Introduction
Diabetic nephropathy (DN) is by far the most common
cause of end-stage renal disease (ESRD) in industrial countries,
making up approximately 45% of all new ESRD cases in the United
States (1). Its pathogenesis is a
chronic and complex process. At the later stages of DN, the gradual
decline of renal function, tubular atrophy and interstitial
fibrosis occurs (2). Several
studies have shown that apoptosis could be considered a vital
component in the processes of DN (3–6).
However, several pathways can induce apoptosis, including the
intrinsic pathway, the extrinsic pathway and the endoplasmic
reticulum stress (ERS) pathway (7).
The ER is the site of lipid biosynthesis, protein
folding and protein maturation in eukaryotic cells. The ER is
extremely sensitive to the factors that affect intracellular energy
levels, the oxidation state, and the calcium concentration. When
the cells receive a shock (such as from hypoxia, and toxic drugs),
the ER environment is destroyed, inducing calcium metabolism
disorder, ER function disorder, an increase in unfolded or
misfolded proteins in the ER, and calcium imbalance. We designated
this state as ERS. One major response of ERS is dissociation of
glucose-regulated protein 78 (GRP78) with transmembrane receptor
which leads to its activation to deal with the accumulated unfolded
proteins (8).
Phosphorylated-extracellular signal-regulated kinase (p-ERK) is
transmembrane protein in the ER, which plays a signal transduction
role. p-ERK is known as an initial, crucial protector for survival
during even mild stress (9).
Slight and medium ERS can protect the cell from death, but severe
ERS induces Caspase-12-dependent cell apoptosis (9). Since the ERS has both protective and
deleterious features, a better understanding of the molecular
pathways of the ERS could reveal novel therapeutic strategies in
chronic renal diseases, including diabetic kidney disease.
Grape seed proanthocyanidin extracts (GSPE) are
derived from grape seeds and have been shown to possess potent
antioxidant, anti-inflammatory, radical-resistance, anti-tumor, and
cardiovascular protecting properties (10–12).
In this study, we treated a streptozotocin (STZ)-induced DN rat
model with GSPE to investigate the effect of GSPE on STZ-induced
DN. In order to ascertain whether a protective effect of GSPE on DN
occurred through the inhibition of ERS-induced apoptosis, We
investigated the protein expression levels of GRP78, p-ERK and
Caspase-12 by Western blotting and immunohistochemical staining. We
used TUNEL kit to detect apoptosis.
Materials and methods
Reagents
Grape seed proanthocyanidin (purity exceeds 96%, Lot
no. G050412) was purchased from Tianjin Jianfeng Natural Product
R&D Co., Ltd. (Tianjin, China). STZ was purchased from Sigma.
TUNEL staining kit (in situ Cell Death Detection kit) was
obtained from Roche Diagnostics, (Indianapolis, IN, USA). Primary
antibodies used in this study include: Rabbit anti-Caspase-12,
rabbit anti-GRP78 (Abcam Ltd., Hong Kong, China), rabbit
anti-p-ERK, rabbit anti-ERK antibodies (Cell Signaling Technology,
Inc., Danvers, MA, USA), mouse anti-β-actin (Santa Cruz
Biotechnology, Inc., Dallas, TX, USA). Anti-mouse and anti-rabbit
secondary antibodies were purchased from Jackson ImmunoResearch
Laboratories, Inc. (West Grove, PA, USA).
Animals
Fifty-five adult male SD rats (8–12 weeks of age),
weighing 120–160 g were provided by the Beijing Vital River
Laboratory Animal Technology Co., Ltd (Beijing, China). The current
study was approved by the Animal Ethics Committee of Shandong
University (no. DWLL-2013-053; Jinan, China).
Forty-five randomly selected rats were given a
high-fat diet (45% fat, 20% protein and 35% carbohydrate, as a
percentage of total kcal; product no. D12451, provided by the
Beijing Vital River Laboratory Animal Technology Co., Ltd.) for one
month, and then intraperitoneally injected with 40 mg/kg STZ
dissolved in pH 4.5 citrate buffer, while the ten remaining rats
were given the same dosage of citrate buffer at pH 4.5 and given a
normal diet (N group). Hyperglycemia was confirmed by measuring the
venous circulating plasma concentration of glucose. Seven days
post-STZ injection, blood samples were obtained from the rat tail
vein after 12 h of fasting, and the glucose concentration was
determined with an automatic analyzer (a Comfore automatic analyzer
purchased from Shanghai). Forty rats showed the diabetic rat
standard of a fasting blood glucose level higher than 300 mg/dl,
while the value in the control group of rats injected with citrate
buffer ranged from 90 to 130 mg/dl. Then, twenty randomly selected
rats were continued on the high-fat diet (DM group) until 16 weeks,
and the other twenty rats received intragastric administration of
250 mg/kg/day GSPE as well as the high-fat diet (GSPE group) until
16 weeks. At the end of the experiment, 16 rats survived in the DM
group and 15 in the GSPE group. Three rats in the GSPE group died
of asphyxiation due to gavage errors. The animals were placed in
individual metabolic cages for 24 h to collect urine samples before
the experimental rats were sacrificed. No difference of total water
intake among groups was observed. Total urinary protein (g/l) was
measured by the sulfosalicylic acid method (722N; China) (13). At the end of the experiments, the
animals were fasted overnight for 18 h and then anesthetized with
intraperitoneal injection of sodium pentobarbitone (60 mg/kg;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and sacrificed. All
animals were weighed before sacrifice. Blood was collected from the
heart before sacrifice, and the serum was separated by
centrifugation (912 × g at 4°C for 20 min) and stored at −80°C
until it was assayed. Each kidney was cut in half by coronal plane
after excision and weighed. Two pieces of kidney were stored at
−80°C for a Western blotting analysis. The other pieces were fixed
in 4% buffered paraformaldehyde at 4°C and embedded in paraffin for
immunohistochemical histopathologic observation, and a terminal
deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL)
assay.
