Introduction
Human colorectal cancer (CRC), one of the most
common malignant tumors in China, is a leading cause of
tumor-associated mortality worldwide (1–3). The
occurrence of CRC is primarily due to age and lifestyle factors
(4,5); however, it is additionally a
consequence of a number of mutations and alterations in critical
oncogenes and suppressor genes (6). Mutations most frequently occur in the
Wnt signaling pathway (7). The Wnt
signaling cascade is implicated in carcinogenesis (8), embryonic development (9), cell proliferation (10) and apoptosis (11). The canonical Wnt pathway is able to
regulate gene transcription, in which β-catenin is involved
(12). Thus, this pathway is
additionally termed the Wnt/β-catenin signaling pathway. Through
the binding of the Wnt protein to the N-terminal extracellular
cysteine-rich domain of a Frizzled (Fz) family receptor,
Wnt/β-catenin signaling begins and leads to the translocation of
accumulated β-catenin into the nucleus, and eventually leads to the
transcription of its target genes, including proto-oncogenes
(12,13). It has been reported that mutation
of β-catenin occurs in 10% of CRC cases (14). Accordingly, factors that may block
the activity of Wnt signaling may be modalities for treating human
CRC.
MicroRNAs (miRNAs), small non-coding RNAs, were
recognized to have regulatory activity in the 2000s (15). Extensive evidence demonstrates that
abnormal expression of miRNAs is a key step in tumor progression,
in which process miRNAs serve as tumor suppressor genes or
oncogenes. miRNAs are able to silence the expression of target
mRNAs by binding to the complementary site in the 3′ untranslated
region (3′ UTR) (16). It has been
claimed that ~60% of genes in humans and other mammals are targets
of miRNAs (17). The association
of miRNAs and CRC was first identified in 2003 (18). Emerging evidence has demonstrated
the role of miRNAs in regulating Wnt signaling, including in CRC
(19). Wnt1, an important
component of Wnt signaling, may serve as a target gene of miRNAs in
multiple cell types (20–22). It has been reported that miRNA
(miR)-200b-3p is a member of the miR-200 miRNA family, which is
downregulated in a wide range of human cancer types including CRC
(23,24). However, the association between
miR-200b-3p and Wnt1 remains unclear.
Thus, the present study sought to examine the effect
of miR-200b-3p on the proliferation and apoptosis of human CRC, and
to clarify the potential associations between miR-200b-3p and Wnt
signaling in the hopes it may provide clues to the mechanism of
tumor progression in CRC.
Materials and methods
Tissue specimens
A total of 45 patients (male, mean age 58.8; range
37–75 years) with CRC were enrolled in the present study, with
written informed consent. None of the patients received
chemotherapy or radiotherapy prior to surgery at Yuhang Branch of
Second Affiliated Hospital of Zhejiang University School of
Medicine (Hangzhou, China) between April 2013 and June 2015.
Patients who were histologically diagnosed with CRC and had
complete clinicopathological data were included. Exclusion criteria
included patients who had familial cancer syndrome and inflammatory
bowel disease, a history of malignancy in other sites or infectious
diseases. The CRC tissues and paracarcinoma tissues (≤5 cm distance
from the tumor site) were obtained during the surgery. All the
protocols in the present study were approved by the Ethics
Committee of the Second Affiliated Hospital of Zhejiang University
School of Medicine.
Cell lines and cell culture
Human CRC cell lines (HCT-8, SW480, LOVO, HT29 and
SW620) and a colonic epithelial cell line (NCM460) were purchased
from the American Type Culture Collection (Manassas, VA, USA). The
cells were maintained in RPM1640 medium (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) containing 10% fetal bovine
serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) at 37°C in the
presence of 5% CO2.
Cell transfection and grouping
The cells were seeded at a density of
4×104 cells/well in a 24-well plate. When cells reached
50% confluence, the human miR-200b-3p mimics (10 nM;
UAAUACUGCCUGGUAAUGAUGA cat. no. HMI0352; Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany), Wnt-1-small interfering (si)RNA (800 ng/µl;
cat. no. HSH059152-CH1; GeneCopoeia, Inc., Rockville, MD, USA),
si-negative control (800 ng/µl; cat. no. CSHCTR001-CH1;
GeneCopoeia, Inc.) or miR-control (10 nM; cat. no. HMC0002;
Sigma-Aldrich; Merck KGaA) was transfected using
Lipofectamine® 2000 reagent (cat. no. 11668019;
Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocols. Following transfection for 48 h, the
cells were collected for subsequent experiments. The experimental
design was as follows: Control group (untreated cells, mock);
negative control group (cells that were transfected with the siRNA
negative control, NC); mimics group (cells that were transfected
with miR-200b-3p mimics); and si-Wnt1 group (cells that were
transfected with si-Wnt1). All the experiments were independently
conducted at least three times.
Cell Counting Kit-8 (CCK-8) assay
The cells were seeded at a density of
1×103 cells/well in a 96-well plate following the
aforementioned treatment. The cell viability was detected at
different time points (12, 24 and 48 h post-siRNA transfection,
respectively). CCK-8 solutions (10 µl) from the CCK-8 kit (cat. no.
C0038; Beyotime Institute of Biotechnology, Haimen, China) were
supplemented into the wells and incubated for 4 h. The absorbance
of the reaction mixtures in each well was detected at 450 nm with a
microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA,
USA).
5(6)-carboxyfluorescein diacetate
succinimidyl ester (CFSE) assay
The cells (1×106 cells/ml) were stained
with 5 µM CFSE (Invitrogen; Thermo Fisher Scientific, Inc.), and
maintained for 10 min at 37°C. The labeling was stopped by adding
medium with 10% FBS. Following a 5 min incubation on ice, the
labeled cells were centrifuged at 700 × g at room temperature for 5
min, re-seeded in 24-well plate (1×105 cells/well) and
treated as described previously in the present study. FACSCanto™
(BD Biosciences, Franklin Lakes, San Jose, CA, USA) was used to
collect the CFSE fluorescence signals. The data were analyzed using
FACSDiva software version 6.1.3 (BD Biosciences).
Flow cytometric analysis of
apoptosis
The apoptosis of CRC cells was measured with the
Annexin V-fluorescein isothiocycanate (FITC)/propidium iodide (PI)
apoptosis detection kit (BioVision, Inc., Milpitas, CA, USA). The
cells in every group were harvested and washed with PBS solution.
The cells were incubated with Annexin V-FITC for 15 min in the dark
at room temperature, and the incubation with PI was in the dark for
5 min at room temperature. The fluorescence intensity was analyzed
using a flow cytometer (FACSCanto™, BD Biosciences). The data was
analyzed with FlowJo software (FlowJo LLC).
