Introduction
Osteosarcoma (OS), the most common type of bone
tumour, occurs in regions with active bone growth and repair
(1). OS commonly affects children
and adolescents and accounts for approximately 5% of all cases of
childhood cancer (2). Patients
with OS are subjected to surgical resection as a primary
therapeutic method, in addition to chemotherapy or radiotherapy
(3). Despite marked development in
the diagnosis and therapy, the therapeutic outcome for patients
with OS remains unfavourable (4).
The 5-year survival rate of patients with OS in early stages is
approximately 65–75%, and it decreases to less than 30% for
patients with local or distant metastasis (5). The most frequent cause of death for
patients with OS is metastasis, especially pulmonary metastasis
(6). Therefore, the detailed
genetic mechanisms of OS should be elucidated to promote the
development of novel therapeutic targets or drug candidates for the
treatment of patients with this aggressive malignant tumour.
MicroRNAs (miRNAs) are an abundant group of
noncoding, single strand and short RNAs transcribed from
nonprotein-coding genes or introns (7). miRNAs are endogenously expressed in
animal and plant cells and implicated in gene regulation by
directly binding to the 3′-untranslated regions (3′-UTRs) of their
genes in a sequence-specific manner, thereby inducing mRNA
degradation and translational suppression; consequently, protein
expression is inhibited (8).
Single miRNA can modulate numerous mRNAs, indicating that miRNAs
may participate in various life processes, such as development,
cell proliferation, differentiation and metabolism (9). miRNAs are dysregulated in a variety
of human malignancies, including OS (10–12).
Dysregulation of miRNAs may play tumor suppressive roles or
oncogenic roles in human cancers, which decrease the expression of
oncogenes and tumor suppressors, respectively (13). Hence, novel miRNAs associated with
OS formation and progression should be investigated to promote the
understanding of OS pathogenesis so that novel effective
therapeutic strategies may be developed.
miR-660-5p (miR-660) has been reported to be
dysregulated in several human cancer types, such as breast cancer
(14), chronic myeloid leukaemia
(15) and Hodgkin lymphoma
(16). However, the expression
pattern, detailed roles and underlying molecular mechanism of
miR-660 in OS remain largely unknown. In our study, miR-660
expression was determined in OS tissues and cell lines, and the
effects of miR-660 on the proliferation and invasion of OS cells
were examined. The regulatory mechanism of the oncogenic roles of
miR-660 in OS was also investigated. Our study may provide novel
insights into the pathogenesis and development of OS and may be
beneficial to the identification of therapeutic target for patients
with OS.
Materials and methods
Patients and tissue specimens
A total of 26 human OS tissues and corresponding
normal adjacent tissues (NATs) were obtained from patients who
suffered from OS and underwent surgical resection at Central
Hospital of Zibo (Zibo, China) between February 2013 and March
2017. None of the patients were treated with chemotherapy or
radiotherapy before the surgery was performed. All of the tissues
were immediately frozen in liquid nitrogen and stored in a super
cold refrigerator at −80°C. The approval for this study was
obtained from the Ethic Committee of Central Hospital of Zibo.
Written informed consent was also provided by the patients with OS
enrolled in this research.
Cell culture and transfection
Human OS cell lines (MG-63, HOS, Saos-2, and U2OS)
and a normal human osteoblast hFOB1.19 were purchased from the
Shanghai Cell Bank of the Chinese Academy of Sciences (Shanghai,
China). All cell lines were grown in RPMI-1640 medium supplemented
with 10% fetal bovine serum, 100 units of penicillin/ml and 100 ng
of streptomycin/ml (all from Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), and maintained at 37°C in a humidified
atmosphere with 5% CO2.
miR-660 inhibitor and negative control miRNA
inhibitor (NC inhibitor) were provided by GenePharma (Shanghai,
China). Small interfering RNA (siRNA) against the expression of
Forkhead box O1 (FOXO1 siRNA) and NC siRNA were produced by Ribobio
(Guangzhou, China). Cells were plated into 6-well culture plates
and transfected with miRNA inhibitor or siRNA when the cells were
grown to approximately 60–70% confluence by using Lipofectamine
2000 (Invitrogen; Thermo Fisher Scientific, Inc.) in accordance
with the manufacturer's instructions. At 6–8 h post-transfection,
the culture medium was removed, and fresh RPMI-1640 medium
containing 10% FBS was added to each well.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from tissue specimens or
culture cells by using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.), in accordance with the manufacturer's protocol.
The miR-660 expression level was detected by utilizing a One-Step
SYBR® PrimeScript™ miRNA RT-PCR kit (Takara Bio, Dalian,
China) with U6 snRNA as an internal reference. To quantify the mRNA
level of FOXO1, we conducted a reverse transcription with a
PrimeScript RT reagent kit (Takara Biotechnology Co., Ltd., Dalian,
China) and quantitative PCR with a SYBR Premix Ex Taq™ kit
(Takara Biotechnology Co., Ltd.). GAPDH served as an internal
control for the mRNA expression of FOXO1. Relative gene expression
was analysed using the 2−∆∆Cq method (17).
Cell Counting Kit-8 (CCK-8) assay
At 24 h post-transfection, the transfected cells
were inoculated into 96-well plates at a density of 3,000
cells/well. In our research, four time points after inoculation (0,
24, 48 and 72 h) were chosen, and CCK-8 assays were carried out at
each time point. A total of 10 µl CCK-8 solution (Dojindo Molecular
Technologies, Inc., Kumamoto, Japan) was added to each well. After
the cells were incubated at 37°C for 2 h, the absorbance at 450 nm
was determined with a SpectraMax Microplate®
Spectrophotometer (Molecular Devices LLC, Sunnyvale, CA, USA).