Assessments of renal function
Blood urea nitrogen (BUN) and serum creatinine (Scr)
were analyzed on a Cobas 8000 (Roche Diagnostics) in Qilu Hospital,
Shandong University. The renal index (RI) was calculated as
follows: both kindey weights (g)/animal's weight (g) ×100.
Histopathologic and
Immunohistochemical staining
The renal pathological changes were determined by
periodic acid-Schiff (PAS) staining. The kidneys were sliced and
fixed in 4% paraformaldehyde for no more than 24 h and then taken
to 0.5% paraformaldehyde for long-term preservation. They were
embedded in paraffin and cut into 4-µm-thick sections for staining
with PAS or immunohistochemical staining.
The PAS-positive area present in the mesangial
region excluding cellular elements indicated mesangial matrix
expansion. The percentage of the PAS-positive area in the
glomerulus was analyzed by using Leica QWin version 3 image
analysis software (Leica Microsystems GmbH, Wetzlar, Germany).
For immunohistochemical analysis, the tissue slices
were microwaved for 10 to 15 min in 0.01% sodium citrate buffer (pH
6.0) for antigen retrieval. The tissue slices were cooled at room
temperature or in ice water and then washed with PBS three times.
The tissue slices were immersed in 0.1% Triton X-100 for 15 min. To
block endogenous peroxidase, all tissue slices were incubated with
3% hydrogen peroxide for 10 min in the dark. The tissue slices were
incubated with 10% goat serum for 60 min at 37°C, then with primary
antibody at 4°C overnight (anti-GRP78 1:200, anti-p-ERK 1:50,
anti-Caspase-12 1:100). The negative control sections were
incubated with PBS instead of the primary antibody. All sections
were incubated with secondary antibodies for 60 min at 37°C and
then stained with DAB and hematoxylin. The stained slides were
analyzed by light microscopy. Brown areas were designated as
positive. A semi-quantitative analysis was performed on the colored
sections using an Image-Pro Plus 5.0.
TUNEL staining
TUNEL staining was performed according to the
manufacturer's instructions. The sections were incubated with
proteinase K at 37°C for 15 min, then washed in PBS for 5 min; the
enzyme solution and label solution were then mixed (1:9) at 37°C
for 60 min in the dark. The sections immersed in label solution
served as negative controls. The sections were then stained with
DAPI for 10 min. TUNEL-positive nuclei were expressed as a
percentage of the total nuclei per field. Ten fields per section
and two sections per kidney were assayed in each experiment.
Proteins sample preparation
The tissue samples were homogenized in TRIzol
(50–100 mg tissue per 1 ml TRIzol). After chloroform was added (0.2
ml chloroform per 1 ml TRIzol), the homogenates were centrifuged at
12,000 × g for 15 min at 4°C. The supernatants were discarded,
isopropanol was added (1.5 ml isopropanol per 1 ml TRIzol) to the
lower phase, and then the samples were centrifuged at 12,000 × g
for 15 min at 4°C. The pellets were washed with 0.3 M guanidine
hydrochloride (2 ml guanidine hydrochloride per 1 ml TRIzol) three
times, then dissolved in 1% SDS (100 µl SDS per 1 ml TRIzol) at
50°C for 30 min. The concentration of protein was determined using
the Pierce BCA assay kit.
Western blotting
Protein (50 µg) was subjected to 10% or 12%
SDS-polyacrylamide gel electrophoresis and transferred to cellulose
acetate membranes. The membranes were blocked at room temperature
for 1 h or more with 5% skim milk, then incubated with the primary
antibodies (anti-GRP78 1:250, anti-p-ERK 1:1,000, anti-ERK 1:1,000,
anti-caspase 12 1:500, anti-β-actin 1:2,500) at 4°C overnight.
After incubation with the secondary antibodies at room temperature
for 1 h, the membranes were soaked with enhanced chemiluminescence
(ECL) reagent and exposed to X-ray film. Quantification of the
luminosity of each identified protein band was performed using
Adobe Photoshop software (Adobe Photoshop 7.0; Adobe, San Jose, CA,
2002). The Western blotting analysis used a ratio of GRP78, p-ERK,
and Caspase-12 in the N group to the other groups.
Statistical analysis
Data were displayed as means ± standard deviation.
Differences between groups were analyzed using the post hoc test
used for multiple comparisions following one-way ANOVA. P<0.05
was considered to indicate a statistically significant
difference.
Results
GSPE protects against STZ-induced
DN
As shown in Table
I, the blood glucose in the DM group and the GSPE group was
higher during the entire experiment, and it was statistically
significant compared with the control group. As shown in Table II, 10 rats survived in the N
group, 16 rats survived in the DM group, and 15 rats survived in
the GSPE group at the end of the study. The BUN level and Scr had
no significant changes among the three groups, but the 24-h urinary
albumin level and the RI increased in the DM group compared with
the N group. Compared with the DM group, the level of 24-h urine
albumin and RI significantly decreased in the GSPE group.
 | Table I.Blood glucose of animals. |
Table I.
Blood glucose of animals.
|
| Blood glucose
(mmol/l) |
|---|
|
|
|
|---|
| Group | 4 weeks | 8 weeks | 12 weeks | 16 weeks |
|---|
| N | 7.05±0.73 | 7.24±0.46 | 7.33±0.47 | 7.04±1.06 |
| D | 7.74±1.09 |
25.28±4.50a |
24.23±4.21a |
26.49±3.14a |
| G | 7.74±1.09 |
26.15±4.38a |
24.14±5.03a |
24.89±3.05a |
 | Table II.Animal data and biochemical
parameters. |
Table II.
