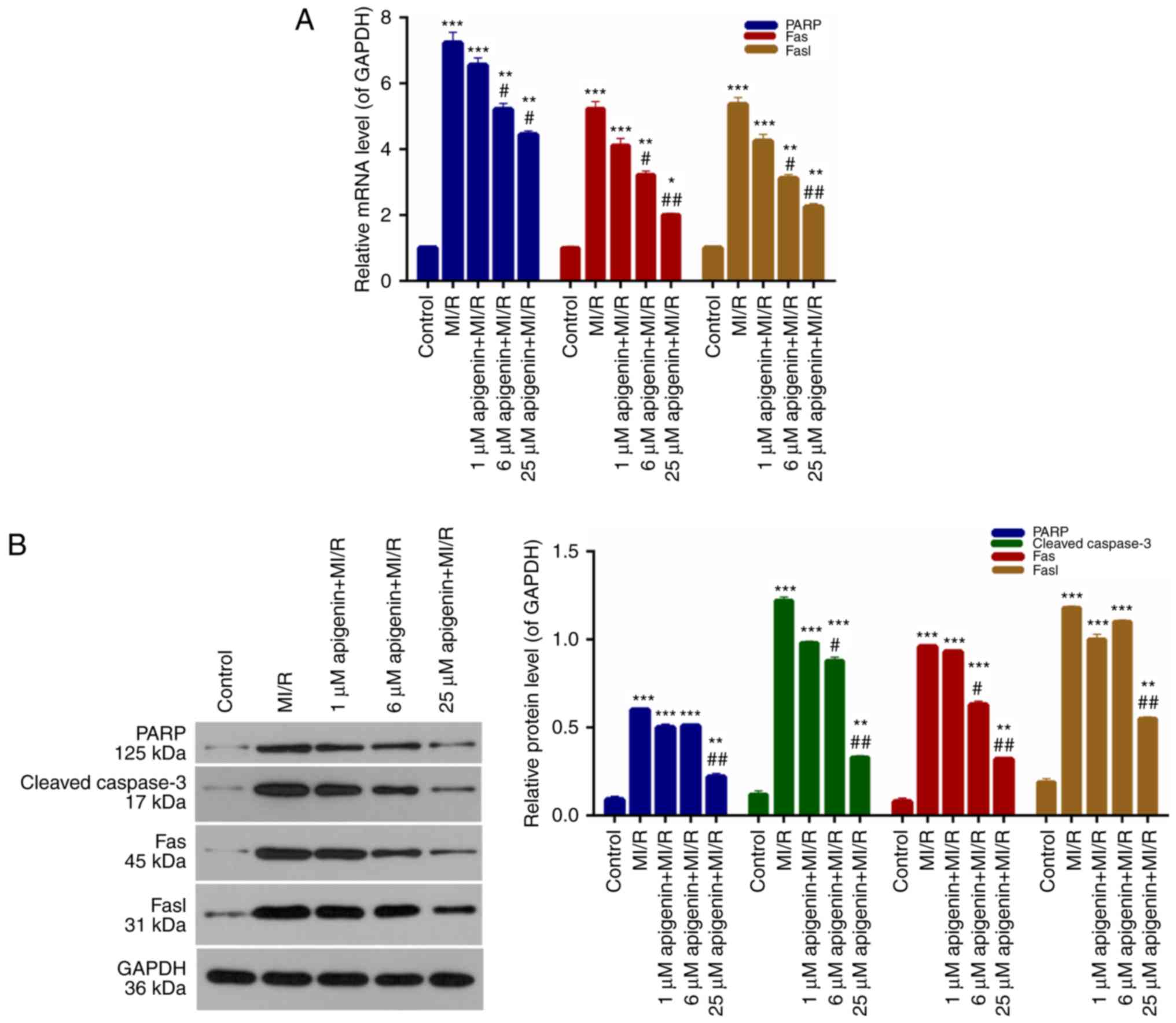 | Figure 6.Apigenin regulates the expression
levels of apoptosis-associated proteins. (A) Reverse
transcription-quantitative polymerase chain reaction and (B)
western blot analysis were conducted to evaluate the expression
levels of PARP, caspase-3, Fas, and Fasl in H9C2 cells treated with
MI/R, and 1, 6 and 25 µM apigenin + MI/R. *P<0.05, **P<0.01
and ***P<0.001 vs. control; #P<0.05 and
