Introduction
Preeclampsia, generally defined as the development
of hypertension and proteinuria after 20 weeks of gestation in a
previously normotensive woman, remains a leading cause of fetal and
maternal mortality and morbidity (1,2).
Preeclampsia is the second greatest cause of maternal mortality and
affects 7–10% of pregnant women worldwide (3,4). The
etiology of preeclampsia begins in early pregnancy, when inadequate
or defective placentation results in deficient maternal spiral
artery remodeling and insufficient placental perfusion. This causes
fetal hypoxia, which results in the release of multiple cytokines
and growth factors into the maternal circulation, in turn leading
to systemic maternal endothelial dysfunction, vascular
inflammation, and the clinical manifestations of preeclampsia. Even
though considerable data have been accumulated on the
pathophysiology of preeclampsia, the precise underlying molecular
mechanisms remain elusive.
During normal placentation, trophoblasts from the
developing embryo invade the uterine sub-endometria and decidual
spiral arteries, converting them into larger competent blood
vessels to enhance blood flow to the embryo. Initial steps in this
process require the trophoblast to detach from the extracellular
matrix and invade the maternal arteries in a process tightly
controlled by specific proteases. Recent studies have shown that
shallow trophoblast invasion into the endometrium, resulting in
insufficient spiral artery remodeling in the decidua, is the
initial cellular pathology in preeclampsia, suggesting a possible
molecular defect in proteolysis.
One class of proteases likely to be involved in
placentation and preeclampsia is the high-temperature requirement A
(HTRA) serine proteases, identified as important ATP-independent
chaperones and cellular quality-control factors. Microarray data
for the four known HTRA genes, after comparing normotensive
pregnancy vs. preeclampsia, showed that HTRA1 and HTRA4 were
significantly upregulated in preeclampsia compared with normal
pregnancy (5). In a previous study
of normal human placenta, HTRA1 expression was detected in
trophoblast subtypes and in both uterine endometrial glands and
decidual cells at the maternal-trophoblast interface during
placentation (6). In mice,
trophoblast invasion is coordinated with decidua regression and, as
the process of placentation occurs, HtrA1 is highly expressed in
both trophoblasts and in the decidua capsularis at its interface
with the trophoblast during active cell invasion and regression
(7). HtrA1 expression in human
placenta and its role in relation to trophoblasts were also
analyzed (8). Recently, increased
expression of HTRA4 was reported in the sera and placentas of
patients with preeclampsia, demonstrating that aberrant placental
HTRA4 expression may be linked to the onset of preeclampsia
(5). These observations indicate
that increased HTRA1 or HTRA4 expression may be a manifestation of
a functional defect in trophoblast migration and invasion and may
be associated with preeclampsia.
Drawing on this background, we conducted an in-depth
investigation of the expression of HTRA1 and HTRA4 in human
placentas during physiologically normal and pathological
pregnancies. Furthermore, we explored the role of HTRA1 and HTRA4
in cell cycle progression and migration in a trophoblast line.
Materials and methods
Ethical statement
The study protocol was approved by the Ethics
Committees of the Shanghai First Maternity and Infant Hospital,
Tongji University School of Medicine (Shanghai, China), and all
participants provided written informed consent.
Patients and sample collection
A total of 20 placental biopsies, at different
gestational ages in the third trimester, were obtained from
patients with preeclamptic (n=10) and normotensive pregnancies
(n=10). Severe preeclampsia was defined as patient blood pressure
higher than 160/110 mmHg, platelets <100,000 µl, elevated serum
transaminase levels [alanine aminotransferase (ALT) or aspartate
aminotransferase (AST)], persistent headache or other cerebral or
visual disturbance, and persistent epigastric pain (9). The diagnosis of severe preeclampsia
was based on strict criteria from the American College of
Obstetricians and Gynecologists (ACOG 2013). Patient
characteristics are shown in Table
I.
 | Table I.Characteristics of subjects with
normotensive pregnancies and severe preeclampsia. |
Table I.
Characteristics of subjects with
normotensive pregnancies and severe preeclampsia.
| Variable | Normotensive
(n=10) | sPE (n=10) | P-value |
|---|
| Maternal age
(years) |
29.4±1.1 |
29.1±1.3 | 0.86 |
| Gestational age
(week) |
38.9±0.4 |
38.0±0.6 | 0.23 |
| Systolic BP
(mmHg) |
109.7±3.3 | 168.5±3.7 | <0.001 |
| Diastolic BP
(mmHg) |
69.5±2.2 |
100.2±2.05 | <0.001 |
| Fetal weight (g) | 3,435.6±81.3 |
2,718.0±126.1 | <0.001 |
| Proteinuria (g/24
h) | 0.12±0.02 | 2.9±0.2 | <0.001 |
All of the placental tissue samples in the study
were obtained immediately after delivery. A central area of
chorionic tissue was dissected, and the maternal decidua and
amniotic membranes were removed. Sections of placental villi (1×1×1
cm) were taken from the four different central quadrants on the
maternal face of the placenta. After prompt washing with sterile
phosphate-buffered saline (PBS), tissues were immediately frozen
and stored in liquid nitrogen until use for RNA and protein
extraction. The remaining portions were fixed in 10% buffered
formalin for immunohistochemistry (IHC).