Animal data and biochemical
parameters.
| Group | n | RI | Pro
(×10−3 g) | BUN (mmol/l) | Scr (umol/l) |
|---|
| N | 10 | 0.64±0.036 | 5.48±1.36 | 6.28±0.43 | 50.2±7.79 |
| D | 16 |
1.07±0.074a |
44.82±5.39a | 5.84±0.81 | 49.63±7.21 |
| G | 15 |
0.98±0.085a,b |
35.12±6.89a,b | 5.79±0.70 | 48.00±5.29 |
The histopathology results are shown in Fig. 1. PAS staining indicated the
presence of glomerular hypertrophy and thickening of the glomerular
basement membrane in the DM group, which showed that the diabetic
rats suffered from diabetic nephrology. In the GSPE group, the
glomerular changes were significantly reduced, which indicated that
the GSPE had a protective effect against diabetic nephrology.
Quantitative analysis of the percentage of the PAS-positive area in
the glomeruli is summarized in the histogram. The extracellular
matrix (ECM) accumulation was significantly higher in the glomeruli
of the D group than that of the N group (P<0.05), and the G
group was significantly shorter than that of the D group
(P<0.05).
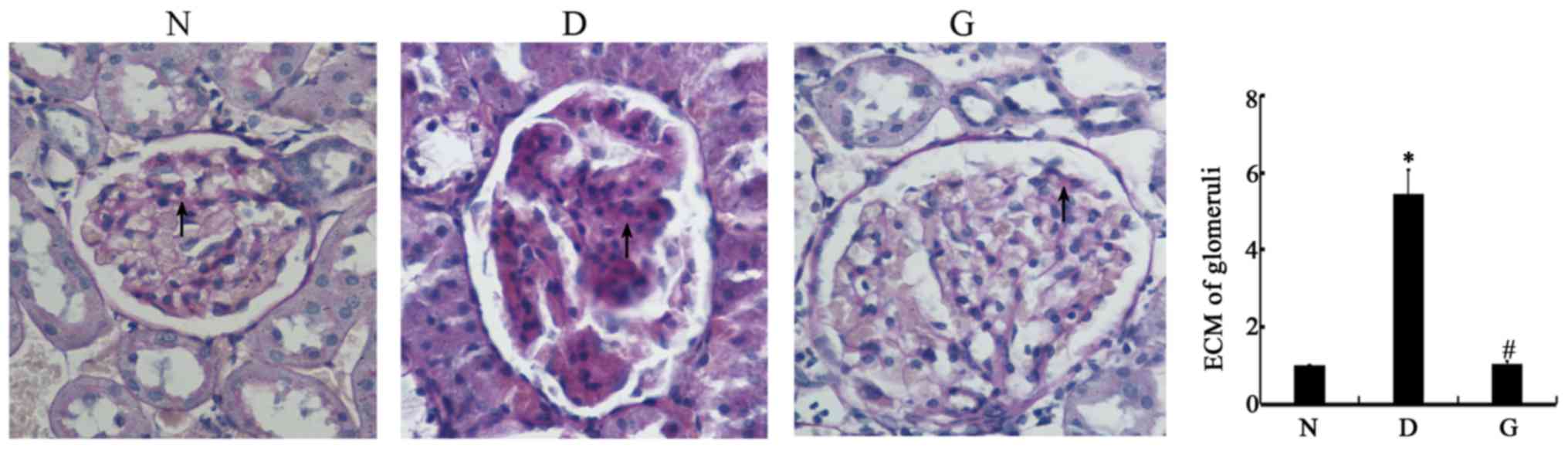 | Figure 1.GSPE protects Streptozotocin-induced
diabetic nephropathy. Animals were divided into three group: The
control group (N), the DM group (D), the GSPE group (G), Sections
were stained with PAS and were examined by microscopy. Original
magnification, ×400. The glomerular hypertrophy, thickness of the
glomerular basement membrane in the D group can be found. In G
group, the change of glomerular was significant reduced. The
histogram means the relative ECM accumulation of each group to the
N group, N group was set to 1. The ECM in the D group is 5.46±0.61,
and in the G group is 1.04±0.09. Higher ECM accumulation was
observed in the kidneys of D group rats compared with that of the N
group, Lower ECM accumulation was observed in the kidneys of the G
group rats compared with the D group. *P<0.05 compared with the
N group; #P<0.05 compared with the D group. DM,
diabetes mellitus; GSPE, grape seed proanthocyanidin extracts; PAS,