Luciferase reporter assay
The bioinformatics analysis was performed using
TargetScan (http://www.targetscan.org/vert_71/) and miRNA.org (http://34.236.212.39/microrna/home.do). The
pLightSwitch-Wnt1-3′UTR marine RenSP luciferase reporter (cat. no.
s812430) and Cypridina TK control vector (pTK-CLuc Vector)
(cat. no. 32036) were purchased from Active Motif (Shanghai,
China). The corresponding mutation vector was constructed as
p-mut-Wnt1-3′UTR (CAGUAUU mutated to TACUCUC) using the MutBEST kit
(Takara Bio, Inc., Otsu, Japan; cat. no. R401). The cells were
plated in a 6-well plate (1×105 cells/well) and cultured
overnight. Using Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.), the Cypridina TK control vector
was co-transfected with pLightSwitch-Wnt1-3′UTR vector or
p-mut-Wnt1-3′UTR vector into cells, respectively. Following 48 h,
the luciferase activity was measured with a LightSwitch™ Dual Assay
kit (Active Motif; cat. no. 32035) on a microplate reader (Bio-Rad
Laboratories, Inc.). The Cypridina luciferase activity
served as the control.
Caspase-3 activity
The cells in each group were harvested and
re-suspended in lysis buffer provided by a caspase-3 assay kit
(R&D systems, Inc., Minneapolis, MN, USA; cat. no. BF3100).
Following centrifugation at 600 × g for 5 min at room temperature,
the supernatant was mixed with reaction buffer and caspase-3
substrate in a 96-well plate, which was incubated for 2 h at 37°C.
The absorbance at 405 nm was read with a microplate reader (Bio-Rad
Laboratories, Inc.).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) was used to extract the total miRNA from tissues
and cell lines, according to the manufacturer's protocol. The
temperature protocol for the RT reaction was 25°C for 10 min, 42°C
for 50 min and 70°C for 15 min. The concentration and the integrity
were determined using a Nanodrop (Thermo Fisher Scientific, Inc.).
cDNA was synthesized using 1 µg RNA with the PrimeScript RT Reagent
kit (Takara Bio Inc.), according to the manufacturer's protocol.
SYBR-Green PCR (Takara Bio Inc.) was used to perform the qPCR on
the ABI 7500HT System (Thermo Fisher Scientific, Inc.). The primer
pairs used were as follows: Wnt1 (forward),
5′-TGGCTGGGTTTCTGCTACG-3′; Wnt1 (reverse),
5′-CCCGGATTTTGGCGTATC-3′; miR-200b (forward),
5′-GCGGCTAATACTGCCTGGTAA-3′; miR-200b (reverse),
5′-GTGCAGGGTCCGAGGT-3′; Ki67 (forward),
5′-ACTGCAGCAGATGGAACTAGG-3′; Ki67 (reverse),
5′-AGAACAGTAGCGTGATGTTTGG-3′; β-catenin (forward),
5′-GCATGGGTCAGAAGGATTCCT-3′; β-catenin (reverse),
5′-TCGTCCCAGTTGGTGACGAT-3′; β-actin (forward),
5′-GCTGAGAACGGGAAGCTTGT-3′; β-actin (reverse),
5′-GCCAGGGGTGCTAAGCAG-3′; U6 (forward), 5′-CTCGCTTCGGCAGCACA-3′;
and U6 (reverse), 5′-AACGCTTCACGAATTTGCGT-3′. The reactions were
conducted using the following thermocycling conditions: Initial
denaturation at 95°C for 5 min; 40 cycles of 95°C for 15 sec, and
60°C for 1 min; 72°C for 10 min for extension. The expression of
target genes was calculated using the 2−ΔΔCq method
(25). The expression fold change
was relative to the internal housekeeping genes β-actin and U6.
Western blot analysis
Total protein from the cells was isolated using
radioimmunoprecipitation assay buffer with protease inhibitors
(Roche Diagnostics, Indianapolis, IN, USA). Proteins from tissue
were isolated by a mechanical homogenizer for 5 min at 3,000 r/min.
The concentrations of the proteins were quantified using a
bicinchoninic acid kit (Beyotime Institute of Biotechnology). An
equal amount of protein (25 µg) was loaded and separated by 8%
SDS-PAGE. The proteins were electrotransferred to polyvinylidene
difluoride membranes (EMD Millipore, Billerica, MA, USA). Bovine
serum albumin (5%) (Beijing Solarbio Science & Technology Co.,
Ltd., Beijing, China) was added to block the non-specific antigens
for 2 h at room temperature. The membranes were incubated with
primary antibodies overnight at 4°C, and probed with secondary
antibodies at room temperature for 1.5 h. The antibodies were as
follows: Wnt1 (cat. no. sc-514531; 1:500; Santa Cruz Biotechnology,
Inc.); β-catenin (cat. no. 2698; 1:1,000; Cell Signaling
Technology, Inc., Danvers, MA, USA); Ki67 (cat. no. ab16667;
1:1,000 dilution; Abcam, Cambridge, UK); β-actin (cat. no. 3700;
1:1,000; Cell Signaling Technology, Inc.;); horseradish
peroxidase-conjugated secondary antibody (cat. no. sc-2954; 1:200;
Santa Cruz Biotechnology, Inc.). Following incubation, the
membranes were washed with PBS containing 0.01% Tween-20. The bands
were developed using enhanced chemiluminescence (ECL; Invitrogen;
Thermo Fisher Scientific), following the manufacturer's protocol,
with the LS45/55 chemiluminescence instrument (PerkinElmer, Inc.,
Waltham, MA, USA). In brief, the blot was incubated with working
solution (provided by the ECL kit) by mixing equal parts of
Detection Reagents 1 and 2 at room temperature for 1 min. Following
removal of the working solution, the protected membranes in clear
plastic wrap were placed in a film cassette, and subsequent X-ray
exposure was performed. The intensity of the developed bands was
calculated using Quantity One software version 4.6 (Bio-Rad
Laboratories, Inc.).
Statistical analysis
GraphPad Prism version 6.0 (GraphPad Software, Inc.,
La Jolla, CA, USA) was used to perform the data analysis. A
two-tailed Student's t-test or one-way analysis of variance
followed by Dunnett's multiple comparison test was performed to
calculate the significant difference. Pearson's correlation
coefficient (r) was used to measure correlation. P<0.05 was
considered to indicate a statistically significant difference. The
data are presented as the mean ± standard deviation. The
experiments were repeated independently ≥3 times.
Results
Expression of miR-200b-3p and Wnt1 in
CRC tissues
The dysregulation of miR-200b-3p and Wnt1 has been
reported in tumorigenesis (26,27).