IHC
Tissue biopsy specimens were embedded in paraffin
and sectioned for IHC. After drying in an oven at 60°C for 2 h,
tissue sections were deparaffinized in xylene and dehydrated
through a graded alcohol series. Endogenous peroxides were quenched
by immersing the tissue sections in 3% hydrogen peroxide in
methanol for 10 min. After heat induction in citric acid for 15 min
and blocking with 1% bovine serum albumin for 20 min, the slides
were incubated at 4°C overnight with a rabbit polyclonal anti-HTRA1
at 1:100 dilution (ab65915; Abcam, Cambridge, UK) or anti-HTRA4 at
1:50 dilution (bs-20063R; Bioss, Beijing, China). After washing
with PBS, the sections were subsequently incubated with IHC
detection reagent (8114P; Cell Signaling Technology, Inc., Danvers,
MA, USA) for 1 h at room temperature. Staining was completed by
incubation with diaminobenzidine tetrahydrochloride, and tissues
were counterstained with hematoxylin. Photomicrographs were taken
with an inverted microscope.
Cell culture and transfection
The immortalized first-trimester human trophoblast
line (HTR-8) (10), originally
obtained from Dr. Charles H. Graham (Queen's University, Kingston,
ON, Canada), has been reported to exhibit a number of
characteristics similar to those of parental trophoblasts. The
cells were cultured in Dulbecco's modified Eagle's medium: Nutrient
Mixture F-12 (DMEM/F12) (SH30023; HyClone; GE Healthcare Life
Sciences, Logan, UT, USA) supplemented with 10% (v/v) fetal bovine
serum (FBS) and antibiotics (100 U/ml penicillin and 100 mg/ml
streptomycin) at 37°C in a 95% air and 5% CO2
water-saturated atmosphere. To induce hypoxia, the cells were
exposed to 1% O2, 5% CO2 and 94%
N2 in a multi-gas incubator (Sanyo, Osaka, Japan).
For transient expression of HTRA1 and HTRA4, HTR-8
cells were divided into three groups: Cells transduced with
negative control adenovirus (HTR-8/vector), cells transduced with
HTRA1-adenovirus (HTR-8/HTRA1) (AH891211; ViGene Biosciences,
Rockville, MD, USA) or cells transduced with HTRA4-adenovirus
(HTR-8/HTRA4) (AH894511; ViGene Biosciences). When HTR-8 cells were
50–70% confluent, they were transduced with specific or negative
control adenovirus for 6 h, and these transfected cells were
cultured in DMEM/F12 (HyClone; GE Healthcare Life Sciences) at 37°C
for further analysis.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the placental villous
tissues and cells using TRIzol and an RNA Simple Total RNA kit
(DP419; Tiangen Biotech, Beijing, China) according to the
manufacturer's instructions. RNA was quantified by UV absorption
measurement and 1 µg RNA was reverse transcribed into cDNA in a
20-µl reaction volume using the PrimeScript RT reagent kit (FP205;
Takara Bio, Inc., Otsu, Japan) for 15 min at 37°C, 5 sec at 85°C.
RT-qPCR was performed using SYBR PreMix Ex Taq (Takara Bio, Inc.).
The RT-qPCR reaction conditions were: Incubation at 95°C for 30
sec, followed by 40 cycles of 95°C for 15 sec and 60°C for 20 sec.
The PCR products were subjected to a melting curve analysis to
confirm the amplification specificity. Levels of mRNA were
normalized to β-actin and evaluated using the 2−ΔΔCq
method (11). PCR primers for
HTRA1, HTRA4, and the housekeeping gene β-actin were obtained from
a previous report. The following primer sequences were used: HTRA1:
5′-GGACTACATCCAGACCGAC-3′ and 5′-TGGGACTCCGTGAGGAAC-3′; HTRA4:
5′-GTCAGCACCAAACAGCG-3′ and 5′-GGAGATTCCATCAGTCACCC-3′; and
β-actin: 5′-AACTCCATCATGAAGTGTGACG-3′ and
5′-GATCCACATCTGCTGGAAGG-3′.
Western blotting
Tissues and cells were lysed using
radioimmunoprecipitation assay (RIPA) lysis buffer (P0013B;
Beyotime Institute of Biotechnology, Haimen, China) containing
protease inhibitors (10 mM EDTA and 4 mM sodium pyrophosphate) and
further lysed by sonication after pulverizing placental specimens
in liquid nitrogen using a mortar and pestle. Following
centrifugation, the protein concentration of each supernatant was
determined with a bicinchoninic acid (BCA) Protein Assay kit
(23225; Thermo Fisher Scientific, Inc., Waltham, MA, USA). Equal
amounts of proteins (100 µg) were boiled at 100°C for 10 min,
separated by sodium dodecyl sulfate polyacrylamide gel
electrophoresis (10% polyacrylamide) and electro-transferred onto
polyvinylidene difluoride membranes. After blocking with 5% non-fat
milk in Tris-buffered saline [including 0.1% (w/v) Tween-20] at
room temperature for 1 h, membranes were first probed with rabbit
polyclonal anti-HTRA1 (ab38610; Abcam) or with rabbit polyclonal
anti-HTRA4 (ab65915; Abcam), followed by reprobing with an antibody
against glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (ab37168;
Abcam) as a loading control. Proteins were detected using enhanced
chemiluminescence (ECL) (NCI4106; Thermo Fisher Scientific, Inc.).
Finally, the immunoreactive signals were quantified using a
densitometer.
Proliferation assay
To assess proliferation rates, we used the BrdU
incorporation assay to analyze DNA synthesis. After transfection
for 30 h, the cells were trypsinized, resuspended, and grown on
6-well plates covered with 10×10-mm polylysine-coated coverslips.