##P<0.01 vs. MI/R. MI/R, myocardial
ischemia-reperfusion; PARP, poly ADP-ribose polymerase; Fas, tumor
necrosis factor receptor superfamily member 6; Fasl, tumor necrosis
factor ligand superfamily member 6. |
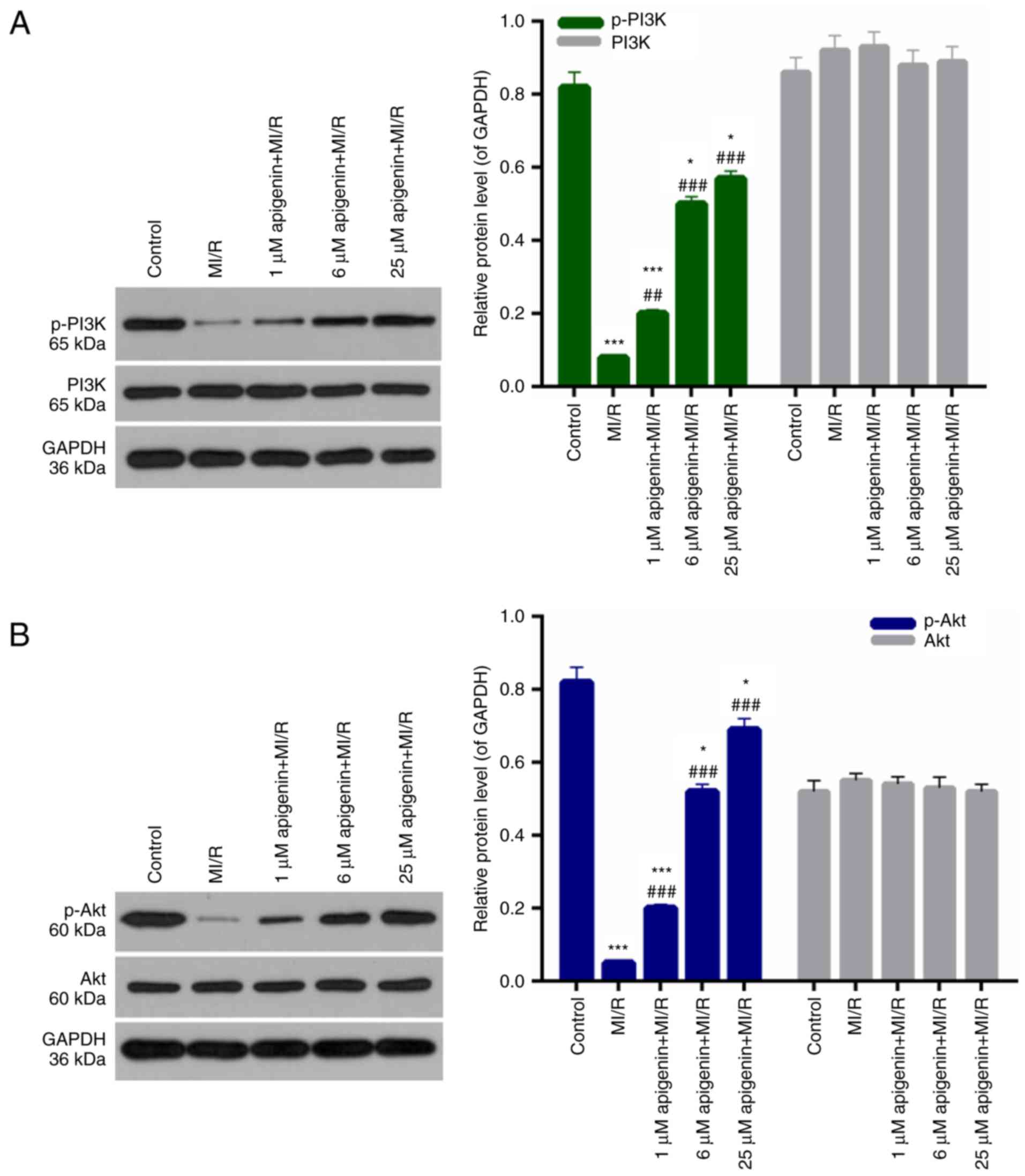
Apigenin affects the PI3K/Akt
pathway
In addition, the mechanisms of apigenin in
protecting H9C2 cells against MI/R induced injury were examined.