Thus, the expression of miR-200b-3p and Wnt1 in patients with CRC
was investigated. According to the RT-qPCR results from carcinoma
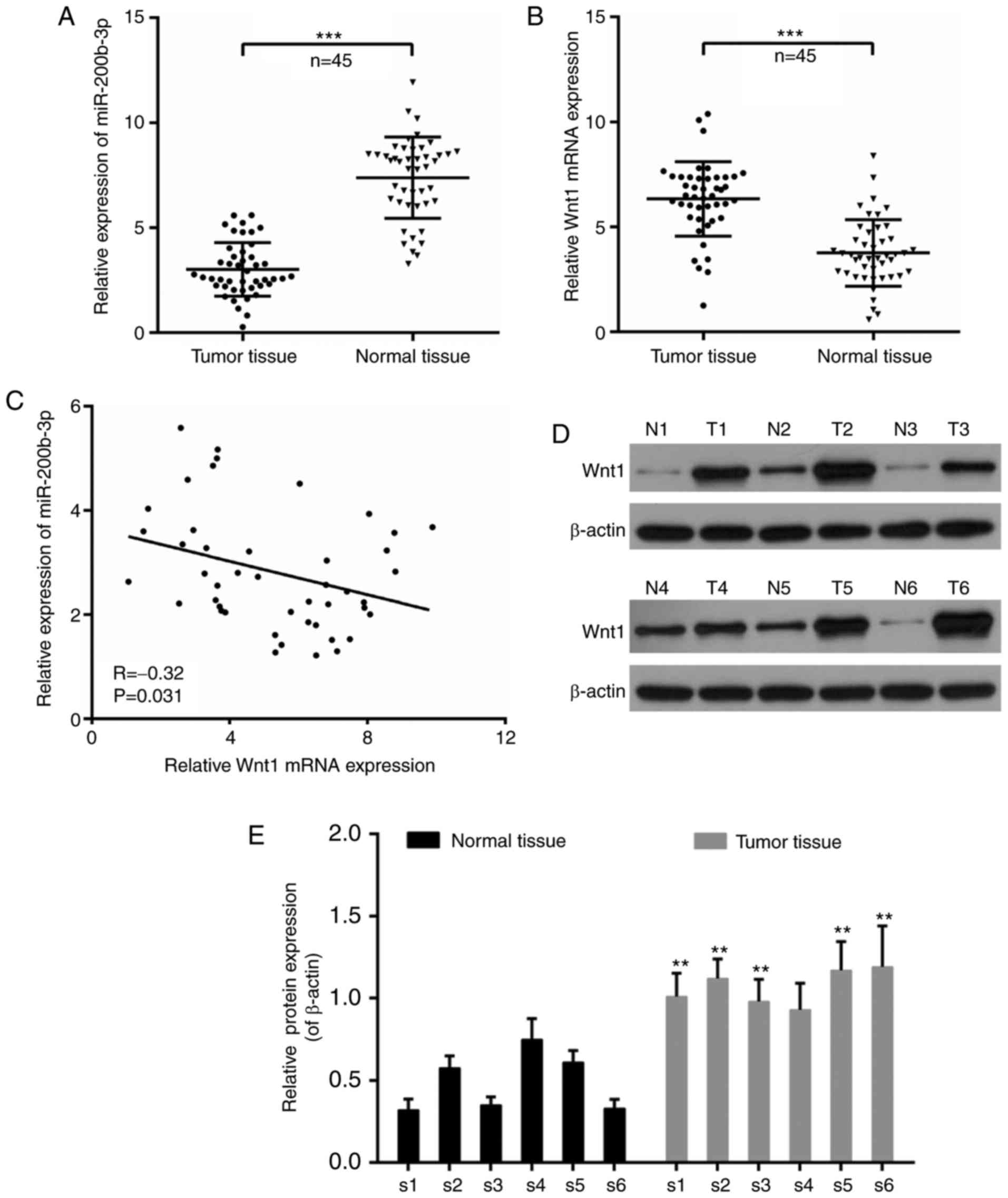
tissues and paracarcinoma tissues, it is noteworthy that the
expression of miR-200b-3p was significantly decreased in tumor
tissues compared with that in paracarcinoma tissues (P<0.001;
Fig. 1A). By contrast, the
expression of Wnt1 was significantly increased in tumor tissues
compared with that in normal tissues (P<0.001; Fig. 1B). Moreover, the expression of
miR-200b-3p and Wnt1 was negatively correlated in CRC (r=−0.32;
P=0.031; Fig. 1C). In addition,
six cases were randomly selected to determine the expression of
Wnt1 at the translational level. It was demonstrated that the
expression of Wnt1 was increased in tumor tissues compared with
that in normal tissues (Fig. 1D),
with the exception that there was no significant difference in the
fourth case (P<0.01; Fig.
1.E).
Expression of miR-200b-3p and Wnt1 in
CRC cell lines
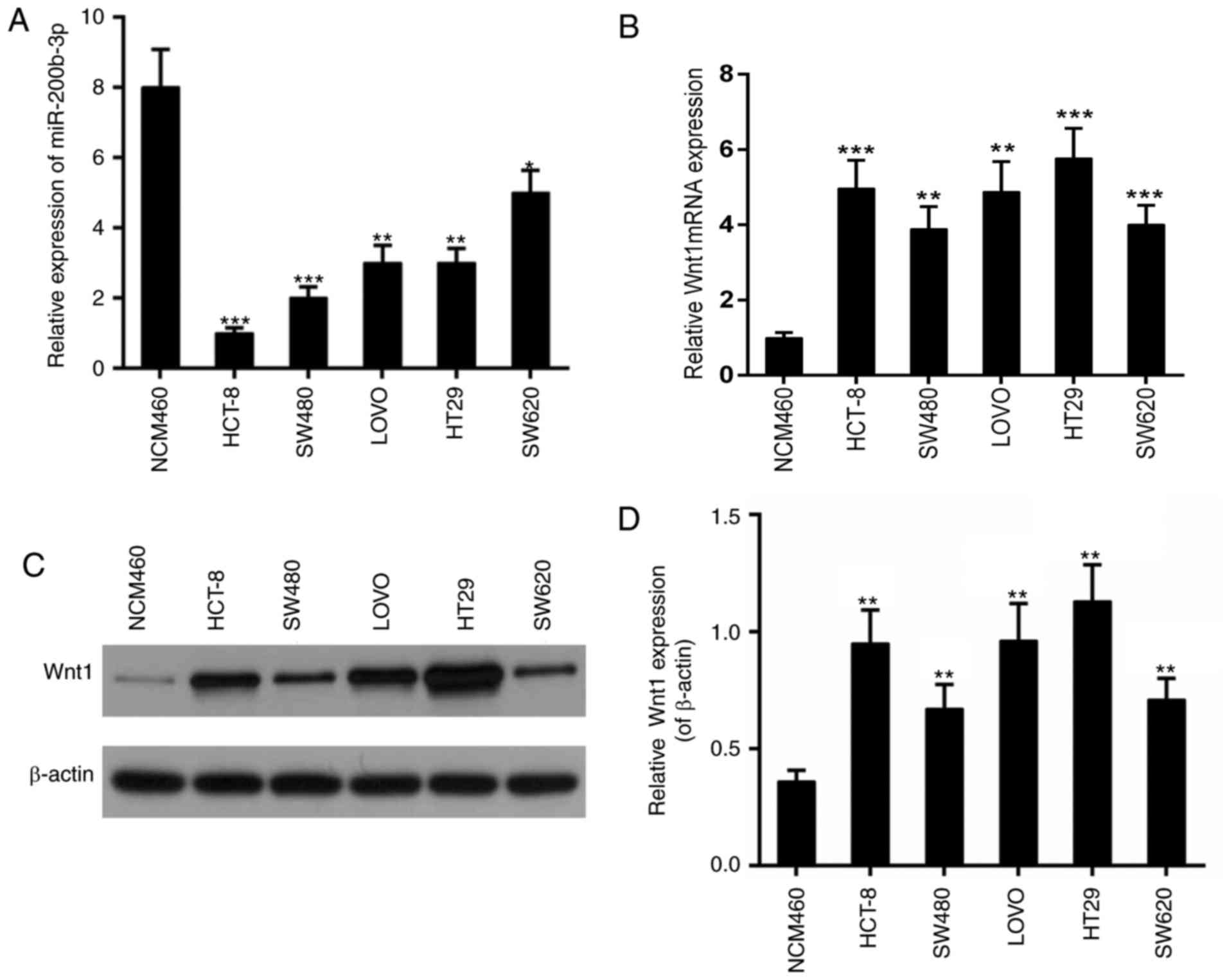
Subsequently, the expression of miR-200b-3p and Wnt1
was examined in different CRC lines including HCT-8, SW480, LOVO,
HT29 and SW620. The colonic epithelial cell line NCM460 was used as
a control. The results revealed that the expression of miR-200b-3p
was decreased in all these CRC lines (Fig. 2A); whereas, the expression of Wnt1
was increased at the transcriptional and the translational level
(Fig. 2B-D). The most marked
alteration in the expression of miR-200b-3p and the expression of
Wnt1 was observed in HCT-8. Thus, HCT-8 was selected for the
subsequent experiments.