periodic acid-Schiff; ECM, extracellular matrix. |
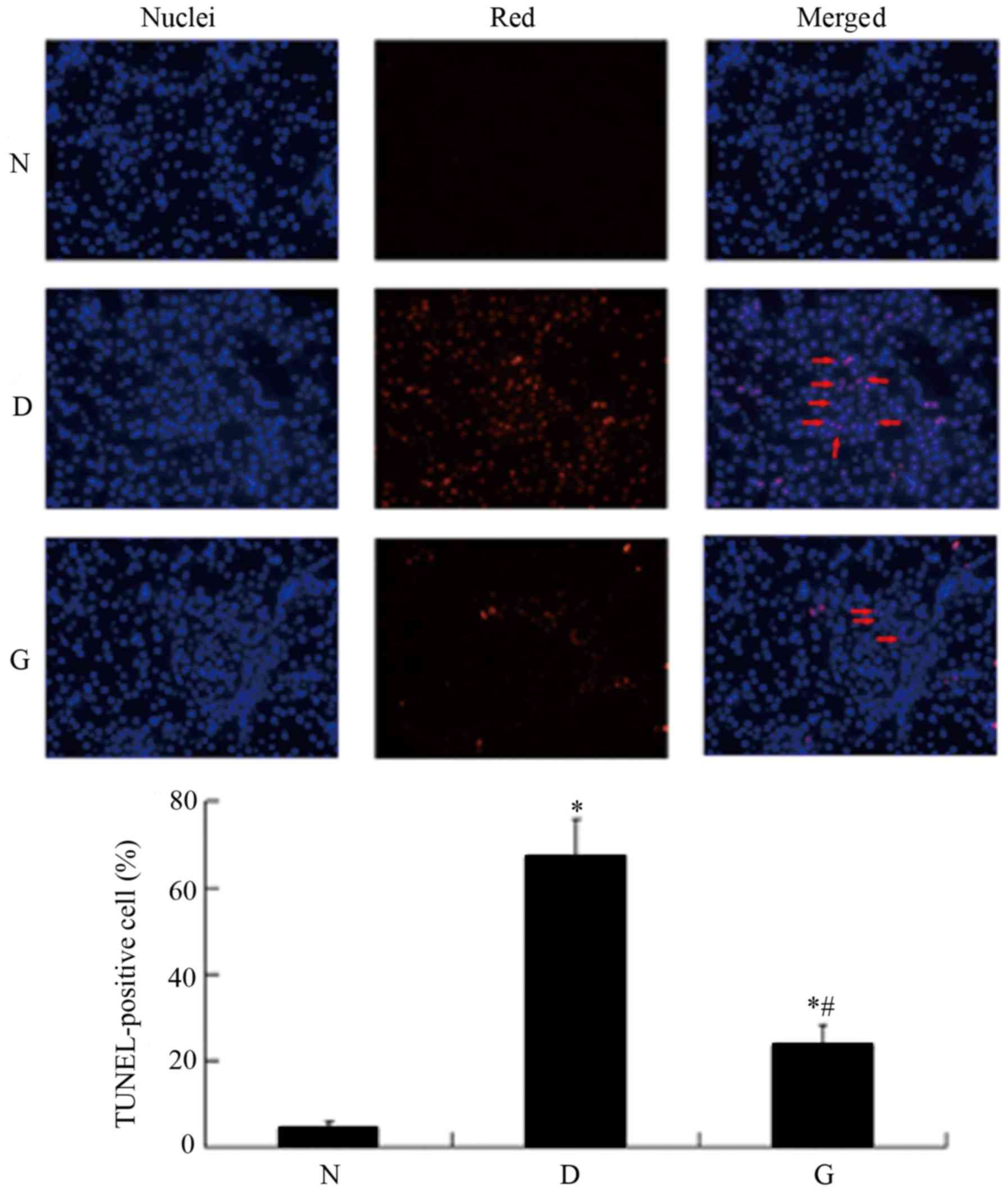
GSPE inhibits apoptosis in the DM
group rats
To assess whether GSPE inhibited cell apoptosis in
DN, the tissue sections were labeled with an in situ TUNEL
assay. As shown in Fig. 2,
apoptotic cells were visible with a red color. The DM group had
high expression of TUNEL-positive cells compared with the N group
(P<0.05). In the GSPE group the TUNEL-positive cells were
significantly reduced compared with the DM group (P<0.05). In
the N group and the GSPE group, little apoptosis was evident.
GSPE inhibits the expression of GRP78,
p-ERK and caspase-12
Apoptosis occurred in the cells with DN. To
investigate whether GSPE protects against DN by attenuating
ERS-induced apoptosis, we determined the expression of p-ERK,
GRP78, and Caspase-12, important participants in ERS-induced
apoptosis, by Western blotting and immunohistochemical methods. The
two approaches showed that GRP78, p-ERK and Caspase-12 were highly
expressed in the DM group, while their levels were significantly
reduced in the GSPE group (P<0.05; Figs. 3 and 4). These proteins showed limited
expression in the N group.
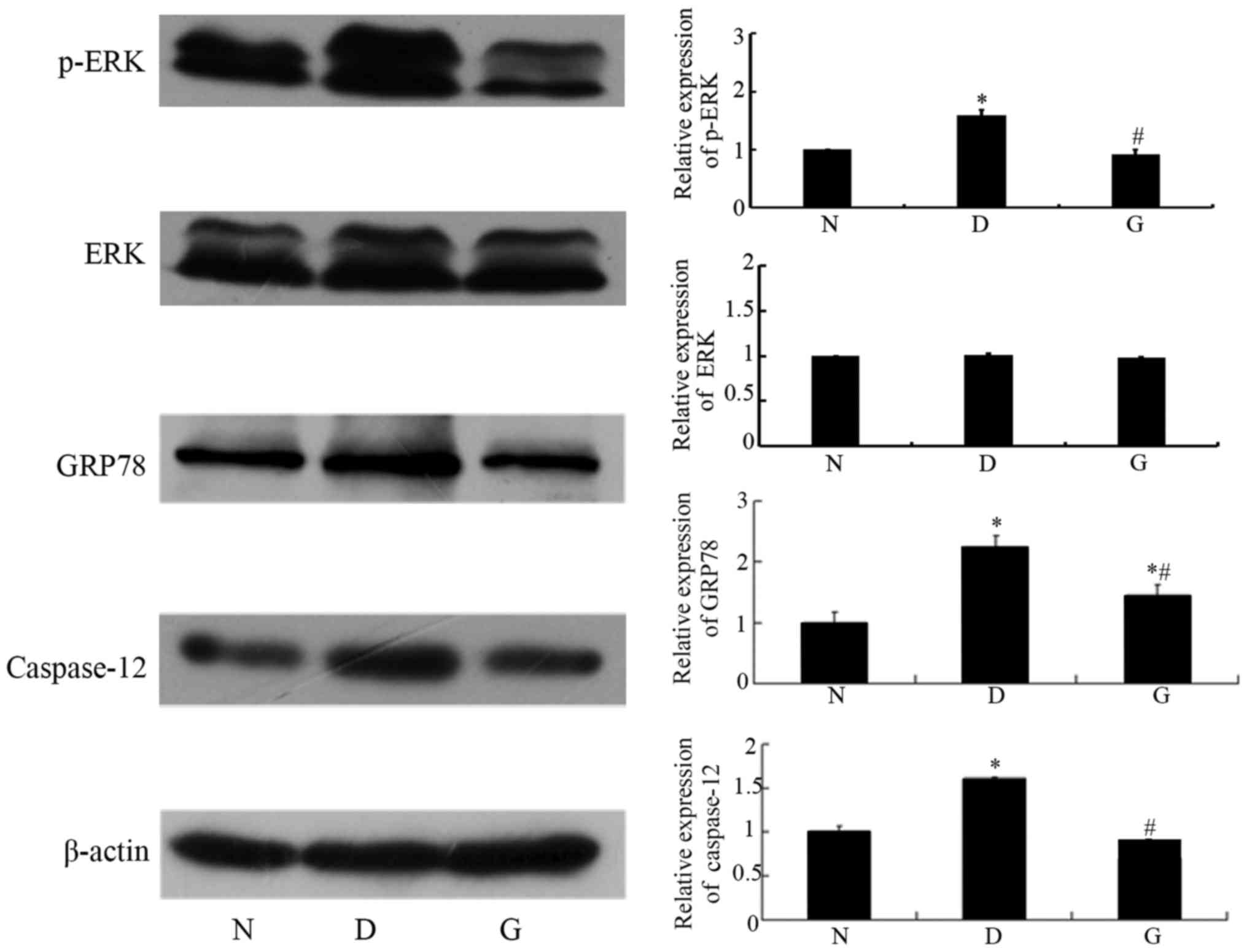 | Figure 3.GSPE inhibits the expression of
GRP78, p-ERK and Caspase-12. Western blotting analysis for GRP78,
p-ERK, and Caspase-12, and quantification of corresponding protein
levels. Animals were divided into three group: The control group
(N), the DM group (D), the GSPE group (G). Data in the expression
of GRP78, p-ERK, ERK and Caspase-12 are expressed as mean ± SD
levels relative to β-actin. The histogram means the relative
protein expression of each group to the N group, N group was set to
1. In the relative expression of ERK, D group was 1.01±0.02, G
group was 0.98±0.01. No significant difference was observed between
the two groups (P>0.05). In the relative expression of p-ERK, D
group was 1.59±0.09, G group was 0.92±0.08. This indicates that
p-ERK levels changed without any effect on the level of normal ERK.
