Introduction
Salivary gland adenoid cystic carcinoma (SACC) is
one of the most common types of malignant tumor, and has unique
clinical manifestations, as it is exclusively observed in the head
and neck (1). It accounts for ~10
to 15% of salivary gland tumors. Pathologically, SACC is a basaloid
tumor comprised of epithelial and myoepithelial cells in variable
morphologic configurations, including tubular, cribriform and solid
patterns (2,3). It is characterized by the indolent
yet persistent growth of salivary gland epithelium, perineural
invasion, local recurrence and distant metastasis (4). The 5-year survival rate of patients
with highly metastatic SACC is <20%. At present, surgical
excision combined with postoperative radiotherapy is the routine
treatment used (5,6). However, the potent invasiveness and
metastasis of SACC prevents complete eradication, which leads to
poor prognosis and low survival rates. Therefore, novel approaches
to treat SACC are required.
Simvastatin inhibits the rate-limiting step in the
mevalonate (MVA) pathway and is applied clinically to reduce serum
cholesterol levels and lower the incidence of cardiovascular and
cerebrovascular events (7). In
addition to its lipid-lowering effects, simvastatin is used to
treat hypercholesterolemia, atherosclerosis, coronary heart disease
and stroke (8,9). However, simvastatin has additionally
been demonstrated to inhibit tumor cell proliferation, promote
apoptosis and suppress invasion via cholesterol-independent
pleiotropic effects (10). The
efficacy and molecular mechanisms underlying its actions in SACC-83
cells have not yet been fully elucidated.
Several oncogenes and tumor suppressor genes have
been suggested to be involved in SACC (11). Survivin is overexpressed in a
number of cancer types, including lung, prostate and Merkel cell
carcinoma (12–15). Survivin overexpression in cancer
patients is an unfavorable prognostic marker and is correlated with
decreased survival in several malignancies (16,17).
In addition, overexpression of survivin is associated with an
increased risk of recurrence, lymph node invasion and metastasis in
cervical cancer, squamous cell cancer of the tongue, primary
laryngeal carcinoma (18–20). In order to investigate the role of
survivin in SACC cells treated with simvastatin in the present
study, the human SACC cell line, SACC-83, was used to assess cell
proliferation, apoptosis and survivin expression following
simvastatin exposure.
Materials and methods
Cells, antibodies and reagents
The human SACC cell line, SACC-83, was provided by
the Shanghai No. 9 People's Hospital (Shanghai, China). Simvastatin
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), the primary
antibodies against survivin (cat. no. BS9870M; Biogot Technology
Co., Ltd., Nanjing, China) and β-actin (cat. no. Ta-09; Beijing
Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing, China)
were commercial products.
Cell culture
Prior to the experiment, simvastatin was dissolved
in anhydrous ethanol to obtain a final concentration of 2 µmol/ml,
followed by aseptic filtration and storage at −20°C until the
commencement of the experiment. SACC-83 cells were cultured in
RPMI-1640 medium (Sigma-Aldrich; Merck KGaA) supplemented with 10%
fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), 100 mg/ml streptomycin and 100 units/ml
penicillin in a humidified atmosphere of 5% CO2 and
37°C. Cells were passaged when 90% confluent.
Cell proliferation assay
A Cell Counting Kit (CCK)-8 assay (Nanjing KeyGen
Biotech Co., Ltd., Nanjing, China) was performed to determine cell
proliferation in the logarithmic phase and to delineate a growth
curve. SACC-83 cells were plated at a density of 2×103
cells/well in 96-well plates and the outer wells were filled with
sterile phosphate-buffered saline (PBS) which were cultured until
adherence occurred. This was followed by treatment with simvastatin
using a gradient of concentrations (0, 10, 20, 30, 40 and 50 µM)
for 24 and 48 h. CCK-8 solution (10 µl) was subsequently added to
each well of the 96-well plate, which was then incubated for 1 h at
37°C. The optical density values were determined using an enzyme
standard instrument set at an absorbance of 460 nm (BioTek
Instruments, Inc., Winooski, VT, USA), and the half maximal
inhibitory concentration (IC50) was then calculated.
Colony forming assay
SACC-83 cells were cultured at a density of 500
cells/well in 6-well petri dish at 37°C in saturated humidity and
5% CO2 for 48 h. Following the removal of non-adherent
cells, SACC-83 were cultured in the presence of simvastatin at
varying concentrations (0, 10, 20, 30, 40 and 50 µM) in RPMI-1640
medium. Ten days following this, adherent cells were washed twice
with PBS, followed by fixation with 4% paraformaldehyde for 10 min
and 0.5% crystal violet staining at room temperature for 20 min to
calculate colony counts.
Cell cycle analysis
SACC-83 cells (2×105 cells/well) were
seeded in six-well plates and treated with 50 µM simvastatin for 24
and 48 h. The cells were harvested, washed twice in PBS and fixed
in 70% ice-cold ethanol at 4°C overnight. The cells were then
centrifuged at 2,000 × g for 5 min at room temperature, washed
twice in cold PBS and centrifuged again under the same conditions.
Cells were suspended in 250 ml PBS and staining with 10 ml
propidium iodide (Nanjing KeyGen Biotech Co., Ltd.) and 10 ml RNase
A (Nanjing KeyGen Biotech Co., Ltd.) for 30 min at room
temperature. The proliferation index was defined as the proportion
of cells in S and G1 phases from the total cell
population. The cells in each group underwent flow cytometry (Facs
Canto II, BD Biosciences) analysis to calculate the percentage of
cells in G0/G1, S and G2/M phases
by FlowJo Diva (FlowJo LLC, Ashland, OR, USA).
Cell apoptosis analysis
SACC-83 cells (2×105 cells/well) were
seeded in 6-well plates and treated with simvastatin (0, 10, 30 and
50 µM) for 48 h. Cells were then harvested and washed twice with
PBS. Cells were subsequently resuspended with 500 µl binding buffer
(Nanjing KeyGen Biotech Co., Ltd.), and stained with 5 µl PI
(Nanjing KeyGen Biotech Co., Ltd.) and 5 µl Annexin V-fluorescein
isothiocyanate (Nanjing KeyGen Biotech Co., Ltd.) and incubated at
room temperature for 10 min in the dark. In each group,
1×105 cells were analyzed by flow cytometry using BD
FACSDiva version 8.0.1 software (Facs Canto II, BD
Biosciences).
Western blot analysis
Total protein was separated by SDS-PAGE and western
blotting was performed. Briefly, following treatment of cells with
varying simvastatin concentrations (0, 10, 20, 30, 40 and 50 µM)
for 48 h and following treatment with 50 µM simvastatin for 24, and
48 h, each group were washed twice with cold PBS and collected at
room temperature. Total protein was extracted using protein lysis
solution containing RIPA buffer and 1% phenyl methane
sulfonyluoride (PMSF; Beyotime Institute of Biotechnology, Haimen,
China). Protein concentrations were measured using a BCA Protein
assay kit (Beyotime Institute of Biotechnology). Followed by
protein (20 µg) separation on a 12.5% SDS-PAGE gel, proteins were
then transferred to polyvinylidene difluoride membranes (Merck
Millipore) for electrophoresis at a voltage of 60 V for 4 h.