Wnt1 may be a direct target of
miR-200b-3p
It is widely accepted that miRNAs exert function by
silencing target mRNAs (28).
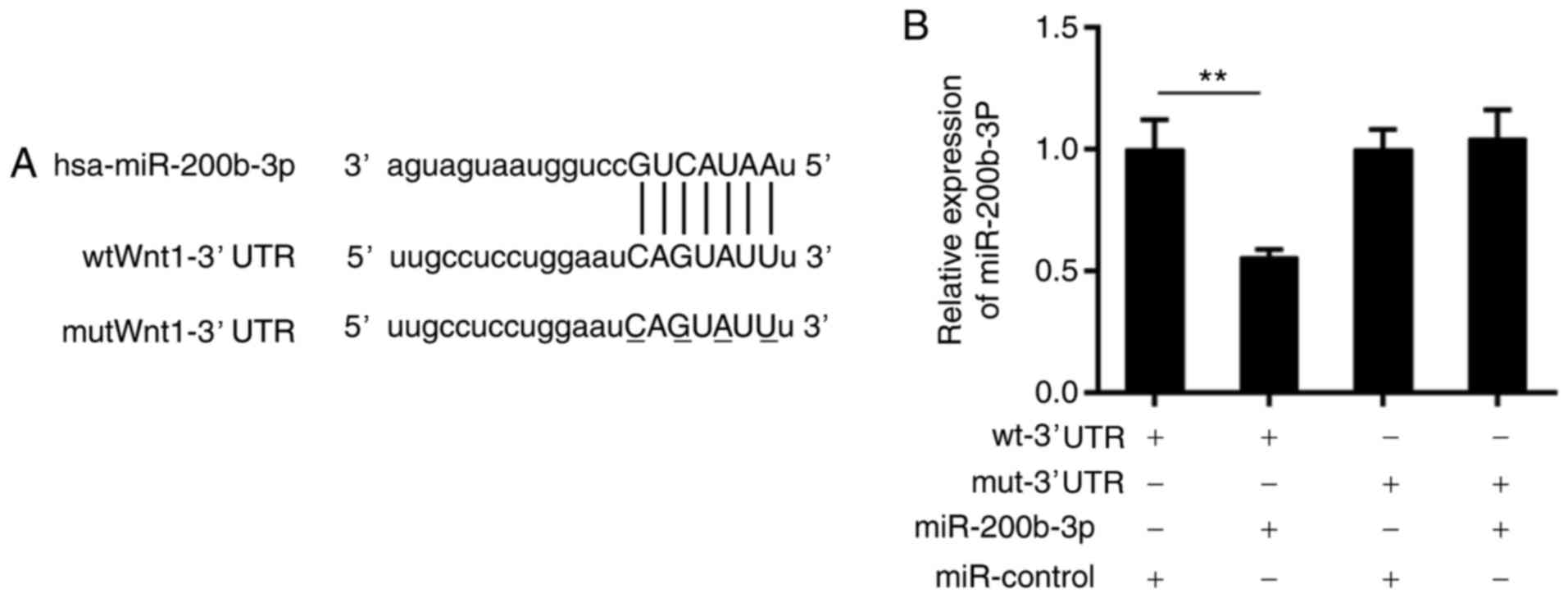
Therefore, a bioinformatics analysis was performed using TargetScan
(http://www.targetscan.org/vert_71/)
and miRNA.org (http://34.236.212.39/microrna/home.do). The data from
the two databases displayed that there was a potential binding site
for miR-200b-3p in the 3′ UTR of the Wnt1 mRNA sequence (Fig. 3A). To verify this prediction, a
luciferase reporter assay was conducted. It was revealed that the
luciferase activity of wild-type Wnt1-3′UTR was significantly
suppressed compared with the wild-type Wnt1-3′UTR and miR-control,
by the presence of the miR-200b-3p mimics (P<0.01), which was
not altered in the mutant Wnt1-3′UTR group (Fig. 3B).