Transwell invasion assay
After 24 h of transfection, the transfected cells
were collected and suspended in FBS-free RPMI-1640 medium. A total
of 1×105 cells were seeded on the upper surface of
Matrigel-coated Transwell chambers (BD Biosciences, Franklin Lakes,
NJ, USA). RPMI-1640 medium (600 µl) containing 20% FBS was added to
the lower chambers. The chambers were incubated at 37°C with 5%
CO2 for 24 h. The non-invasive cells that remained on
the upper surface of the chambers were removed with a cotton swab.
The invasive cells on the lower surface of the chamber were fixed
with 4% paraformaldehyde, stained with 0.05% crystal violet and
photographed under an inverted microscope (Olympus Corporation,
Tokyo, Japan). The number of invasive cells was counted in five
randomly selected visual fields (magnification, 200×) from each
Transwell chamber.
Prediction of miR-660 target genes and
luciferase reporter assay
The putative targets of miR-660 were predicted using
TargetScan (http://www.targetscan.org) and Pictar
(http://www.pictar.org/).
The FOXO1 3′-UTR containing wild-type (Wt) and
mutant-type (Mut) predicted miR-660 binding sequences was generated
by GenePharma, cloned into the pGL3 plasmid (Promega Corporation,
Madison, WI, USA), and named as pGL3-FOXO1-Wt-3′-UTR and
pGL3-FOXO1-Mut-3′-UTR, respectively. Cells were seeded into 24-well
plates and cotransfected using Lipofectamine 2000 with miR-660
inhibitor or NC inhibitor, and pGL3-FOXO1-Wt-3′-UTR or
pGL3-FOXO1-Mut-3′-UTR. 48 h after transfection, luciferase
activities were detected using the Dual-Luciferase Reporter Assay
System (Promega Corporation). The firefly luciferase activity was
normalized to Renilla luciferase activity.
Western blotting analysis
Total protein was extracted using
radioimmunoprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology, Inc., Shanghai, China) supplemented with 0.1 mg/ml
phenylmethylsulfonyl fluoride, 1 mM sodium orthovanadate and 1
mg/ml aprotinin (all from Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany). The concentration of total protein was quantified using
the BCA Protein Assay kit (Beyotime Institute of Biotechnology,
Inc.). Equal amounts of protein were separated by 10% sodium
dodecyl sulfate polyacrylamide gel electrophoresis before being
transferred to polyvinylidene difluoride (PVDF) membranes (EMD
Millipore, Billerica, MA, USA). Subsequent to block with 5% non-fat
milk for 1 h, the membranes were incubated overnight at 4°C with
the following primary antibodies: Rabbit anti-human FOXO1
monoclonal antibody (ab52857; Abcam, Cambridge, UK) and rabbit
anti-human GAPDH monoclonal antibody (ab128915; Abcam). Afterwards,
the membranes were washed thrice with Tris-buffered saline