In the relative expression of GRP78, D group was 2.22±0.21, G group
was 1.44±0.19. In the relative expression of Caspase-12, D group
was 1.60±0.03, G group was 0.70±0.01. Compared with the N group,
the expressions of GRP78, p-ERK and Caspase-12 in the D group were
significantly increased. Compared with the D group, the expression
of GRP78, p-ERK and Caspase-12 were significantly decreased in the
G group. *P<0.05 compared with the N group;
#P<0.05 compared with the D group. DM, diabetes
mellitus; GSPE, grape seed proanthocyanidin extracts; p-ERK,
phosphorylated-extracellular signal-regulated kinase; GRP78,
glucose-regulated protein 78. |
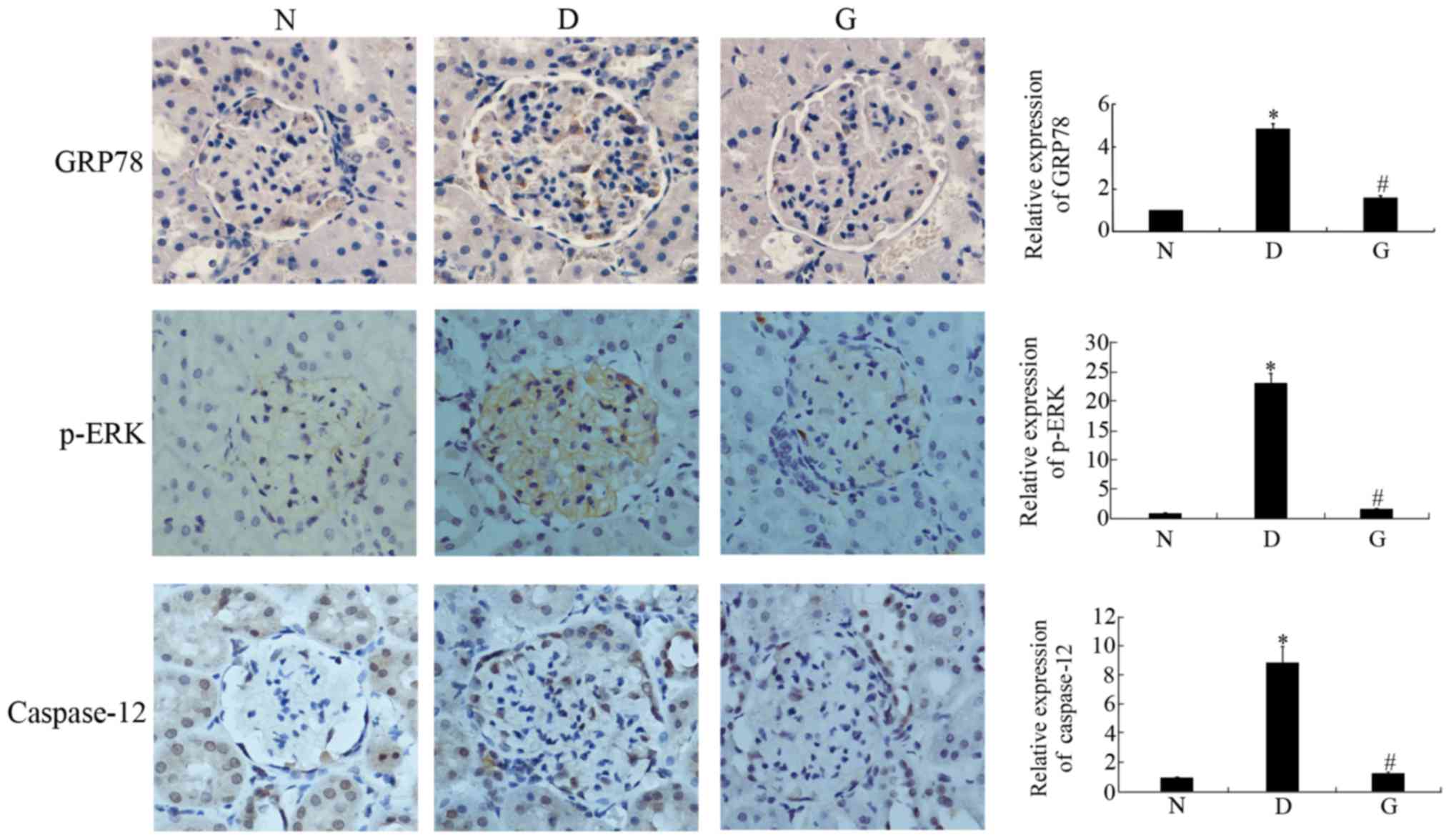 | Figure 4.GSPE inhibits the expression of
GRP78, p-ERK and Caspase-12. Immunohistochemical staining of GRP78,
p-ERK and Caspase-12 of kidney and their measurement of the
intensity of corresponding protein in the immunohistochemical
staining. Animals were divided into three group: the control group
(N), the DM group (D), the GSPE group (G). The grown granules
reprehensive the positive cells. Original magnification, ×1,000.
The expression of GRP78, p-ERK and Caspase-12 were significantly
increased in D group compared with N group and decreased in G group
compared with D group. Data are presented as means ± SD. The
histogram means the relative protein expression of each group to
the N group, N group was set to 1. In the relative expression of
GRP78, D group was 4.85±0.24, G group was 1.57±0.11. In the
relative expression of p-ERK, D group is 23.3±1.60, G group is
1.70±0.06. In the relative expression of Caspase-12, D group was
8.90±1.10, G group was 1.30±0.09. *P<0.05 compared with the N
group; #P<0.05 compared with the D group. DM,
diabetes mellitus; GSPE, grape seed proanthocyanidin extracts;
p-ERK, phosphorylated-extracellular signal-regulated kinase; GRP78,
glucose-regulated protein 78. |
Discussion
Diabetes mellitus (DM) is a multifactorial chronic
metabolic disease characterized by hyperglycaemia (14). DM can be divided by the etiology
type into type 1 diabetes (T1DM), type 2 diabetes (T2DM) and other
special types of diabetes, and approximately 90–95% of diabetic
patients have T2DM. T2DM is the most common form of diabetes, and
it is caused by genetic and environmental factors. Patients with
T2DM develop DN after 15 years (15). DN is one of the most serious
complications of diabetes, and it is by far the most common cause
of ESRD in industrial countries (16). It has been shown that several
genetic and environmental factors probably contribute to its
development, although the precise mechanisms are unknown (17). Accumulating evidence suggests that
ERS-induced apoptosis may play an important role in the progression
of DM and its complications (14).
In our study, we first established a model of type 2
diabetes. The establishment of a T2DM model can be induced with a
high-fat diet for a period of time to induce insulin resistance,
and then a small dose of intraperitoneal injection of STZ is given
to destroy the islets, resulting in insulin secretion
deficiency-induced hyperglycemia disease, in order to mimic the
pathogenesis of human type 2 diabetes (18). The high-fat diet and low-dose
STZ-induced rat model was initially developed by Reed et al
(19) and modified by Srinivasan
et al (20), and it closely
mimics the natural history of human metabolic syndrome and T2DM. In
this study, the DM group showed a significant increase in the level
of 24-h urine albumin and RI compared with the N group. The
histopathology results showed that the DM group had significant
structural glomerular damage compared with the N group. The number
of TUNEL-positive cells was significantly increased in the DM group
compared with the N group. However, compared with the DM group, the
GSPE group showed a significant decline in the level of 24-h urine
albumin and RI. The histopathology results also showed that the
GSPE group had very little glomerular damage. These observations
are consistent with previous studies (12). It has been shown that GSPE is safe
in animals with normal kidneys (12). Compared with the DM group, the GSPE
group had a significant decline in the number of TUNEL-positive
cells, which showed that GSPE can inhibit apoptosis in DN, reduce
the generation of proteinuria and delay the development of kidney
damage. This phenomenon is associated with the antioxidant
activities of GSPE, for which it has been shown that oxidative
stress proteins can be down-regulated by GSPE in DN (11,12).
Recent studies have shown that GSPE has powerful antioxidant
activity. Its antioxidative activity is much stronger than that of
vitamin C and vitamin E (12).
Additionally, GSPE has an anti-apoptosis function in diabetes
(21). Some recent studies have
found that apoptosis caused by the ERS pathway plays an important
role (4,9). Studies have even shown that apoptosis
induced by ERS is involved in diabetic kidney disease (4). However, whether GSPE can protect
against STZ-induced DN by attenuating ERS-induced apoptosis is
unknown, so we investigated it in the present study.
A variety of factors, including ischemia, hypoxia,
glucose starvation, heat shock and Ca2+ overload, can
disturb ER function and result in ERS (9). Gentle ERS can protect cells from
damage, but prolonged or severe stress leads to apoptosis (22–24).
ERS is evident in various renal diseases, including primary
glomerulonephritides, acute kidney injury, chronic kidney disease,
renal fibrosis, glomerulopathies associated with genetic mutations
and DN. The induction of ERS may be cytoprotective, or it may be
cytotoxic by activating apoptosis (25,26).
In mammalian cells, there are three major arms of the ERS: PERK,
inositol-requiring protein-1 (IRE1), and activating transcription
factor-6 (ATF6) pathways. In response to ERS, the pERK pathway
rapidly attenuates protein translation, the IRE1 pathway
upregulates expression of factors involved in ER-associated
degradation in order to degrade unfolded proteins, while the ATF6
pathway increases expression of ER chaperones such as GRP78/BiP and
calreticulin in order to refold unfolded proteins accumulated in
the ER. These pathways are designed to relieve the accumulation of
misfolded ER proteins; however, when these pathways are overwhelmed
by sustained ERS, then initiates proapoptotic pathways (25,27–30).
GRP78, which is localized in the ER, is an important molecular
chaperone, and it has been used extensively as an indicator of the
induction of ERS (31). p-ERK is
known as an initial, crucial protector for survival during even
mild stress (9). p-ERK is
transmembrane protein in the ERS, which plays a signal transduction
role. In the inactive state, p-ERK and two other ERS sensors (IRE-1
and ATF6) are associated with the ER chaperone GRP78/BiP. When
unfolded and/or misfolded proteins increase, they dissociate from
GRP78/BiP and activate downstream molecules (14). Caspase-12 plays a key role in
ERS-induced apoptosis (32).
