Introduction
Gastric cancer (GC) is the third most common
malignant solid cancer and a global public health concern (1,2). As
in other cancers, GC is characterized by uncontrolled
proliferation, extensive invasion and distant metastasis, primarily
due to multiple risk factor-induced genetic alterations (3–5).
Certain developments have been achieved in the diagnosis and
therapy of GC over previous decades, but the long-term prognosis of
patients with GC has not markedly improved (6). The primary reason for poor prognosis
is late diagnosis at an advanced stage of the disease due to
insufficient understanding of the mechanisms underlying GC
(7). Therefore, it is necessary to
improve early diagnosis and develop effective treatment for GC.
Trophinin associated protein (TROAP; also termed
tastin) was first described as a soluble cytoplasmic protein that
forms a complex with trophinin and bystin, and participates in
early embryo implantation by mediating cellular invasion and
proliferation (8–11). Human TROAP is composed of 778 amino
acid residues rich in proline, with a basic N terminus and an
acidic C terminus. TROAP is abundant in testes, bone marrow and
thymus tissues (12). It contains
five putative cyclin recognition sites that could target the
mitotic kinase to appropriate substrates. A previous study has
demonstrated that TROAP is essential for spindle assembly and
monopolar spindle formation, which serve an important role in
maintaining structural and dynamic features of centrosomes during
mitosis (13). A microarray
analysis based on genomics data from the Genomics Institute of the
Novartis Research Foundation database demonstrated that TROAP is
upregulated in human cancer cell lines, including HeLa and Jurkat.
A previous study indicated that TROAP expression is also
upregulated in prostate cancer tissues (14). The above data strongly suggest that
increased TROAP expression may serve a role in GC proliferation and
metastasis.
To validate this hypothesis, the expression pattern
of TROAP was determined using the Oncomine database and GC cell
lines. The effect of dysregulation of TROAP expression on patient
overall survival was predicted using the Kaplan-Meier plotter.
Furthermore, the role of TROAP in GC cell lines was investigated by
analyzing cell proliferation, cell cycle distribution and invasive
capability.
Materials and methods
Oncomine microarray database
analysis
Expression of TROAP was retrieved from the Oncomine
database (https://www.oncomine.org/resource/login.html). A
combined filter was used to display corresponding datasets to
determine the differential expression of TROAP between GC and their
normal counterparts by defining the cancer type was GC, data type
as mRNA and analysis type as cancer vs. normal analysis. The
original data, including the Cui gastric (15), Cho gastric (16), Chen gastric (17) and Wang gastric (18) datasets were further analyzed and
results visualized using the GraphPad Prism software (version 5.0;
GraphPad Software, Inc., La Jolla, CA, USA) (15–18).
Kaplan-Meier overall survival
analysis
The prognostic value of the TROAP gene in GC was
analyzed using the Kaplan-Meier plotter (http://kmplot.com/analysis/). Samples have been
classified as either, increased or lower expression levels compared
with the median expression level of TROAP in GC tissues. Patient
survival information was compared using a Kaplan-Meier survival
plot. The hazard ratios with 95% confidence intervals and log rank
P-values were calculated using independent sample t test. In order
to reduce the false discovery rate, P<0.01 was considered to
indicate a statistically significant difference.
Cell lines and culture conditions
Human GC cell lines (AGS, SGC-7901, MGC80-3 and
BGC-823) and a normal gastric mucosa epithelial cell line GES-1
were obtained from the American Type Culture Collection (Manassas,
VA, USA). AGS cells were cultured in F12 medium (Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) containing 10% fetal bovine serum
(FBS; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA). The
remaining cell lines were cultured in RPMI-1640 medium (HyClone; GE
Healthcare Life Sciences, Logan, UT, USA) supplemented with 10% FBS
(Gibco; Thermo Fisher Scientific, Inc.). All cell lines were
incubated in a humidified atmosphere with 5% CO2 at
37°C.
Cell transfection
For the knockdown of TROAP (NCBI reference sequence
NM_005480), two sequences targeting TROAP (TROAP-shRNA1:
5′-CCGGCCTCCAACTCTGACCTCATATCTCGAGATATGAGGTCAGAGTTGGAGGTTTTTG-3′
and TROAP-shRNA2:
5′-CCGGGCCCTGTGTTTCATTCCAGTTCTCGAGAACTGGAATGAAACACAGGGCTTTTTG-3′),
and a scrambled negative control (NC) sequence
(5′-TTCTCCGAACGTGTCACGT-3′) were designed and synthesized by
Sigma-Aldrich (Merck KGaA). These stem-loop-stem oligo small
hairpin RNAs (shRNAs) were synthesized, annealed and ligated into a
pLKO.1-TRC vector (Addgene, Inc., Cambridge, MA, USA) between the
Age1 and EcoRI sites. Ligation was confirmed by DNA
sequencing (Shanghai Sangong Pharmaceutical Co., Ltd., Shanghai,
China). Lentiviral particles were generated by transient
transfection of 293T cells (Cell Bank of Chinese Academy of
Science, Shanghai, China) with TROAP-shRNA (or a scrambled
sequence) plasmid (from Professor ZhangXu lab; Institute of
Genetics and Developmental Biology, Chinese Academy of Sciences,
Beijing, China) at a multiplicity of infection of 45 using
Lipofectamine 2000 transfection reagent (Thermo Fisher Scientific,
Inc.), according to the manufacturer's protocol. Lentivirus
particles expressing TROAP-shRNA1, TROAP-shRNA2 or a
scrambled sequence were named shTROAP-1, shTROAP-2 and NC,
respectively. At 72 h after transfection, lentiviral particles were
harvested by ultracentrifugation (50,000 × g at 4°C for 15 min).
For cell transfection, SGC-7901 and MGC80-3 cell lines were seeded
in 24-well plates and transfected with constructed lentiviruses
containing shTROAP-1, shTROAP-2 or NC, respectively. Subsequently,
knockdown efficiency of TROAP was examined by western blot analysis
4 days after transfection.
Western blot analysis
Cells were lysed using a radioimmunoprecipitation
assay lysis buffer (Beyotime Institute of Biotechnology, Haimen,
China) supplemented with a Protease Inhibitor Cocktail
(Sigma-Aldrich; Merck KGaA) for protein extraction. Protein
concentration was determined by bicinchoninic acid protein assay
kit (Beyotime Institute of Biotechnology). A total of 30 µg protein
samples were separated by 10% SDS-PAGE and transferred to
polyvinylidene fluoride membranes (EMD Millipore, Billerica, MA,
USA). Western blot analysis was performed by incubation in 5%
skimmed milk blocking reagent at room temperature for 1 h, followed
by primary antibody against TROAP (1:1,000; cat. no. ab14531,
Abcam, Cambridge, UK) and GAPDH (1:50,000; cat. no. 10494-1-AP,
ProteinTech Group, Inc., Chicago, IL, USA) at 4°C overnight.
Membranes were washed with NaCl/Tris-Tween (Shanghai Sangong
Pharmaceutical Co., Ltd.) and incubated with a horseradish
peroxidase-conjugated secondary antibody (1:5,000; cat. no.
SC-2054; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) for 2 h
at room temperature. The blots were detected using an enhanced
chemiluminescence reagent (Amersham; GE Healthcare, Chicago, IL,
USA). GAPDH was used as an internal control.
Cell proliferation assay
MTT assay (Sigma-Aldrich; Merck KGaA) was used to
evaluate the proliferation rate of lentivirus-shRNA infected cells
according to manufacturer's protocol. Cells were seeded into
96-well plates at a density of 1×104 cells/well and
cultured in RPMI-1640 medium supplemented with 10% FBS. Cell
proliferation was determined at 24, 48 and 72 h by adding 20 µl MTT
(5 mg/ml) to each well, followed by incubation for 4 h. The
reaction was terminated by removal of the supernatant and addition
of 100 µl dimethyl sulfoxide (Sigma-Aldrich; Merck KGaA). The
absorbance was measured at a wavelength of 490 nm using an ELISA
microplate reader. Analysis of each sample was repeated three
times.
Cell cycle analysis
Flow cytometry was used to analyze cell cycle in GC
cells after 4 days after transfection. Briefly, cells were seeded
into 6-well plates, harvested by trypsinization and fixed in
ice-cold 70% ethanol overnight. Fixed cells were subsequently
stained with 200 µl propidium iodide at 4°C for 30 min. Cellular
DNA content from each sample was determined using a BD FACSCaliber
flow cytometer and ModFit LT software (Version 3.2; BD Biosciences,
San Jose, CA, USA). Analysis of each sample was repeated three
times.
Cell migration and invasion
assays
Transwell assay was used to detect the migration and
invasion of GC cells 96 h following transfection. For cell
migration, cells (1×105 cells/well) in 200 µl serum-free
medium were seeded in 24-well plates with an 8-mm pore membrane
insert (Corning Incorporated, Corning, NY, USA) in the upper
chamber. A total of 500 µl 10% FBS-containing medium was used as a
chemoattractant in the lower chamber. After 24 h incubation, cells
that migrated into the lower side of the membrane were stained with
0.1% crystal violet for 10 min at 37°C. For the cell invasion
assay, the procedure was similar to the cell migration assay,
except that the upper chamber was pre-coated with Matrigel (Corning
Incorporated). Stained cells at the bottom of the membrane were
observed and counted under a fluorescent microscope (Olympus CH-2,
Olympus Corporation, Tokyo, Japan). Experiments were performed
three times.
Statistical analysis
All data were analyzed using SPSS software (version
18.0; SPSS, Inc., Chicago, IL, USA) and GraphPad Prism software
(version 5.0) and expressed as the mean ± standard deviation of
three independent experiments. The statistical difference among
groups was evaluated by one-way analysis of variance or two-tailed
Student's t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
TROAP expression is upregulated in GC
tissues
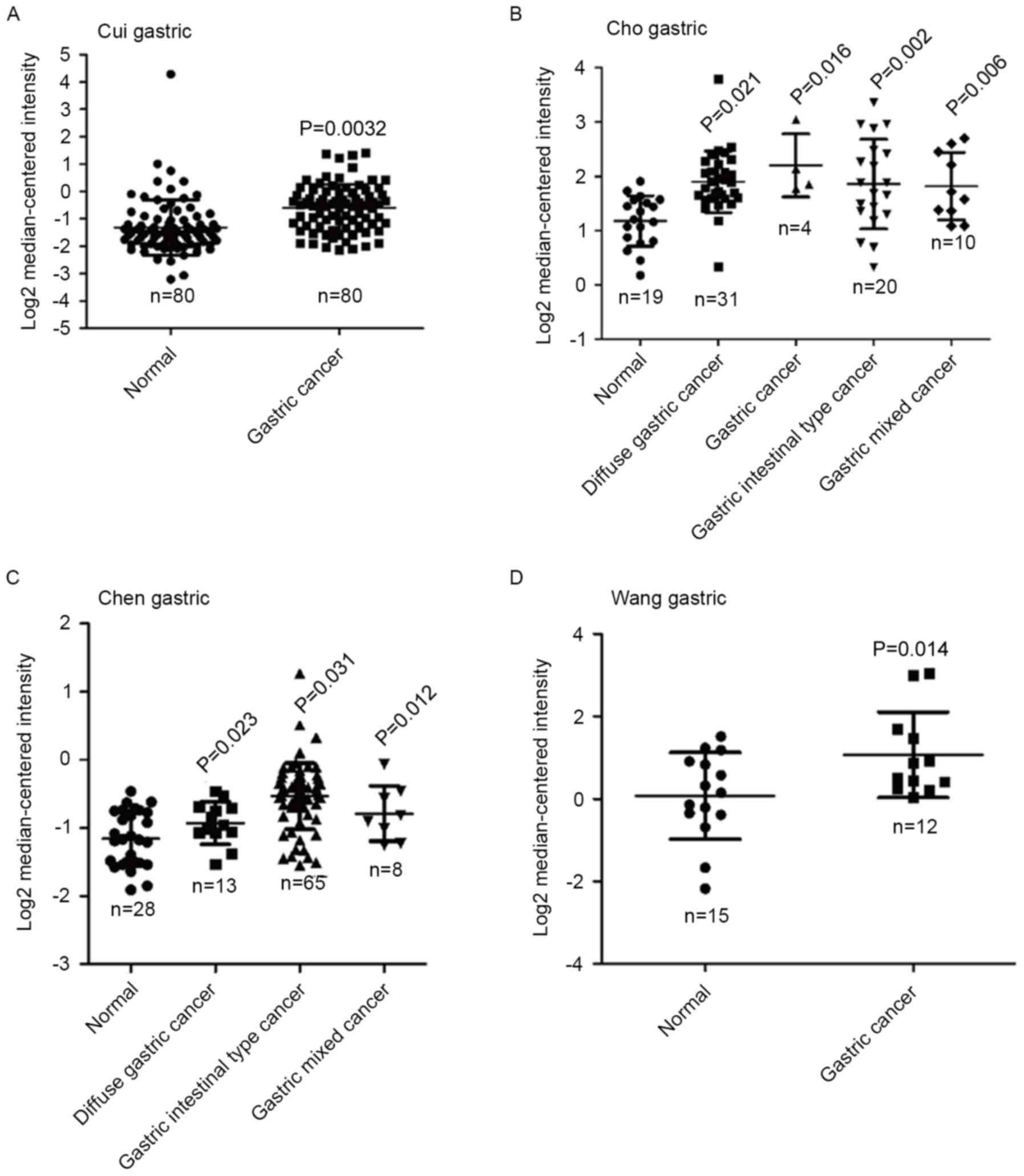
Using the Oncomine database, differential mRNA
expression of TROAP was determined between GC tissues and normal
gastric tissues. The results demonstrated that the expression of
TROAP was significantly elevated in gastric cancer (n=80; P=0.0032)
compared with normal tissues (n=80) in the Cui gastric dataset
(Fig. 1A). In the Cho gastric
dataset, TROAP expression was significantly increased in diffuse
gastric cancer (n=31; P=0.021), gastric cancer (n=4; P=0.016),
gastric intestinal type cancer (n=20; P=0.002) and gastric mixed
cancer (n=10; P=0.006) compared with normal tissues (n=19; Fig. 1B). Similarly, TROAP expression was
significantly increased in diffuse gastric cancer (n=13; P=0.023),
gastric intestinal type cancer (n=65; P=0.031) and gastric mixed
cancer (n=8; P=0.012) in the Chen gastric datasets (Fig. 1C). Data derived from the Wang
gastric dataset indicated that TROAP expression was also
upregulated in gastric cancer (n=15; P=0.014) compared with normal
tissues (n=12; Fig. 1D). The
observations suggest that TROAP expression is dysregulated in
GC.
Elevated expression of TROAP predicts
poor survival in patients with GC
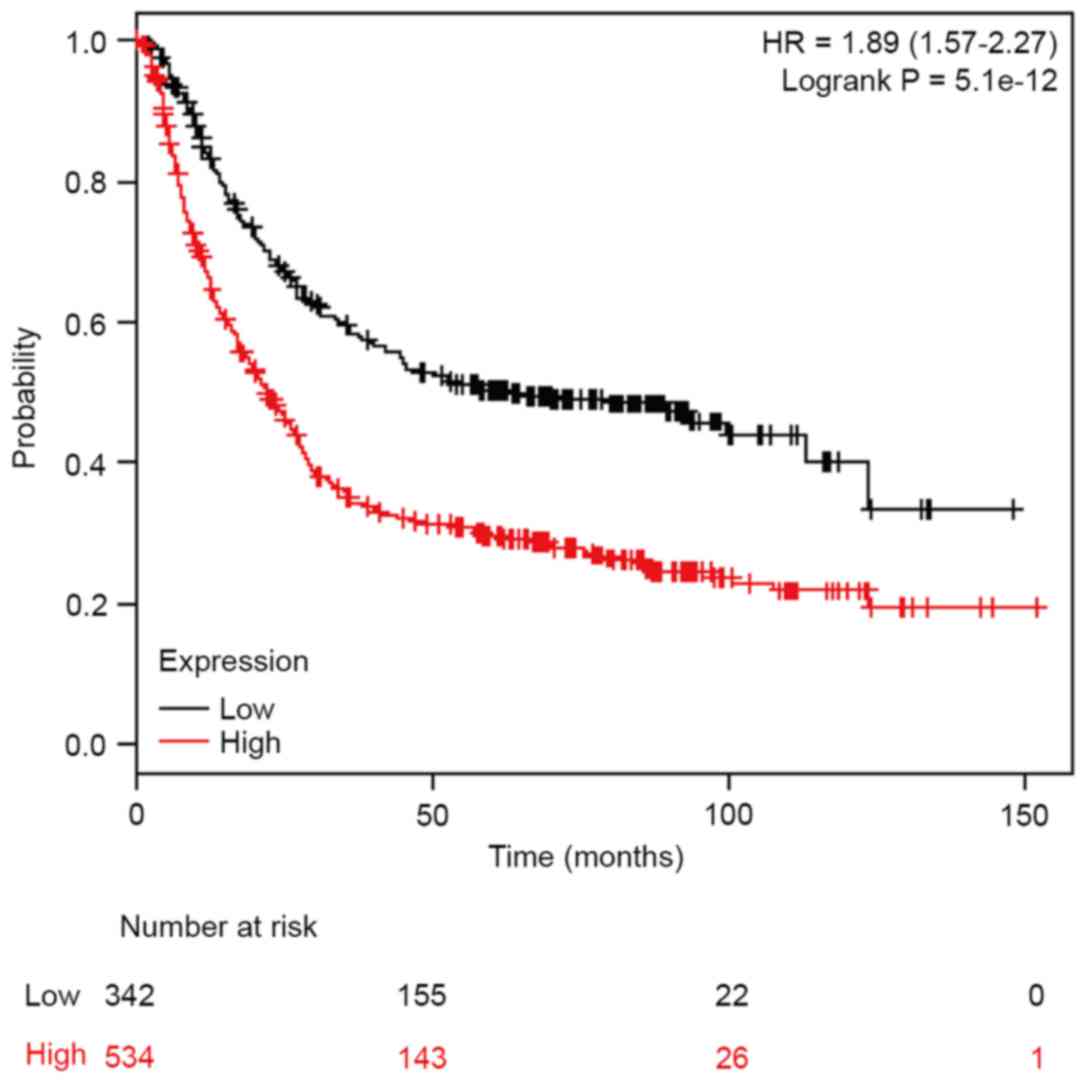
Furthermore, association between TROAP mRNA
expression levels and overall survival in patients with GC was
assessed using the Kaplan-Meier plotter (http://kmplot.com/analysis/). Elevated levels of TROAP
expression were associated with lower survival rates in patients
(hazard ratio=1.89, 95% confidence interval=1.57–2.27,
P=5.1×10−12; Fig. 2).
The results demonstrated that TROAP may act as a prognostic
oncogene for patients with GC.
Efficiency of shRNA knockdown of TROAP
determined by western blot analysis
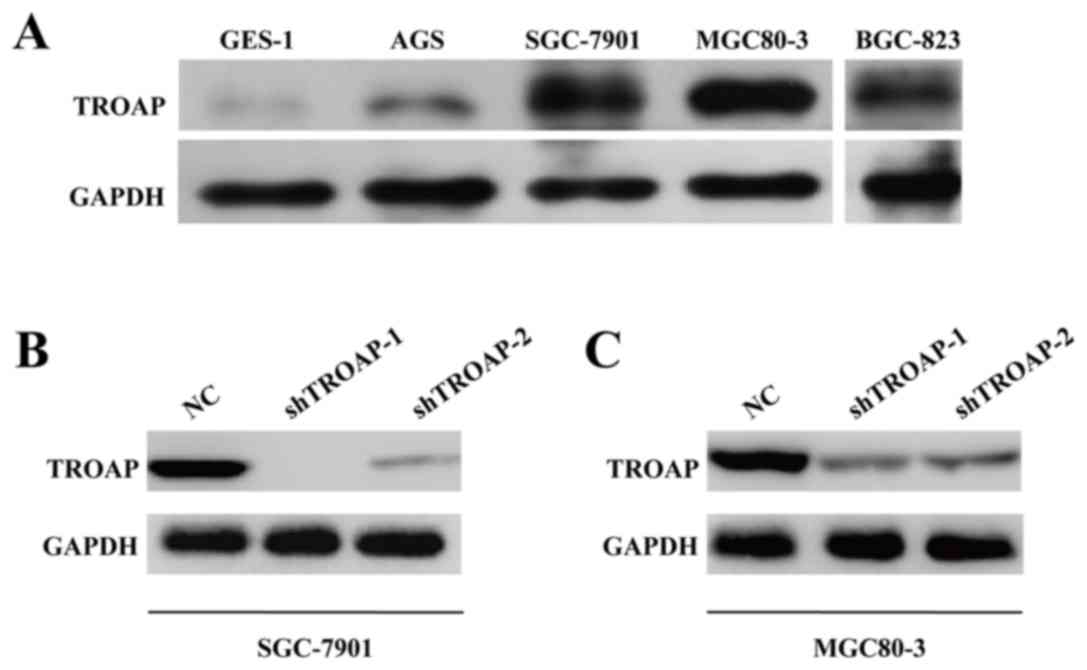
To further determine the potential oncogenic role of
TROAP in GC, expression of TROAP was determined in several GC cell
lines and a normal gastric cell line, GES-1. The expression level
of TROAP protein was upregulated in all GC cells compared with
GES-1 cells (Fig. 3A). SGC-7901
and MGC80-3 cells demonstrated markedly elevated TROAP expression.
Therefore, both of SGC-7901 and MGC80-3 cell lines were cultured
and transfected with NC or shTROAP. The expression levels of TROAP
protein were decreased in shTROAP groups compared with NC groups in
SGC-7901 and MGC80-3 cells (Fig. 3B
and C, respectively). Notably, shTROAP-1 produced a stronger
knockdown efficiency compared with shTROAP-2 in both cells and
therefore shTROAP-1 was selected for the subsequent
loss-of-function assays.
TROAP knockdown suppresses cell
proliferation and cell cycle progression in GC
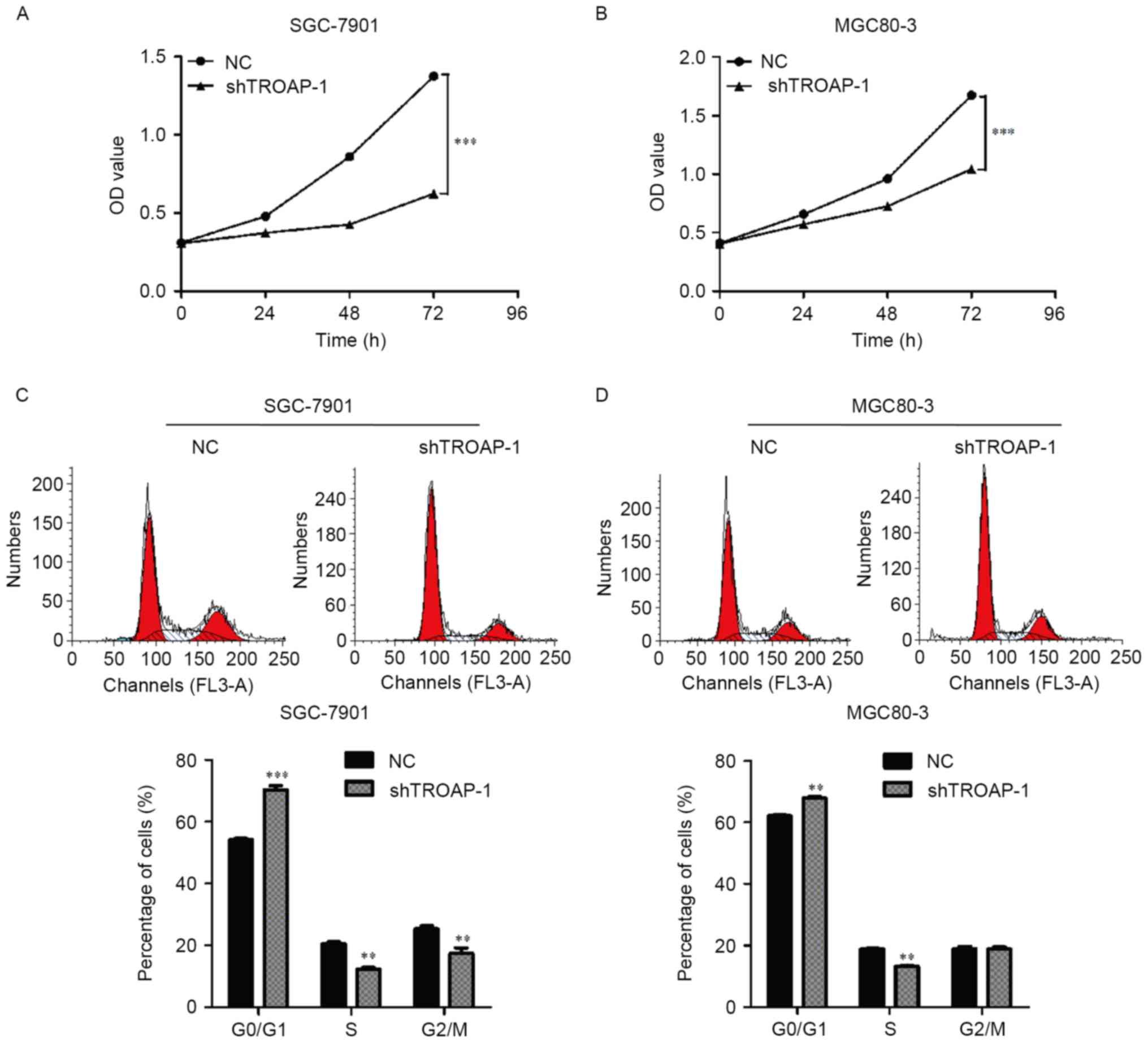
Proliferation rates of SGC-7901 and MGC80-3 cells
transfected with shTROAP-1 were significantly reduced compared with
the respective NC groups on the 72 h of the experiment (P<0.001;
Fig. 4A and B). To determine the
mechanism underlying the inhibition of cell growth, cell cycle
distribution was detected in SGC-7901 and MGC80-3 cells following
lentiviral transfection. The number of cells in the G0/G1 phase was
significantly increased, while the number of cells in the G2/M
phase was decreased in SGC-7901 cells transfected with shTROAP-1
compared with the NC group (P<0.05; Fig. 4C). Similar results were observed in
MGC80-3 cells treated with shTROAP-1 (Fig. 4D). These results indicated that the
cell cycle was arrested in the G0/G1 phase following TROAP
knockdown.
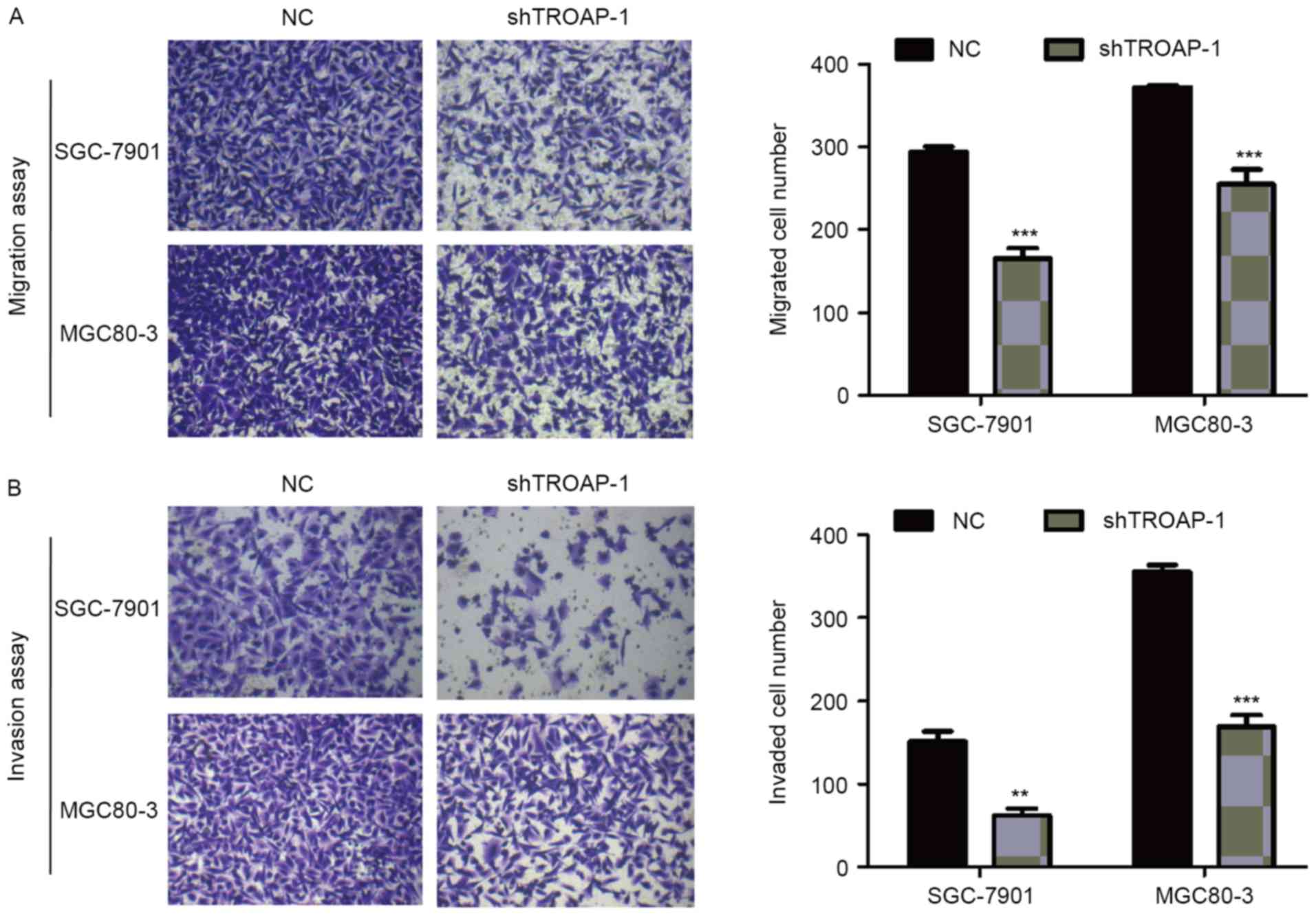
TROAP knockdown inhibits cell
migration and invasion capacity in GC
Transwell assay was used to detect the effect of
TROAP knockdown on cell migration and invasion. The number of cells
that migrated to the lower membrane in the SGC-7901/shTROAP-1 and
MGC80-3/shTROAP-1 groups was decreased markedly compared with the
respective NC groups (P<0.05; Fig.
5A). In addition, TROAP knockdown significantly impaired the
invasion ability of SGC-7901 and MGC80-3 cells (P<0.05; Fig. 5A). As expected, knockdown of TROAP
was associated with reduced migration and invasion abilities of GC
cells.
Discussion
The role of TROAP in malignancy has attracted the
interest of various research groups. TROAP, a soluble cytoplasmic
protein, was reported to be overexpressed in HeLa and Jurkat cells
and prostate cancer tissues, suggesting that TROAP is a
tumor-associated gene (14). In
the present study, mRNA expression of TROAP was significantly
upregulated in GC tissues compared with normal tissues and
upregulation of TROAP was associated with lower survival rates
predicted using an analysis of public online databases. In
addition, the effect of TROAP on the biological behavior of GC
cells was investigated. The results of the present study
demonstrated that TROAP may have an oncogenic effect on GC.
Deregulated growth is a primary requirement for
cancer development (19) and is
closely associated with dysregulation of the cell cycle, which is
composed of distinct sequential phases (G0/G1, S and G2/M)
(19–21). TROAP is a cycling protein essential
for cell cycle progression and its endogenous levels are tightly
controlled during mitosis. Furthermore, TROAP contains multiple
potential sites for serine/threonine phosphorylation by highly
active protein kinases, including cyclin-dependent kinase 1,
polo-like kinase and mitogen-activated protein kinases during
mitosis (20). Consistent with the
above evidence, in the present study reduced expression of TROAP by
shRNA significantly suppressed cell proliferation of SGC-7901 and
MGC80-3 by inhibiting G1 to S cell cycle transition. Resulting
abnormal spindle assembly caused by overexpression of TROAP may in
theory lead to genomic imbalances and contribute to oncogenesis
(21,22). The above data indicate that TROAP
may contribute to gastric carcinogenesis.
In addition to cell proliferation, invasion is also
a key step associated with the progression of tumor cells in target
microenvironments. Therefore, a Transwell migration/invasion assay
was performed to investigate the role of TROAP in GC cell motility.
The results of the present study indicated that reduced expression
of TROAP markedly decreased the migration and invasion ability of
GC cell lines (SGC-7901 and MGC80-3). Previous studies demonstrated
that TROAP and two other cytoplasmic proteins, trophinin and bystin
are the components of an adhesion molecule complex required for the
initial attachment of an embryo to the uterus (11,23).
Furthermore, overexpression of TROAP has been reported to promote
gallbladder cancer invasion and metastasis (24). TROAP is necessary for the cell
adhesion function of trophinin as it creates sites for efficient
adhesion to the cell surface (9).
TROAP act as a promoter of tumor migration and invasion during GC
progression.
In conclusion, the present study provided evidence
that TROAP serves a role in GC cells by promoting cell
proliferation, cell cycle progression and invasion in vitro.
Combined analysis of cell lines and datasets from a public
database, demonstrated that TROAP overexpression may be used as a
predictor of poor survival in patients with GC. The molecular
mechanisms of TROAP in GC remain to be elucidated. The present
study may be used for future application of TROAP-targeted therapy
in preclinical and clinical studies of gastric cancer
treatment.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
KJ and PM conceived and designed the experiments,
and wrote the manuscript. KJ, QM and PM performed the experiments
and analyzed the data.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ajani JA, Bentrem DJ, Besh S, D'Amico TA,
Das P, Denlinger C, Fakih MG, Fuchs CS, Gerdes H, Glasgow RE, et
al: Gastric cancer, version 2.2013: Featured updates to the NCCN
guidelines. J Natl Compr Canc Netw. 11:531–546. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Zeng H, Zhang S and He J:
Annual report on status of cancer in China, 2011. Chin J Cancer
Res. 27:2–12. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen DL, Ju HQ, Lu YX, Chen LZ, Zeng ZL,
Zhang DS, Luo HY, Wang F, Qiu MZ, Wang DS, et al: Long non-coding
RNA XIST regulates gastric cancer progression by acting as a
molecular sponge of miR-101 to modulate EZH2 expression. J Exp Clin
Cancer Res. 35:1422016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cai Y, Yi M, Chen D, Liu J, Guleng B, Ren
J and Shi H: Trefoil factor family 2 expression inhibits gastric
cancer cell growth and invasion in vitro via interactions with the
transcription factor Sp3. Int J Mol Med. 38:1474–1480. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lynch HT, Grady W, Suriano G and Huntsman
D: Gastric cancer: New genetic developments. J Surg Oncol.
90:114–133. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ju J, Wang N, Wang X and Chen F: A novel
all-trans retinoic acid derivative inhibits proliferation and
induces differentiation of human gastric carcinoma xenografts via
up-regulating retinoic acid receptor β. Am J Transl Res. 7:856–865.
2015.PubMed/NCBI
|
|
7
|
Yu B, Lv X, Su L, Li J, Yu Y, Gu Q, Yan M,
Zhu Z1 and Liu B: MiR-148a Functions as a tumor suppressor by
targeting CCK-BR via inactivating STAT3 and Akt in human gastric
cancer. PLoS One. 11:e01589612016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fukuda MN, Sato T, Nakayama J, Klier G,
Mikami M, Aoki D and Nozawa S: Trophinin and tastin, a novel cell
adhesion molecule complex with potential involvement in embryo
implantation. Genes Dev. 9:1199–1210. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Suzuki N, Zara J, Sato T, Ong E, Bakhiet
N, Oshima RG, Watson KL and Fukuda MN: A cytoplasmic protein,
bystin, interacts with trophinin, tastin, and cytokeratin and may
be involved in trophinin-mediated cell adhesion between trophoblast
and endometrial epithelial cells. Proc Natl Acad Sci USA.
95:5027–5032. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fukuda MN and Nozawa S: Trophinin, tastin,
and bystin: A complex mediating unique attachment between
trophoblastic and endometrial epithelial cells at their respective
apical cell membranes. Semin Reprod Endocrinol. 17:229–234. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Suzuki N, Nakayama J, Shih IM, Aoki D,
Nozawa S and Fukuda MN: Expression of trophinin, tastin, and bystin
by trophoblast and endometrial cells in human placenta. Biol
Reprod. 60:621–627. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nadano D, Nakayama J, Matsuzawa S, Sato
TA, Matsuda T and Fukuda MN: Human tastin, a proline-rich
cytoplasmic protein, associates with the microtubular cytoskeleton.
Biochem J. 364:669–677. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang S, Liu X, Yin Y, Fukuda MN and Zhou
J: Tastin is required for bipolar spindle assembly and centrosome
integrity during mitosis. FASEB J. 22:1960–1972. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dhanasekaran SM, Barrette TR, Ghosh D,
Shah R, Varambally S, Kurachi K, Pienta KJ, Rubin MA and Chinnaiyan
AM: Delineation of prognostic biomarkers in prostate cancer.
Nature. 412:822–826. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cui J, Chen Y, Chou WC, Sun L, Chen L, Suo
J, Ni Z, Zhang M, Kong X, Hoffman LL, et al: An integrated
transcriptomic and computational analysis for biomarker
identification in gastric cancer. Nucleic Acids Res. 39:1197–1207.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cho JY, Lim JY, Cheong JH, Park YY, Yoon
SL, Kim SM, Kim SB, Kim H, Hong SW, Park YN, et al: Gene expression
signature-based prognostic risk score in gastric cancer. Clin
Cancer Res. 17:1850–1857. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen X, Leung SY, Yuen ST, Chu KM, Ji J,
Li R, Chan AS, Law S, Troyanskaya OG, Wong J, et al: Variation in
gene expression patterns in human gastric cancers. Mol Biol Cell.
14:3208–3215. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang Q, Wen YG, Li DP, Xia J, Zhou CZ, Yan
DW, Tang HM and Peng ZH: Upregulated INHBA expression is associated
with poor survival in gastric cancer. Med Oncol. 29:77–83. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vermeulen K, Van Bockstaele DR and
Berneman ZN: The cell cycle: A review of regulation, deregulation
and therapeutic targets in cancer. Cell Prolif. 36:131–149. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mayor T, Meraldi P, Stierhof YD, Nigg EA
and Fry AM: Protein kinases in control of the centrosome cycle.
FEBS Lett. 452:92–95. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Brinkley BR: Managing the centrosome
numbers game: From chaos to stability in cancer cell division.
Trends Cell Biol. 11:18–21. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Doxsey SJ: Centrosomes as command centres
for cellular control. Nat Cell Biol. 3:E105–E108. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ayala GE, Dai H, Li R, Ittmann M, Thompson
TC, Rowley D and Wheeler TM: Bystin in perineural invasion of
prostate cancer. Prostate. 66:266–272. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chang XZ, Yu J, Zhang XH, Yin J, Wang T
and Cao XC: Enhanced expression of trophinin promotes invasive and
metastatic potential of human gallbladder cancer cells. J Cancer
Res Clin Oncol. 135:581–590. 2009. View Article : Google Scholar : PubMed/NCBI
|