The p-PI3K, PI3K, p-Akt, and Akt level in H9C2 cells was measured.
Among the H9C2 cells from MI/R groups, the level of p-PI3K was
significantly decreased compared with the control (Fig. 7A; P<0.001). However, significant
increases were observed in the p-PI3K level in MI/R-induced H9C2
cells treated with apigenin at different concentrations (Fig. 7A; P<0.01). Furthermore, there
was no significant difference in the expression of PI3K in H9C2
cells between each treatment group (Fig. 7A; P>0.05). Additionally, the
level of p-Akt in H9C2 cells was significantly downregulated by
MI/R injury (Fig. 5B; P<0.001).
The decreased level of p-Akt in MI/R-induced cardiomyocytes was
significantly recovered upon treatment with apigenin (Fig. 5B; P<0.001). Additionally, there
was no significant difference in the expression level of Akt in
H9C2 cells among the treatment groups (Fig. 7B; P>0.05).
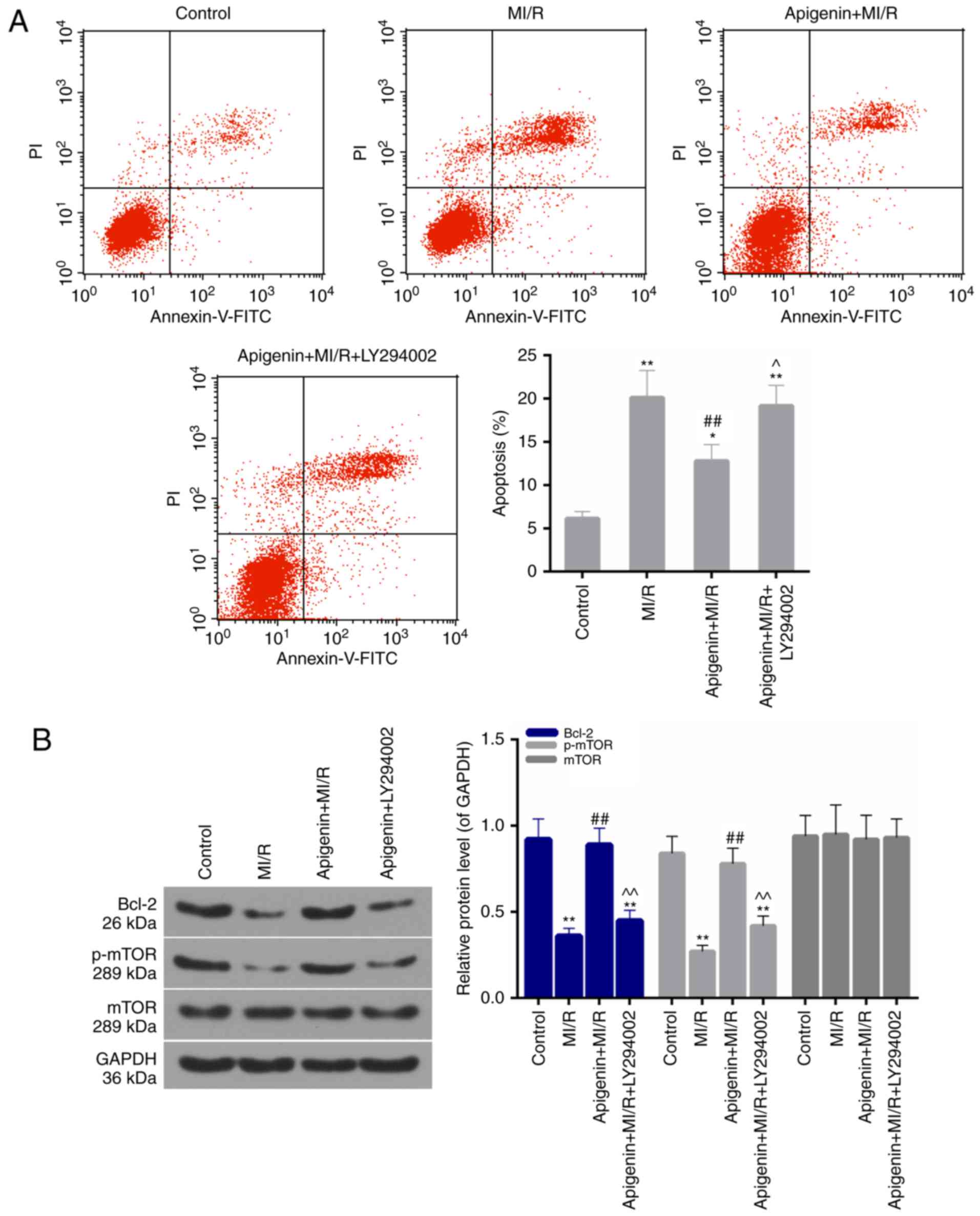
LY294002 reverses the anti-apoptotic
effect of apigenin
To further confirm the role of PI3K/Akt in the
present study model, LY294002 was used to block the activity of
PI3K. It was revealed that the apoptosis rate was significantly
increased in the MI/R + apigenin + LY294002 group compared with the
MI/R + apigenin group (Fig. 8A;
P<0.05). Furthermore, the expression of Bcl-2 and p-mTOR
decreased upon treatment with LY294002 compared with the apigenin +
MI/R group (Fig. 8B;
P<0.01).