After incubating with 60 µM BrdU reagent (KGA326; Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) for 12 h, the cells were fixed with
cold acetone-methanol (1:1, v/v) for 10 min and denatured with 4
mol/l HCl for 10 min, followed by three washes with PBS. The cells
were incubated with 1 M Tris-HCl (pH 8.0) with mouse anti-BrdU
(1:1,000; Sigma-Aldrich; Merck KGaA) and Cy3-labeled secondary
antibody (1:300; Jackson ImmumoResearch Laboratories, Inc., West
Grove, PA, USA). The BrdU+ cells were visualized by
immunofluorescence. Nuclei were stained with 1 µg/ml DAPI (Dojindo
Molecular Technologies, Inc., Kumamoto, Japan) and the images for
cell counting were obtained from at least 10 randomly selected
fields.
Cell cycle assay
A Cell Cycle Detection kit (KAG511; Keygen, Nanjing,
China) was used to monitor the cell cycle. HTR-8 cells
(1×106) were fixed in ice-cold 70% ethanol overnight at
4°C 48 h after transfection, and then incubated with 100 µg/ml
RNase A at 37°C for 30 min. After staining with 25 µg/ml propidium
iodide for 30 min, the cells were subjected to
fluorescence-activated cell sorting and analyzed using Cell Quest
Pro Software (BD Biosciences, Franklin Lakes, NJ, USA.
Migration assay
A transwell chamber assay was performed to analyze
cell migration ability. HTR-8 cells (5×104) transfected
with different adenoviruses for 30 h were trypsinized, resuspended,
and seeded into the upper 8-µm pore-size transwell chamber (BD
Biosciences) with DMEM/F12 medium (300 µl) containing 1% FBS, and
the bottom chambers were filled with 800 µl medium containing 10%
FBS. After 16 h at 37°C, cells that migrated to the lower chambers
were stained with calcein AM (0.2 µM; C3100MP; Invitrogen; Thermo
Fisher Scientific, Inc.) for 30 min and visualized with an inverted
microscope mounted with a charge-coupled device (CCD) camera. The
cells were randomly counted under the microscope (magnification,
×100) in four representative quadrants on each plate.
Statistical analysis
All experiments were carried out in three
independent replicates. Data are expressed as the mean ± standard
error of the mean and differences between groups were determined
using Student t-tests. P<0.05 was considered to indicate a
statistically significant difference. SPSS version 17.0 software
(SPSS, Inc., Chicago, IL, USA) was used to analyze the data, and
GraphPad Prism version 5.0 software (GraphPad Software, Inc., La
Jolla, CA, USA) was used to create the graphs.
Results
Localization of HTRA1 and HTRA4
proteins in human placenta
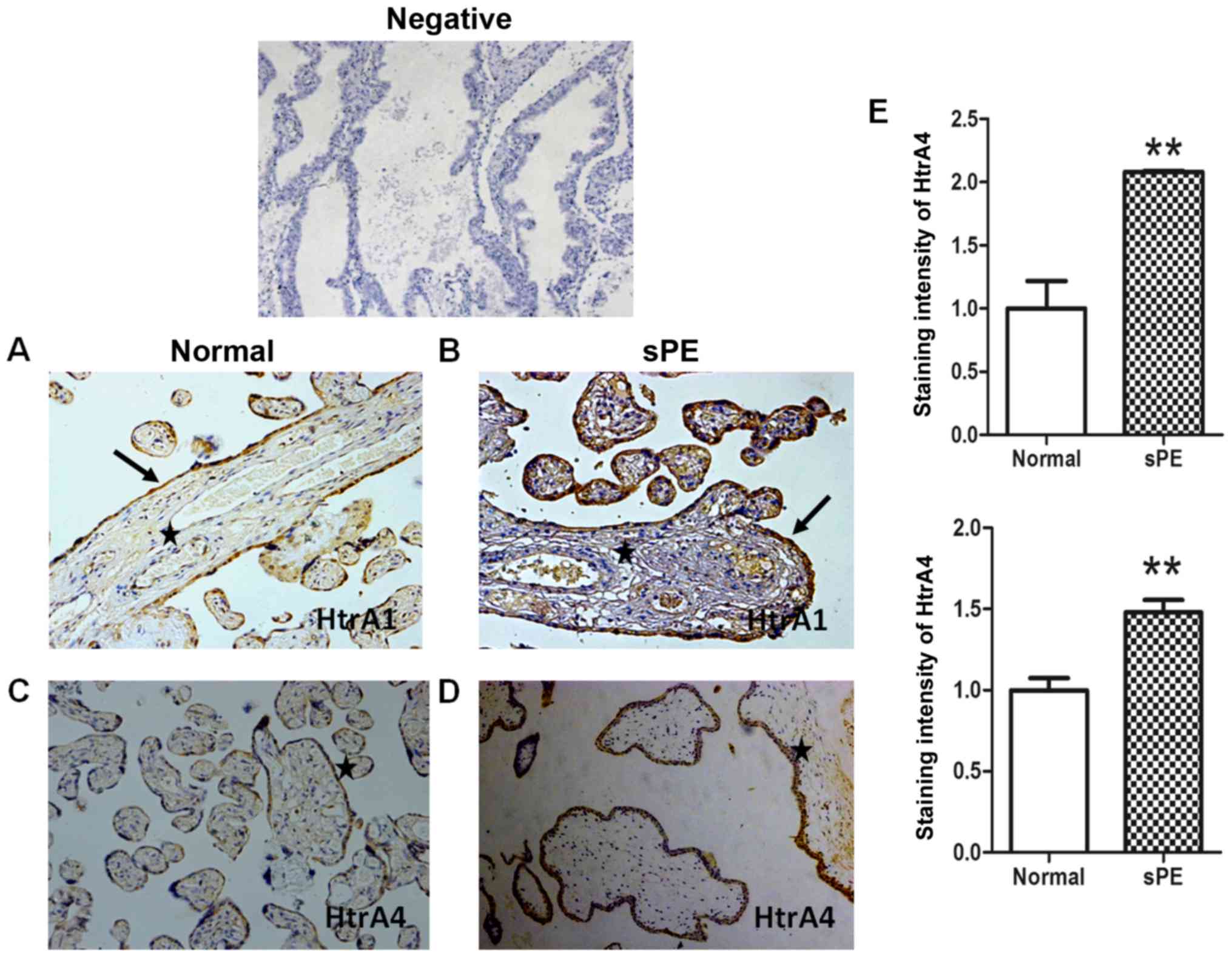
First, we localized HTRA1 and HTRA4 proteins in
human placenta tissues from normal and severe preeclamptic patients
by IHC. In the third trimester, both HTRA1 (Fig. 1A and B) and HTRA4 (Fig. 1C and D) mainly localized to the
cytoplasm of syncytiotrophoblasts in the placental villus. In
addition, we observed weak staining for HTRA1 in the fetal vessel
endothelium. Upregulation of the HtrA1 and HtrA4 in
syncytiotrophoblasts from severe preeclampsia were confirmed with
the use of quantitative analysis (Fig.