containing 0.1% Tween-20 (TBST) and incubated with Goat anti-rabbit
horseradish peroxidase-conjugated IgG secondary antibodies
(ab205718; Abcam) followed by visualization using an enhanced
chemiluminescence system (EMD Millipore). GAPDH served as a
normalization control.
Statistical analysis
Statistical analysis was performed using Statistical
Package for Social Sciences version 19.0 (IBM Corp., Armonk, NY,
USA). Data were shown as mean ± standard deviation and analysed
with Student's t-test or one-way ANOVA plus multiple comparisons
combined with Tukey's post hoc test. The relationship between
miR-660 and FOXO1 mRNA in OS tissues was examined through
Spearman's correlation analysis. P<0.05 was considered to
indicate a statistically significant difference.
Results
miR-660 expression is frequently
upregulated in OS tissues and cell lines
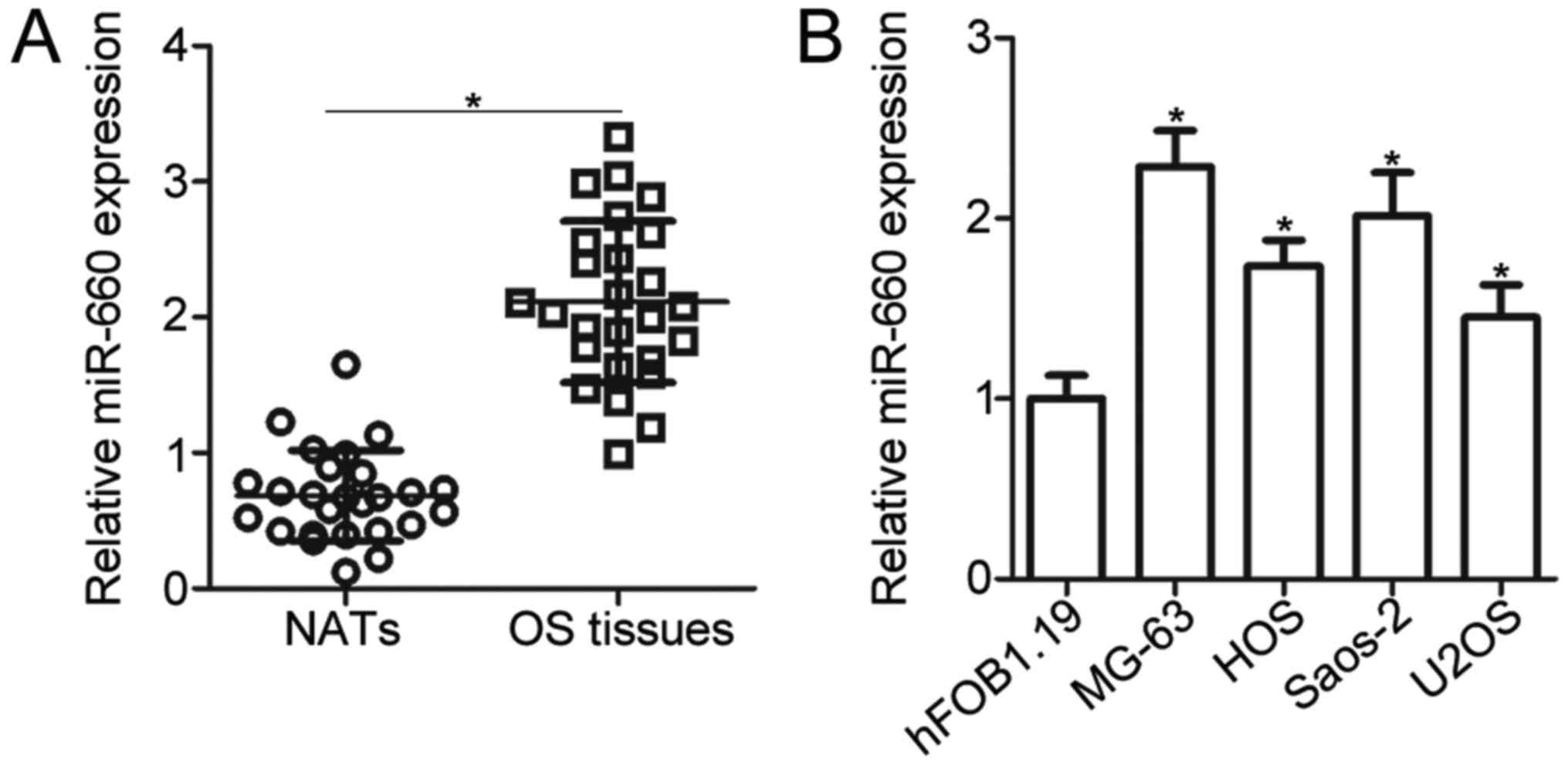
To evaluate the miR-660 expression level in OS,
RT-qPCR was performed to measure the miR-660 expression in 26 pairs
of OS tissues and the corresponding NATs. The results showed that
the miR-660 expression level was significantly higher in the OS
tissues than that in the NATs (P<0.05; Fig. 1A). We next sought to examine
whether miR-660 upregulation also occurred in OS cell lines.
miR-660 was overexpressed in all of the four human OS cell lines,
namely, MG-63, HOS, Saos-2 and U2OS, compared with that in the
normal human osteoblast hFOB1.19 (P<0.05; Fig. 1B). These results suggested that
miR-660 is upregulated in OS tissues and cell lines.
miR-660 inhibition restricts the
proliferative and invasive abilities of OS cells
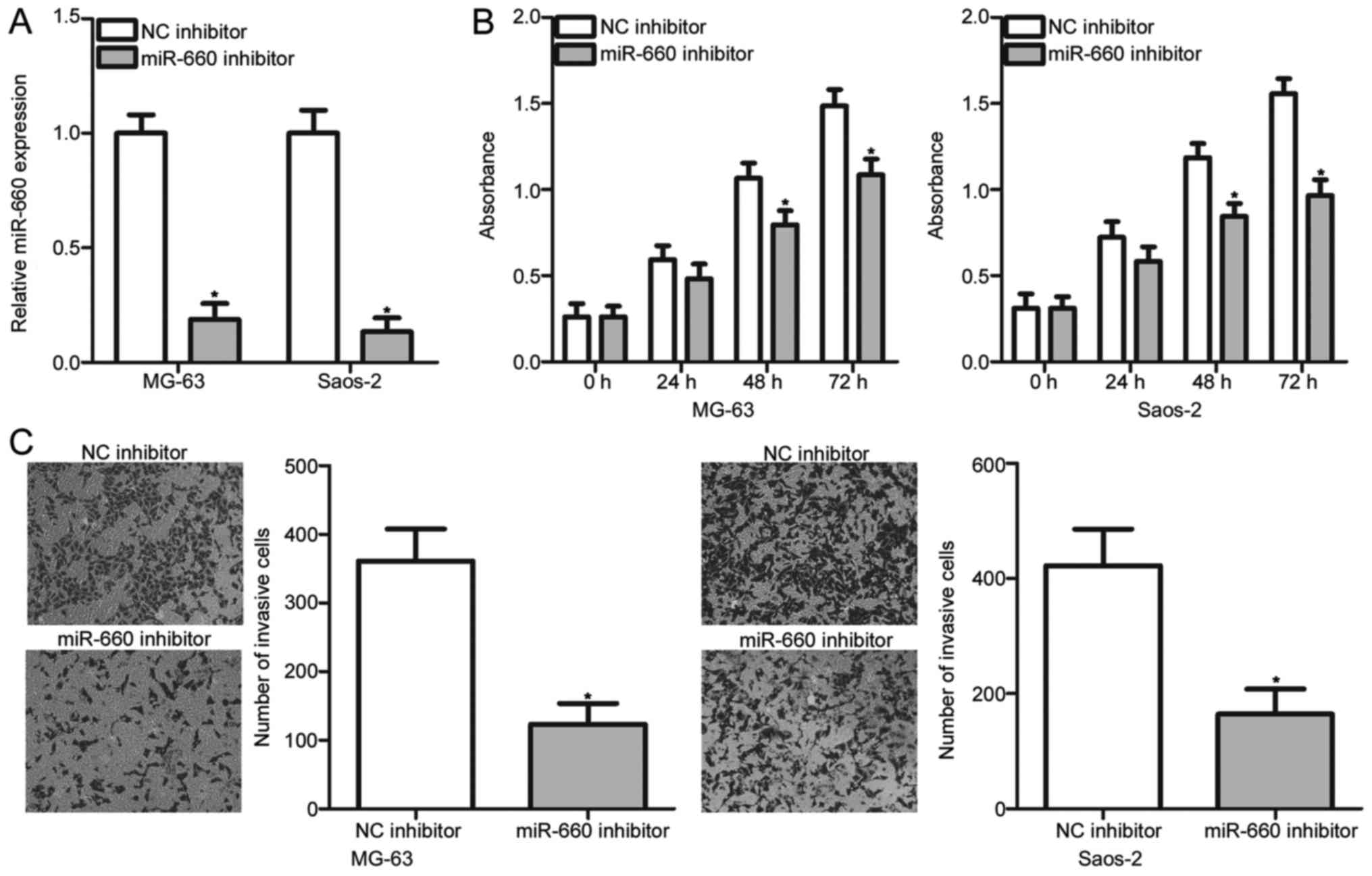
To illustrate the biological roles of miR-660 in the
development of OS, miR-660 inhibitor was introduced to MG-63 and
Saos-2 cells to decrease the endogenous miR-660 level. The
transfection efficiency was evaluated through RT-qPCR, and the
results demonstrated that miR-660 was markedly downregulated in
miR-660 inhibitor-transfected MG-63 and Saos-2 cells (P<0.05;
Fig. 2A). The transfected MG-63
and Saos-2 cells were tested to examine their proliferation rate
through the CCK-8 assay. The CCK-8 assay revealed that miR-660
downregulation reduced the proliferation of MG-63 and Saos-2 cells
compared with that in the NC inhibitor groups (P<0.05; Fig. 2B). Transwell invasion assay was
performed to determine the effect of miR-660 inhibition on OS cell
invasion. In Fig. 2C, the invasion
abilities of the MG-63 and Saos-2 cells transfected with miR-660
inhibitor were significantly lower than those of the cells
transfected with NC inhibitor (P<0.05; Fig. 2C). These results suggested that
miR-660 may play an oncogenic role in OS progression.