Discussion
Myocardial ischemia is common in patients with CHD
(28,29). Myocardial ischemia and hypoxia lead
to myocardial apoptosis and necrosis, resulting in heart damage.
Early restoration of blood flow and reperfusion is important for
the primary treatment of CHDs (30). Secondary impairment caused by
reperfusion, including arrhythmia, myocardial stunning and
myocardial energy metabolism disorders, has become a major clinical
problem. There are numerous potential factors contributing to CHD,
including increased levels of oxygen free radicals, neutrophil
infiltration, calcium overload, complement involvement, myocardial
apoptosis, gene expression changes and the impact of enzymes
(31). However, to recover the
dying myocardium and reduce the myocardial infarction area, MI/R is
necessary (32). Therefore, the
prevention and therapy for MI/R injury and search for effective
therapeutic agents are of particular significance.
Apigenin was previously hypothesized to be involved
in the protection of anoxia/reoxygenation-induced myocardium injury
in a previous study (33).
Therefore, apigenin was selected as the focus for the present
study, and its role and mechanism in MI/R injury of cardiomyocytes
was examined. In the present study, the cytotoxicity of apigenin
was evaluated. A CCK-8 assay was performed to determine the cell
viability of H9C2 cells. The present results demonstrated that
apigenin had limited cytotoxicity to H9C2 cells. Previous studies
suggested that apigenin exhibits the function of suppressing
oxidative stress in early brain injury and Parkinson's disease
(34,35). LDH, MDA and CAT are considered to
represent oxidative stress markers, and are involved in the
regulation of the intracellular redox state (36). Therefore, the expression levels of
LDH, MDA and CAT in H9C2 cells following I/R injury and apigenin
treatment at different concentrations was assessed. Apigenin
reduced the LDH and MDA contents in MI/R-induced H9C2 cells.
Furthermore, apigenin markedly enhanced the activity of CAT in
MI/R-induced H9C2 cells. ATPase is a type of protease that exists
in tissue cells and organelle membranes, which serves an important
role in material transport, energy conversion and information
transfer (37). In particular,
Na+K+-ATPase and Ca2+-ATPase serve
crucial roles in the modulation of oxidative stress (38,39).
Therefore, the activities of Na+K+-ATPase and
Ca2+-ATPase in H9C2 cells treated with MI/R injury and
apigenin at different concentrations were examined. It was observed
that apigenin may enhance the activities of
Na+K+-ATPase and Ca2+-ATPase in
MI/R-induced H9C2 cells. Therefore, apigenin may be involved in
reducing oxidative stress in MI/R injury.
Previous studies have suggested that ROS content in
cells is associated with typical characteristics of oxidative
stress (40,41). In the current study, MI/R resulted
in ROS overproduction in H9C2 cells, whereas, apigenin markedly
reduced the ROS production in MI/R-induced H9C2 cells. These
results suggested that apigenin alleviated the oxidative stress in
H9C2 cells that was induced by MI/R injury, potentially by
regulating the levels of LDH, MDA, CAT, ATPases and ROS. Therefore,
it was demonstrated that apigenin may reduce the oxidative stress
in H9C2 cells induced by MI/R injury.