1E).
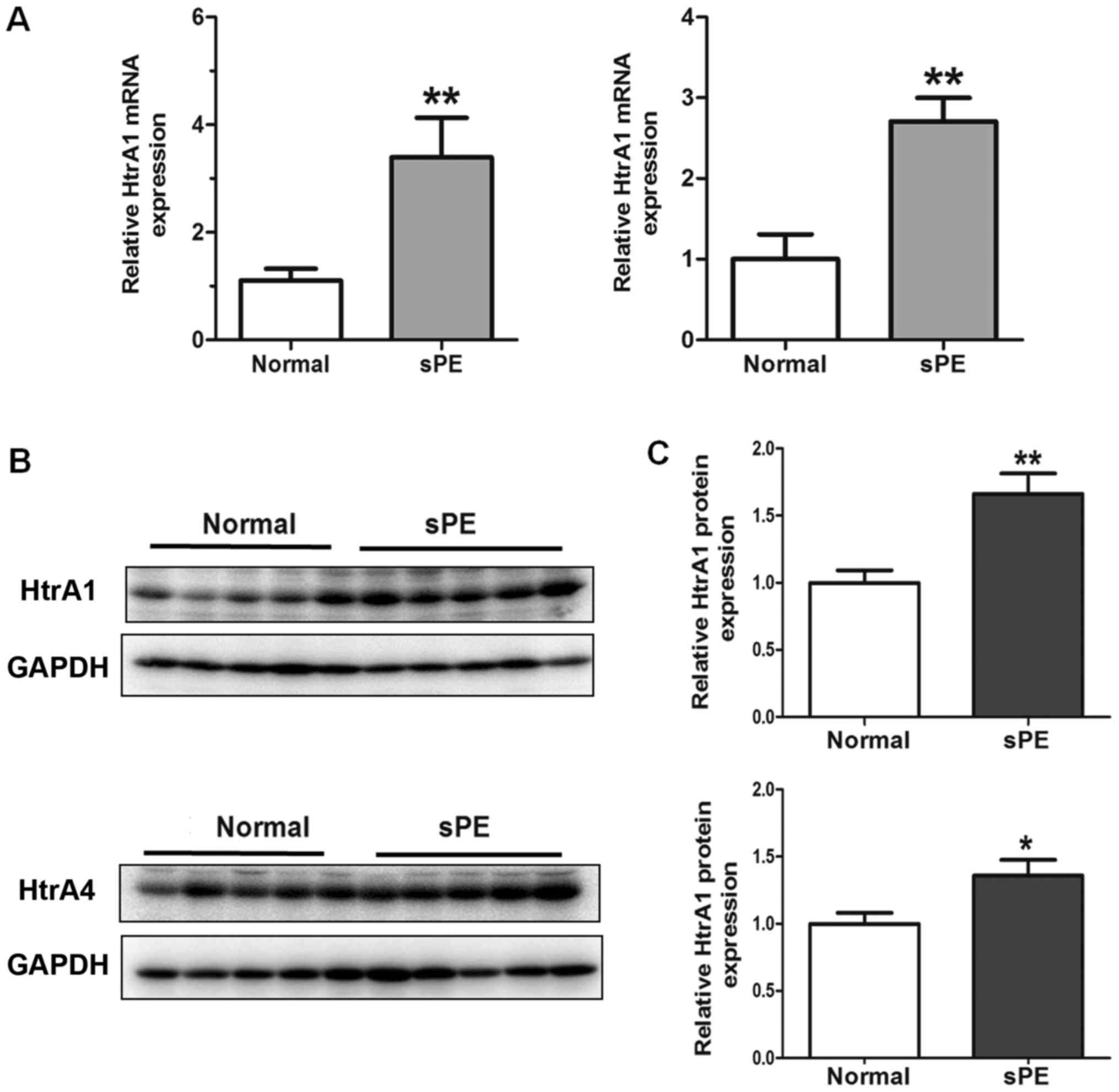
Upregulated expression of HTRA1 and
HTRA4 in human severe preeclamptic placentas
Next, RT-qPCR and western blotting were performed to
compare the expression levels of HTRA1 and HTRA4 in different
placental samples. We validated that the expression of HTRA1 and
HTRA4 at both the mRNA (Fig. 2A)
and protein (Fig. 2B and C)
levels, after normalization with GAPDH, were significantly elevated
in the severe preeclamptic placentas in the third trimester of
gestation compared to the control group. In western blotting, both
HTRA1 and HTRA4 antibodies detected a single band at ~51 kDa in
HTR-8 cells and in human placental tissues.