FOXO1 is a direct target gene of
miR-660 in OS cells
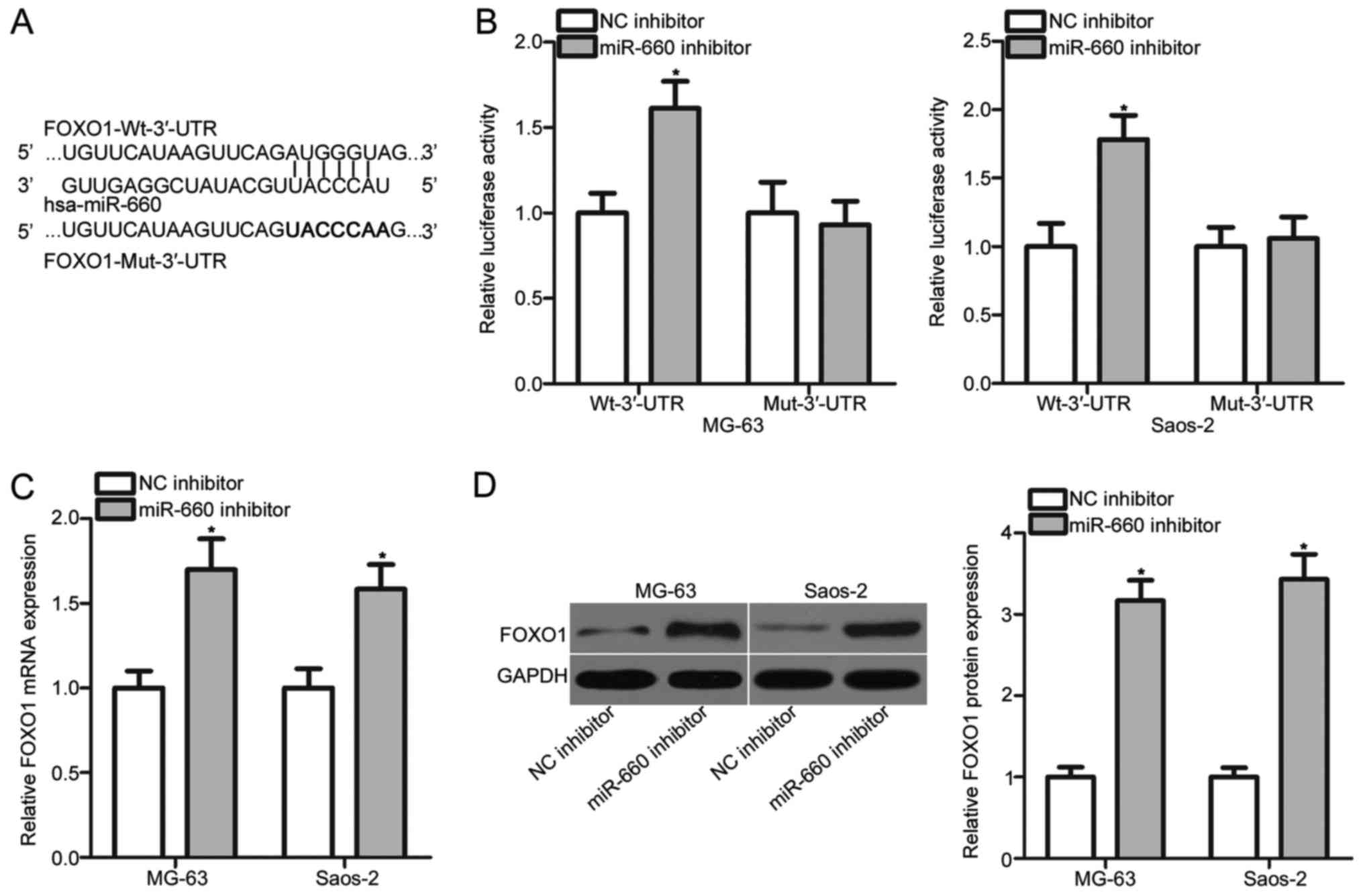
Bioinformatics analysis was performed to predict the
potential targets of miR-660 and to explore the mechanisms
underlying the oncogenic roles of miR-660 in OS. A putative binding
site for miR-660 was observed in the 3′-UTR of FOXO1 (Fig. 3A). FOXO1 was selected for further
experimental confirmation because FOXO1 played tumour suppressive
roles in the onset and progression of OS (18–20).