Following this and blocking in 5% nonfat dry milk for 2 h at room
temperature, membranes were incubated with primary antibodies
against survivin (cat. no. BS9870M; 1:1,000; Biogot Technology Co.,
Ltd., Nanjing, China) overnight at 4°C. Subsequently, the blots
were washed three times in PBS and incubated with a horseradish
peroxidase-conjugated Goat anti-rabbit IgG (cat. no. ZB-2301;
1:5,000; Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd.)
for 2 h at room temperature. After washing three times, the
membranes were immersed in BCIP/NBT alkaline phosphatase color
development kit (C3206 Beyotime Institute of Biotechnology) for
coloration and blots were quantified using ImageJ 1.49 software
(National Institutes of Health, Bethesda, MD, USA).
Cell invasion assays
To assess the invasive capacity of
simvastatin-treated cells, Matrigel invasion assays were performed.
Pore filters (8-mm) were inserted in 24-well plates (Sigma-Aldrich;
Merck KGaA) coated with Matrigel (BD Biosciences, Franklin Lakes,
NJ, USA) and the cells were then incubated with 0, 10, 30 and 50 µM
simvastatin for 48 h. Cells (1×105) were harvested and
resuspended in RPMI-1640 medium containing 1% FBS, before they were
added to the upper chambers and incubated for 24 h at 37°C. Each
lower chamber was filled with 500 µl RPMI-1640 medium supplemented
with 10% FBS. At the end of incubation, the non-invasive cells on
the upper surface of the membrane were carefully removed using a
cotton swab. The invaded cells on the lower surface of the membrane
were fixed with 4% formaldehyde for 20 min and stained with 0.1%
crystal violet for 5 min at room temperature. Subsequently, the
invaded cells on the lower surface of the membrane were visualized
in three randomly selected fields under a light microscope (Olympus
BH-2; Olympus Corporation, Tokyo, Japan; magnification, ×200). All
assays were performed in triplicate, and the mean count of invaded
cells was used for analysis.
Statistical analysis
Each experiment was conducted in triplicate.
Statistical analysis was performed using SPSS 13.0 for Windows
(SPSS, Inc., Chicago, IL, USA). Data are presented as the mean ±
standard deviation, and one-way analysis of variance and Dunnett's
post-hoc tests were employed to determine statistically significant
differences among groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
Effect of simvastatin on the
proliferation of SACC-83 cells
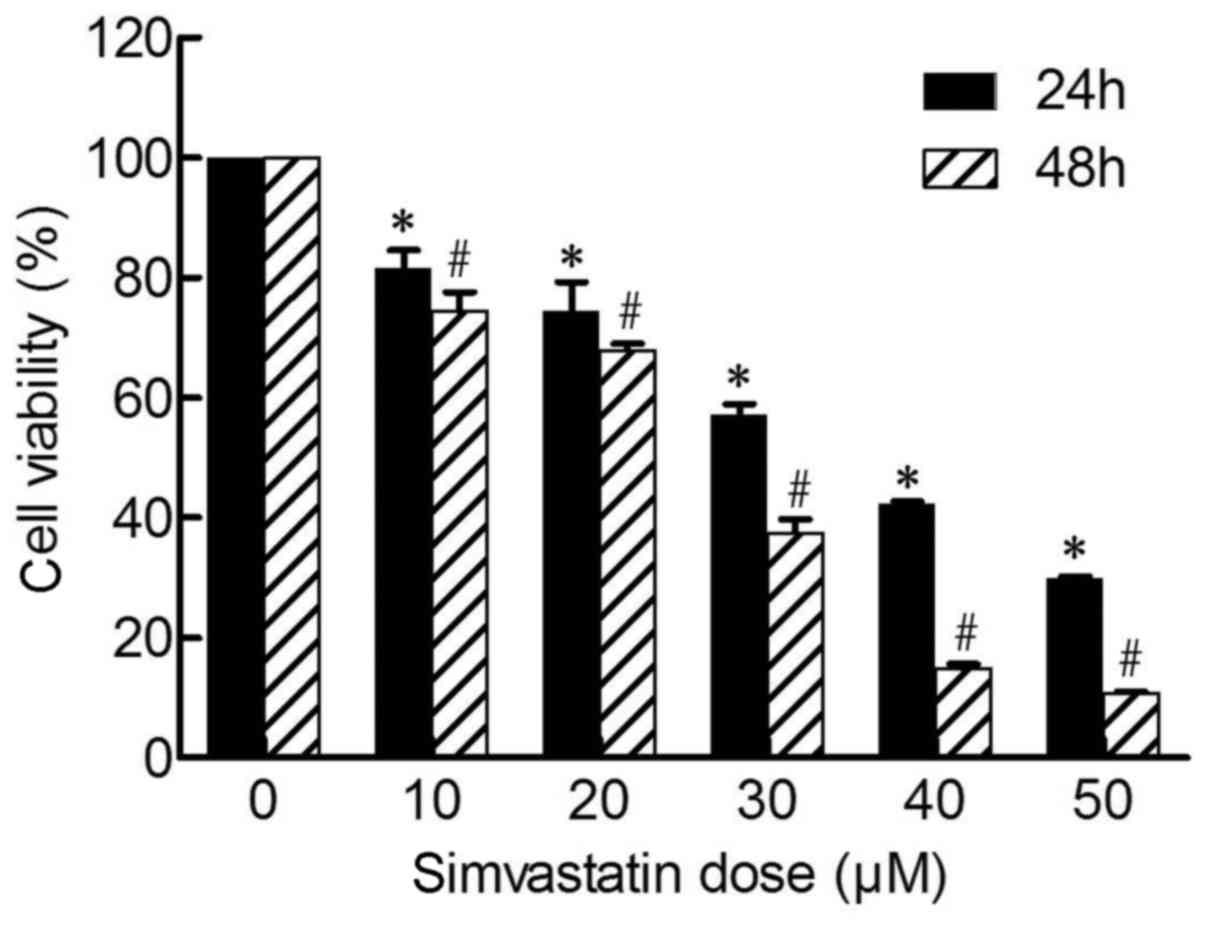
As shown in Fig. 1,
following treatment of cells with varying concentrations of
simvastatin (0, 10, 20, 30, 40 and 50 µM) for 24 and 48 h, the
proliferation of SACC-83 cells was significantly inhibited in a
time-(24 to 48 h) and dose-dependent (10 to 50 µM) manner
(P<0.05), whereas a modest level of inhibition was observed at
concentrations <10 µM (data not shown). The IC50
value of simvastatin was 22.9 µM at 48 h, and the inhibitory rate
at 50 µM was 89.19±0.21% at 48 h (Fig.