HTRA1 regulates proliferation and cell
cycle in HTR-8 cells
Western blotting of HTR-8 cells derived from
first-trimester trophoblast culture revealed that both HTRA1 and
HTRA4 were minimally expressed. After transient transfection with
control and specific adenoviruses, respectively, for 24, 48, 72,
and 96 h, western blotting indicated higher expression levels of
HTRA1 and HTRA4 (Fig. 3A) in
transfected cells.
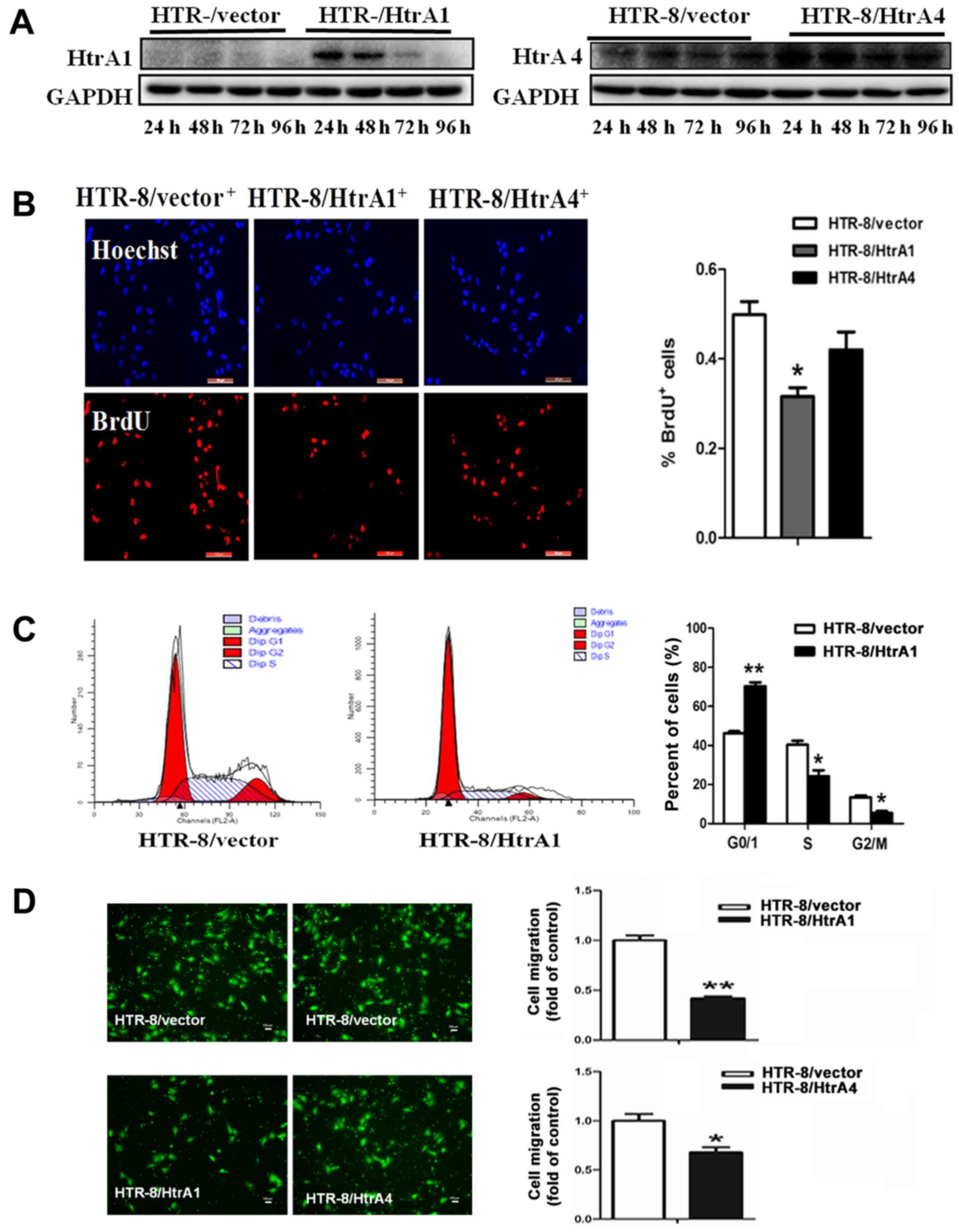 | Figure 3.Western blotting for HtrA1 and HtrA4
in cells. (A) Expression of HtrA1 and HtrA4 in transfected HTR-8
cells was increased at 24, 48 and 72 h when compared with the
control groups. (B) Proliferating cells of HTR-8/vector,
HTR-8/HtrA1, and HTR-8/HtrA4 were labeled with BrdU, and identified
with anti-BrdU (red) and counterstained with Hoechst to visualize
nuclei (blue). Scale bars, 100 µm. The percentage of BrdU-positive
of HTR-8/HtrA1 in total Hoechst-stained cells was decreased when
compared with the control groups. (C) Flow cytometric analysis
demonstrated that the proportion of G0/1-phase cells increased, and
the proportion of S and G2/M-phase cells decreased in the
HTR-8/HtrA1 cells. Data represent the average percentage of cells
in each phase of the cell cycle in three independent triplicate
experiments. (D) The migratory activities of transfected cells were
estimated based on the number of cells that migrated through the
filter of the chamber (scale bars, 100 µm; magnification, ×100).
Quantitation indicated that the number of migrating cells in the
HtrA1 and HtrA4-transfected groups was less than the number
observed in the control groups. Results are presented as the mean ±
standard error mean (n=3). *P<0.05 and **P<0.01 vs.