Luciferase reporter assay was subsequently carried out in the MG-63
and Saos-2 cells to confirm this prediction. miR-660 inhibition led
to a significant increase in the luciferase activities of
pGL3-FOXO1-Wt-3′-UTR in the MG-63 and Saos-2 cells (P<0.05), but
no significant change was occurred in the pGL3-FOXO1-Mut-3′-UTR
group (Fig. 3B). RT-qPCR and
Western blot analysis confirmed that the mRNA (P<0.05; Fig. 3C) and protein (P<0.05; Fig. 3D) expression levels of FOXO1 were
upregulated in the MG-63 and Saos-2 cells transfected with miR-660
inhibitor. These results demonstrated that FOXO1 is a direct target
of miR-660 in OS.
FOXO1 is downregulated in OS tissues
and negatively correlated with miR-660 expression
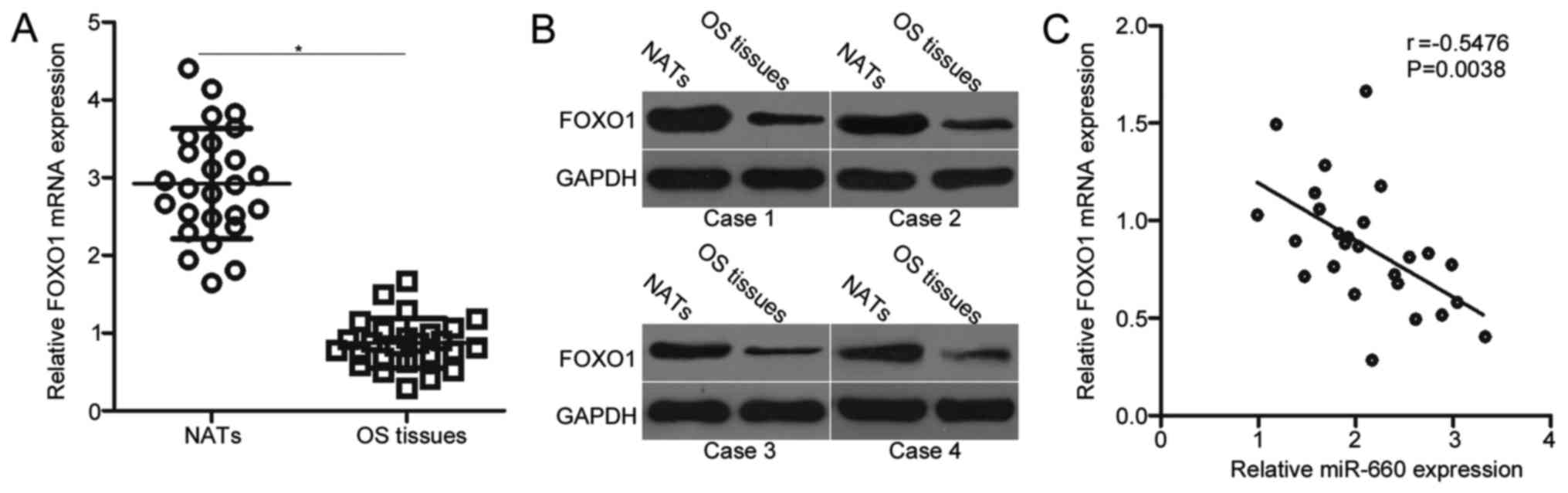
Considering that FOXO1 is identified as a direct
target of miR-660, we next sought to further examine the
relationship between miR-660 and FOXO1 in OS. Firstly, RT-qPCR was
conducted to detect the mRNA level of FOXO1 in 26 pairs of OS
tissues and NATs. The mRNA expression level of FOXO1 was weakly
expressed in the OS tissues in comparison with that in the NATs
(Fig. 4A, P<0.05). Secondly,
the protein expression of FOXO1 was determined in several pairs of
OS tissues and NATs. In Fig. 4B,
the FOXO1 protein was downregulated in the OS tissues relative to
that in the NATs. An inverse association between miR-660 and FOXO1
mRNA was also validated in the OS tissues (r=−0.5476, P=0.0038;
Fig. 4C).
FOXO1 knockdown abolishes the
functions of miR-660 in OS cells
On the basis of our findings described above, we
hypothesised that FOXO1 mediated the oncogenic roles of miR-660 in
OS cells. A series of rescue experiments was conducted to test this
hypothesis. Firstly, we transfected MG-63 and Saos-2 cells with NC
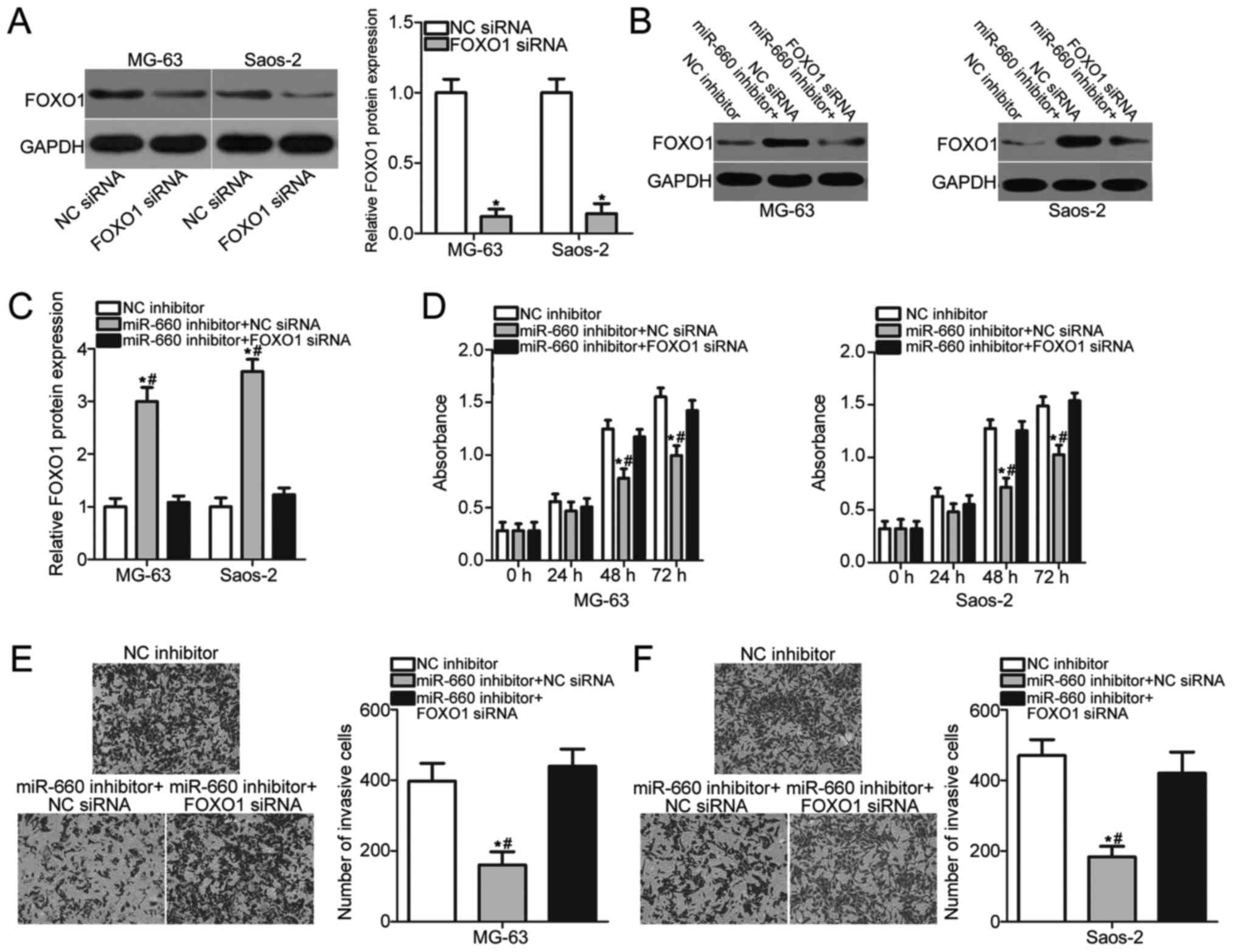
siRNA or FOXO1 siRNA and detected the protein level of FOXO1. As
shown in Fig. 5A, FOXO1 expression
was efficiently knocked down in MG-63 and Saos-2 cells after
transfection with FOXO1 siRNA (P<0.05). Next, MG-63 and Saos-2
cells were transfected with miR-660 inhibitor and FOXO1 siRNA or NC
siRNA. Western blot analysis indicated that the FOXO1 protein
expression was restored by FOXO1 siRNA co-transfection in MG-63 and
Saos-2 cells (P<0.05; Fig. 5B and
C). Furthermore, CCK-8 and Transwell invasion assays revealed
that FOXO1 knockdown could abrogate the effects of miR-660
inhibition on the proliferation (P<0.05; Fig. 5D) and invasion (P<0.05; Fig. 5E and F) of MG-63 and Saos-2 cells.
Thus, our data evidently demonstrated that miR-660 probably
performs an oncogenic function in OS at least partially by
regulating FOXO1 expression.
Discussion
The abnormal expression of miRNAs has been observed
in OS, and these differently expressed miRNAs contribute to the
occurrence and development of OS by regulating various biological
behaviours, such as cell proliferation, apoptosis, cycle,