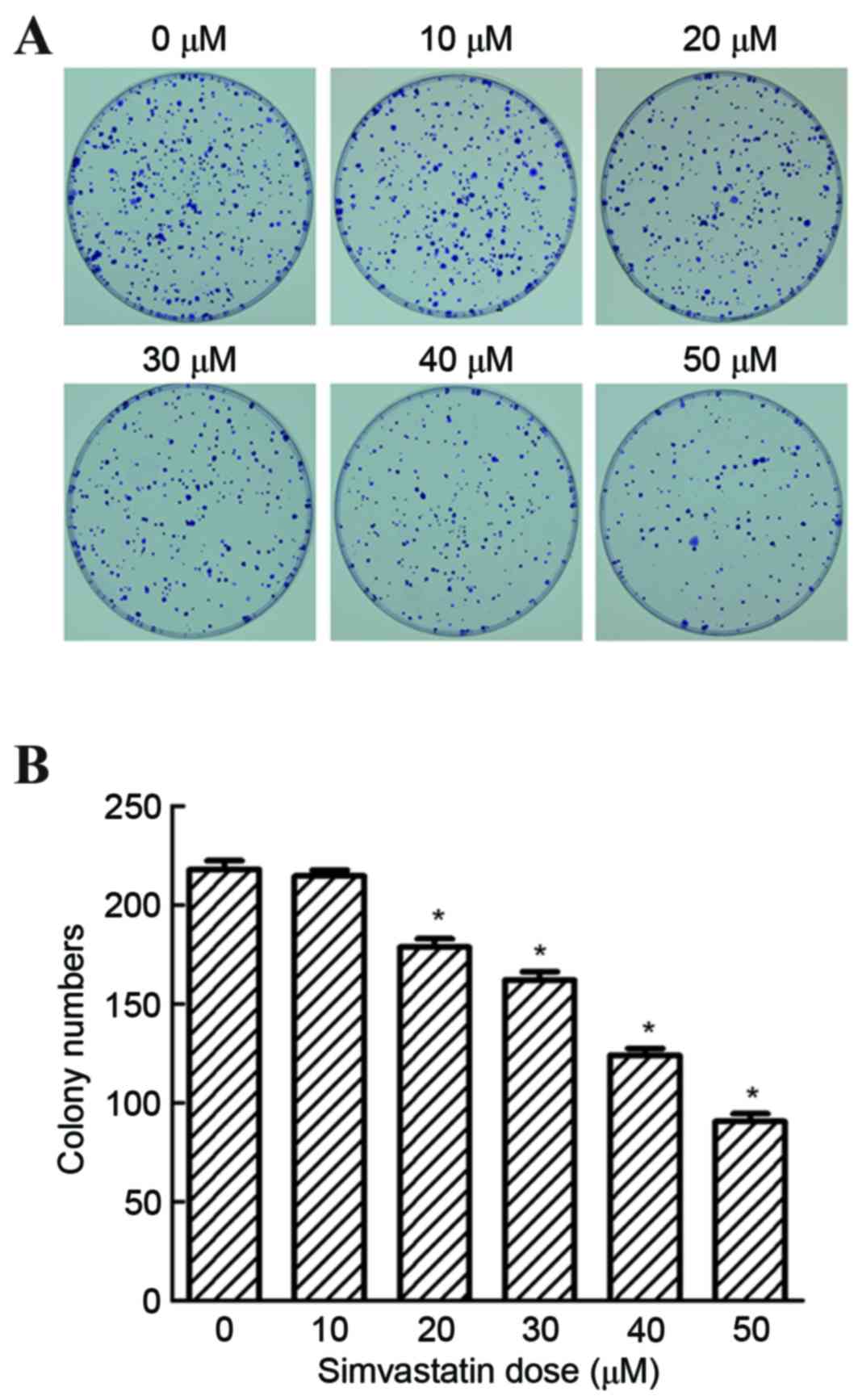
1). Following treatment of cells with 0 to 50 µM simvastatin,
the colony forming ability of SACC-83 cells was significantly
reduced at 20, 30, 40 and 50 µM concentrations, when compared with
untreated controls (P<0.05; Fig.
2). These results demonstrated that simvastatin significantly
suppressed the colonization of SACC-83 cells in a dose-dependent
manner, indicating that simvastatin may inhibit the proliferation
of SACC-83 cells.
Effect of simvastatin on SACC-83 cell
cycle and survivin expression
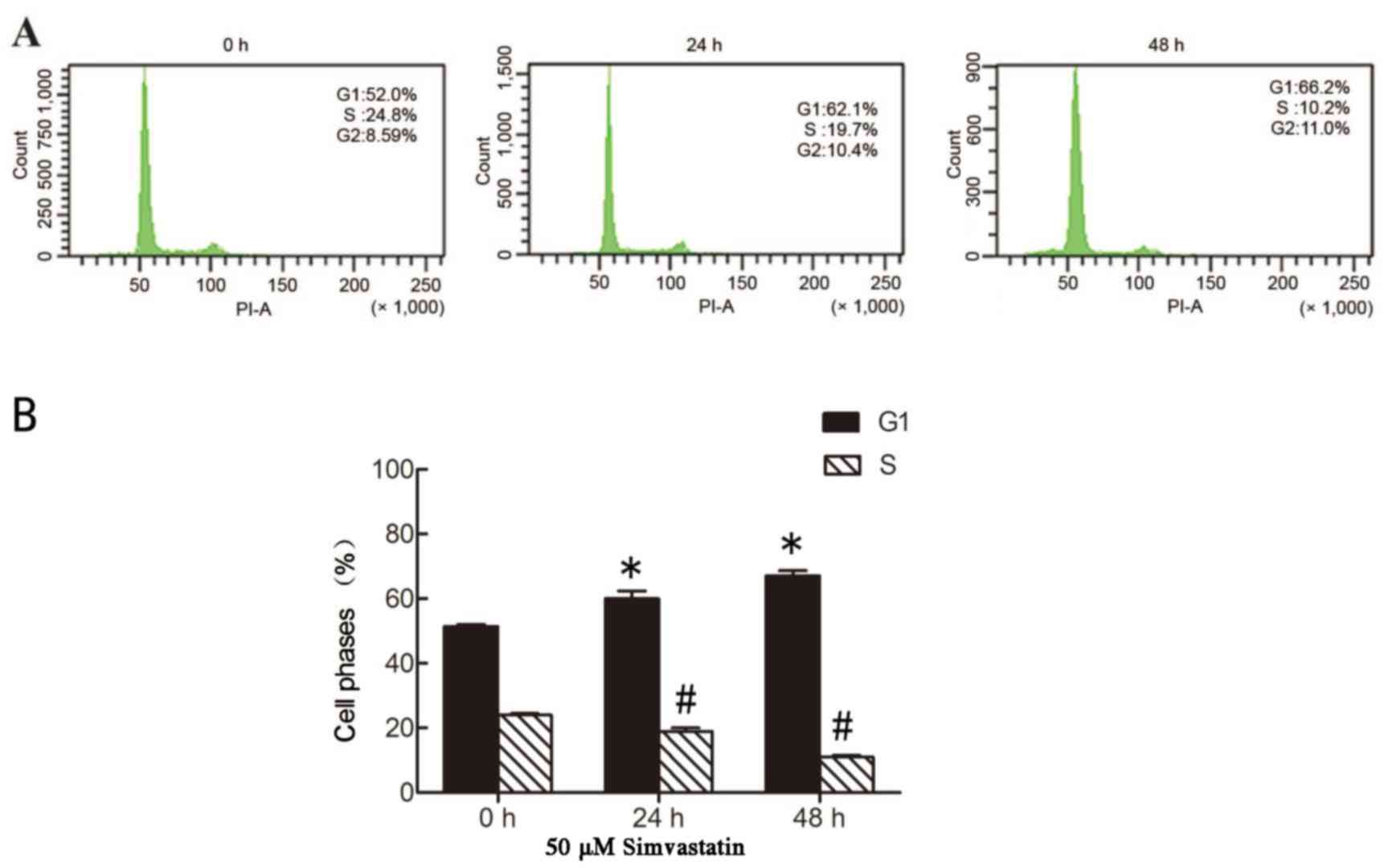
As simvastatin was demonstrated to inhibit the
proliferation of SACC-83 cells, the effect of simvastatin on cell
cycle distribution was subsequently investigated by flow cytometry
analysis. As shown in Fig. 3,
concomitant with its inhibition of the proliferation of SACC-83
cells, simvastatin treatment significantly induced cell cycle
arrest in G1 phase following incubation for 24 and 48 h,
with the percentage of cells in G1 phase increasing from
52 to 62.1 and 66.2% at these time points, respectively
(P<0.05). By contrast, the percentage of cells in S phase
significantly decreased from 24.8 to 10.2% at 48 h following
simvastatin treatment (P<0.05; Fig.