HTR-8/vector. HtrA, high-temperature requirement A; BrdU,
5-bromo-2′-deoxyuridine. |
To directly determine the effects of HTRA1 or
HTRA4-induced HTR-8 cell proliferation, the rates of DNA synthesis
in HTR-8/vector, HTR-8/HTRA1, and HTR-8/HTRA4 cells were quantified
using BrdU incorporation assays. HTR-8/HTRA1 proliferation was
reduced by 18% compared with control cells. However, ectopic HTRA4
expression had no effect on the growth of HTR-8 cells (Fig. 3B). Furthermore, flow cytometric
analysis indicated that the percentage of HTR-8/HTRA1 cells in the
G0/G1 phase increased significantly compared with that of control
cells, concomitant with decreases in S-phase and G2/M-phase cells
(Fig. 3C).
Both HTRA1 and HTRA4 attenuate
migration of HTR-8 cells
To investigate whether HTRA1 and HTRA4 affect the
migration activity of HTR-8 cells, transwell cell migration
experiments were conducted. The mean counts of migrating cells in
both the HTRA1 and HTRA4-transfected HTR-8 cells were significantly
lower than in the control group (Fig.
3D). These observations indicate that increased HTRA1 and HTRA4
expression may inhibit trophoblast migration during placental
development.
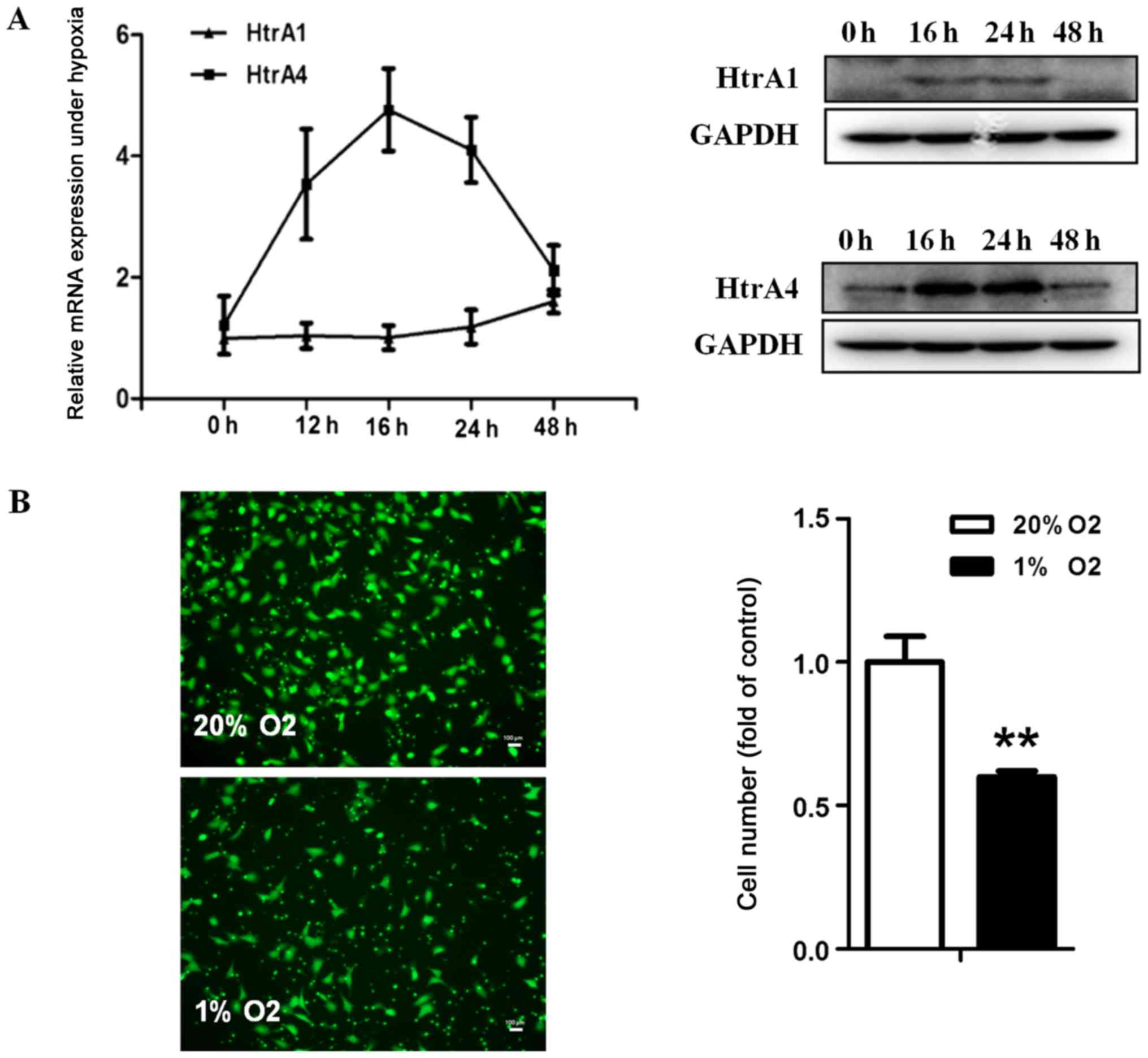
Hypoxia-induced HTRA1 and HTRA4
expression and attenuated HTR-8 cell migration
As development of preeclampsia is tightly associated
with placental hypoxia, HTR-8 cells were incubated for 0, 16, 24,
and 48 h under normoxic or hypoxic conditions, and the endogenous
expression of HTRA1 and HTRA4 was determined by RT-qPCR and western
blotting. Interestingly, our studies indicated that hypoxic
treatment had a significantly positive effect on HTRA1 and HTRA4
levels in HTR-8 cells at 16 and 24 h (Fig. 4A).