Mitochondrial dysfunction has been reported to
participate in the induction of apoptosis and has even been
suggested to be important in the apoptotic pathway (42,43).
Indeed, opening of the mitochondrial permeability transition pore
was hypothesized to induce depolarization of the transmembrane
potential, release of apoptogenic factors and loss of oxidative
phosphorylation (44).
Furthermore, a previous study demonstrated that a close association
exists between the mitochondria and oxidative stress (45). As apigenin may reduce the oxidative
stress in H9C2 cells induced by MI/R injury, it was investigated
whether apigenin affected the MMP of H9C2 cells subjected to MI/R
injury. According to the present results, the MMP level in H9C2
cells was decreased upon MI/R injury, and apigenin markedly
increased the MMP level in MI/R-induced H9C2 cells. Additionally,
it has been reported that apigenin attenuated the apoptosis of
adriamycin-induced cardiomyocytes (46). However, the impact of apigenin on
myocardial apoptosis induced by MI/R injury remains unclear.
As apigenin reduced the oxidative stress and
enhanced the MMP level in MI/R-induced H9C2 cells, it was
hypothesized that apigenin may suppress myocardial apoptosis
induced by MI/R injury. The apoptotic capacity of H9C2 cells
treated with MI/R and apigenin at different concentrations was
studied. The FCM data indicated that apigenin markedly decreased
the apoptotic capacity of MI/R-induced H9C2 cells. Furthermore, the
apoptosis-associated mechanisms in MI/R-induced H9C2 cells that
affected by apigenin were investigated. The present results
demonstrated that apigenin significantly downregulated the
expression levels of PARP, cleaved caspase-3, Fas, and Fasl in
MI/R-induced H9C2 cells. Therefore, apigenin suppressed the
MI/R-induced apoptosis of H9C2 cells and modulated the expression
levels of oxidative stress markers, MMP and apoptosis-associated
proteins.
Previous studies suggested that the PI3K/Akt pathway
is involved in the apoptotic process of cardiomyocytes (47–49).
However, the role and mechanism of apigenin in the regulation of
the PI3K/Akt pathway in MI/R-induced H9C2 cells remains unclear.
The possible mechanism of the PI3K/Akt pathway in the suppression
of MI/R-induced H9C2 cell apoptosis was investigated. According to
the western blot analysis, it was observed that apigenin enhanced
the phosphorylation of PI3K and Akt in MI/R-induced H9C2 cells.
These results suggested that apigenin affects the phosphorylation
of PI3K and Akt in MI/R-induced H9C2 cells. Furthermore, the
inhibition of PI3K mediated by LY294002 reversed the anti-apoptotic
effect of apigenin by suppressing cellular apoptosis, indicated by
the decreased expression level of Bcl-2, which is an anti-apoptotic
protein. As a downstream target of PI3K/Akt, mTOR may be
phosphorylated. The inhibition of PI3K/Akt/mTOR is associated with
apoptosis (50). Treatment with
LY294002 additionally decreased the phosphorylation of mTOR in the
current study. It was confirmed that apigenin inhibited the
apoptosis of MI/R-induced H9C2 cells by regulating the
phosphorylation levels of PI3K and Akt. The present study
demonstrated that apigenin suppressed the apoptosis of H9C2 cells
subjected to MI/R injury by affecting the PI3K/Akt pathway;
however, the exact molecular mechanisms underlying this apoptotic
effect remain unclear.
In conclusion, the present study demonstrated that
apigenin suppressed the apoptosis of H9C2 cells subjected to MI/R
injury by affecting the PI3K/Akt pathway. The results provided a
novel insight for understanding the pathogenesis of MI/R and the
mechanisms of apigenin in cardiomyocytes. The potential effects of
apigenin on the suppression of myocardial apoptosis suggest that
apigenin may be an effective MI/R therapeutic.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated and/or analyzed during this study
are included in this published article.
Authors' contributions
JZ designed the study. YZ and LL performed the
experiments. ZZ wrote the manuscript and analyzed the data. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
McCullough PA: Coronary artery disease.
