Introduction
Chronic renal failure is a syndrome associated with
serious metabolic disorders and refers to damage caused by various
types of chronic kidney disease (1). Inflammation is one of the most common
characteristics of patients with type II diabetes (2), and is associated with dysfunctional
urinary albumin excretion, endothelial function and cellular
metabolism (3). In addition,
hypertension is often present in patients with type II diabetes,
and the onset of hypertension is a frequent focus of clinical
investigations (4–6). A previous study reported that
inhibition of the renin-angiotensin system was able to markedly
decrease blood pressure by reducing vascular inflammation (7). However, long-term treatment with
antihypertensive drugs may lead to a decline of renal function and
may even cause chronic renal failure in patients with type II
diabetes (8).
The main pathogeneses associated with chronic renal
failure include glomerulonephritis, interstitial nephritis, high
blood pressure, diabetes and nephritis (9). At present, the prevalence of
diabetes-associated chronic renal failure is increasing, which may
be due to the marked rapid increase in patients with diabetes
worldwide (10,11). A previous study noted that patients
with diabetes and end-stage renal failure exhibited prolonged
tissue healing of critical limb ischemia (12). In addition, chronic renal failure
demands specific, continuous and varied care, which may present
burden for family caregivers (13). Therefore, it is vital to
investigate the association between chronic renal failure and type
II diabetes in order to explore novel strategies for the diagnosis
and treatment of patients with chronic renal failure.
Hepatocyte growth factor (HGF) is produced by
mesenchymal cells during organ injury (14). HGF exerts extensive biological
activities and serves as a multifunctional antifibrotic factor that
has a critical role in kidney development, acute injury and
regeneration. HGF is activated by proteolytic cleavage at the site
of injury, thus resulting in generation of the biological HGF
protein (15). A previous study
reported that serum levels of HGF were correlated with quality of
life in patients undergoing hemodialysis (16). Furthermore, biologically active HGF
is able to suppress fibrosis, and a molecular basis for
HGF-mediated regression of renal fibrosis has previously been
elaborated on (17,18). Therefore, HGF may be regarded as a
local acute phase protein associated with chronic renal
failure.
The present study aimed to investigate whether the
expression and function of HGF were decreased and associated with
inflammation in mice with type II diabetes-induced chronic renal
failure. The results demonstrated that treatment with HGF markedly
relieved chronic renal failure by decreasing the inflammatory
response. Notably, the results revealed that HGF may improve
chronic renal failure via the nuclear factor (NF)-κB signaling
pathway. These preclinical data may provide important information
for doctors and clinicians in the treatment of patients with type
II diabetes-induced chronic renal failure.
Materials and methods
Ethics statement
The present study was carried out in strict
accordance with the recommendations in the Guide for the Care and
Use of Laboratory Animals of Southern Medical University
(Guangzhou, China). The study was approved by the ethics committee
of Zhujiang Hospital, Southern Medical University. All surgical
procedures and methods of euthanasia were conducted to minimize
suffering.
Type II diabetes-induced chronic renal
failure mouse model
HLA-A2 mice with type II diabetes-induced renal
failure (age, 16–18 weeks; n=120) were purchased from The Jackson
Laboratory (Bar Harbor, ME, USA). All mice were feed under
pathogen-free conditions and were given free access to food and
water, and housed in a temperature-controlled facility at 23±1°C
and relative humidity of 50±5% with a 12-h light/dark cycle. The
mice were divided into three groups (n=30/group), which received
treatment with HGF (10 mg/kg), aldosterone (positive control; 10
mg/kg, Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) or PBS (10
mg/kg) once a day for 30 days with healthy mice (n=30) as a
control. Following 30-day treatment, the entire right kidney was
removed, following a right flank incision under IV sodium
pentobarbital (35 mg/kg) anesthesia, for subsequent analysis. The
mice were sacrificed by cervical dislocation.