Accordingly, we further investigated cell migration
ability in hypoxia and discovered that the migration ability of
HTR-8 cells was significantly impaired after hypoxic treatment for
26 h. These results suggest that the inhibition of trophoblast
migration by hypoxia may be due to the upregulation of HTRA1 and
HTRA4 expression during placentation (Fig. 4B).
Discussion
Successful pregnancy depends on a coordinated series
of gene expression in the placenta and embryo. Placentation
requires trophoblasts to proliferate, migrate and invade the uterus
and its spiral arterioles. The root cause of preeclampsia is
reduced placental perfusion commonly caused by abnormal
placentation and aberrant trophoblast functions.
In the present study, we have demonstrated that mRNA
and protein levels of both HTRA1 and HTRA4 were upregulated in the
villous tissues of placentas complicated preeclampsia by in late
gestation, compared with the placentas of normotensive pregnant
women. Consistent with the fact that HTRA1 and HTRA4 can be
secreted, levels are also elevated in the sera of patients with
preeclampsia (5,12). These results independently
confirmed our findings. In addition, HTRA4 was found to be
upregulated in unexplained fetal growth restriction, which shares a
common etiological pathway with preeclampsia; thus, HTRA4 may
trigger preeclampsia via a pathway common to fetal growth
restriction.
Our results showed that ectopic expression of HTRA1
or HTRA4 in HTR-8 cells inhibited their migration. Interestingly,
several studies have indicated that HtrA1, as a tumor suppressor
downregulated in ovarian cancer (13) and in melanoma metastases, induces
cell death and inhibits proliferation and invasion (14). Thus, HTRA1 seems to have two
paradoxical roles. HTRA family proteases share similar molecular
architecture. Human HTRA1 and HTRA4 are composed of variable N
termini, a protease domain, and one PDZ domain. Their role in cell
migration is probably not dependent on their protease activity, but
on the PDZ domain or the N terminal regions, which contain signal
peptides and a fragment of insulin-like growth factor binding
protein (15). Moreover, a wide
variety of hormones differ in variety and quantity during
pregnancy. Therefore, to investigate the roles of these domains in
trophoblast migration in the placenta, further experiments
involving various deletion constructs of HTRA1 and HTRA4 are
needed.
We identified HTRA1 as an inhibitory factor in
trophoblast proliferation and progression past the G1/S checkpoint
of the cell cycle. Previous studies have implicated intracellular
HTRA1 in the proliferation of multiple cell types, and it may
mediate these effects by inhibiting a number of proteins such as
tuberous sclerosis complex 2 (TSC-2), participating in increasing
cell growth and proliferation (16), insulin-like growth factor binding
protein 5 (17), and transforming
growth factor-β. Therefore, HTRA1 may suppress trophoblast
proliferation through its effects on cell cycle progression.
Furthermore, we demonstrated that hypoxia (1%
O2) induced significant overexpression of both HTRA1 and
HTRA4 and inhibited the chemotactic cell migration of HTR-8 cells.
The upregulation of HTRA1 and HTRA4 expression may be secondary to
placental ischemia and hypoxia in preeclamptic pregnancies.
However, due to complicated alterations of various factors in
trophoblasts in hypoxia, it is uncertain whether overproduction of
HTRA1 or HTRA4 directly leads to defects in HTR-8 cell migration.
Oxidative stress and endoplasmic reticulum stress in trophoblasts
play key intermediary roles in generating preeclampsia (18–20).
Hypoxia may precipitate endoplasmic reticulum stress and lead to
increased generation of reactive oxygen species (21). HTRA1 does not appear to be
inducible by heat shock, but may be regulated by a more general
stress pathway (22). HTRA1 or
HTRA4, as essential chaperones, may be upregulated to stabilize
misfolded or mislocalized proteins to enhance cell survival in
preeclamptic placentas. Therefore, more experiments on HTRA1
and HTRA4 are needed to determine their roles in trophoblast
migration during hypoxia.
In conclusion, HTRA1 and HTRA4 are upregulated in
severe preeclamptic placentas, which may be linked to disease
onset. However, the specific mechanisms responsible for the
elevation of HTRA1 or HTRA4 in preeclampsia and their roles in
trophoblasts under hypoxia are not yet understood. Whether
overexpression may be a primary step or secondary to placental
ischemia and hypoxia or abnormal cytokine expression remains
unclear. Thus, further studies are needed to elucidate the roles of
HTRA1 and HTRA4 proteins in trophoblast function and to investigate
the potential therapeutic utility of targeting this pathway.
Acknowledgements
The authors would like to thank Mrs. Xin Luo, Mrs.
Huihui Wang, Mrs. Fangyuan Wang, Mrs. Yifei Chen and Mrs. Hui Wang
(Experimental Center of International Peace Maternity & Child
Health Hospital Affiliated to Shanghai Jiao Tong University School
of Medicine) for their technical assistance.
Funding
The present study was supported in part by a grant
from the National Natural Science Foundation of China (grant no.
81771659).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
QZ and KW obtained funding and designed the present
study. ChL and FX carried out the experiments and drafted the
manuscript. YH, SZ, CfL, CqL and TD acquired, analyzed and
interpreted the data. TD supervised the enrollment of patients,
collected clinical data and revised the manuscript for important
intellectual content.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committees of the Shanghai First Maternity and Infant Hospital,
Tongji University School of Medicine (Shanghai, China), and all
participants provided written informed consent.