Effect of miR-200b-3p on the cell
viability, proliferation and apoptosis of CRC cells
The effect of miR-200b-3p on CRC cells was detected
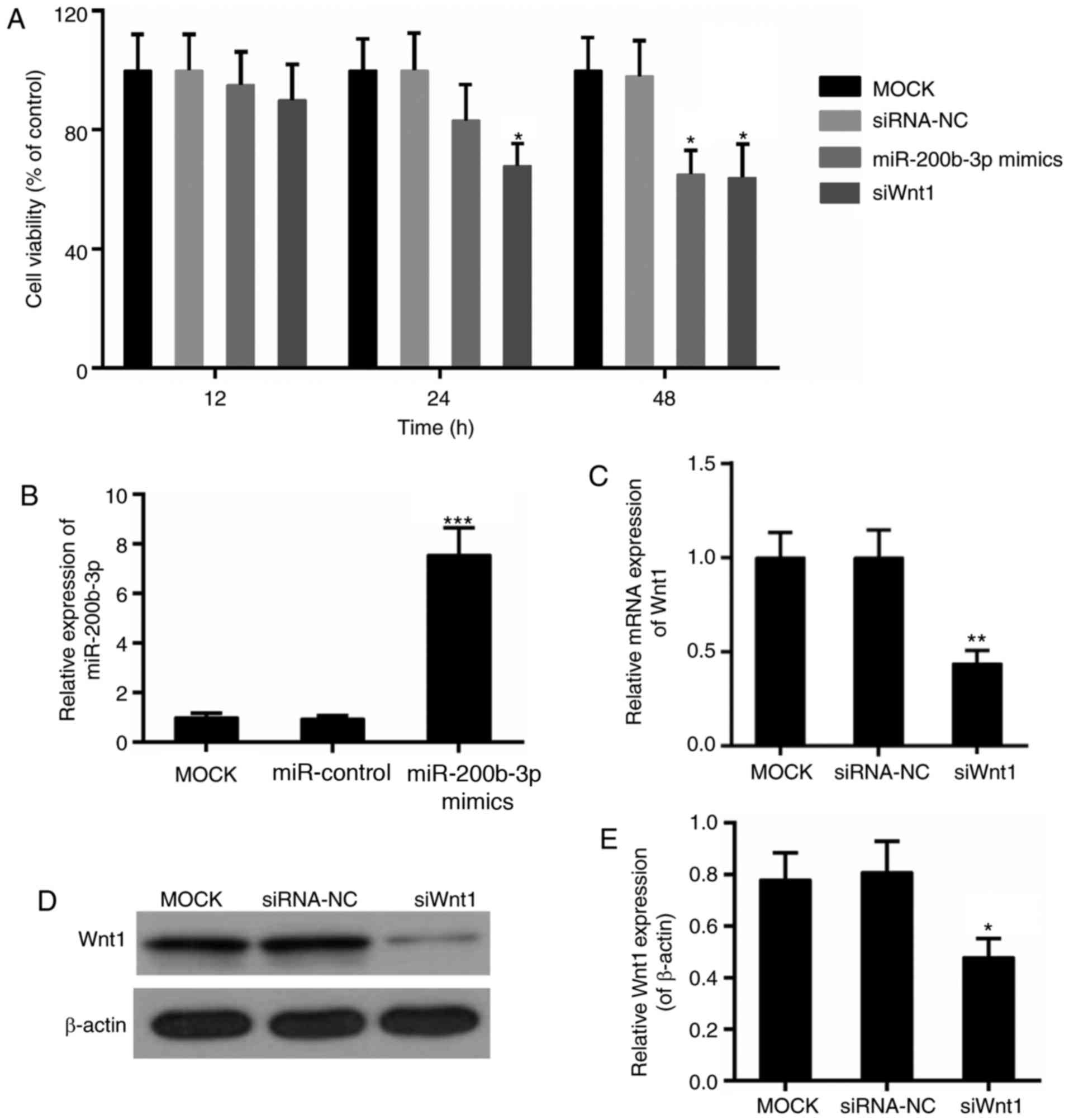
with regards to cell viability, cell proliferation and apoptosis.
The cell viability assay revealed that the Wnt1 interference
suppressed the viability of CRC cells significantly, beginning at
24 h (P<0.05; Fig. 4A). By
contrast, the significant decline in cell viability in the
miR-200b-3p mimics group was observed following transfection for 48
h (P<0.05; Fig. 4A). In
addition, the transfection efficiency of miR-200b-3p and Wnt1 was
verified, respectively (Fig.
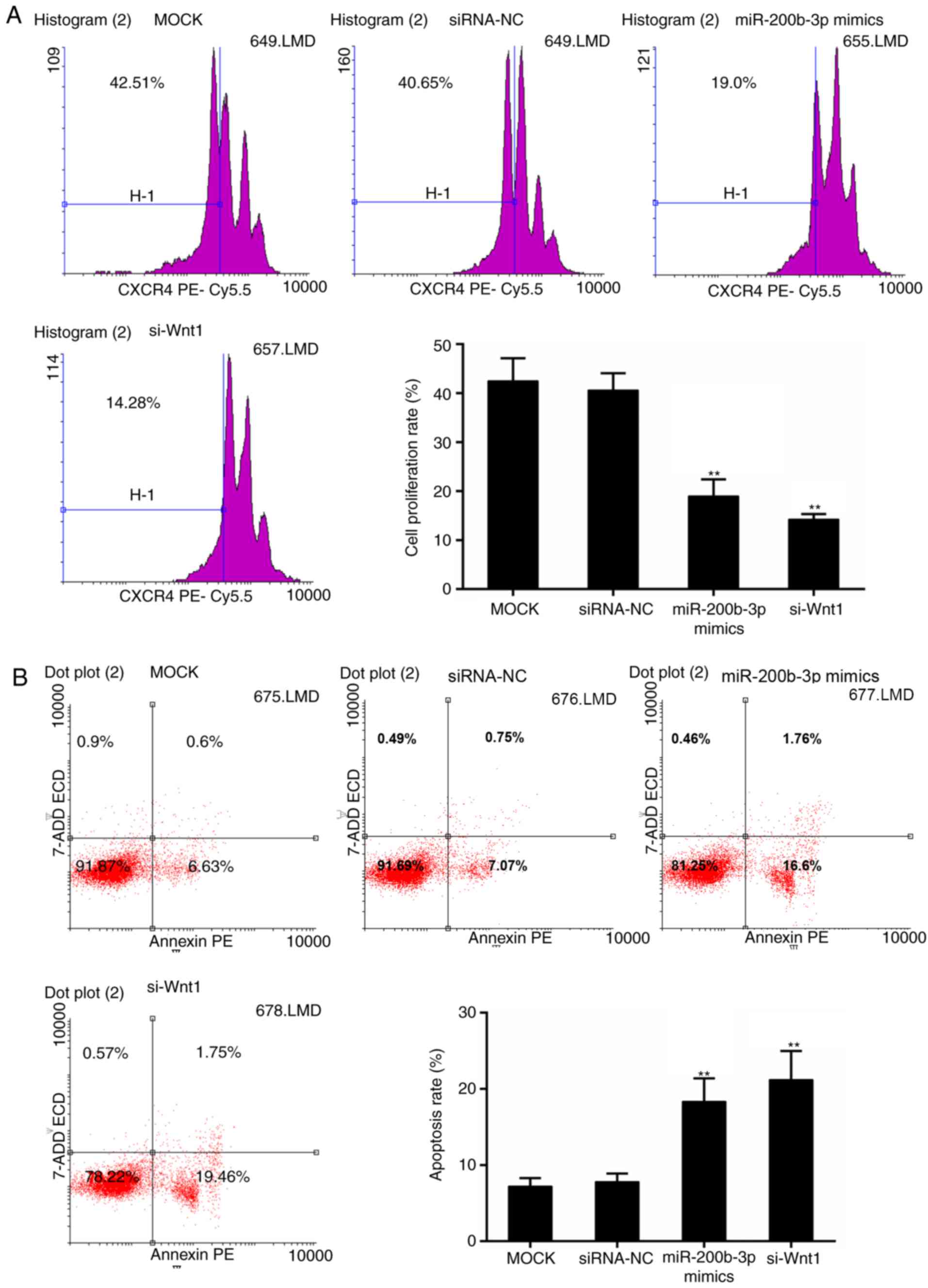
4B-E). Furthermore, the results from the CFSE assay revealed
that cell proliferation was significantly inhibited in the
miR-200b-3p mimics group and si-Wnt1 group compared with that in
the control group, respectively (P<0.01; Fig. 5A). By contrast, the apoptosis level
was significantly higher with the presence of miR-200b-3p mimics
and si-Wnt1 compared with that in the control group, respectively
(P<0.01; Fig. 5B).