migration, invasion and metastasis (21–23).
Therefore, an comprehensive understanding of the detailed roles of
aberrantly expressed miRNAs in OS progression may be favourable to
the identification of promising therapeutic strategies for the
treatment of patients with OS. In our current study, miR-660 was
upregulated in OS tissues and cell lines compared with that in NATs
and normal human osteoblast hFOB1.19, respectively. miR-660
downregulation prohibited cell proliferative and invasive abilities
in OS. FOXO1 was identified as a direct target of miR-660 in OS.
FOXO1 was downregulated in OS tissues, and this downregulation was
inversely correlated with the miR-660 expression level. Moreover,
FOXO1 knockdown abrogated the oncogenic effects of miR-660 on the
proliferation and invasion of OS cells. Based on these data, our
conclusion was that targeting miR-660 may be an effective
therapeutic target to inhibit the growth and metastasis of OS.
miR-660 has been reported to be upregulated in
breast cancer tissues and cell lines (14). The overall survival of patients
with breast cancer and with a high miR-660 level is shorter than
that of patients with low miR-660 (24). miR-660 knockdown attenuates cell
proliferation, migration and invasion, promotes apoptosis and
induces G1 arrest in the cell cycle (14). Salati found that miR-660 expression
is highly expressed in chronic myeloid leukaemia. Ectopic miR-660
expression can protect chronic myeloid leukaemia cells from
apoptosis caused by tyrosine kinase inhibitors and lead to
resistance to tyrosine kinase inhibitors (15). Aberrantly overexpressed miR-660 is
also observed in classical Hodgkin lymphoma (16). However, miR-660 is downregulated in
lung cancer tissues, cell lines and plasma. Restoration miR-660
expression plays tumour suppressive roles in lung cancer
progression by inhibiting cell growth and metastasis in
vitro and in vivo and by promoting cell apoptosis in
vitro (25,26). These conflicting studies suggested
that the expression patterns and roles of miR-660 in human
malignancy exhibit tissue specificity and may be developed as a
potential therapeutic target for anticancer therapy.
Several miR-660 targets, including TFCP2 (14) in breast cancer, TET2 (15), EPAS1 (15) in chronic myeloid leukaemia and MDM2
(25) in lung cancer, have been
identified. In our study, FOXO1 was demonstrated to be a direct
target of miR-660 in OS. FOXO1, a member of the Forkhead box (FOX)
family, is a transcription factor and underexpressed in various
human cancers, such as gastric cancer (27), breast cancer (28), lung cancer (29) and prostate cancer (30). FOXO1 expression is closely related
to clinicopathological features and prognosis in human cancers. For
instance, FOXO1 is downregulated in bladder cancer. Decreased FOXO1
expression is significantly correlated with tumour stage and grade.