Caspase-12 is localized only in the ER, and it is a marker of
apoptosis. It has been demonstrated that Caspase-12 mediated
apoptosis was specific to the ER, and Caspase-12 cannot be
activated when apoptosis occurs via membrane or mitochondrial
targets (33). In brief, GRP78 and
p-ERK are markers of ERS, and Caspase-12 is a marker of ERS-induced
apoptosis (34).
In our study, the expression of ERS markers,
including GRP78, p-ERK and Caspase-12, was upregulated in DN. GRP78
and p-ERK are two different proteins that lead to ERS. The increase
of the two proteins in diabetic nephropathic rats showed that ERS
had indeed occurred. The results of the TUNEL assay indicated the
occurrence of apoptosis in the diabetic nephropathic kidneys.
Compared with the normal group, the expression of Caspase-12 in the
DM group and the GSPE group was significantly increased, while it
was significantly decreased in the GSPE group compared with the DM
group. It has been demonstrated that Caspase-12-mediated apoptosis
is specific to the ER, and Caspase-12 cannot be activated when
apoptosis occurs via membrane or mitochondrial targets (35). Therefore, it was shown that
ERS-mediated apoptosis occurred via the Caspase-12 pathway in the
DM group. GRP78, p-ERK, and Caspase-12 were significantly lower in
the GSPE group, which showed that GSPE has a protective effect
against DN, which is achieved by attenuating the ERS-mediated
apoptosis via the Caspase-12 pathway.
A large number of evidence suggests that ERS plays
an important role in the development and progression of DN, and
that reducing ERS may thwart the kidney disease progression
(30). In this study, we concluded
that GSPE can protect the renal function in DN. The mechanism is
related to the attenuation of ERS-induced apoptosis by the
Caspase-12 pathway in STZ-induced DN. It may be a novel therapeutic
approach in DN. In our study, we only selected a few representative
proteins, such as GRP78, p-ERK, and Caspase-12. We hope to be able
to investigate other ERS markers such as the splicing of XBP1, the
cleavage of ATF6, ATF4 and CHOP induction, the phosphorylation of
eIF2 alpha etc in further study to find out whether there are other
mechanisms exist in DN, in order to better serve the clinic.
Acknowledgements
The authors gratefully acknowledge the assistance of
Tao Peng in contributing to the revision of the language of the
article.
Funding
This study was supported by Projects of Medical and
Health Technology Development Program in Shandong Province (grant
no. 2017WS305) and the Science Foundation of Shandong University
(grant no. 260110175616015).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
ZG, GL, ZH and XL participated to the conception and
design of the study, data analysis interpretation and drafting of
the manuscript. ZG, WS, BC and PZ contributed to data acquisition
and analysis. ZG and XL participated in the drafting of the
manuscript and substantive revisions of the important contents of
the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The current study was approved by the Animal Ethics
Committee of Shandong University, Jinan, Shandong, China (no.
DWLL-2013-053).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
GSPE
|
grape seed proanthocyanidin
extracts
|
|
STZ
|
Streptozotocin
|
|
DN
|
diabetic nephropathy
|
|
ER
|
endoplasmic reticulum
|
|
GRP78
|
glucose-regulated protein 78
|
|
p-ERK
|
phosphorylated-extracellular
signal-regulated kinase
|
|
BUN
|
blood urea nitrogen
|
|
Scr
|
serum creatinine
|
|
RI
|
renal index
|
|
ESRD
|
end stage renal disease
|
References
|
1
|
Najafian B and Mauer M: Progression of
diabetic nephropathy in type 1 diabetic patients. Diabetes Res Clin
Pract. 83:1–8. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gilbert RE and Cooper ME: The
tubulointerstitium in progressive diabetic kidney disease: More
than an aftermath of glomerular injury? Kidney Int. 56:1627–1637.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Verzola D, Gandolfo MT, Ferrario F,
Rastaldi MP, Villaggio B, Gianiorio F, Giannoni M, Rimoldi L,
Lauria F, Miji M, et al: Apoptosis in the kidneys of patients with
type II diabetic nephropathy. Kidney Int. 72:1262–1272. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu GH, Sun Y, Li Z, Song T, Wang H, Zhang
Y and Ge Z: Apoptosis induced by endoplasmic reticulum stress
involved in diabetic kidney disease. Biochem Biophys Res Commun.
370:651–656. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kumar D, Zimpelmann J, Robertson S and
Burns KD: Tubular and interstitial cell apoptosis in the
streptozotocin-diabetic rat kidney. Nephron Exp Nephrol.
96:e77–e88. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liang SH, Zhang W, McGrath BC, Zhang P and
Cavener DR: PERK (eIF2alpha kinase. is required to activate the
stress-activated MAPKs and induce the expression of immediate-early
genes upon disruption of ER calcium homoeostasis. Biochem J.
393:201–209. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pabla N and Dong Z: Cisplatin
nephrotoxicity: Mechanisms and renoprotective strategies. Kidney
Int. 73:994–1007. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schroder M and Kaufman RJ: The mammalian
unfolded protein response. Annu Rev Biochem. 74:739–789. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Szegezdi E, Logue SE, Gorman AM and Samali
A: Mediators of endoplasmic reticulum stress-induced apoptosis.
EMBO Rep. 7:880–885. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cheng M, Gao HQ, Xu L, Li BY, Zhang H and
Li XH: Cardioprotective effects of grape seed proanthocyanidins
extracts in streptozocin induced diabetic rats. J Cardiovasc
Pharmacol. 50:503–509. 2007.PubMed/NCBI
|
|
11
|
Li BY, Cheng M, Gao HQ, Ma YB, Xu L, Li
XH, Li XL and You BA: Back-regulation of six oxidative stress
proteins with grape seed proanthocyanidin extracts in rat diabetic
nephropathy. J Cell Biochem. 104:668–679. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li XH, Xiao YL, Gao HQ, Li B, Xu L, Cheng
M, Jiang B and Ma Y: Grape seed proanthocyanidins ameliorate
diabetic nephropathy via modulation of levels of AGE, RAGE and
CTGF. Nephron Exp Nephrol. 111:e31–e41. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Song Y, Li C and Cai L: Fluvastatin
prevents nephropathy likely through suppression of connective
tissue growth factor-mediated extracellular matrix accumulation.