3). A previous study demonstrated that survivin serves a key
role in the regulation of cell proliferation, and is overexpressed
during the G2/M phase and rapidly declines during
G1 phase (21).
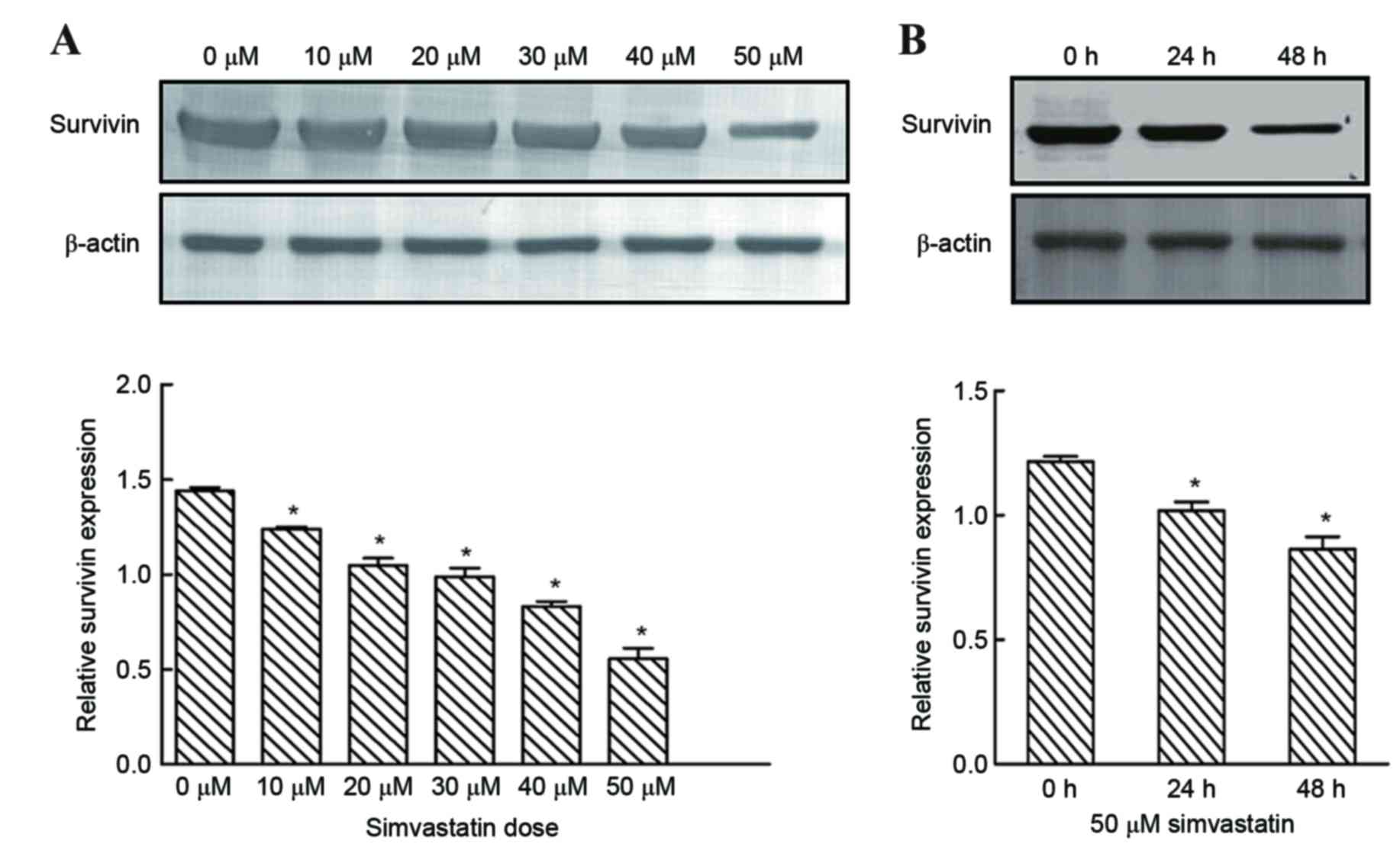
Following treatment of cells with varying simvastatin
concentrations for 48 h and following treatment with 50 µM
simvastatin for 24, and 48 h, survivin expression was significantly
reduced when compared with untreated controls (Fig. 4). By contrast, survivin was
expressed at high levels in untreated SACC-83 cells (Fig. 4). These results suggest that
simvastatin may mediate G1 phase arrest of SACC-83
cells, in part, via a survivin-mediated pathway.