Renal cell analysis
Renal cells were isolated from experimental mice as
described previously (19) with
type II diabetes-induced chronic renal failure following treatment
with HGF (10 mg/kg), aldosterone (10 mg/kg) or PBS. Serum was
collected from 10 ml blood using centrifugation at 6,000 × g for 10
min at 4°C. Serum and urine samples were collected to analyze
inflammatory factors and biochemical indexes. Renal cells also
underwent immunohistochemical staining and western blotting.
Analysis of blood lipid levels
Serum samples were obtained from blood using
centrifugation at 4,000 × g for 10 min at 4°C. Concentration of TC,
TG, HDL-C, LDL-C and VLDL-C were measured using the Roche Cobas
6000 analytical system according to the manufacturer's protocols
(20).
ELISA
A total of 1 ml blood was drawn from each mouse and
centrifuged at 4,000 × g for 15 min at 4°C. To determine protein
concentration, interleukin (IL)-1 (MLB00C; Bio-Rad Laboratories,
Inc., Hercules, CA, USA), IL-8 (YJ1012360; Shanghai YiJi Industrial
Co., Ltd.), IL-10 (DY417), HGF (MHG00; both Bio-Rad Laboratories,
Inc.), monocyte chemoattractant protein (MCP)-1 (DCP00; Bio-Rad)
and IL-6 (M6000B; Bio-Rad Laboratories, Inc.), blood urea nitrogen
(0–012300; Shanghai Jianglai Biotechnology Co., Ltd.), plasma
creatinine concentrations (KGE005; Bio-Rad Laboratories, Inc.),
total serum protein (KA3946; Shanghai YaoYun Biotechnology Co.
Ltd.), parathyroid hormone (6031) and C-reactive protein (MCRP00;
both Bio-Rad Laboratories, Inc.) ELISA kits were used to determine
serum concentration levels. The kits were used according to the
manufacturer's protocols. The final results were recorded at 450 nm
using an ELISA plate reader.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from renal cells prior to
(healthy mice) or following treatment with HGF (10 mg/kg),
aldosterone (10 mg/kg) or the same dose of PBS using RNAeasy Mini
kit (Qiagen, Inc., Valencia, CA, USA). Subsequently, 1 µg total RNA
was transcribed into cDNA using a RT kit (Qiagen, Inc.) according
to manufacturer's protocol and quality was confirmed by
electrophoresis. cDNA (10 ng) was then subjected to qPCR (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) using the SYBR-Green Master
Mix system. All forward and reverse primers were synthesized by
Invitrogen (Thermo Fisher Scientific, Inc., Waltham, MA, USA) and
are detailed in Table I.
Thermocycling conditions were as follows: Pre-denaturation at 95°C
for 120 sec, denaturation at 95°C for 30 sec and annealing at 57°C
for 10 sec for 45 cycles. Relative mRNA expression levels were
determined according to the 2−ΔΔCq method (21). The results are expressed as n-fold
compared with the control (β-actin).
 | Table I.Sequences of primers used in the
present study. |
Table I.
Sequences of primers used in the
present study.