Exp Mol Pathol. 76:66–75. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
van der Kallen CJ, van Greevenbroek MM,
Stehouwer CD and Schalkwijk CG: Endoplasmic reticulum
stress-induced apoptosis in the development of diabetes: Is there a
role for adipose tissue and liver? Apoptosis. 14:1424–1434. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dronavalli S, Duka I and Bakris GL: The
pathogenesis of diabetic nephropathy. Nat Clin Pract Endocrinol
Metab. 4:444–452. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu Y and Freedman BI: Genetics of
progressive renal failure in diabetic kidney Disease. Kidney Int
Suppl. S94–S97. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Luo ZF, Feng B, Mu J, Qi W, Zeng W, Guo
YH, Pang Q, Ye ZL, Liu L and Yuan FH: Effects of 4-phenylbutyric
acid on the process and development of diabetic nephropathy induced
in rats by streptozotocin: Regulation of endoplasmic reticulum
stress-oxidative activation. Toxicol Appl Pharmacol. 246:49–57.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Negis Y, Aytan N, Ozer N, Ogru E, Libinaki
R, Gianello R, Azzi A and Zingg JM: The effect of tocopheryl
phosphates on atherosclerosis progression in rabbits fed with a
high cholesterol diet. Arch Bioch Biophysics. 450:63–66. 2006.
View Article : Google Scholar
|
|
19
|
Reed MJ, Meszaros K, Entes LJ, Claypool
MD, Pinkett JG, Gadbois TM and Reaven GM: A new rat model of type 2
diabetes: The fat-fed, streptozotocin-treated rat. Metabolism.
49:1390–1394. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Srinivasan K, Viswanad B, Asrat L, Kaul CL
and Ramarao P: Combination of high-fat diet-fed and low-dose
streptozotocin-treated rat: A model for type 2 diabetes and
pharmacological screening. Pharmacol Res. 52:313–320. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cedó L, Castell-Auví A, Pallarès V, Blay
M, Ardévol A, Arola L and Pinent M: Grape seed procyanidin extract
modulates proliferation and apoptosis of pancreatic beta-cells.
Food Chem. 138:524–530. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Choi AY, Choi JH, Yoon H, Hwang KY, Noh
MH, Choe W, Yoon KS, Ha J, Yeo EJ and Kang I: Luteolin induces
apoptosis through endoplasmic reticulum stress and mitochondrial
dysfunction in Neuro-2a mouse neuroblastoma cells. Eur J Pharmacol.
668:115–126. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tsutsumi S, Gotoh T, Tomisato W, Mima S,
Hoshino T, Hwang HJ, Takenaka H, Tsuchiya T, Mori M and Mizushima
T: Endoplasmic reticulum stress response is involved in
nonsteroidal anti-inflammatory drug-induced apoptosis. Cell Death
Differ. 11:1009–1016. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Song S, Chew C, Dale BM, Traum D, Peacock
J, Yamazaki T, Clynes R, Kurosaki T and Greenberg S: A requirement
for the p85 PI3K adapter protein BCAP in the protection of
macrophages from apoptosis induced by endoplasmic reticulum stress.
J Immunol. 187:619–625. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cybulsky AV: Endoplasmic reticulum stress,
the unfoldedprotein response and autophagy in kindey diseases. Nat
Rev Nephrol. 13:681–696. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Taniguchi M and Yoshida H: Endoplasmic
reticulum stress in kidney function and disease. Curr Opin Nephrol
Hypertens. 24:345–350. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim R, Emi M, Tanabe K and Murakami S:
Role of the unfolded protein response in cell death. Apoptosis.
11:5–13. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Masanori K: Endoplasmic reticulum stress
in the kidney. Clin Exp Nephrol. 12:317–325. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cunard R and Sharma K: The endoplasmic
reticulum stress response and diabetic kidney disease. Am J Physiol
Renal Physiol. 300:F1054–F1061. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fan Y, Lee K, Wang N and He JC: The role
of endoplasmic reticulum stress in diabetic nephropathy. Curr Diab
Rep. 17:172017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lakshmanan AP, Thandavarayan RA,
Palaniyandi SS, Sari FR, Meilei H, Giridharan VV, Soetikno V,
Suzuki K, Kodama M and Watanabe K: Modulation of
AT-1R/CHOP-JNK-Caspase12 pathway by olmesartan treatment attenuates
ER stress-induced renal apoptosis in streptozotocin-induced
diabetic Mice. Eur J Pharm Sci. 44:627–634. 2011.PubMed/NCBI
|
|
32
|
Szegezdi E, Fitzgerald U and Samali A:
Caspase-12 and ER-stress-mediated apoptosis: The story so far. Ann
NY Acad Sci. 1010:186–194. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ohse T, Inagi R, Tanaka T, Ota T, Miyata
T, Kojima I, Ingelfinger JR, Ogawa S, Fujita T and Nangaku M:
Albumin induces endoplasmic reticulum stress and apoptosis in renal
proximal tubular cells. Kidney Int. 70:1447–1455. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gao Z, Liu G, Hu Z, Li X, Yang XD, Jiang B
and Li XH: Grape seed proanthocyanidin extracts protect against
cisplatin-induced nephrotoxicity by attenuating endoplasmic
reticulum stress-induced apoptosis. Mol Med Rep. 9:801–807. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nakagawa T, Zhu H, Morishima N, Li E, Xu
J, Yankner BA and Yuan J: Caspase-12 mediates
endoplasmic-reticulum-specific apoptosis and cytotoxicity by
amyloid-beta. Nature. 403:98–103. 2000. View Article : Google Scholar : PubMed/NCBI
|