The survival time of patients with bladder cancer and high FOXO1
levels is longer than that of patients with low FOXO1 expression
(31,32). FOXO1 plays important roles in
carcinogenesis and cancer progression by regulating several
biological functions, including cancer cell proliferation,
differentiation, apoptosis, cycle, migration, invasion, metastasis
and angiogenesis (33–35). These findings suggested that FOXO1
may be an excellent target for human cancer therapy.
FOXO1 expression is generally low in OS tissues and
cell lines. Functional analysis revealed that FOXO1 might play
tumour suppressive roles in OS oncogenesis by inhibiting cell
proliferation, survival, colony formation, migration and invasion
(18–20). Previous studies demonstrated that
FOXO1 can be targeted by multiple miRNAs in human cancers. For
example, miR-374a and miR-135b directly target FOXO1 and promote
cell proliferation and invasion in OS (18,36).
Furthermore, FOXO1 is directly targeted by miR-196a in
hepatocellular carcinoma (37),
miR-223 in breast cancer (38),
miR-215 in gastric cancer (39)
and miR-132 in laryngeal squamous cell carcinoma (40), and therefore to be involved in
regulating the tumorigeneis and tumor development of these specific
tumor types. Hence, miRNA-based targeted therapy of FOXO1 may be an
attractive therapeutic strategy for patients with malignant
diseases.
In summary, this study revealed that the miR-660
expression level increased in OS tissues and cell lines, and its
downregulation prohibited OS cell proliferation and invasion. FOXO1
was confirmed as a direct target gene of miR-660 in OS. On the
basis of these results, we proposed the hypothesis that the
inhibition of miR-660 or the restoration of FOXO1 expression might
be a novel therapeutic method for patients with OS. However, there
are several limitations in our study. We did not analyse the effect
of miR-660 inhibition on OS tumor growth in vivo.
Additionally, we could not conclude that FOXO1 was the primary or
only target of miR-660 in OS. In our future research, intensive
studies are necessary to overcome these limitations.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
XW, PZ and HG designed the present study. PZ and HG
performed RT-qPCR and Transwell invasion assays. QL and XC
conducted the CCK-8 assay, western blot analysis and luciferase
reporter assay. XW performed the bioinformatics analysis and
statistical analysis. All authors read and approved the final
draft.
Ethics approval and consent to
participate
The present study was approved by the Research
Ethics Committee of Central Hospital of Zibo (Zibo, China), and was
performed in accordance with the Declaration of Helsinki and the
guidelines of the Ethics Committee of Central Hospital of Zibo.
Written informed consent was obtained from all patients for the use
of their clinical tissues.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Valery PC, Laversanne M and Bray F: Bone
cancer incidence by morphological subtype: A global assessment.
Cancer Causes Control. 26:1127–1139. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Meyers PA, Schwartz CL, Krailo M,
Kleinerman ES, Betcher D, Bernstein ML, Conrad E, Ferguson W,
Gebhardt M, Goorin AM, et al: Osteosarcoma: A randomized,
prospective trial of the addition of ifosfamide and/or muramyl
tripeptide to cisplatin, doxorubicin, and high-dose methotrexate. J
Clin Oncol. 23:2004–2011. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Marina N, Gebhardt M, Teot L and Gorlick
R: Biology and therapeutic advances for pediatric osteosarcoma.
Oncologist. 9:422–441. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Geller DS and Gorlick R: Osteosarcoma: A
review of diagnosis, management, and treatment strategies. Clin Adv
Hematol Oncol. 8:705–718. 2010.PubMed/NCBI
|
|
5
|
Gorlick R, Janeway K, Lessnick S, Randall
RL and Marina N: COG Bone Tumor Committee: Children's oncology
Group's 2013 blueprint for research: Bone tumors. Pediatr Blood
Cancer. 60:1009–1015. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Di Fiore R, Drago-Ferrante R, Pentimalli
F, Di Marzo D, Forte IM, Carlisi D, De Blasio A, Tesoriere G,
Giordano A and Vento R: Let-7d miRNA shows both antioncogenic and
oncogenic functions in Osteosarcoma-derived 3AB-OS cancer stem
cells. J Cell Physiol. 231:1832–1841. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nat Rev Cancer. 6:857–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Carrington JC and Ambros V: Role of
microRNAs in plant and animal development. Science. 301:336–338.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shukla GC, Singh J and Barik S: MicroRNAs:
Processing, maturation, target recognition and regulatory
functions. Mol Cell Pharmacol. 3:83–92. 2011.PubMed/NCBI
|
|
12
|
Kushlinskii NE, Fridman MV and Braga EA:
Molecular mechanisms and microRNAs in osteosarcoma pathogenesis.
Biochemistry (Mosc). 81:315–328. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jansson MD and Lund AH: MicroRNA and
cancer. Mol Oncol. 6:590–610. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shen Y, Ye YF, Ruan LW, Bao L, Wu MW and
Zhou Y: Inhibition of miR-660-5p expression suppresses tumor
development and metastasis in human breast cancer. Genet Mol Res.
16:2017. View Article : Google Scholar
|
|
15
|
Salati S, Salvestrini V, Carretta C,
Genovese E, Rontauroli S, Zini R, Rossi C, Ruberti S, Bianchi E,
Barbieri G, et al: Deregulated expression of miR-29a-3p, miR-494-3p
and miR-660-5p affects sensitivity to tyrosine kinase inhibitors in
CML leukemic stem cells. Oncotarget. 8:49451–49469. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Paydas S, Acikalin A, Ergin M, Celik H,
Yavuz B and Tanriverdi K: Micro-RNA (miRNA) profile in Hodgkin
lymphoma: Association between clinical and pathological variables.
Med Oncol. 33:342016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pei H, Jin Z, Chen S, Sun X, Yu J and Guo
W: MiR-135b promotes proliferation and invasion of osteosarcoma
cells via targeting FOXO1. Mol Cell Biochem. 400:245–252. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang J and Sun G: FOXO1-MALAT1-miR-26a-5p
feedback loop mediates proliferation and migration in osteosarcoma
cells. Oncol Res. 25:1517–1527. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Guan H, Tan P, Xie L, Mi B, Fang Z, Li J,
Yue J, Liao H and Li F: FOXO1 inhibits osteosarcoma oncogenesis via
Wnt/β-catenin pathway suppression. Oncogenesis. 4:e1662015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kafchinski LA and Jones KB: MicroRNAs in
osteosarcomagenesis. Adv Exp Med Biol. 804:119–127. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gang L, Qun L, Liu WD, Li YS, Xu YZ and
Yuan DT: MicroRNA-34a promotes cell cycle arrest and apoptosis and
suppresses cell adhesion by targeting DUSP1 in osteosarcoma. Am J
Transl Res. 9:5388–5399. 2017.PubMed/NCBI
|
|
23
|
Xiao Y, Zhao Q, Du B, Chen HY and Zhou DZ:
MicroRNA-187 inhibits growth and metastasis of osteosarcoma by
downregulating S100A4. Cancer Invest. 36:1–9. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Krishnan P, Ghosh S, Wang B, Li D,
Narasimhan A, Berendt R, Graham K, Mackey JR, Kovalchuk O and
Damaraju S: Next generation sequencing profiling identifies
miR-574-3p and miR-660-5p as potential novel prognostic markers for
breast cancer. BMC Genomics. 16:7352015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fortunato O, Boeri M, Moro M, Verri C,
Mensah M, Conte D, Caleca L, Roz L, Pastorino U and Sozzi G:
Mir-660 is downregulated in lung cancer patients and its
replacement inhibits lung tumorigenesis by targeting MDM2-p53
interaction. Cell Death Dis. 5:e15642014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Borzi C, Calzolari L, Centonze G, Milione
M, Sozzi G and Fortunato O: mir-660-p53-mir-486 Network: A new key
regulatory pathway in lung tumorigenesis. Int J Mol Sci. 18:pii:
E222. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ko YS, Cho SJ, Park J, Kim Y, Choi YJ, Pyo
JS, Jang BG, Park JW, Kim WH and Lee BL: Loss of FOXO1 promotes
gastric tumour growth and metastasis through upregulation of human
epidermal growth factor receptor 2/neu expression. Br J Cancer.
113:1186–1196. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wu Y, Elshimali Y, Sarkissyan M, Mohamed
H, Clayton S and Vadgama JV: Expression of FOXO1 is associated with
GATA3 and Annexin-1 and predicts disease-free survival in breast
cancer. Am J Cancer Res. 2:104–115. 2012.PubMed/NCBI
|
|
29
|
Maekawa T, Maniwa Y, Doi T, Nishio W,
Yoshimura M, Ohbayashi C, Hayashi Y and Okita Y: Expression and
localization of FOXO1 in non-small cell lung cancer. Oncol Rep.
22:57–64. 2009.PubMed/NCBI
|
|
30
|
Zhang H, Pan Y, Zheng L, Choe C, Lindgren
B, Jensen ED, Westendorf JJ, Cheng L and Huang H: FOXO1 inhibits
Runx2 transcriptional activity and prostate cancer cell migration
and invasion. Cancer Res. 71:3257–3267. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kim TH, Jo SW, Lee YS, Kim YJ, Lee SC, Kim
WJ and Yun SJ: Forkhead box O-class 1 and forkhead box G1 as
prognostic markers for bladder cancer. J Korean Med Sci.
24:468–473. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang Y, Jia L, Zhang Y, Ji W and Li H:
Higher expression of FOXOs correlates to better prognosis of
bladder cancer. Oncotarget. 8:96313–96322. 2017.PubMed/NCBI
|
|
33
|
Kim SY, Ko YS, Park J, Choi Y, Park JW,
Kim Y, Pyo JS, Yoo YB, Lee JS and Lee BL: Forkhead transcription
factor FOXO1 inhibits angiogenesis in gastric cancer in relation to
SIRT1. Cancer Res Treat. 48:345–354. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhao Y, Wang Y and Zhu WG: Applications of
post-translational modifications of FoxO family proteins in
biological functions. J Mol Cell Biol. 3:276–282. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dong T, Zhang Y, Chen Y, Liu P, An T,
Zhang J, Yang H, Zhu W and Yang X: FOXO1 inhibits the invasion and
metastasis of hepatocellular carcinoma by reversing ZEB2-induced
epithelial-mesenchymal transition. Oncotarget. 8:1703–1713.
2017.PubMed/NCBI
|
|
36
|
He W, Feng L, Xia D and Han N: MiR-374a
promotes the proliferation of human osteosarcoma by downregulating
FOXO1 expression. Int J Clin Exp Med. 8:3482–3489. 2015.PubMed/NCBI
|
|
37
|
Yang L, Peng F, Qin J, Zhou H and Wang B:
Downregulation of microRNA-196a inhibits human liver cancer cell
proliferation and invasion by targeting FOXO1. Oncol Rep.
38:2148–2154. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wei YT, Guo DW, Hou XZ and Jiang DQ:
miRNA-223 suppresses FOXO1 and functions as a potential tumor
marker in breast cancer. Cell Mol Biol (Noisy-le-Grand).
63:113–118. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zang Y, Wang T, Pan J and Gao F: miR-215
promotes cell migration and invasion of gastric cancer cell lines
by targeting FOXO1. Neoplasma. 64:579–587. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lian R, Lu B, Jiao L, Li S, Wang H, Miao W
and Yu W: MiR-132 plays an oncogenic role in laryngeal squamous
cell carcinoma by targeting FOXO1 and activating the PI3K/AKT
pathway. Eur J Pharmacol. 792:1–6. 2016. View Article : Google Scholar : PubMed/NCBI
|