Effect of simvastatin on SACC-83 cell
apoptosis
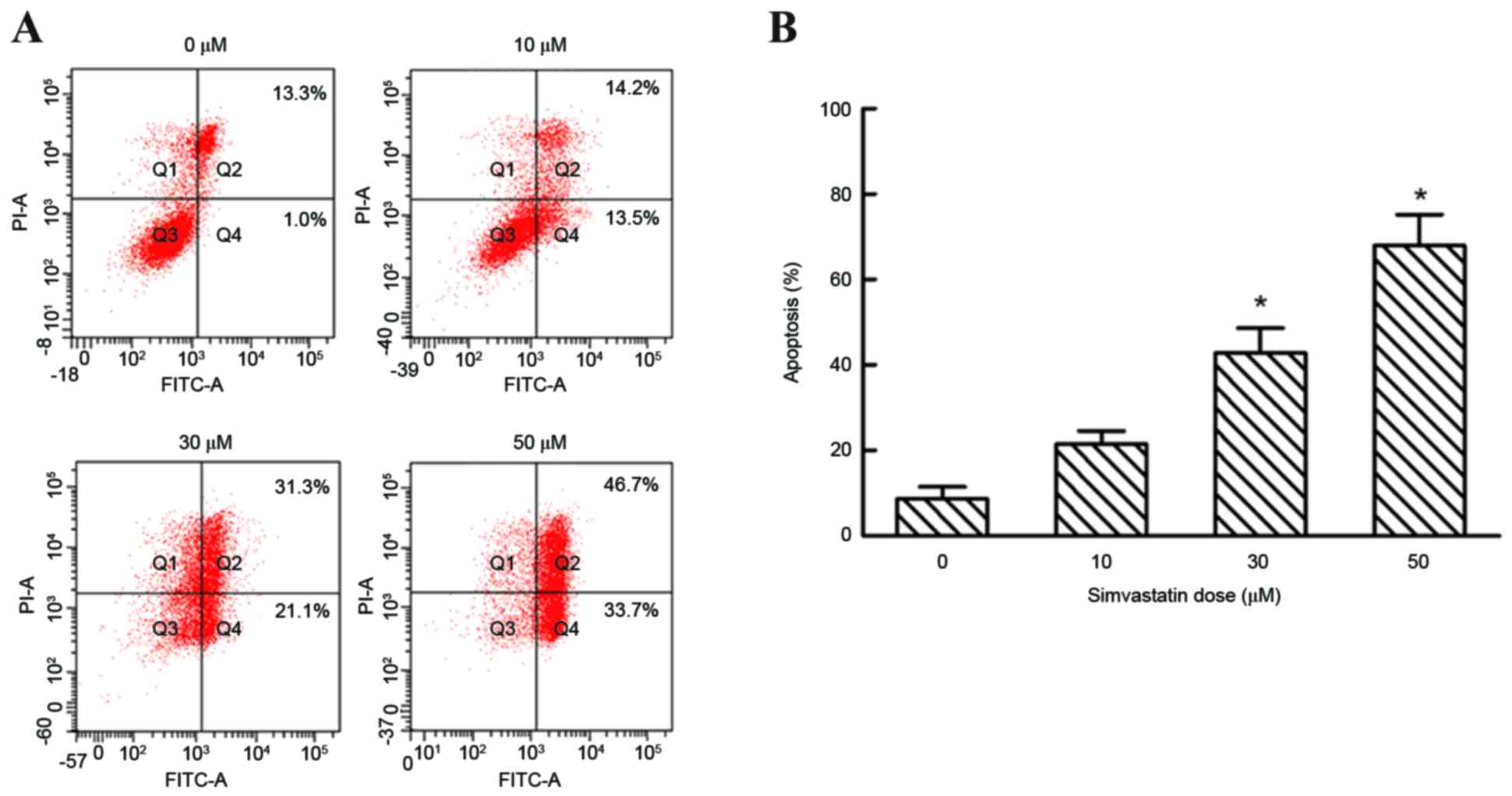
Flow cytometry analysis was performed to evaluate
the effect of simvastatin on cell apoptosis. Simvastatin at
concentrations of 0, 10, 30 and 50 µM increased the percentage of
apoptotic cells to 8.63±4.93, 21.43±5.43, 42.80±10.08 and
67.97±12.50%, respectively, following 48 h of exposure in early and
late apoptotic cells (P<0.05; Fig.
5). By contrast, lower concentrations of simvastatin (10 µM)
demonstrated no significant effects on the number of apoptotic of
SACC-83 cells when compared with untreated controls (10 µM,
21.34±5.43%; Fig. 5).
Effects of simvastatin on the
invasiveness of SACC-83 cells
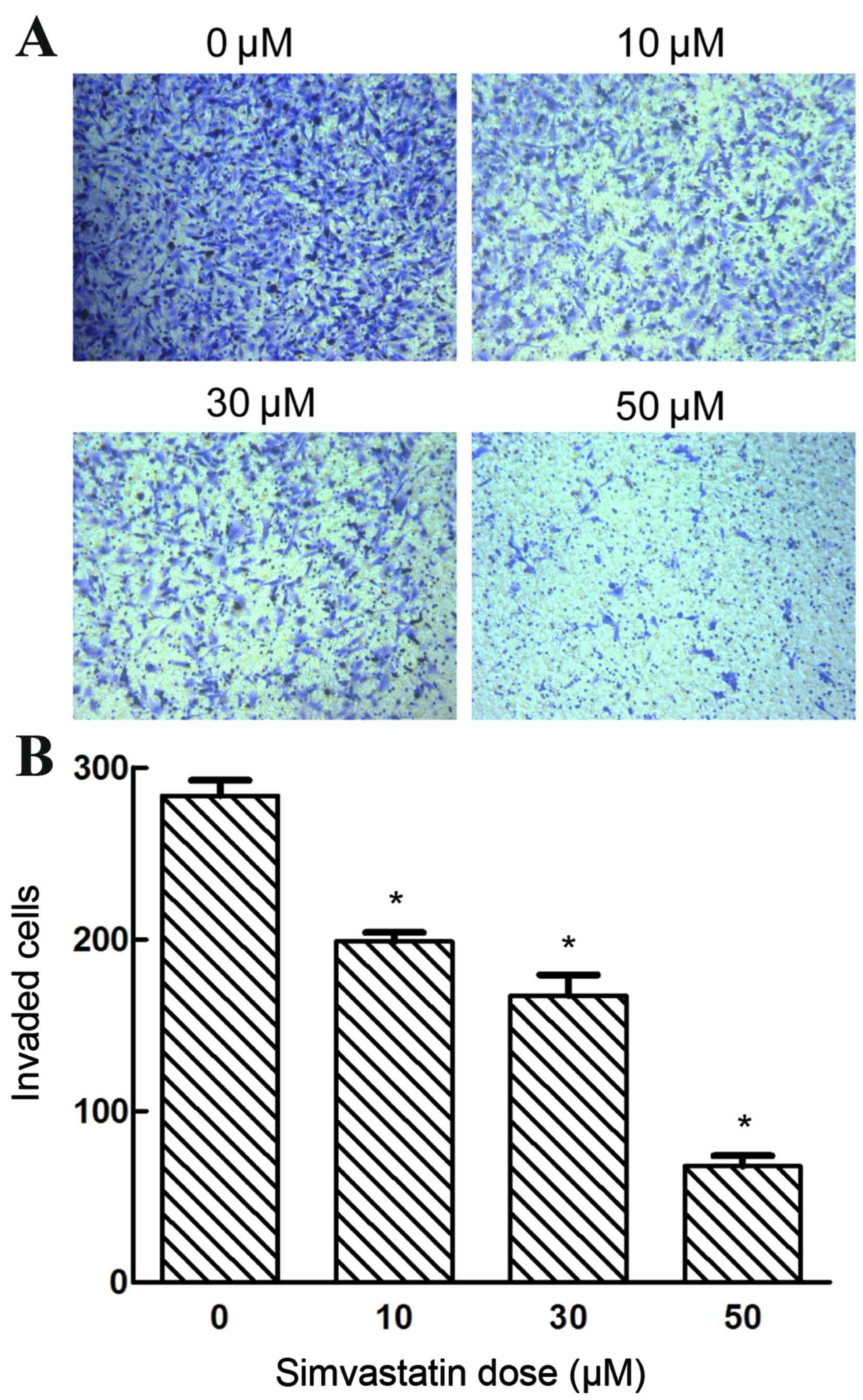
The present study investigated whether simvastatin
exposure affected the invasive capacity of SACC-83 cells. Treatment
with varying concentrations of simvastatin (0, 10, 30 and 50 µM)
significantly suppressed the invasive ability of SACC-83 cells in a
dose-dependent manner (P<0.05; Fig.