|
| Sequence |
|---|
|
|
|
|---|
| Gene | Reverse | Forward |
|---|
| p53 |
5′-TTAAGCTTTTTGCGTTCGGGCTGGGAGC-3′-3′ |
5′-ATGGTGGCATGAACCTGTGG-3′ |
| Bid |
5′-CGACGAGGTGAAGACATCCT-3′ |
3′-AGCAGAGATGGTGCATGAC-3′ |
| Ccl2 |
5′-CCCCAGTCACCTGCTGTTAT-3′ |
5′-TGGAATCCTGAACCCACTTC-3′ |
| Ccl5 |
5′-TGTACAATACGCGCTACAGCTCCA-3′ |
5′-ATGCACTCGTTCTGGTCTGCGTTA-3′ |
| Icam-1 |
5′-GCCCTTGCCTCTGAGTAGTG-3′ |
5′-CCAACCAAATGAAGCCAAG-3′ |
| TNFα |
5′-ATGTTGTAGCAAACCCTCAA-3′ |
5′-AGGACCTGGGAGTAGATGA-3′ |
| β-actin |
5′-CCTTCCTGGGCATGGAGTCCT-3′ |
5′-GGAGCAATGATCTTGATCTTC-3′ |
Western blot analysis
Renal cells from experimental mice were homogenized
in lysis buffer containing protease-inhibitor (Sigma-Aldrich; Merck
KGaA) and were centrifuged at 6,000 × g at 4°C for 10 min. The
supernatant was then used to analyze protein expression. To detect
proteins of interest, Protein concentration was measured by a BCA
protein assay kit (Thermo Fisher Scientific, Inc.). Protein samples
(20 µg/lane) were resolved by 15% SDS-PAGE and then transferred
onto polyvinylidene fluoride membranes (Merck KGaA) according to
the manufacturer's protocol. For western blotting, primary
antibodies: HGF (ab83760; 1:2,000), IKK-α (ab32041; 1:2,000), IKK-β
(ab7547; 1:2,000), NF-κB inhibitor α (IκBα; ab133478; 1:2,000) and
β-actin (ab8827; 1:2,000; all from Abcam) were added to the
polyvinylidene fluoride membranes following blocking with 5%
skimmed milk for 1 h at 37°C. Subsequently, membranes were
incubated with horseradish peroxidase-labeled secondary goat
anti-rabbit antibodies (ab150077; 1:2,000; Abcam) for 24 h at 4°C.
Blots were visualized using a chemiluminescence detection system
(Pierce™ Fast Western Blot kits, SuperSignal™ West Femto; Thermo
Fisher Scientific, Inc.).
NF-κB activity
Renal cells (1×106 cells/well) were
seeded in 6-well plates for 12 h at 37°C. NF-κB-luc plasmids
(Qiagen, Inc.) containing the response element were transfected
into the renal cells, following replacement with MEM medium
(Invitrogen; Thermo Fisher Scientific, Inc.) 12 h after
transfection, the cells were then treated with various
concentrations of apigenin. Cell lysate (NF-κB) was collected on a
6-well white plate after 48 h, and luciferase activity was detected
using a luciferase reporter assay as described previously (22).
Immunohistochemical staining
Immunohistochemical staining was performed according
to the avidin-biotin-peroxidase technique. Tissues were fixed with
10% formaldehyde for 2 h at 37°C, then embedded in paraffin and
sectioned at 4 µm. Epitope retrieval using Epitope Retrieval kit
(cat. no. GTX30934; GeneTex, Inc., Irvine, CA, USA) was performed
for further analysis. The paraffin-embedded sections were incubated
with hydrogen peroxide (3%) for 10–15 min at 37°C, and were then
blocked with regular blocking solution (5% skimmed milk) for 10–15
min at 37°C. Finally, the sections were incubated with anti-HGF
(ab83760; 1:1,000), anti-CD3 (ab16669; 1:1,000), anti-kidney injury
molecule (KM)-1 (ab47634; 1:1,000), anti-IL-18 (ab52914; 1:1,000),
anti-cluster of differentiation (CD)86 (ab119857; 1:1,000; all from
Abcam) at 4°C for 12 h. All sections were washed three times with
PBS at room temperature and were incubated with secondary
antibodies horseradish peroxidase-conjugated anti-rabbit IgG
secondary antibody (cat. no. 1721019; 1:5,000; Bio-Rad
Laboratories, Inc.) for 1 h at 37°C. For hematoxylin and eosin
(H&E) assay, tissue sections (4 µm) were stained with H&E
for 2 h at 37°C. Then 6 random fields of view were observed under a
fluorescent microscope (Olympus Corporation, Tokyo, Japan) at
magnification, ×40.
TUNEL assay. For analysis the apoptosis of renal
cells in experimental mice after treatment with HGF, aldosterone or
PBS, the terminal deoxynucleotidyl transferase (TdT)-mediated dUTP
nick end labeling (TUNEL) assay (Biotool) were used to detect
TUNEL-positive cells. The procedures were performed by previous
study (23). