Introduction
Diabetes mellitus (DM) can lead to various vascular
complications, and thus, cause cardiovascular dysfunction, which is
often coupled with high morbidity and mortality rates. It has been
reported that hyperglycemia plays an essential role in the
pathogenesis of diabetic vascular complications (1,2).
Apoptosis is a prominent phenotype in the hyperglycemia injury
model, which affects the function of vascular endothelium during
tissue damage (3,4). Vascular endothelial dysfunction is
recognized as the main pathophysiological basis of diabetic
angiopathy. Hyperglycemia-induced oxidative stress, an important
feature of diabetes, is one of the major consequences of diabetes
that may lead to production of reactive oxygen species (ROS) and
may also cause mitochondrial dysfunction (5). Additionally, high glucose (HG) levels
can induce apoptotic response in endothelial cells, thereby leading
to endothelial injury (6).
Numerous evidences indicate a link between ROS generation and high
glucose-induced apoptosis in endothelial cells (7,8).
Production of ROS is a part of the mitochondrial respiratory chain,
which can cause oxidative damage and aging (9). During the progress of diabetes
mellitus, excessive ROS production induces the loss of
mitochondrial membrane potential (MMP) along with an increase in
mitochondrial membrane permeabilization (10). Subsequently, amounts of important
proteases or proteins that are directly related to apoptosis are
released from the mitochondria into the cytosol, which initiates
the apoptotic cascade and accelerates apoptosis (11,12).
The protein, Omi/HtrA2, is recognized as a pro-apoptotic serine
protease, which is located in the mitochondrial inter-membrane
space. It is reported that Omi/HtrA2 promotes cell apoptosis mainly
through the caspase-dependent pathways and caspase-independent
pathways. In the former pathway, Omi/HtrA2 is transferred into the
cytoplasm. After wards, Omi/HtrA2 activates the caspase cascade by
binding to the IAPs (inhibitor of apoptosis proteins), which
finally induces cell apoptosis. The latter relies on the activity
of protease itself to cause apoptosis (13,14).
Recently, the use of traditional Chinese medicine or
combined therapy of Chinese and Western medicine in the treatment
of diabetes has increasingly attracted attention. Obtusifolin is an
anthraquinone compound obtained from the seeds of Cassia
obtusifolia, which is a traditional Chinese medicine. It is
reported that obtusifolin can lower blood pressure and reduce serum
cholesterol levels. Meanwhile, its anti-oxidant, anti-fungal, and
neuroprotective activities have also been reported (15–17).
However, the potential effects of obtusifolin on
high-glucose-induced injury have not yet been clearly illustrated
and the underlying mechanisms remain obscure.
Our results have demonstrated that obtusifolin can
protect human umbilical vein endothelial cells (HUVECs) from
oxidative stress and mitochondrial apoptosis, which may otherwise
be induced by high glucose treatment. The protective effect may be
attributed to the inhibition of the release of Omi/HtrA2 into the
cytosol. Our research would provide molecular clues to provide
basis for further theoretical research and will be an evidence for
the potential of obtusifolin to be a potent agent in the treatment
of diabetes mellitus.
Materials and methods
Cell culture
HUVECs were obtained from the American Type Culture
Collection (ATCC; Manassas, VA, USA). Cells were cultured in
low-glucose Dulbecco's modified Eagle's medium (DMEM; Invitrogen
Life Technologies, Carlsbad, CA, USA) that was supplemented with
10% fetal bovine serum, 100 U/ml penicillin and 100 µg/ml
streptomycin (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). The
cells were then incubated in a humidified atmosphere with 5%
CO2 at 37°C.
Cell treatment
Six treatment groups were designed for the
subsequent experiments: i) Control group (control), where cells
were incubated with 5.5 mM glucose; ii) mannitol group, where cells
were treated with 33 mM mannitol for 48 h; iii) high glucose group
(H-Glu), where cells were incubated with 33 mM glucose for 48 h;
iv) obtusifolin pretreatment groups, where cells were preserved in
the medium containing obtusifolin (Solarbio, Beijing, China) at
different doses (5, 7.5 and 10 mg/ml) for 6 h prior to the
treatment with 33 mM glucose for 48 h (H-Glu+5, H-Glu+7.5,
H-Glu+10). After incubation, HUVECs were harvested for subsequent
experiments.
Cell viability assay
For the cell viability assay, 1×104
HUVECs were seeded in a 96-well plate. After being serum-starved
overnight, the cells were treated with glucose (11–33 mM) for 12,
24, and 48 h, separately. Following this, CCK-8 solution was added
to the 96-well plate. The plate was incubated in a CO2
incubator at 37°C for 4 h and then measured according to the
protocol provided by the manufacturers of the CCK-8 detection kit
(Cwibo, China).
Flow cytometer analysis for cellular
ROS
The HUVECs were treated as above. Total and
mitochondrial-specific ROS production was measured using CM-H2DCFDA
probe (Invitrogen Life Technologies) and mitoSOX dye (Molecular
Probes, Eugene, OR, USA), respectively. Briefly, cells were
harvested and rinsed in pre-warmed phosphate-buffered solution
(PBS) containing CM-H2DCFDA or mitoSOX in the dark for 20 min at
37°C. The levels of ROS were determined using a BD FACSCalibur flow
cytometer (Becton-Dickinson, San Jose, CA, USA) according to the
manufacturer's instructions.
Flow cytometry analysis for MMP
The MMP in HUVECs was determined following the JC-1
dye staining method (Beyotime Institute of Biotechnology, Shanghai,
China). The cells seeded in 6-well plates were treated as above and
stained using the fluorescent dye, JC-1, at 37°C for 20 min in the
dark. The cells were resuspended in PBS and analyzed using flow
cytomety (Becton-Dickinson). The results then were calculated as
previously described (18) and
expressed as the proportion of cells with a low MMP.
Flow cytometry analysis for
apoptosis
A fluorescein isothiocyanate (FITC)-Annexin V
apoptosis detection kit (Invitrogen Life Technologies) was used to
quantify the numbers of apoptotic cells. Cells in the 96-well plate
(at a density of 1×104 cells/well) were treated as
instructed by the manufacturer's instructions and then washed with
PBS. They were then stained with Annexin V and PI for 20 min at
room temperature. The level of apoptosis was detected by measuring
the fluorescence of cells using flow cytometer
(Becton-Dickinson).
Immunofluorescence assay
Firstly, HUVECs were fixed using methanol. To block
unspecific antigens, cells were incubated with 5% fetal bovine
serum (FBS), 0.1% Triton X-100 in 1X PBS buffer. Then, HUVECs were
incubated with anti-factor VIII antibody (Abcam, Cambridge, UK) at
4°C overnight after washing with 1X PBS thrice. After removal of
the antibody solution, HUVECs were incubated with secondary
FITC-conjugated anti-mouse antibody (Sigma-Aldrich; Merck KGaA) at
37°C for 30 min, and their nuclei were stained with DAPI for 15
min. The HUVECs were then mounted on a slide and observed under a
fluorescence microscope (Olympus, Tokyo, Japan).
Antioxidant enzyme activity assays and
malondialdehyde levels
The HUVECs seeded in a 6-well plate (at a density of
1×105 cells/well) were treated as described above.
According to the manufacturer's instructions, the activity of
superoxide dismutase (SOD) and catalase (CAT) was measured using
the SOD assay kit (Beyotime Institute of Biotechnology) and the CAT
assay kit (Solarbio), respectively. Malondialdehyde (MDA) levels
were detected using a malondialdehyde assay kit (Solarbio).
Mitochondrial respiratory chain
activities assays
HUVECs (1×105) were seeded and treated as
described above. The enzyme activities of complex I (NADH
dehydrogenase) and complex III (cytochrome bc1 complex) were
examined using spectrophotometric method as previously described
(19). Briefly, cells were lysed
with 100 mM Tris-HCl (pH 7.4) buffer containing 250 mM sucrose and
2 mM EDTA. The activity of complexes I and III was detected by
measuring the reduction of 2,6-dichlorophenolindophenol (DCPIP) at
520–600 nm (extinction coefficient, 19.1 mM/cm) and cytochrome
c at 550–540 nm (extinction coefficient, 19.0 mM/cm),
respectively.
Quantitative polymerase chain reaction
(qPCR) analysis
Total RNA was isolated using TRIzol reagent
(Invitrogen Life Technologies). cDNA was synthesised with 1 µg RNA
and GoScript™ reverse transcriptase (Promega, Madison, WI, USA)
according to the manufacturer's instructions. qPCR assays were
performed using power SYBR green PCR master mix and ABI 7500 Fast
Real-Time PCR system (Applied Biosystems; Thermo Fisher Scientific,
Inc., Waltham, MA, USA). The mRNA relative expression was
calculated with the 2−ΔΔCt method (20). The primer sequences were as
follows: PARP forward, 5′-GTGCCAACTACTGCCATACG-3′ and reverse,
5′-GCTATCATCAGACCCTCCCC-3′; caspase-3 forward,
5′-CATCGCTCTTGAAGACCAGC-3′ and reverse, 5′-AGTCCAGTTCTGTACCACGG-3′;
caspase-9 forward, 5′-AAGTGACCCTCCCAAGTAGC-3′ and reverse,
5′-GTTCTGGCCAGGTCTCTTCT-3′; XIAP forward,
5′-TGTCCCTTTGATTACGGGCT-3′ and reverse, 5′-AAGCCTGTAATCCCAGCACT-3′;
GAP DH forward, 5′-CACAGTCCATGCCATCACTG-3′ and reverse,
5′-ATCTCGCTCCTGGAAGATGG-3′.
Immunoblot analysis
Whole cell proteins were extracted using total
protein extraction kit (Solarbio). Then, samples were separated by
running them on sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE) before transferring to polyvinylidene
fluoride (PVDF) membrane (Millipore, Billerica, MA, USA). After
blocking non-specific antigen, the membrane was incubated at 4°C
overnight with primary antibodies for PARP, XIAP, caspase-3/-9, and
GAPDH (Cell Signaling Technology, Inc., Danvers, MA, USA). The
membrane was then incubated with horseradish peroxidase-conjugated
goat anti-rabbit secondary antibody (Santa Cruz Biotechnology,
Inc., Santa Cruz, CA, USA) for 1 h. The blots were developed by
enhanced chemiluminescence substrate (FD, China).
Statistical analysis
Data are presented as mean ± standard deviation (SD)
and analyzed by one-way analysis of variance (ANOVA) or t-test.
P-value <0.05 was considered to indicate a statistically
significant difference.
Results
Establishment of hyperglycemia injury
model
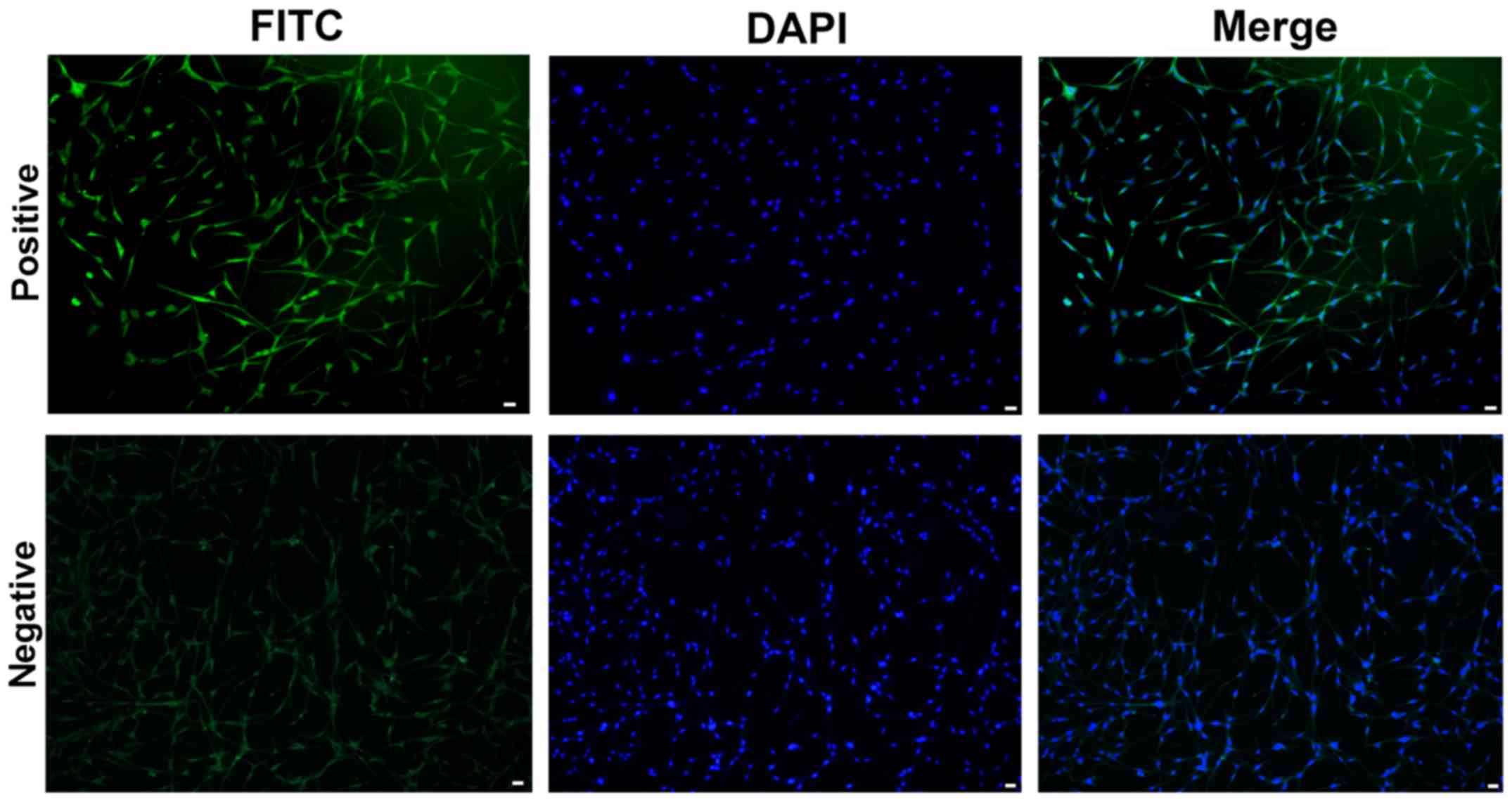
First, the phenotype of HUVECs was identified using
immunofluorescence staining (Fig.
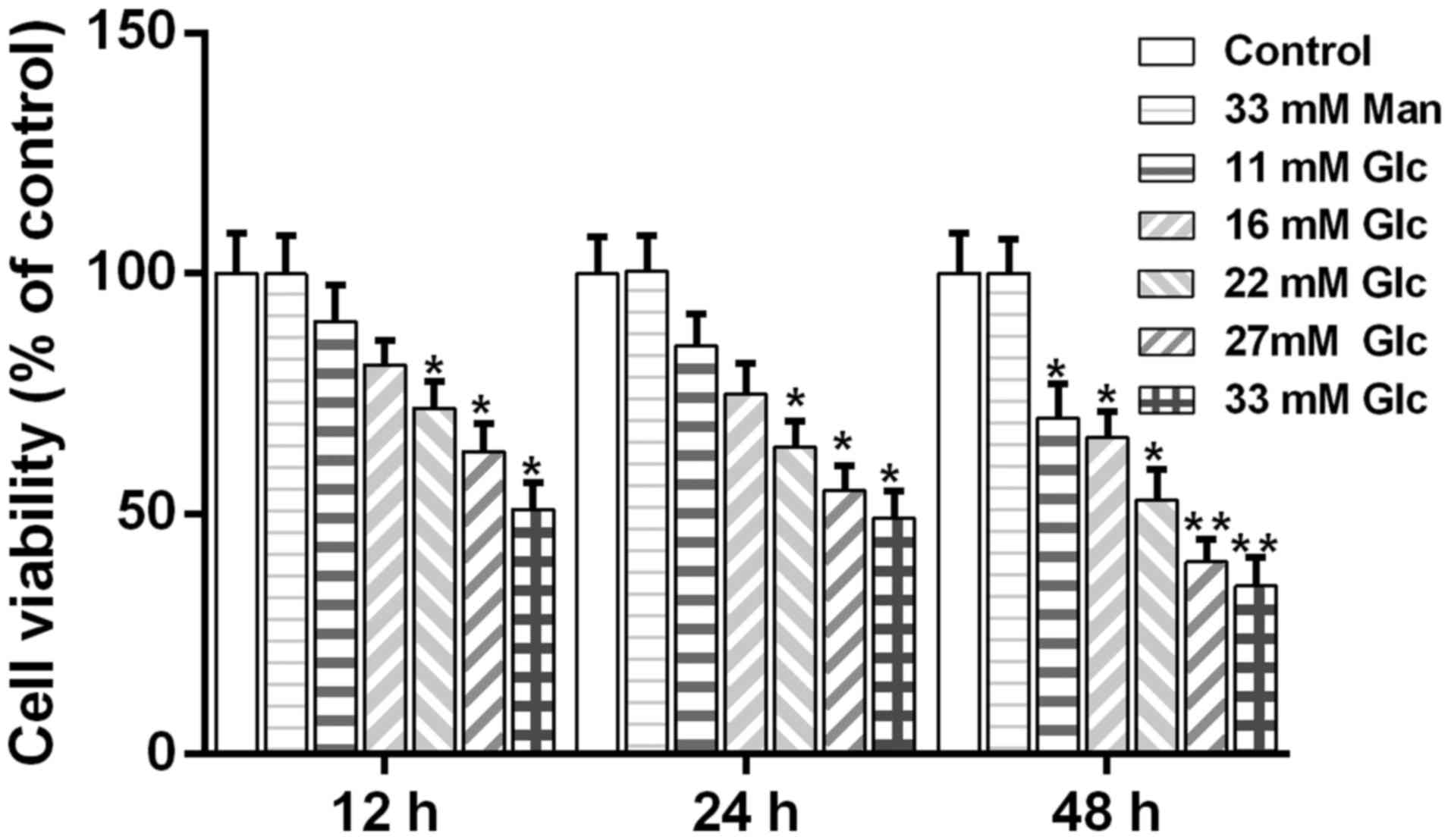
1). Then, the CKK-8 detection showed that the cell viability of
HUVECs declined gradually with an increase in the concentration of
glucose. In contrast, cell viability was not affected by high
mannitol content (Fig. 2). In this
study, we employed 33 mM glucose to stimulate HUVECs for
establishment of the hyperglycemia injury model.
Obtusifolin protects HUVECs from
oxidative stress
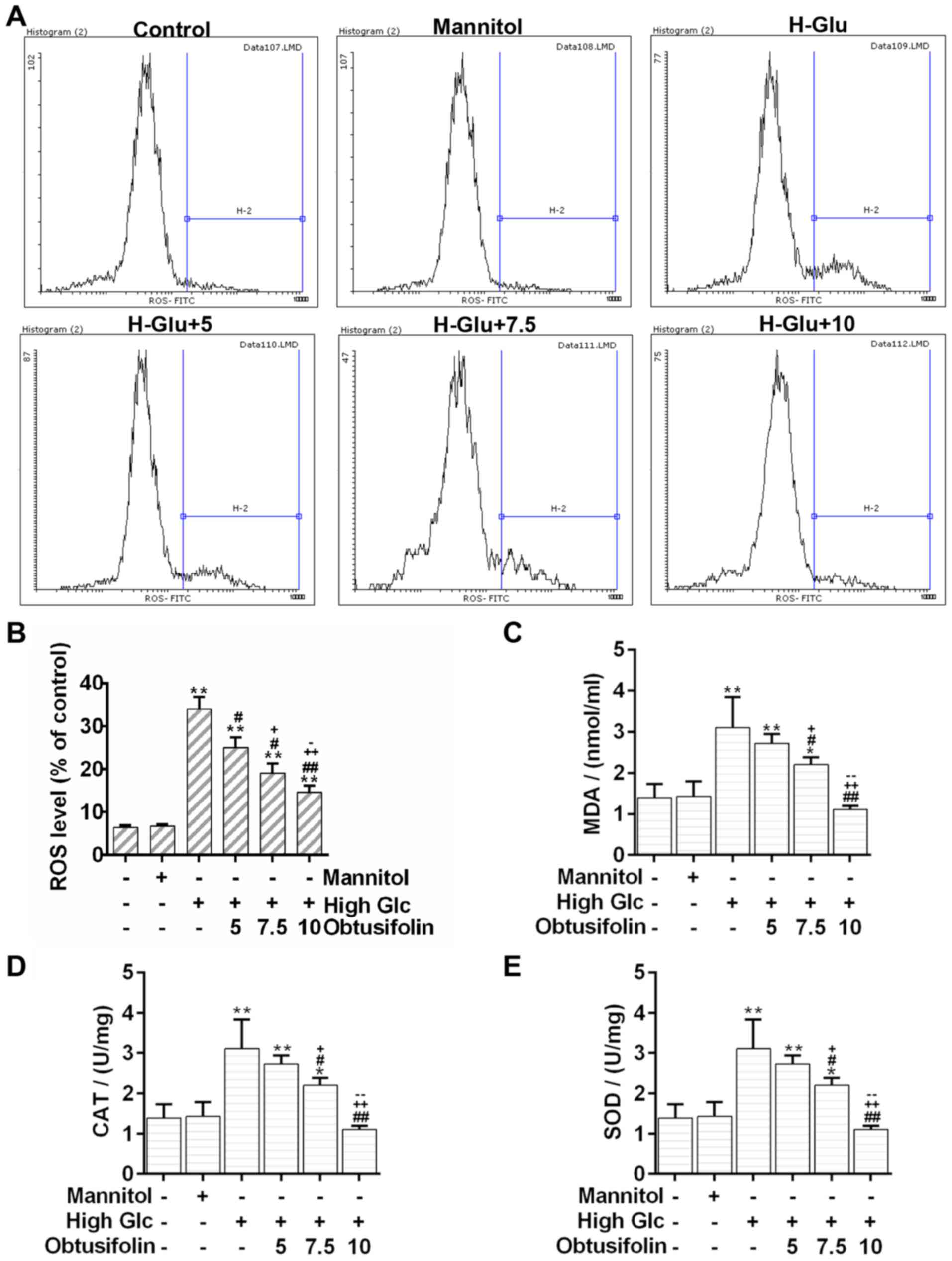
Oxidative stress is an important mediator of
vascular complications in DM (21). The flow cytometry analysis
displayed that cellular ROS was triggered under the high glucose
environment. Conversely, the production of cellular ROS was reduced
significantly after pretreatment with obtusifolin (Fig. 3A and B). Moreover, our results
showed that the levels of MDA were significantly lower in the
obtusifolin pretreatment groups than in the model group (Fig. 3C). Nevertheless, the activity
detection assays displayed that both the CAT and SOD activities
significantly increased in the obtusifolin pretreatment groups as
compared to activities in the model group (Fig. 3D and E). Results further suggested
that obtusifolin could protect HUVECs from oxidative stress in in a
dose dependent manner.
Obtusifolin attenuates high
glucose-induced mitochondrial dysfunction in HUVECs
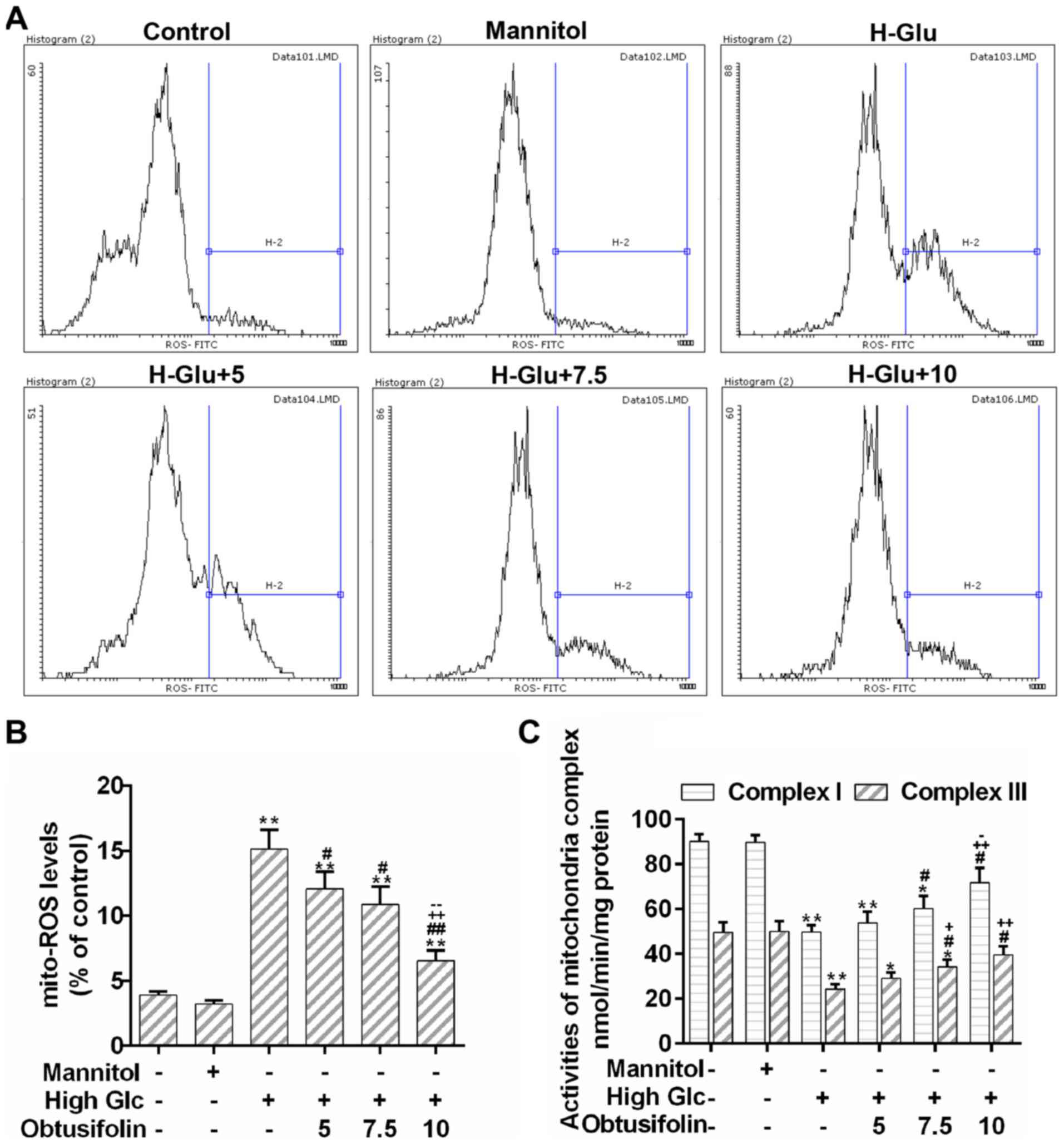
According to results of the flow cytometry analysis,
the production of mitochondrial ROS caused by hyperglycemia was
obviously decreased by the pretreatment with obtusifolin, although
there was no significant difference between the medium
concentration group and the low one (Fig. 4A and B). Moreover, the activities
of mitochondrial respiratory chain complex I/III in the obtusifolin
pretreatment groups were effectively recovered as compared to those
in the model group (Fig. 4C).
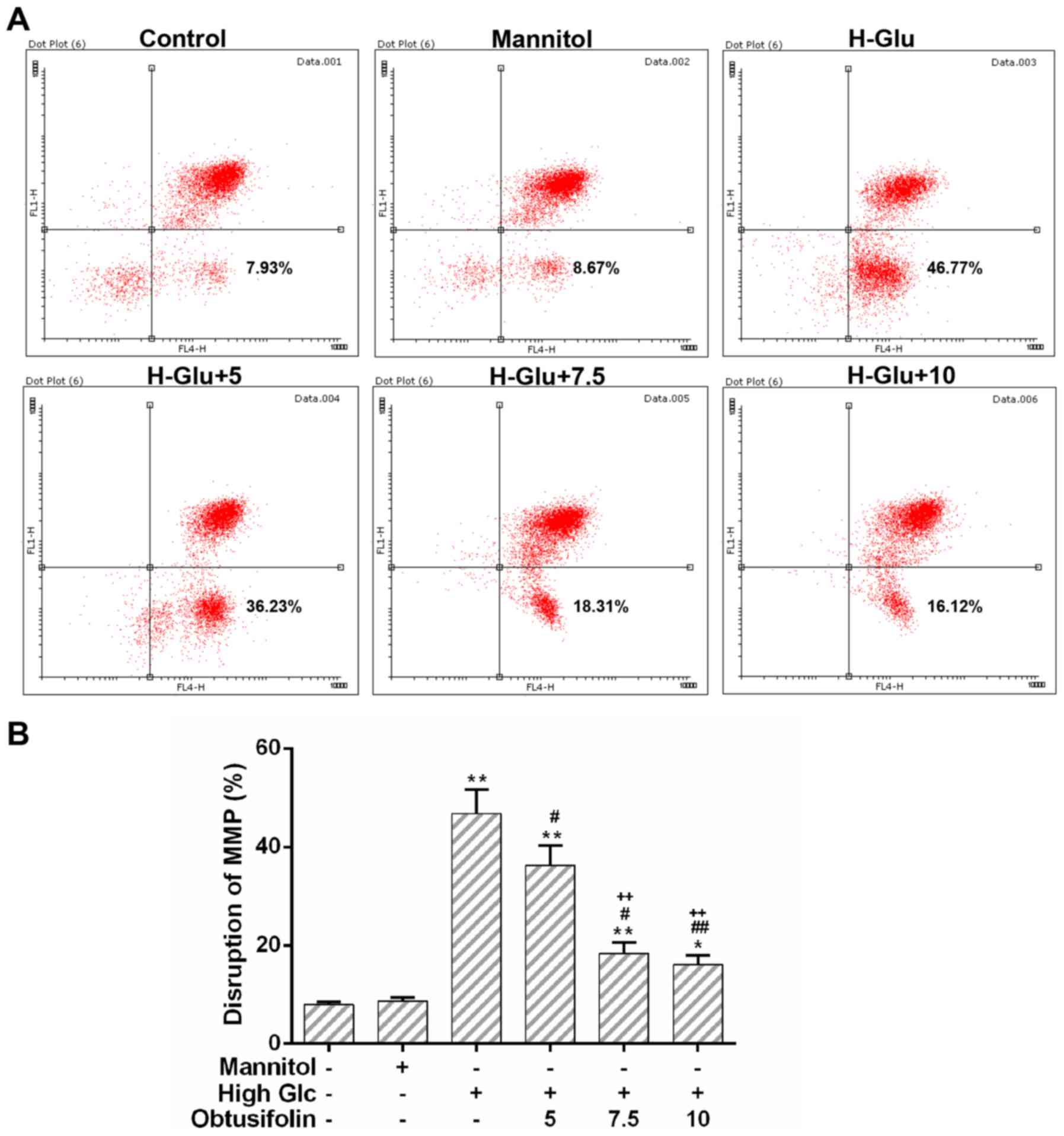
Furthermore, the MMP of HUVECs was recovered significantly after
pretreatment with obtusifolin as compared to that in the model
group (Fig. 5A and B). No
significant difference was observed between the high concentration
obtusifolin treatment group and the medium one with regard to the
activity of mitochondrial respiratory chain complex III and the
MMP. Nevertheless, the declined tendency of mitochondrial
dysfunction in the obtusifolin pretreatment groups was still
obvious.
Obtusifolin protects cells against
high glucose-induced apoptosis
Mitochondrial dysfunction could activate
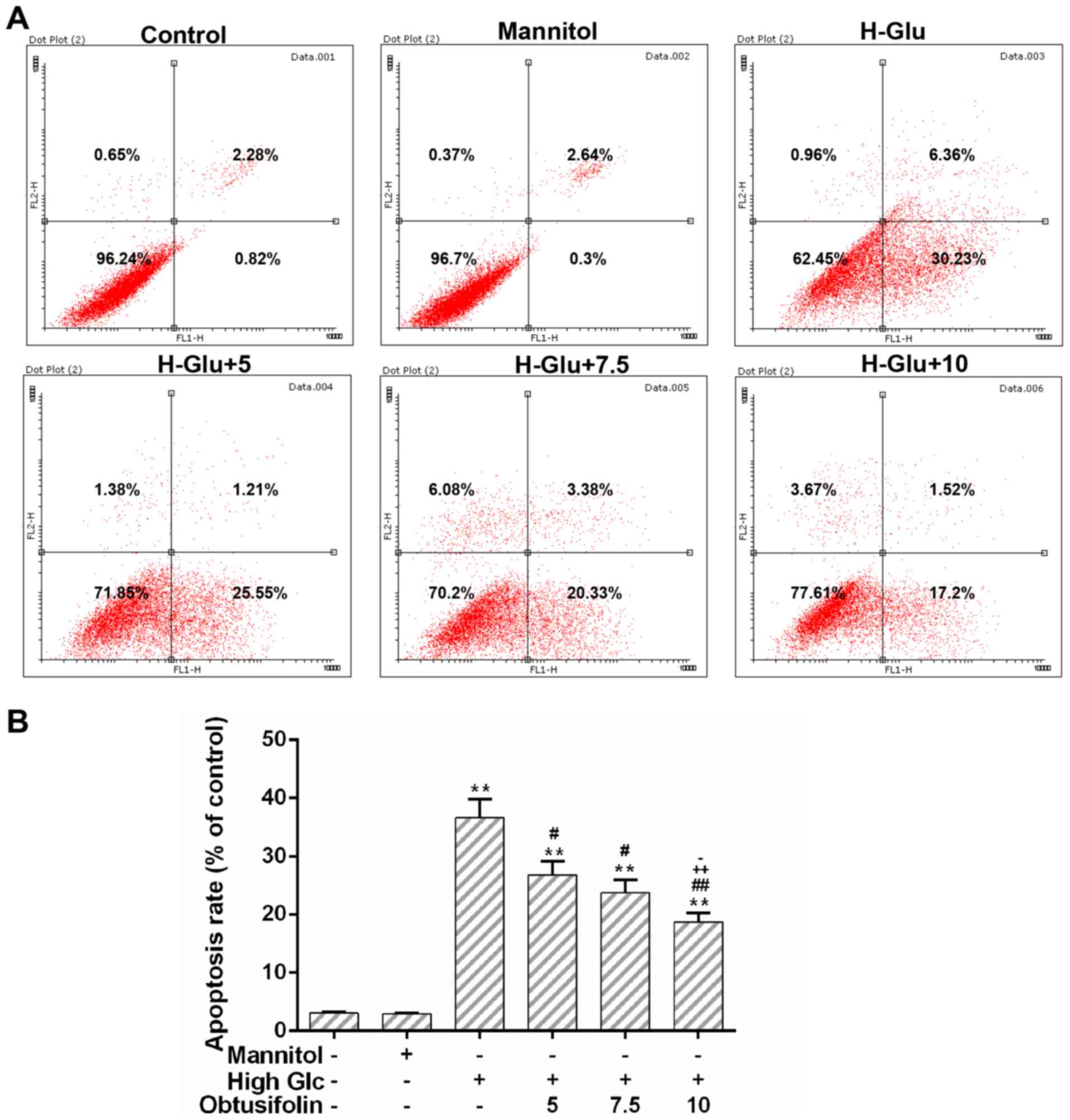
mitochondria-dependent apoptosis pathway (22). The flow cytometry analysis showed
that cell apoptosis caused by hyperglycemia was significantly
inhibited in the obtusifolin pretreatment groups (Fig. 6A and B). In addition, our results
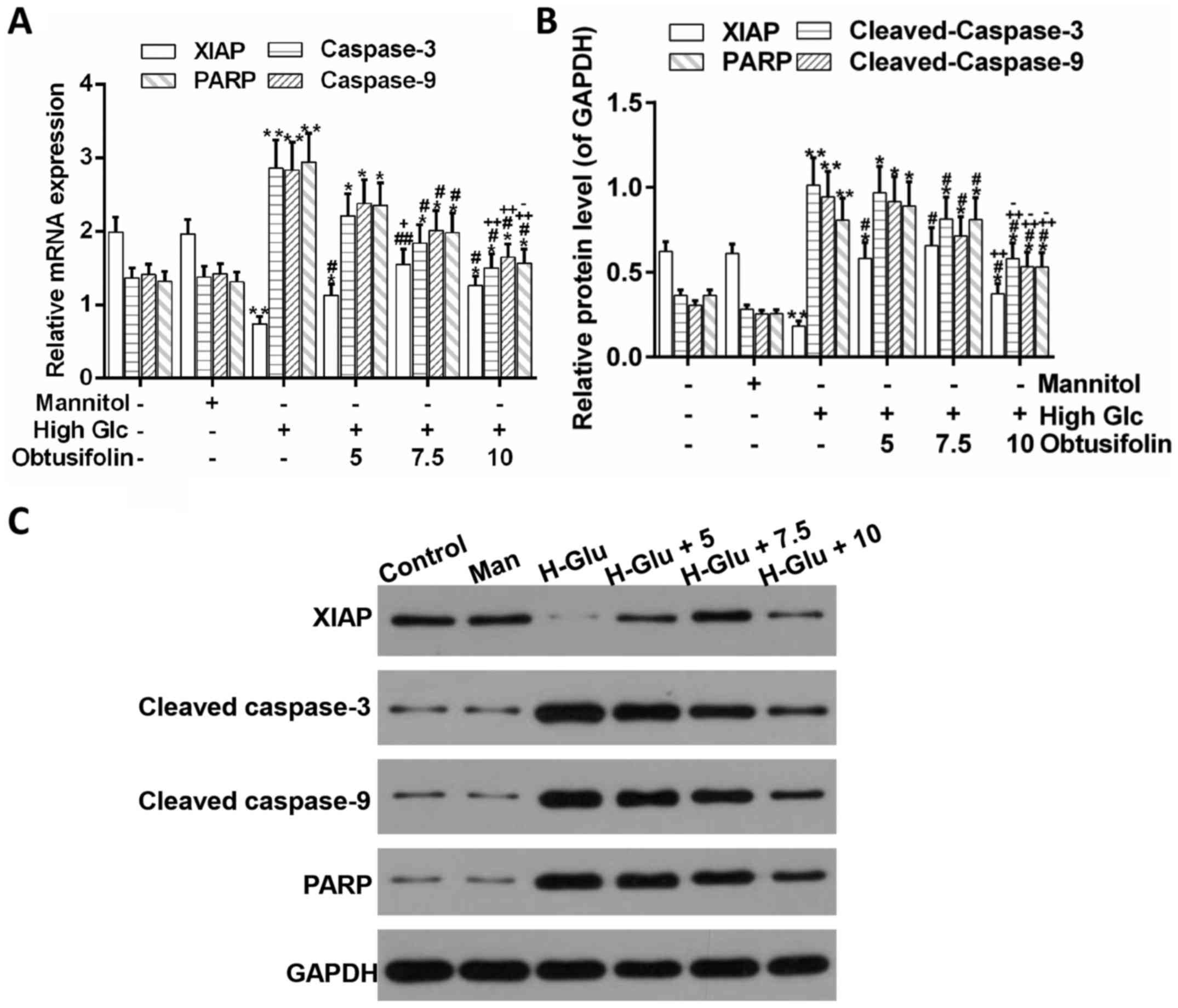
showed that the expression of XIAP, an inhibitor of apoptosis
proteins (23), was higher in the
medium concentration btusifolin pretreatment group than in the
model group according to the RT-PCR and western blot analyses.
Meanwhile, the expression of PARP and caspase-3/-9, as
pro-apoptotic proteins (24,25),
was significantly downregulated in the obtusifolin pretreatment
groups as compared to in the model group (Fig. 7A-C). Conclusively, it was suggested
that obtusifolin protected HUVECs against high glucose-induced
apoptosis.
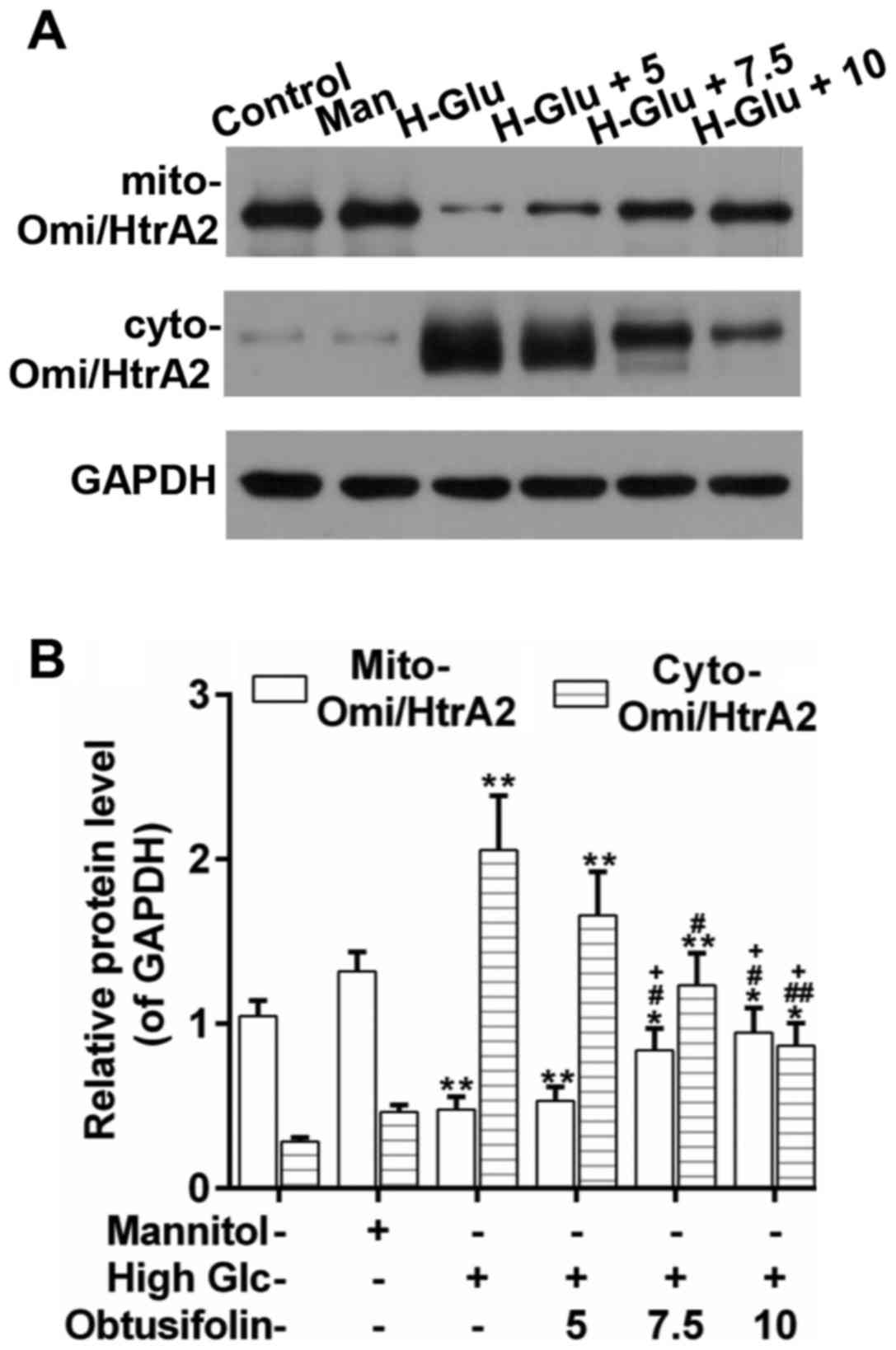
Obtusifolin prevents the release of
Omi/HtrA2 into the cytosol
Omi/HtrA2 is released into the cytosol during
apoptosis and promotes caspase pathway activation, which is a
mitochondrial serine protease that resides in the mitochondria of
healthy cells (14). Western blot
analysis revealed that Omi/HtrA2 was released substantially into
the cytosol under the high glucose condition. Interestingly, the
release of Omi/HtrA2 was inhibited effectively in the obtusifolin
pretreatment groups as compared to in the model group. Also, there
was significant difference between the high concentration group and
the low one (Fig. 8A and B).
Discussion
Macrovascular complications commonly occur in
patients with diabetes mellitus, whose mortality accounts for more
than 50% of all diabetes-related deaths (26). It has been reported that
diabetes-associated hyperglycemia can induce apoptosis, which is a
critical event in the onset and progression of diabetes mellitus
(27,28). Overproduction of ROS is a major
cause of high glucose-induced apoptosis of endothelial cells
(7). Moreover, oxidative stress
could be attributed to the disruption of redox homeostasis
(29). The bioactivity of
obtusifolin has received much attention with regard to some
physiological disorders (17).
However, the effect of obtusifolin in the treatment of endothelial
dysfunction and its possible molecular mechanisms still needs to be
validated. Therefore, exploration of the potential effect of
obtusifolin would provide new insights into its promising
theoretical and translational value. In addition, it is possible
that it will provide valuable reference for informed clinical
application of other Chinese medicines as well.
Primary cells share great similarity with living
cells in their genetic backgrounds. Moreover, HUVECs, as primary
cells, originate from the fetal umbilical cord, which is not only
abundant but also easy to obtain and work upon. These HUVECs have
been used in the study of cardiovascular disease before (30,31);
thus, they were selected to set up an in vitro model in the
present study.
In this investigation, cell viability was observed
to decrease by glucose treatment in a dose-dependent manner.
Hyperglycemia injury model was set up through high glucose
treatment of HUVECs (Figs. 1 and
2). Our results showed that
obtusifolin can decline the cellular ROS levels significantly
(Fig. 3A and B). In addition, when
accompanied by the relief of oxidative stress, the production of
MDA-an end product of fatty acid peroxidation (32)-decreased in the obtusifolin
pretreatment groups (Fig. 2C).
Meanwhile, obtusifolin can help recover the activities of CAT and
SOD (Fig. 2D), which are the main
enzymes in the antioxidant defense system (33). Notably, obtusifolin can help
maintain the balance between ROS-generation and ROS-removal in
HUVECs. To investigate the origin of the increasing ROS generation
and to evaluate if mitochondria are the main source of ROS, we
determined the production of mitochondria-specific ROS by flow
cytometry analysis. The results showed that mitochondria-specific
ROS production was inhibited by the pretreatment with obtusifolin
as compared to that in the model group (Fig. 4A and B). Furthermore, obtusifolin
can increase the activities of mitochondrial respiratory chain
complex I/III (Fig. 4C). The
excessive ROS production is a critical step towards the opening of
the mitochondrial membrane channel, which in turn, promotes the ROS
production as well as oxidative stress, and thus, finally leads to
apoptosis (34–36). Our results showed that obtusifolin
could reverse the loss of the MMP (Fig. 5A and B). It was also implied by our
results that obtusifolin plays an important role in the prevention
of ROS generation and cellular oxidative stress. In addition,
apoptosis induced by high glucose was inhibited in the obtusifolin
pretreatment groups (Fig. 6A and
B). Caspase-3/-9 have been recognized as apoptosis-related
genes by extensive studies (37,38).
Caspase-9 belongs to initiators of the cell apoptosis, which is
able to cleave pro-caspase-3 to active caspase-3 (also named
cleaved caspase-3) (39). Upon its
activation, caspase-3, which is a death protease frequently
activated by extrinsic (death ligand) and intrinsic (mitochondrial)
pathways, serves as the executor of apoptosis and then activates
the apoptosis signals to downstream targets, including PARP
(37,38). By contrast, XIAP is considered to
be an inhibitor of apoptosis proteins (23). In this study, the expression of
XIAP was upregulated, whereas the expression of cleaved-PARP and
cleaved-caspase-3/-9 was downregulated by the pretreatment with
obtusifolin (Fig. 7A-C). One point
that is worth consideration here is that the expression of XIAP was
not higher in the high concentration obtusifolin pretreatment group
than in the medium one. This can be explained by the fact that if
the concentration of glucose is too high, treatment with
obtusifolin may have toxic side effects on HUVECs, which may be due
to the structure of anthraquinone in obtusifolin (40,41).
Additionally, we found that obtusifolin could prevent the release
of Omi/HtrA2 into the cytosol effectively (Fig. 8A-C). These results suggested that
obtusifolin protected HUVECs against high glucose-induced
mitochondrial apoptosis mainly through the regulation of the
location of Omi/HtrA2.
It is known that Omi/HtrA2 can enhance caspase
activity through multiple pathways (14). Thus, exploring the connections
between obtusifolin and related signaling pathways would provide
clues for the treatment of diabetes mellitus. Furthermore, whether
obtusifolin can inhibit apoptosis through the caspase-independent
pathway is worth investigating in detail in the future. Some
studies have reported that endoplasmic reticulum stress pathway is
involved in hyperglycemia-induced apoptosis (42); however, it has not been illustrated
in this study. Besides, the protective effects of obtusifolin
demonstrated in this study are largely from in vitro data.
Thus, future explorations are needed to precisely define the
effects of obtusifolin in vivo.
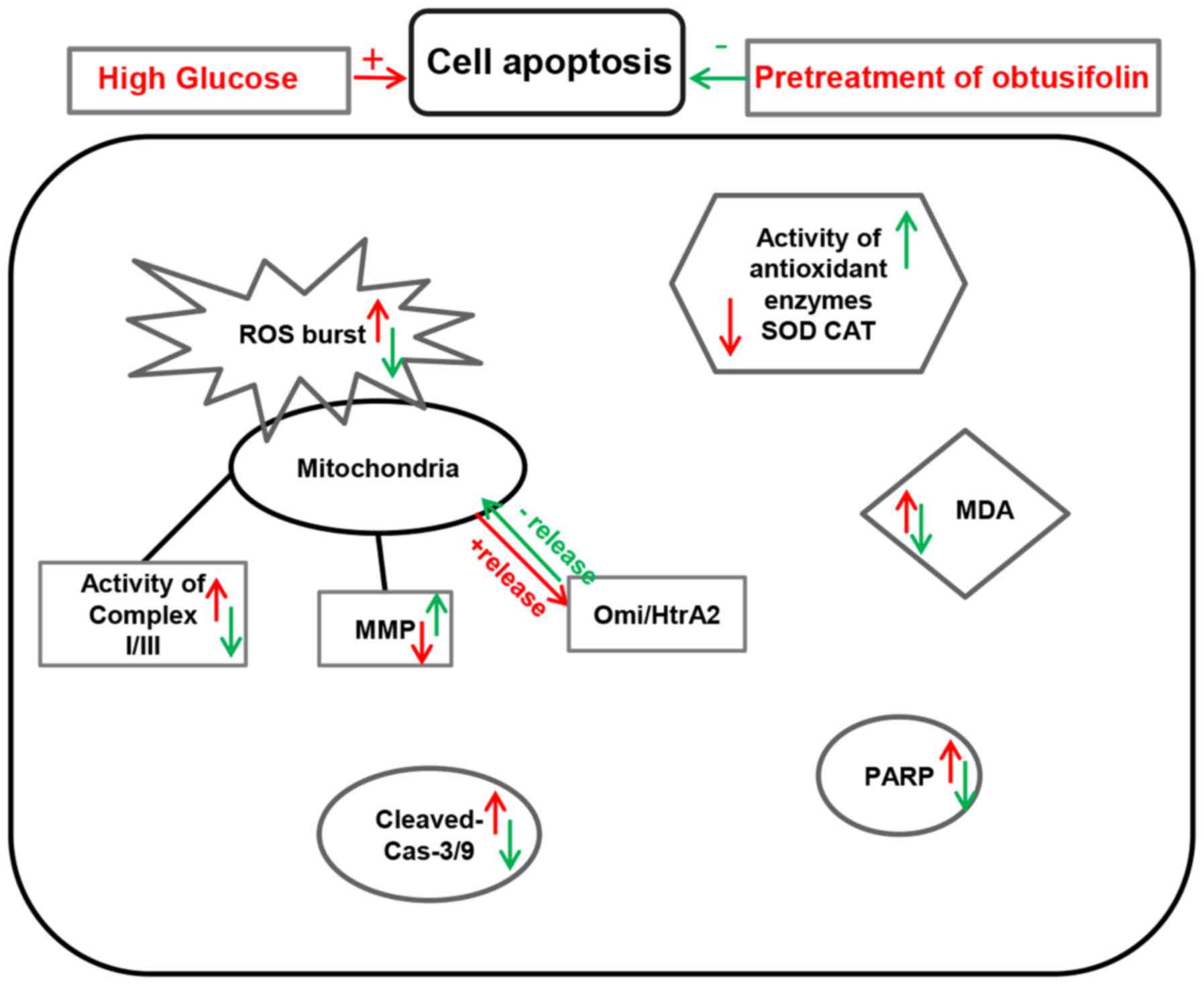
In conclusion, it was obvious that the protective
effect of obtusifolin was enhanced with the increasing
concentration. As shown in Fig. 9,
obtusifolin protected HUVECs against high glucose-induced oxidative
stress and mitochondrial dysfunction mainly through inhibition of
ROS production and modulation of the activities of related enzymes.
Meanwhile, obtusifolin could protect cells against high
glucose-induced apoptosis in HUVECs, which was mainly due to aid in
regulation of the release of Omi/HtrA2 into the cytosol. Based on
these data, our study would provide effective therapeutic
strategies in the treatment of DM.
References
|
1
|
Costantino S, Paneni F and Cosentino F:
Hyperglycemia: A bad signature on the vascular system. Cardiovasc
Diagn Ther. 5:403–406. 2015.PubMed/NCBI
|
|
2
|
King GL, Shiba T, Feener EP and Nayak R:
Cell culture model for the study of vascular complications of
diabetes: The effect of high glucose levels on metabolism and
growth of vascular cells. Hyperglyc Diab Vas Dis. 162–177.
1992.
|
|
3
|
Ceriello A, Quagliaro L, D'Amico M, Di
Filippo C, Marfella R, Nappo F, Berrino L, Rossi F and Giugliano D:
Acute hyperglycemia induces nitrotyrosine formation and apoptosis
in perfused heart from rat. Diabetes. 51:1076–1082. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Baumgartner-Parzer SM, Wagner L,
Pettermann M, Grillari J, Gessl A and Waldhäusl W:
High-glucose-triggered apoptosis in cultured endothelial cells.
Diabetes. 44:1323–1327. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Evans JL, Goldfine ID, Maddux BA and
Grodsky GM: Oxidative stress and stress-activated signaling
pathways: A unifying hypothesis of type 2 diabetes. Endocr Rev.
23:599–622. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang D, Wang Q, Yan G, Qiao Y, Sun L, Zhu
B, Tang C and Gu Y: High glucose and interleukin 1β-induced
apoptosis in human umbilical vein endothelial cells involves in
down-regulation of monocarboxylate transporter 4. Biochem Biophys
Res Commun. 466:607–614. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhao H, Ma T, Fan B, Yang L, Han C, Luo J
and Kong L: Protective effect of trans-δ-viniferin against
high glucose-induced oxidative stress in human umbilical vein
endothelial cells through the SIRT1 pathway. Free Radic Res.
50:68–83. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Du X, Stocklauser-Färber K and Rösen P:
Generation of reactive oxygen intermediates, activation of
NF-kappaB, and induction of apoptosis in human endothelial cells by
glucose: Role of nitric oxide synthase? Free Radic Biol Med.
27:752–763. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Magalang UJ, Rajappan R, Hunter MG, Kutala
VK, Kuppusamy P, Wewers MD, Marsh CB and Parinandi NL: Adiponectin
inhibits superoxide generation by human neutrophils. Antioxid Redox
Signal. 8:2179–2186. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Surico D, Farruggio S, Marotta P, Raina G,
Mary D, Surico N, Vacca G and Grossini E: Human chorionic
gonadotropin protects vascular endothelial cells from oxidative
stress by apoptosis inhibition, cell survival signalling activation
and mitochondrial function protection. Cell Physiol Biochem.
36:2108–2120. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen W, Zheng G, Yang S, Ping W, Fu X,
Zhang N, Wang DW and Wang J: CYP2J2 and EETs protect against
oxidative stress and apoptosis in vivo and in vitro following lung
ischemia/reperfusion. Cell Physiol Biochem. 33:1663–1680. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Prudent J and McBride HM: Mitochondrial
dynamics: ER actin tightens the Drp1 noose. Curr Biol.
26:R207–R209. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vaux DL and Silke J: HtrA2/Omi, a sheep in
wolf's clothing. Cell. 115:251–253. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Suzuki Y, Takahashi-Niki K, Akagi T,
Hashikawa T and Takahashi R: Mitochondrial protease Omi/HtrA2
enhances caspase activation through multiple pathways. Cell Death
Differ. 11:208–216. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ju MS, Kim HG, Choi JG, Ryu JH, Hur J, Kim
YJ and Oh MS: Cassiae semen, a seed of Cassia obtusifolia,
has neuroprotective effects in Parkinson's disease models. Food
Chem Toxicol. 48:2037–2044. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Patil UK, Saraf S and Dixit VK:
Hypolipidemic activity of seeds of Cassia tora Linn. J
Ethnopharmacol. 90:249–252. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tang Y and Zhong Z: Obtusifolin treatment
improves hyperlipidemia and hyperglycemia: Possible mechanism
involving oxidative stress. Cell Biochem Biophys. 70:1751–1757.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ding F, Shao ZW, Yang SH, Wu Q, Gao F and
Xiong LM: Role of mitochondrial pathway in compression-induced
apoptosis of nucleus pulposus cells. Apoptosis. 17:579–590. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Frazier AE and Thorburn DR: Biochemical
analyses of the electron transport chain complexes by
spectrophotometry. Methods Mol Biol. 837:49–62. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ha H and Lee HB: Reactive oxygen species
as glucose signaling molecules in mesangial cells cultured under
high glucose. Kidney Int Suppl. 77:S19–S25. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Soon BH, Murad Abdul NA, Then SM, Bakar
Abu A, Fadzil F, Thanabalan J, Haspani Mohd MS, Toh CJ, Tamil Mohd
A, Harun R, et al: Mitochondrial DNA mutations in grade II and III
glioma cell lines are associated with significant mitochondrial
dysfunction and higher oxidative stress. Front Physiol. 8:2312017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rajcan-Separovic E, Liston P, Lefebvre C
and Korneluk RG: Assignment of human inhibitor of apoptosis protein
(IAP) genes xiap, hiap-1 and hiap-2 to chromosomes Xq25 and
11q22-q23 by fluorescence in situ hybridization. Genomics.
37:404–406. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Boulares AH, Zoltoski AJ, Yakovlev A, Xu M
and Smulson ME: Roles of DNA fragmentation factor and poly
(ADP-ribose) polymerase in an amplification phase of tumor necrosis
factor-induced apoptosis. J Biol Chem. 276:38185–38192.
2001.PubMed/NCBI
|
|
25
|
Julien O and Wells JA: Caspases and their
substrates. Cell Death Differ. 24:1380–1389. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Stehouwer CD: Vascular complications in
diabetes mellitus: Role of endothelial dysfunction. Ned Tijdschr
Geneeskd. 140:870–874. 1996.(In Dutch). PubMed/NCBI
|
|
27
|
Altabas V: Diabetes, endothelial
dysfunction and vascular repair: What should a diabetologist keep
his eye on? Int J Endocrinol. 2015:8482722015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Caballero AE: Endothelial dysfunction in
obesity and insulin resistance: A road to diabetes and heart
disease. Obes Res. 11:1278–1289. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Baynes JW: Role of oxidative stress in
development of complications in diabetes. Diabetes. 40:405–412.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Agnoletti L, Bachetti T, Bastianon D,
Francolini G and Ferrari R: Rosuvastatin stimulates eNOS and
inhibits apoptosis in HUVECs exposed to sera from cardiovascular
diseases patients. J Mol Cell Cardio. 42 Suppl:S2262007. View Article : Google Scholar
|
|
31
|
Udvardy M, Posan E, Harsfalvi J, Kaplar M,
Batar P and Altorjay I: 88. In vitro clot-lysis in the presence of
cultured human umbilical vein endothelial cells, experiences in
diabetes mellitus and liver cirrhosis. Fibrinolysis. 10 Suppl
1:S271996. View Article : Google Scholar
|
|
32
|
Tsikas D: Assessment of lipid peroxidation
by measuring malondialdehyde (MDA) and relatives in biological
samples: Analytical and biological challenges. Anal Biochem.
524:13–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sharafati-Chaleshtori R, Shirzad H,
Rafieian-Kopaei M and Soltani A: Melatonin and human mitochondrial
diseases. J Res Med Sci. 22:22017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zorov DB, Filburn CR, Klotz LO, Zweier JL
and Sollott SJ: Reactive oxygen species (ROS)-induced ROS release:
A new phenomenon accompanying induction of the mitochondrial
permeability transition in cardiac myocytes. J Exp Med.
192:1001–1014. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Barateiro A, Vaz AR, Silva SL, Fernandes A
and Brites D: ER stress, mitochondrial dysfunction and calpain/JNK
activation are involved in oligodendrocyte precursor cell death by
unconjugated bilirubin. Neuromolecular Med. 14:285–302. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Vincent AM, Russell JW, Low P and Feldman
EL: Oxidative stress in the pathogenesis of diabetic neuropathy.
Endocr Rev. 25:612–628. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chai WS, Zhu XM, Li SH, Fan JX and Chen
BY: Role of Bcl-2 family members in caspase-3/9-dependent apoptosis
during Pseudomonas aeruginosa infection in U937 cells. Apoptosis.
13:833–843. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Seol JG, Park WH, Kim ES, Jung CW, Hyun
JM, Lee YY and Kim BK: Potential role of caspase-3 and −9 in
arsenic trioxide-mediated apoptosis in PCI-1 head and neck cancer
cells. Int J Oncol. 18:249–255. 2001.PubMed/NCBI
|
|
39
|
Yin Q, Park HH, Chung JY, Lin SC, Lo YC,
da Graca LS, Jiang X and Wu H: Caspase-9 holoenzyme is a specific
and optimal procaspase-3 processing machine. Mol Cell. 22:259–268.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xue W and Warshawsky D: Metabolic
activation of polycyclic and heterocyclic aromatic hydrocarbons and
DNA damage: A review. Toxicol Appl Pharmacol. 206:73–93. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Haruna K, Kanezaki H, Tanabe K, Dai WM and
Nishimoto S: Effects of structural modification on the DNA binding
properties and photo-induced cleavage reactivity of propargylic
sulfones conjugated with an anthraquinone structure. Bioorg Med
Chem. 14:4427–4432. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gwak H, Kim S, Dhanasekaran DN and Song
YS: Resveratrol triggers ER stress-mediated apoptosis by disrupting
N-linked glycosylation of proteins in ovarian cancer cells. Cancer
Lett. 371:347–353. 2016. View Article : Google Scholar : PubMed/NCBI
|