Effect of miR-200b-3p on the
expression of Ki67, cleaved caspase-3 and β-catenin
Caspase-3 is an important protein implicated in
multiple apoptosis pathways (29),
while Ki67 is recognized to be a cell proliferation-associated
protein (30). As an important
component of the canonical Wnt pathway, β-catenin is dysregulated
in numerous carcinomas, including CRC (31); thus, the effect of miR-200b-3p on
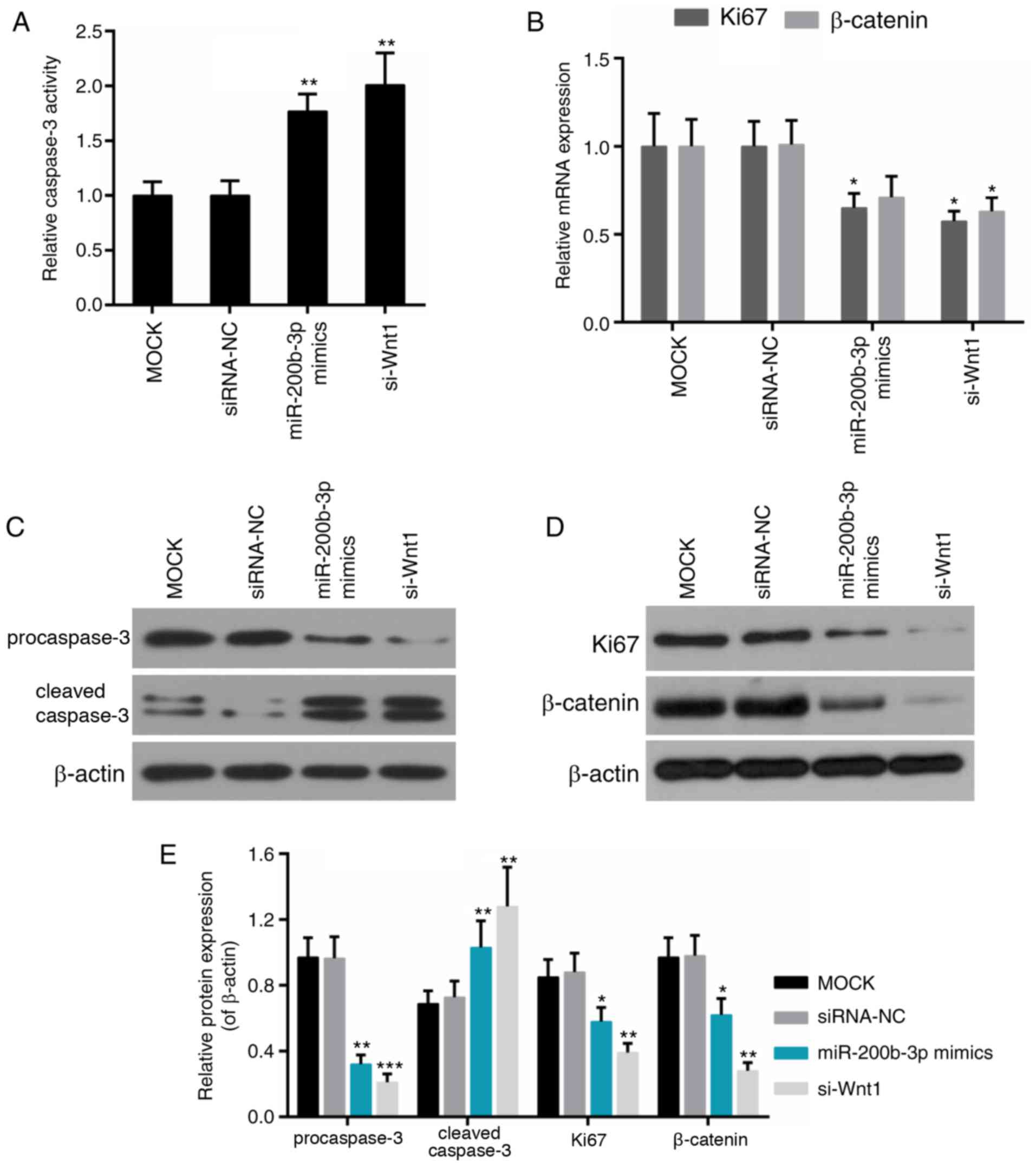
their expression was further examined. It was demonstrated that
caspase-3 activity was elevated significantly in the miR-200b-3p
mimics group and the si-Wnt1 group compared with that in the
control group, respectively (P<0.01; Fig. 6A). Expression of Ki67 was reduced
in the miR-200b-3p mimics group; the expression of Ki67 and
β-catenin was decreased in si-Wnt1 group compared with that in the
control group (Fig. 6B).
Furthermore, the protein level of pro-caspase-3 and cleaved
caspase-3 was decreased and increased in the miR-200b-3p mimics
group and the si-Wnt1 group compared with that in the control
group, respectively (Fig.
6C-E).
Discussion
The present study illustrated the role of
miR-200b-3p in the proliferation and apoptosis of CRC, and
highlighted the importance of increased expression of Wnt1 in CRC
progression.
In the present study, miR-200b-3p was frequently
decreased in the CRC tissues and cell lines, compared with the
paracarcinoma tissues or normal cells. Decreased expression of
miR-200b has been identified to be closely associated with poor
survival in ovarian carcinomas (32). Another study also indicated that
miR-200b may be a prognostic marker for breast cancer (33). These results indicated the
potential of miR-200b as a valuable biomarker for tumors. Wnt1, as
an important ligand involved in Wnt/β-catenin signaling (27), is generally upregulated in human
cancer (34). Similarly, the
expression of Wnt1 was elevated in the CRC tissues and cell lines
in the present study. Furthermore, the protein expression of Wnt1
was increased in approximately all tumor tissues from patients with
CRC. Additionally, the expression of miR-200b-3p and Wnt1 was
negatively correlated. It was indicated that decreased miR-200b-3p
and elevated Wnt1 expression may be critical in the progression of
CRC.
In consideration of the regulatory machinery of
miRNAs, it was proposed that the expression of Wnt1 may be
downregulated by miR-200b-3p. Thus, bioinformatics analysis was
undertaken to confirm this hypothesis. According to the data from
available online databases, it was revealed that there was a
complementary sequence of miR-200b-3p in the 3′UTR of Wnt1.
Subsequently, a luciferase reporter assay was performed to confirm
this prediction. The presented data revealed that the luciferase
activity of wild-type Wnt1-3′UTR was reduced by miR-200b-3p mimics,
whereas the activity of mutant Wnt1-3′UTR was not affected. It was
indicated that Wnt1 may be a direct target of miR-200b-3p.
Therefore, there was further speculation that miR-200b-3p may be a
tumor suppressor of CRC by regulating Wnt signaling.
To confirm the effect of miR-200b-3p on CRC and the
underlying mechanism, the proliferation and apoptosis behavior of
CRC following miR-200b-3p transfection was examined. It was
demonstrated that the viability of CRC cells and CSFE fluorescence
intensity was decreased via miR-200b-3p. Cellular apoptosis in CRC
was increased in the miR-200b-3p mimics group compared with the
control group. A previous study reported that miR-200b-3p may
inhibit the metastasis of prostate cancer (26). The data presented in the present
study combined with previous results from another study
demonstrated the tumor suppressive ability of miR-200b-3p (35).
Sequential activation of caspases is the hallmark
event in cellular apoptosis. Caspase-3, as an executioner caspase,
is at the convergence of multiple apoptosis pathways (29,36,37).
Furthermore, Ki67, as a strictly cell proliferation-associated
factor (30), is present in all
active phases in cell cycle progression and is inactive in the G0
phase (38). The activity of these
molecules, which are associated with apoptosis and cell
proliferation, was investigated. The present data demonstrated that
the activity of caspase-3 was enhanced by miR-200b-3p. The
expression of Ki67 was deduced and cleaved caspase-3 was enhanced
in the presence of miR-200b-3p mimics. Furthermore, the abnormal
activation of Wnt/β-catenin signaling is frequently identified in
CRC (39,40). Therefore, the effect of miR-200b-3p
on the expression of β-catenin was examined in the present study.
The protein expression level of β-catenin was decreased via
miR-200b-3p, although its mRNA expression was reduced slightly. It
was suggested that miR-200b-3p decreased the activity of β-catenin.
In addition, the inactive status of Wnt signaling may lead to the
degradation of β-catenin (41).
Thus, the decreased β-catenin protein expression level may be in
part due to the silencing of Wnt1 that was mediated through
miR-200b-3p. Taken together, it was suggested that miR-200b-3p may
suppress the tumor progression of CRC by inhibiting Wnt/β-catenin
signaling. The present study proposed that Wnt1 and miR-200b-3p may
serve as potential biomarkers for predicting the occurrence of CRC
and candidate modalities for targeted therapy for CRC.
A limitation of the present study was that the
direct binding of miR-200b-3p to Wnt1 requires validation by
in-depth investigations. A previous study reported that histone
deacetylase 1/4 and specificity protein 1 (Sp1) may regulate the
miR-200b expression, thereby affecting chemoresistance and
epithelial-mesenchymal transition, respectively (42,43).
However, another previous study claimed that Sp1 was a target of
miR-200b (33). Thus, future
examination of the upstream regulator may be interesting and
valuable for understanding the molecular mechanisms underlying the
progression of CRC. In addition, the miR-200 family has been
demonstrated to have an anti-tumor effect (44). Thus, determining the combined
effect of miR-200b-3p and other family members on CRC may inspire
another promising strategy for the treatment of CRC.
In conclusion, the present study uncovered valuable
clues underpinning the mechanism of tumor progression in CRC. The
present data revealed that miR-200b-3p and Wnt1 serve important
roles in the progression of CRC cells. Wnt1 may be a direct target
of miR-200b-3p. Furthermore, miR-200b-3p suppressed proliferation
and induced apoptosis in CRC by regulating the activity of
caspase-3, Ki67 and β-catenin. These results may have clinical
implications for patients with CRC.
Acknowledgements
Not applicable.
Funding
The present study was supported by Zhejiang
Provincial Medical Science and Technology Project (grant no.
2017KY565).
Availability of data and materials
All data generated and/or analyzed during this study
are included in this published article.
Authors' contributions
LC wrote the main manuscript. LC, XW, YZ and JZ
performed the experiments. LC and QL designed the study. XW and YZ
performed data analysis. All authors reviewed the manuscript.
Ethics approval and consent to
participate
All the protocols in the present study were approved
by the Ethics Committee of the Second Affiliated Hospital of
Zhejiang University School of Medicine and written informed consent
was obtained.
Consent for publication
Informed consent was obtained from all participants
for the publication of their data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vaiopoulos AG, Athanasoula KC and
Papavassiliou AG: Epigenetic modifications in colorectal cancer:
Molecular insights and therapeutic challenges. Biochim Biophys
Acta. 1842:971–980. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Watson AJM and Collins PD: Colon cancer: A
civilization disorder. Dig Dis. 29:222–228. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee IM, Shiroma EJ, Lobelo F, Puska P,
Blair SN and Katzmarzyk PT: Lancet Physical Activity Series Working
Group: Effect of physical inactivity on major non-communicable
diseases worldwide: An analysis of burden of disease and life
expectancy. Lancet. 380:219–229. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fearon ER, Pardoll DM, Itaya T, Golumbek
P, Levitsky HI, Simons JW, Karasuyama H, Vogelstein B and Frost P:
Interleukin-2 production by tumor cells bypasses T helper function
in the generation of an antitumor response. Cell. 60:397–403. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Basu S, Haase G and Ben-Ze'ev A: Wnt
signaling in cancer stem cells and colon cancer metastasis.
F1000Res. 19:52016.
|
|
8
|
Giles RH, van Es JH and Clevers H: Caught
up in a wnt storm: Wnt signaling in cancer. Biochim Biophys Acta.
1653:1–24. 2003.PubMed/NCBI
|
|
9
|
Capdevila J and Belmonte lzpisúa JC:
Extracellular modulation of the Hedgehog, Wnt and TGF-beta
signalling pathways during embryonic development. Curr Opin Genet
Dev. 9:427–433. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tanaka S, Terada K and Nohno T: Canonical
Wnt signaling is involved in switching from cell proliferation to
myogenic differentiation of mouse myoblast cells. J Mol Signal.
6:122011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen S, Guttridge DC, You Z, Zhang Z,
Fribley A, Mayo MW, Kitajewski J and Wang CY: WNT-1 Signaling
Inhibits Apoptosis by Activating β-Catenin/T Cell Factor–Mediated
Transcription. J Cell Biol. 152:87–96. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Logan CY and Nusse R: The Wnt signaling
pathway in development and disease. Annu Rev Cell Dev Biol.