6). These results indicate the potency of simvastatin in
inhibiting the invasiveness of SACC-83 cells.
Discussion
SACC develops in the major and minor salivary glands
and is dispersed to the oral and oropharyngeal mucosa,
tracheobronchial tree and the esophagus. At present, focal
recurrence, distal metastases, or these outcomes together occur in
the majority of patients (22),
with 40 to 60% of SACC patients developing distant metastases in
the lungs, bone and soft tissues (23). There is currently no available
systemic therapy that effectively inhibits SACC progression.
Therefore, the aim of current research is to identify strategies
that target these refractory tumors.
Previous studies have demonstrated the potential of
simvastatin in the treatment of human cancers (10,24).
Simvastatin competitively functions as a potent and specific
inhibitor of 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA)
reductase, and its use in clinical practice to treat
hypercholesterolemia and hyperlipidemia is well established
(7). An increasing number of
studies have indicated that statins may improve the outcome of
anti-cancer treatments (25–27).
HMG-CoA reductase is involved in the transformation of HMG-CoA into
MVA. MVA, coupled with its downstream products, is involved in
essential biological processes, including cell membrane composition
(28–30), glycoprotein synthesis,
intracellular signal transduction and cell cycle regulation. In
addition, the MVA pathway has been implicated in tumorigenesis,
including cell survival, proliferation, migration and invasion
(31–33). In cancer patients, the suppression
of genes involved in the MVA pathway is associated with favorable
prognoses (34,35). However, the mechanisms by which
simvastatin downregulates the MVA pathway to activate
apoptosis-associated signaling remains poorly understood. A
previous study highlighted the critical role of the non-canonical
regulation of Rho guanosine 5′-diphosphate hydrolases, and the
involvement of the downstream superoxide-mediated activation of the
c-Jun N-terminal kinase pathway in simvastatin-mediated anticancer
activities (10,36). Therefore, in addition to their
lipid lowering, anti-inflammatory, antithrombotic and anti-oxidant
properties, as a result of the involvement of HMG-CoA reductase in
cholesterol synthesis and growth control, statins may demonstrate
chemopreventative effects against cancer. However, the underlying
molecular mechanisms remain unknown.
Survivin is an inhibitor of apoptosis proteins, and
is overexpressed in malignant tumors and repressed in normal
tissues. This suggests that survivin may be a novel target for
tumor diagnosis, prognosis and anti-cancer therapies (16). In addition, survivin is a key
factor in a number of cancers (37), and its gene encodes a
multifunctional protein involved in the regulation of the cell
cycle and inhibition of apoptosis pathways (38,39).
However, the role of survivin in regulating these processes is
currently unclear. At the transcriptional level, survivin
expression has been demonstrated to involve cell-cycle-dependent
element/cell cycle gene homology region G1 repressor elements in
the baculoviral IAP repeat-containing 5 promoter (40).
The CCK-8 and colony-forming experiments performed
in the present study demonstrated that simvastatin significantly
inhibited the proliferation of SACC-83 cells in a time- and
concentration-dependent manner. Cancer cell proliferation is
usually accompanied by cell cycle arrest, and cell cycle
progression is modulated by cyclin-dependent kinase inhibitors
(41,42). In the present study, an increased
number of SACC-83 cells were arrested at the G1 phase
following simvastatin treatment; however, further investigation is
required to elucidate the underlying mechanisms involved. Cancer
pathogenesis is associated with cell proliferation as well as
abnormal apoptosis (43). Cell
apoptosis is mediated by specific signaling molecules and
controlled by the programmed process of cellular self-destruction,
which is regulated by relevant genes (44). In the present study, simvastatin
demonstrated the ability to induce apoptosis of SACC cells. Flow
cytometry analysis revealed that, at 48 h post-simvastatin
treatment, a significant increase in the rate of cell apoptosis was
observed. Degradation of the extracellular matrix is crucial during
cell invasion, and is usually mediated by extracellular proteases,
notably matrix metalloproteinases (MMPs). Therefore, inhibition of
MMPs may facilitate the prevention of the metastasis of cancerous
cells (45). The mRNA and protein
expression levels of MMPs have been reported to be reduced
following exposure to increasing concentrations of simvastatin in
squamous cells (46). Thus,
simvastatin may reduce the expression of MMPs, which validates the
inhibited invasion and metastasis of tumor cells. In the present
study, simvastatin demonstrated a significant inhibitory effect on
the invasive capability of SACC-83 cells, which may be attributable
to its effect on MMP expression.
In the present study, simvastatin-induced apoptosis
was characterized by the expression of survivin. A total of 68.4%
in patients with SACC exhibit overexpression of survivin, and
patients with negative survivin expression demonstrate
significantly higher postoperative 5-year survival rates (47). In the present study, survivin
expression decreased in a concentration-dependent manner following
exposure of SACC-83 cells to simvastatin. The results of previous
studies indicate that survivin is closely associated with
tumorigenesis and the prognosis of adenoid cystic carcinoma (AdCC)
(10,48). Therefore, targeting survivin may be
an effective and novel approach to suppress the growth and improve
the prognosis of AdCC, which is consistent with the results of
previous studies. Altered survivin expression may be directly
implicated in the carcinogenic process, and due to its role in
multiple cellular networks, survivin has been extensively studied
as a potential anti-cancer drug target (49). In addition, reduced survivin
expression reportedly serves a crucial role in lovastatin-induced
apoptosis of SW480 colon cancer cells. Simvastatin induces
apoptosis via survivin downregulation (50), and may activate specific signaling
cascades to downregulate survivin expression by transcriptional,
post-transcriptional or post-translational mechanisms (51,52).
In addition, the downregulation of survivin by simvastatin may be
mediated by nuclear factor (NF)-κB and additional transcription
factors, such as hypoxia inducible factor-1α and signal transducer
and activator of transcription 3, with simvastatin as an inhibitor
of NF-κB-dependent anti-apoptotic gene expression (53). The authors of the present study
hypothesize that simvastatin may be attributable to the
downregulation of survivin expression. Therefore, further
investigation is required to elucidate the potential effects of
simvastatin on these transcription factors and/or their
interactions.