Clin J Am Soc Nephrol. 2:611–616. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
GBD 2015 DALYs and HALE Collaborators:
Global, regional, and national disability-adjusted life-years
(DALYs) for 315 diseases and injuries and healthy life expectancy
(HALE), 1990–2015: A systematic analysis for the Global Burden of
Disease Study 2015. Lancet. 388:1603–1658. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Arslan F, Lai RC, Smeets MB, Akeroyd L,
Choo A, Aguor EN, Timmers L, van Rijen HV, Doevendans PA,
Pasterkamp G, et al: Mesenchymal stem cell-derived exosomes
increase ATP levels, decrease oxidative stress and activate
PI3K/Akt pathway to enhance myocardial viability and prevent
adverse remodeling after myocardial ischemia/reperfusion injury.
Stem Cell Res. 10:301–312. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Iwasaki K: Myocardial ischemia is a key
factor in the management of stable coronary artery disease. World J
Cardiol. 6:130–139. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hausenloy DJ and Yellon DM: Myocardial
ischemia-reperfusion injury: A neglected therapeutic target. J Clin
Invest. 123:92–100. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shin IW, Jang IS, Lee SM, Park KE, Ok SH,
Sohn JT, Lee HK and Chung YK: Myocardial protective effect by
ulinastatin via an anti-inflammatory response after regional
ischemia/reperfusion injury in an in vivo rat heart model. Korean J
Anesthesiol. 61:499–505. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Larue L and Bellacosa A:
Epithelial-mesenchymal transition in development and cancer: Role
of phosphatidylinositol 3′-kinase/AKT pathways. Oncogene.
24:7443–7454. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fliss H and Gattinger D: Apoptosis in
ischemic and reperfused rat myocardium. Circ Res. 79:949–956. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Saraste A, Pulkki K, Kallajoki M,
Henriksen K, Parvinen M and Voipio-Pulkki LM: Apoptosis in human
acute myocardial infarction. Circulation. 95:320–323. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dhanasekaran A, Gruenloh SK, Buonaccorsi
JN, Zhang R, Gross GJ, Falck JR, Patel PK, Jacobs ER and Medhora M:
Multiple antiapoptotic targets of the PI3K/Akt survival pathway are
activated by epoxyeicosatrienoic acids to protect cardiomyocytes
from hypoxia/anoxia. Am J Physiol Heart Circ Physiol.
294:H724–H735. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wu W, Lee WL, Wu YY, Chen D, Liu TJ, Jang
A, Sharma PM and Wang PH: Expression of constitutively active
phosphatidylinositol 3-kinase inhibits activation of caspase 3 and
apoptosis of cardiac muscle cells. J Biol Chem. 275:40113–40119.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vander Heiden MG, Plas DR, Rathmell JC,
Fox CJ, Harris MH and Thompson CB: Growth factors can influence
cell growth and survival through effects on glucose metabolism. Mol
Cell Biol. 21:5899–5912. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Henshall DC, Araki T, Schindler CK, Lan
JQ, Tiekoter KL, Taki W and Simon RP: Activation of
Bcl-2-associated death protein and counter-response of Akt within
cell populations during seizure-induced neuronal death. J Neurosci.
22:8458–8465. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bartling B, Tostlebe H, Darmer D, Holtz J,
Silber RE and Morawietz H: Shear stress-dependent expression of
apoptosis-regulating genes in endothelial cells. Biochem Biophys
Res Commun. 278:740–746. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rodriguez-Mateos A, Vauzour D, Krueger CG,
Shanmuganayagam D, Reed J, Calani L, Mena P, Del Rio D and Crozier
A: Bioavailability, bioactivity and impact on health of dietary
flavonoids and related compounds: An update. Arch Toxicol.
88:1803–1853. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Singh JP, Selvendiran K, Banu SM,
Padmavathi R and Sakthisekaran D: Protective role of Apigenin on
the status of lipid peroxidation and antioxidant defense against
hepatocarcinogenesis in Wistar albino rats. Phytomedicine.
11:309–314. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Birt DF, Walker B, Tibbels MG and Bresnick
E: Anti-mutagenesis and anti-promotion by apigenin, robinetin and
indole-3-carbinol. Carcinogenesis. 7:959–963. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Funakoshi-Tago M, Nakamura K, Tago K,
Mashino T and Kasahara T: Anti-inflammatory activity of
structurally related flavonoids, apigenin, luteolin and fisetin.