20:781–810. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cadigan KM and Nusse R: Wnt signaling: A
common theme in animal development. Genes Dev. 11:3286–3305. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dai X, Wang L, Zhang L, Han Y, Yang G and
Li L: The expression and mutation of beta-catenin in colorectal
traditional serrated adenomas. Indian J Pathol Microbiol.
55:288–293. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pasquinelli AE, Reinhart BJ, Slack F,
Martindale MQ, Kuroda MI, Maller B, Hayward DC, Ball EE, Degnan B,
Muller P, et al: Conservation of the sequence and temporal
expression of let-7 heterochronic regulatory RNA. Nature.
408:86–89. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang XJ, Reyes JL, Chua NH and Gaasterland
T: Prediction and identification of Arabidopsis thaliana microRNAs
and their mRNA targets. Genome Biol. 5:E652004. View Article : Google Scholar
|
|
17
|
Lewis BP, Burge CB and Bartel DP:
Conserved seed pairing, often flanked by adenosines, indicates that
thousands of human genes are microRNA targets. Cell. 120:15–20.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Michael MZ, SM OC, van Holst Pellekaan NG,
Young GP and James RJ: Reduced accumulation of specific microRNAs
in colorectal neoplasia. Mol Cancer Res. 1:882–891. 2003.PubMed/NCBI
|
|
19
|
Fan D, Lin X, Zhang F, Zhong W, Hu J, Chen
Y, Cai Z, Zou Y, He X, Chen X, et al: MicroRNA 26b promotes
colorectal cancer metastasis by down-regulating pten and wnt5a.
Cancer Sci. 109:354–362. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hashimi ST, Fulcher JA, Chang MH, Gov L,
Wang S and Lee B: MicroRNA profiling identifies miR-34a and miR-21
and their target genes JAG1 and WNT1 in the coordinate regulation
of dendritic cell differentiation. Blood. 114:404–414. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huang S, Zhang L, Yang P, Chen P and Xie
Y: HCV core protein-induced down-regulation of microRNA-152
promoted aberrant proliferation by targeting Wnt1 in HepG2 cells.
PLOS One. 9:e817302014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Si W, Li Y, Shao H, Hu R, Wang W, Zhang K
and Yang Q: MiR-34a Inhibits Breast Cancer Proliferation and
Progression by Targeting Wnt1 in Wnt/β-catenin Signaling Pathway.
Am J Med Sci. 352:191–199. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gregory PA, Bert AG, Paterson EL, Barry
SC, Tsykin A, Farshid G, Vadas MA, Khew-Goodall Y and Goodall GJ:
The miR-200 family and miR-205 regulate epithelial to mesenchymal
transition by targeting ZEB1 and SIP1. Nat Cell Biol. 10:593–601.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kinzler KW and Vogelstein B: Lessons from
hereditary colorectal cancer. Cell. 87:159–170. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Williams LV, Veliceasa D, Vinokour E and
Volpert OV: miR-200b inhibits prostate cancer EMT, growth and
metastasis. PLoS One. 8:e839912013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wei W, Chua MS, Grepper S and So SK:
Blockade of Wnt-1 signaling leads to anti-tumor effects in
hepatocellular carcinoma cells. Mol Cancer. 8:762009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Carthew RW and Sontheimer EJ: Origins and
Mechanisms of miRNAs and siRNAs. Cell. 136:642–655. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Porter AG and Janicke RU: Emerging roles
of caspase-3 in apoptosis. Cell Death Differ. 6:99–104. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Scholzen T and Gerdes J: The Ki-67
protein: From the known and the unknown. J Cell Physiol.
182:311–322. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gao ZH, Lu C, Wang MX, Han Y and Guo LJ:
Differential β-catenin expression levels are associated with
morphological features and prognosis of colorectal cancer. Oncol
Lett. 8:2069–2076. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Leskela S, Leandro-Garcia LJ, Mendiola M,
Barriuso J, Inglada-Perez L, Munoz I, Martinez-Delgado B, Redondo
A, de Santiago J, Robledo M, et al: The miR-200 family controls
beta-tubulin III expression and is associated with paclitaxel-based
treatment response and progression-free survival in ovarian cancer
patients. Endocr Relat Cancer. 18:85–95. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yao Y, Hu J, Shen Z, Yao R, Liu S, Li Y,
Cong H, Wang X, Qiu W and Yue L: MiR-200b expression in breast
cancer: A prognostic marker and act on cell proliferation and
apoptosis by targeting Sp1. J Cell Mol Med. 19:760–769. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
He B, You L, Uematsu K, Xu Z, Lee AY,
Matsangou M, Mccormick F and Jablons DM: A monoclonal antibody
against Wnt-1 induces apoptosis in human cancer cells. Neoplasia.
6:7–14. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Humphries B and Yang C: The microRNA-200
family: Small molecules with novel roles in cancer development,
progression and therapy. Oncotarget. 6:6472–6498. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ghavami S, Hashemi M, Ande SR, Yeganeh B,
Xiao W, Eshraghi M, Bus CJ, Kadkhoda K, Wiechec E, Halayko AJ and
Los M: Apoptosis and cancer: Mutations within caspase genes. J Med
Genet. 46:497–510. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Boatright KM and Salvesen GS: Mechanisms
of caspase activation. Curr Opin Cell Biol. 15:725–731. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bruno S and Darzynkiewicz Z: Cell cycle
dependent expression and stability of the nuclear protein detected
by Ki-67 antibody in HL-60 cells. Cell Prolif. 25:31–40. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Rafael S, Veganzones S, Vidaurreta M, de
la Orden V and Maestro ML: Effect of β-catenin alterations in the
prognosis of patients with sporadic colorectal cancer. J Cancer Res
Ther. 10:591–596. 2014.PubMed/NCBI
|
|
40
|
Jung YS, Jun S, Lee SH, Sharma A and Park
JI: Wnt2 complements Wnt/β-catenin signaling in colorectal cancer.
Oncotarget. 6:37257–37268. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Clevers H: Wnt/beta-catenin signaling in
development and disease. Cell. 127:469–480. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chen DQ, Pan BZ, Huang JY, Zhang K, Cui
SY, De W, Wang R and Chen LB: HDAC 1/4-mediated silencing of
microRNA-200b promotes chemoresistance in human lung adenocarcinoma
cells. Oncotarget. 5:3333–3349. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kolesnikoff N, Attema JL, Roslan S, Bert
AG, Schwarz QP, Gregory PA and Goodall GJ: Specificity protein 1
(Sp1) maintains basal epithelial expression of the miR-200 family:
Implications for epithelial-mesenchymal transition. J Biol Chem.
289:11194–11205. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Manavalan TT, Teng Y, Litchfield LM,
Muluhngwi P, Al-Rayyan N and Klinge CM: Reduced expression of
miR-200 family members contributes to antiestrogen resistance in
LY2 human breast cancer cells. PLOS One. 8:e623342013. View Article : Google Scholar : PubMed/NCBI
|