In conclusion, the present study demonstrated that
simvastatin inhibited the proliferation and invasion of SACC-83
cells. In addition, survivin was downregulated during the process
of simvastatin-induced apoptosis. These results support the current
notions regarding the molecular mechanisms of SACC, and the use of
survivin as a novel therapeutic target. The present study was
performed in vitro; however, the results demonstrate the
potential efficacy of simvastatin for the treatment of SACC, and
may therefore support further extensive investigation into the
efficacy of simvastatin as an antitumor agent.
Acknowledgements
The human SACC cell line, SACC-83, was provided by
the Shanghai No. 9 People's Hospital. The present study was
supported by the ‘Qing Lan Project’ of the Jiangsu Higher Education
Institutions Young and Middle-aged Academic Leaders Funded Project
(grant no. 51934) and the Outstanding Talent Fund Project of Xuzhou
Medical College (grant no. 2013012).
Glossary
Abbreviations
Abbreviations:
|
SACC
|
salivary gland adenoid cystic
carcinoma
|
|
MVA
|
mevalonate
|
References
|
1
|
van Weert S, Bloemena E, van der Waal I,
de Bree R, Rietveld DH, Kuik JD and Leemans CR: Adenoid cystic
carcinoma of the head and neck: A single-center analysis of 105
consecutive cases over a 30-year period. Oral Oncol. 49:824–829.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Moskaluk CA: Adenoid cystic carcinoma:
Clinical and molecular features. Head Neck Pathol. 7:17–22. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gondivkar SM, Gadbail AR, Chole R and
Parikh RV: Adenoid cystic carcinoma: A rare clinical entity and
literature review. Oral Oncol. 47:231–236. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tang Y, Liang X, Zheng M, Zhu Z, Zhu G,
Yang J and Chen Y: Expression of c-kit and Slug correlates with
invasion and metastasis of salivary adenoid cystic carcinoma. Oral
Oncol. 46:311–316. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dodd RL and Slevin NJ: Salivary gland
adenoid cystic carcinoma: A review of chemotherapy and molecular
therapies. Oral Oncol. 42:759–769. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Laurie SA, Ho AL, Fury MG, Sherman E and
Pfister DG: Systemic therapy in the management of metastatic or
locally recurrent adenoid cystic carcinoma of the salivary glands:
A systematic review. Lancet Oncol. 12:815–824. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xu L, Li ZL, Zhao LY, Liu YF, Li GX, Ding
MX, Zhao YQ, Fu Q and Zhao X: Effects of simvastatin on DNA
synthesis in rat cardiac fibroblasts. Nan Fang Yi Ke Da Xue Xue
Bao. 26205–207. (213)2006.(In Chinese). PubMed/NCBI
|
|
8
|
Kavalipati N, Shah J, Ramakrishan A and
Vasnawala H: Pleiotropic effects of statins. Indian J Endocrinol
Metab. 19:554–562. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen W, Garcia JG and Jacobson JR:
Integrin beta4 attenuates SHP-2 and MAPK signaling and reduces
human lung endothelial inflammatory responses. J Cell Biochem.
110:718–724. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhu Y, Casey PJ, Kumar AP and Pervaiz S:
Deciphering the signaling networks underlying simvastatin-induced
apoptosis in human cancer cells: Evidence for non-canonical
activation of RhoA and Rac1 GTPases. Cell Death Dis. 4:e5682013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu J, Shao C, Tan ML, Mu D, Ferris RL and
Ha PK: Molecular biology of adenoid cystic carcinoma. Head Neck.
34:1665–1677. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fukuda S and Pelus LM: Survivin, a cancer
target with an emerging role in normal adult tissues. Mol Cancer
Ther. 5:1087–1098. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ko YH, Roh SY, Won HS, Jeon EK, Hong SH,
Lee MA, Kang JH, Hong YS, Kim MS and Jung CK: Prognostic
significance of nuclear survivin expression in resected adenoid
cystic carcinoma of the head and neck. Head Neck Oncol. 2:302010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dresang LR, Guastafierro A, Arora R,
Normolle D, Chang Y and Moore PS: Response of Merkel cell
polyomavirus-positive merkel cell carcinoma xenografts to a
survivin inhibitor. PLoS One. 8:e805432013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Falleni M, Pellegrini C, Marchetti A,
Oprandi B, Buttitta F, Barassi F, Santambrogio L, Coggi G and
Bosari S: Survivin gene expression in early-stage non-small cell
lung cancer. J Pathol. 200:620–626. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Altieri DC: Survivin, versatile modulation
of cell division and apoptosis in cancer. Oncogene. 22:8581–8589.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Schlette EJ, Medeiros LJ, Goy A, Lai R and
Rassidakis GZ: Survivin expression predicts poorer prognosis in
anaplastic large-cell lymphoma. J Clin Oncol. 22:1682–1688. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kogo R, How C, Chaudary N, Bruce J, Shi W,
Hill RP, Zahedi P, Yip KW and Liu FF: The microRNA-218~Survivin
axis regulates migration, invasion, and lymph node metastasis in
cervical cancer. Oncotarget. 6:1090–1100. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Doğan M, Çağlı S, Yüce İ, Bayram A, Somdaş
MA, Karataş D, Cihan MC, Yüksel F and Güney E: Survivin expression
correlates with nodal metastasis in T1-T2 squamous cell carcinoma
of the tongue. Eur Arch Otorhinolaryngol. 272:689–694. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Marioni G, Bertolin A, Giacomelli L,
Marchese-Ragona R, Savastano M, Calgaro N, Marino F, De Filippis C
and Staffieri A: Expression of the apoptosis inhibitor protein
survivin in primary laryngeal carcinoma and cervical lymph node
metastasis. Anticancer Res. 26:3813–3817. 2006.PubMed/NCBI
|
|
21
|
Altieri DC and Marchisio PC: Survivin
apoptosis: An interloper between cell death and cell proliferation
in cancer. Lab Invest. 79:1327–1333. 1999.PubMed/NCBI
|
|
22
|
Jaso J and Malhotra R: Adenoid cystic
carcinoma. Arch Pathol Lab Med. 135:511–515. 2011.PubMed/NCBI
|
|
23
|
Ding LC, Huang XY, Zheng FF, Xie J, She L,
Feng Y, Su BH, Zheng DL and Lu YG: FZD2 inhibits the cell growth
and migration of salivary adenoid cystic carcinomas. Oncol Rep.
35:1006–1012. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Turrell FK, Kerr EM, Gao M, Thorpe H,
Doherty GJ, Cridge J, Shorthouse D, Speed A, Samarajiwa S, Hall BA,
et al: Lung tumors with distinct p53 mutations respond similarly to
p53 targeted therapy but exhibit genotype-specific statin
sensitivity. Genes Dev. Aug 8–2017.(Epub ahead of print).
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shimoyama S: Statins are logical
candidates for overcoming limitations of targeting therapies on
malignancy: Their potential application to gastrointestinal
cancers. Cancer Chemother Pharmacol. 67:729–739. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Matusewicz L, Meissner J, Toporkiewicz M
and Sikorski AF: The effect of statins on cancer cells-review.