Int Immunopharmacol. 11:1150–1159. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shukla S and Gupta S: Apigenin: A
promising molecule for cancer prevention. Pharm Res. 27:962–778.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yin Y, Guan Y, Duan J, Guo W, Zhu Y, Wei
Q, Guo C, Zhou D, Wang Y, Xi M and Wen A: Cardioprotective effect
of Danshensu against myocardial ischemia/reperfusion injury and
inhibits apoptosis of H9c2 cardiomyocytes via Akt and ERK1/2
phosphorylation. Eur J Pharmacol. 699:219–226. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Doukas J, Wrasidlo W, Noronha G,
Dneprovskaia E, Fine R, Weis S, Hood J, Demaria A, Soll R and
Cheresh D: Phosphoinositide 3-kinase/inhibition limits infarct size
after myocardial ischemia/reperfusion injury. Proc Natl Acad Sci
USA. 103:19866–19871. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhai M, He L, Ju X, Shao L, Li G, Zhang Y,
Liu Y and Zhao H: Icariin acts as a potential agent for preventing
cardiac ischemia/reperfusion injury. Cell Biochem Biophys.
72:589–597. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nishimura S, Akagi M, Yoshida K, Hayakawa
S, Sawamura T, Munakata H and Hamanishi C: Oxidized low-density
lipoprotein (ox-LDL) binding to lectin-like ox-LDL receptor-1
(LOX-1) in cultured bovine articular chondrocytes increases
production of intracellular reactive oxygen species (ROS) resulting
in the activation of NF-kappaB. Osteoarthritis Cartilage.
12:568–576. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kadirve G, Kumar S, Ghosh SK and Perumal
P: Activity of antioxidative enzymes in fresh and frozen thawed
buffalo (Bubalus bubalis) spermatozoa in relation to lipid
peroxidation and semen quality. Asian Pac J Repro. 3:210–217. 2014.
View Article : Google Scholar
|
|
25
|
Qin J, Kang Y, Xu Z, Zang C, Fang B and
Liu X: Dioscin prevents the mitochondrial apoptosis and attenuates
oxidative stress in cardiac H9c2 cells. Drug Res (Stuttg).
64:47–52. 2014.PubMed/NCBI
|
|
26
|
Shen ZY, Shen WY, Chen MH, Shen J, Cai WJ
and Zeng Y: Mitochondria, calcium and nitric oxide in the apoptotic
pathway of esophageal carcinoma cells induced by As2O3. Int J Mol
Med. 9:385–390. 2002.PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Vanamali A: Climate strategy is critical
for india's future. Am Heart J. 163:20–26. 2011.PubMed/NCBI
|
|
29
|
Wei J, Velazquez EJ, Samad Z, Kuchibhatla
M, Martsberger C, Rogers J, Williams R, Kuhn C, Ortel TL, Becker
RC, et al: Responses of mental stress induced myocardial ischemia
to escitalopram treatment: Background, design, and method for the
REMIT Trial. Am Heart J. 163:202012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sirvinskas E, Kinderyte A, Trumbeckaite S,
Lenkutis T, Raliene L, Giedraitis S, Macas A and Borutaite V:
Effects of sevoflurane vs. propofol on mitochondrial functional
activity after ischemia-reperfusion injury and the influence on
clinical parameters in patients undergoing CABG surgery with
cardiopulmonary bypass. Perfusion. 30:590–595. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yuan SM and Jing H: Insights into the
monomers and single drugs of Chinese herbal medicine on myocardial
preservation. Afr J Tradit Complement Altern Med. 8:104–127.
2011.PubMed/NCBI
|
|
32
|
Lu F, Fernandes SM and Davis AE III: The
effect of C1 inhibitor on myocardial ischemia and reperfusion
injury. Cardiovasc Pathol. 22:75–80. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen C, He H, Luo Y, Zhou M, Yin D and He
M: Involvement of Bcl-2 signal pathway in the protective effects of
apigenin on anoxia/reoxygenation-induced myocardium injury. J
Cardiovasc Pharmacol. 67:152–163. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Anusha C, Sumathi T and Joseph LD:
Protective role of apigenin on rotenone induced rat model of
Parkinson's disease: Suppression of neuroinflammation and oxidative
stress mediated apoptosis. Chem Biol Interact. 269:67–79. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Han Y, Zhang T, Su J, Zhao Y, Chenchen
Wang and Li X: Apigenin attenuates oxidative stress and neuronal
apoptosis in early brain injury following subarachnoid hemorrhage.