Patient consent for publication
The patients provided written informed consent prior
to participating in the present study.
Competing interests
All authors declare that they have no any conflict
of interests.
References
|
1
|
Sibai BM, Caritis SN, Thom E, Klebanoff M,
McNellis D, Rocco L, Paul RH, Romero R, Witter F, Rosen M, et al:
Prevention of preeclampsia with low-dose aspirin in healthy,
nulliparous pregnant women. The National Institute of Child Health
and Human Development Network of Maternal-Fetal Medicine Units. N
Engl J Med. 329:1213–1218. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Levine RJ, Hauth JC, Curet LB, Sibai BM,
Catalano PM, Morris CD, DerSimonian R, Esterlitz JR, Raymond EG,
Bild DE, et al: Trial of calcium to prevent preeclampsia. N Engl J
Med. 337:69–76. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chappell LC, Enye S, Seed P, Briley AL,
Poston L and Shennan AH: Adverse perinatal outcomes and risk
factors for preeclampsia in women with chronic hypertension: A
prospective study. Hypertension. 51:1002–1009. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
National high blood pressure education
program working group report on high blood pressure in pregnancy.
Am J Obstet Gynecol. 163:1691–1712. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Inagaki A, Nishizawa H, Ota S, Suzuki M,
Inuzuka H, Miyamura H, Sekiya T, Kurahashi H and Udagawa Y:
Upregulation of HtrA4 in the placentas of patients with severe
pre-eclampsia. Placenta. 33:919–926. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nie G, Hale K, Li Y, Manuelpillai U,
Wallace EM and Salamonsen LA: Distinct expression and localization
of serine protease HtrA1 in human endometrium and first-trimester
placenta. Dev Dyn. 235:3448–3455. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nie GY, Li Y and Salamonsen LA: Serine
protease HtrA1 is developmentally regulated in trophoblast and
uterine decidual cells during placental formation in the mouse. Dev
Dyn. 233:1102–1109. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ajayi F, Kongoasa N, Gaffey T, Asmann YW,
Watson WJ, Baldi A, Lala P, Shridhar V, Brost B and Chien J:
Elevated expression of serine protease HtrA1 in preeclampsia and
its role in trophoblast cell migration and invasion. Am J Obstet
Gynecol. 199(557): e1–e10. 2008.
|
|
9
|
American College of Obstetricians and
Gynecologists; Task Force on Hypertension in Pregnancy:
Hypertension in pregnancy. Report of the American College of
Obstetricians and Gynecologists' Task Force on Hypertension in
Pregnancy. Obstet Gynecol. 122:1122–1131. 2013.PubMed/NCBI
|
|
10
|
Graham CH, Hawley TS, Hawley RG,
MacDougall JR, Kerbel RS, Khoo N and Lala PK: Establishment and
characterization of first trimester human trophoblast cells with
extended lifespan. Exp Cell Res. 206:204–211. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lorenzi T, Marzioni D, Giannubilo S,
Quaranta A, Crescimanno C, De Luca A, Baldi A, Todros T, Tranquilli
AL and Castellucci M: Expression patterns of two serine protease
HtrA1 forms in human placentas complicated by preeclampsia with and
without intrauterine growth restriction. Placenta. 30:35–40. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chien J, Staub J, Hu SI, Erickson-Johnson
MR, Couch FJ, Smith DI, Crowl RM, Kaufmann SH and Shridhar V: A
candidate tumor suppressor HtrA1 is downregulated in ovarian
cancer. Oncogene. 23:1636–1644. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Baldi A, De Luca A, Morini M, Battista T,
Felsani A, Baldi F, Catricalà C, Amantea A, Noonan DM, Albini A, et
al: The HtrA1 serine protease is down-regulated during human
melanoma progression and represses growth of metastatic melanoma
cells. Oncogene. 21:6684–6688. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Oh Y, Nagalla SR, Yamanaka Y, Kim HS,
Wilson E and Rosenfeld RG: Synthesis and characterization of
insulin-like growth factor-binding protein (IGFBP)-7. Recombinant
human mac25 protein specifically binds IGF-I and -II. J Biol Chem.
271:30322–30325. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Campioni M, Severino A, Manente L, Tuduce
IL, Toldo S, Caraglia M, Crispi S, Ehrmann M, He X, Maguire J, et
al: The serine protease HtrA1 specifically interacts and degrades
the tuberous sclerosis complex 2 protein. Mol Cancer Res.
8:1248–1260. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hou J, Clemmons DR and Smeekens S:
Expression and characterization of a serine protease that
preferentially cleaves insulin-like growth factor binding
protein-5. J Cell Biochem. 94:470–484. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Redman CW and Sargent IL: Latest advances
in understanding preeclampsia. Science. 308:1592–1594. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yung HW, Calabrese S, Hynx D, Hemmings BA,
Cetin I, Charnock-Jones DS and Burton GJ: Evidence of placental
translation inhibition and endoplasmic reticulum stress in the
etiology of human intrauterine growth restriction. Am J Pathol.
173:451–462. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Roberts JM and Hubel CA: Is oxidative
stress the link in the two-stage model of pre-eclampsia? Lancet.
354:788–789. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Burton GJ, Yung HW, Cindrova-Davies T and
Charnock-Jones DS: Placental endoplasmic reticulum stress and
oxidative stress in the pathophysiology of unexplained intrauterine
growth restriction and early onset preeclampsia. Placenta. 30 Suppl
A:S43–S48. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ly DH, Lockhart DJ, Lerner RA and Schultz
PG: Mitotic misregulation and human aging. Science. 287:2486–2492.
2000. View Article : Google Scholar : PubMed/NCBI
|