Tumour Biol. 36:4889–4904. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Clendening JW and Penn LZ: Targeting tumor
cell metabolism with statins. Oncogene. 31:4967–4978. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Clendening JW, Pandyra A, Boutros PC, El
Ghamrasni S, Khosravi F, Trentin GA, Martirosyan A, Hakem A, Hakem
R, Jurisica I and Penn LZ: Dysregulation of the mevalonate pathway
promotes transformation. Proc Natl Acad Sci USA. 107:15051–15056.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Goldstein JL and Brown MS: Regulation of
the mevalonate pathway. Nature. 343:425–430. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nowakowski GS, Maurer MJ, Habermann TM,
Ansell SM, Macon WR, Ristow KM, Allmer C, Slager SL, Witzig TE and
Cerhan JR: Statin use and prognosis in patients with diffuse large
B-cell lymphoma and follicular lymphoma in the rituximab era. J
Clin Oncol. 28:412–417. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mokarram P, Alizadeh J, Razban V, Barazeh
M, Solomon C and Kavousipour S: Interconnection of
estrogen/testosterone metabolism and mevalonate pathway in breast
and prostate cancers. Curr Mol Pharmacol. 10:86–114. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ciaglia E, Grimaldi M, Abate M and Bifulco
M: The isoprenoid derivative N6-benzyladenosine (CM223) exerts
antitumor effect in glioma patient-derived primary cells through
the mevalonate pathway. Br J Pharmacol. 174:2287–2301. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shi J, Zhu J, Zhao H, Zhong C, Xu Z and
Yao F: Mevalonate pathway is a therapeutic target in esophageal
squamous cell carcinoma. Tumour Biol. 34:429–435. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jiang P, Mukthavaram R, Chao Y, Nomura N,
Bharati IS, Fogal V, Pastorino S, Teng D, Cong X, Pingle SC, et al:
In vitro and in vivo anticancer effects of mevalonate pathway
modulation on human cancer cells. Br J Cancer. 111:1562–1571. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Freed-Pastor WA, Mizuno H, Zhao X,
Langerød A, Moon SH, Rodriguez-Barrueco R, Barsotti A, Chicas A, Li
W, Polotskaia A, et al: Mutant p53 disrupts mammary tissue
architecture via the mevalonate pathway. Cell. 148:244–258. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Stamatakis K, Cernuda-Morollón E,
Hernández-Perera O and Pérez-Sala D: Isoprenylation of RhoB is
necessary for its degradation. A novel determinant in the complex
regulation of RhoB expression by the mevalonate pathway. J Biol
Chem. 277:49389–49396. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hwang KE, Na KS, Park DS, Choi KH, Kim BR,
Shim H, Jeong ET and Kim HR: Apoptotic induction by simvastatin in
human lung cancer A549 cells via Akt signaling dependent
down-regulation of survivin. Invest New Drugs. 29:945–952. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Boidot R, Végran F and Lizard-Nacol S:
Transcriptional regulation of the survivin gene. Mol Biol Rep.
41:233–240. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Salman T, Argon A, Kebat T, Vardar E,
Erkan N and Alacacıoğlu A: The prognostic significance of survivin
expression in gallbladder carcinoma. APMIS. 124:633–638. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Jaiswal PK, Goel A and Mittal RD:
Survivin: A molecular biomarker in cancer. Indian J Med Res.
141:389–397. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lin S, Shang Z, Li S, Gao P, Zhang Y, Hou
S, Qin P, Dong Z, Hu T and Chen P: Neddylation inhibitor MLN4924
induces G2 cell cycle arrest, DNA damage and sensitizes esophageal
squamous cell carcinoma cells to cisplatin. Oncol Lett.
15:2583–2589. 2018.PubMed/NCBI
|
|
42
|
Yu C, Cao H, He X, Sun P, Feng Y, Chen L
and Gong H: Cyclin-dependent kinase inhibitor 3 (CDKN3) plays a
critical role in prostate cancer via regulating cell cycle and DNA
replication signaling. Biomed Pharmacother. 96:1109–1118. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lara-Padilla E and Caceres-Cortes JR: On
the nature of the tumor-initiating cell. Curr Stem Cell Res Ther.
7:26–35. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Utz PJ and Anderson P: Life and death
decisions: Regulation of apoptosis by proteolysis of signaling
molecules. Cell Death Differ. 7:589–602. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Liu CC, Chang TC, Lin YT, Yu YL, Ko BS,
Sung LY and Liou JY: Paracrine regulation of matrix
metalloproteinases contributes to cancer cell invasion by
hepatocellular carcinoma-secreted 14-3-3σ. Oncotarget.
7:36988–36999. 2016.PubMed/NCBI
|
|
46
|
Yu X, Pan Y, Ma H and Li W: Simvastatin
inhibits proliferation and induces apoptosis in human lung cancer
cells. Oncol Res. 20:351–357. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wang YF, Ma SR, Wang WM, Huang CF, Zhao
ZL, Liu B, Zhang WF, Zhao YF, Zhang L and Sun ZJ: Inhibition of
survivin reduces HIF-1α, TGF-β1 and TFE3 in salivary adenoid cystic
carcinoma. PLoS One. 9:e1140512014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Farnebo L, Jerhammar F, Ceder R, Grafström
RC, Vainikka L, Thunell L, Grénman R, Johansson AC and Roberg K:
Combining factors on protein and gene level to predict
radioresponse in head and neck cancer cell lines. J Oral Pathol
Med. 40:739–746. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Tsuneki M, Kinjo T, Mori T, Yoshida A,
Kuyama K, Ohira A, Miyagi T, Takahashi K, Kawai A, Chuman H, et al:
Survivin: A novel marker and potential therapeutic target for human
angiosarcoma. Cancer Sci. 108:2295–2305. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Chang HL, Chen CY, Hsu YF, Kuo WS, Ou G,
Chiu PT, Huang YH and Hsu MJ: Simvastatin induced HCT116 colorectal
cancer cell apoptosis through p38MAPK-p53-survivin signaling
cascade. Biochim Biophys Acta. 1830:4053–4064. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Hisada T, Ayaori M, Ohrui N, Nakashima H,
Nakaya K, Uto-Kondo H, Yakushiji E, Takiguchi S, Terao Y, Miyamoto
Y, et al: Statin inhibits hypoxia-induced endothelin-1 via
accelerated degradation of HIF-1α in vascular smooth muscle cells.
Cardiovasc Res. 95:251–259. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Hu D, Liu S, Shi L, Li C, Wu L and Fan Z:
Cleavage of survivin by Granzyme M triggers degradation of the
survivin-X-linked inhibitor of apoptosis protein (XIAP) complex to
free caspase activity leading to cytolysis of target tumor cells. J
Biol Chem. 285:18326–18335. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wang YF, Ma SR, Wang WM, Huang CF, Zhao
ZL, Liu B, Zhang WF, Zhao YF, Zhang L and Sun ZJ: Inhibition of
survivin reduces HIF-1α, TGF-β1 and TFE3 in salivary adenoid cystic
carcinoma. PLoS One. 9:e1140512014. View Article : Google Scholar : PubMed/NCBI
|