J Clin Neurosci. 40:157–162. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Naveen P, Kumar NS, Sanjeev G, Gowda KMD
and Madhu LN: Radioprotective effect of Nardostachys
jatamansi against whole body electron beam induced oxidative
stress and tissue injury in rats. J Phar Res. 4:2197–2200.
2011.
|
|
37
|
Thastrup O, Cullen PJ, Drobak BK, Hanley
MR and Dawson AP: Thapsigargin, a tumor promoter, discharges
intracellular Ca2+ stores by specific inhibition of the
endoplasmic reticulum Ca2(+)-ATPase. Proc Natl Acad Sci USA.
87:2466–2470. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Scherer NM and Deamer DW: Oxidative stress
impairs the function of sarcoplasmic reticulum by oxidation of
sulfhydryl groups in the Ca2+-ATPase. Arch Biochem
Biophys. 246:589–601. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang XQ, Xiao AY, Sheline C, Hyrc K, Yang
A, Goldberg MP, Choi DW and Yu SP: Apoptotic insults impair
Na+, K+-ATPase activity as a mechanism of
neuronal death mediated by concurrent ATP deficiency and oxidant
stress. J Cell Sci. 116:2099–2110. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Apel K and Hirt H: Reactive oxygen
species: Metabolism, oxidative stress, and signal transduction.
Annu Rev Plant Biol. 55:373–399. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Guerin P, El Mouatassim S and Menezo Y:
Oxidative stress and protection against reactive oxygen species in
the pre-implantation embryo and its surroundings. Hum Reprod
Update. 7:175–189. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Irwin WA, Bergamin N, Sabatelli P,
Reggiani C, Megighian A, Merlini L, Braghetta P, Columbaro M,
Volpin D, Bressan GM, et al: Mitochondrial dysfunction and
apoptosis in myopathic mice with collagen VI deficiency. Nat Genet.
35:367–371. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
43
|
Borutaite V, Jekabsone A, Morkuniene R and
Brown GC: Inhibition of mitochondrial permeability transition
prevents mitochondrial dysfunction, cytochrome c release and
apoptosis induced by heart ischemia. J Mol Cell Cardiol.
35:357–366. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ly JD, Grubb DR and Lawen A: The
mitochondrial membrane potential (deltapsi(m)) in apoptosis; an
update. Apoptosis. 8:115–128. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ott M, Gogvadze V, Orrenius S and
Zhivotovsky B: Mitochondria, oxidative stress and cell death.
Apoptosis. 12:913–922. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yu W, Sun H, Zha W, Cui W, Xu L, Min Q and
Wu J: Apigenin attenuates adriamycin-induced cardiomyocyte
apoptosis via the PI3K/AKT/mTOR pathway. Evid Based Complement
Alternat Med. 2017:25906762017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Jia Y, Zuo D, Li Z, Liu H, Dai Z, Cai J,
Pang L and Wu Y: Astragaloside IV inhibits doxorubicin-induced
cardiomyocyte apoptosis mediated by mitochondrial apoptotic pathway
via activating the PI3K/Akt pathway. Chem Pharm Bull (Tokyo).
62:45–53. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yang B, Yan P, Gong H, Zuo L, Shi Y, Guo
J, Guo R, Xie J and Li B: TWEAK protects cardiomyocyte against
apoptosis in a PI3K/AKT pathway dependent manner. Am J Transl Res.
8:3848–3860. 2016.PubMed/NCBI
|
|
49
|
Yu W, Zha W, Ke Z, Min Q, Li C, Sun H and
Liu C: Curcumin protects neonatal rat cardiomyocytes against high
glucose-induced apoptosis via PI3K/Akt signalling pathway. J
Diabetes Res. 2016:41585912016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Saiki S, Sasazawa Y, Imamichi Y, Kawajiri
S, Fujimaki T, Tanida I, Kobayashi H, Sato F, Sato S and Ishikawa
K: Caffeine induces apoptosis by enhancement of autophagy via
PI3K/Akt/mTOR/p70S6K inhibition. Autophagy. 7:176–187. 2011.
View Article : Google Scholar : PubMed/NCBI
|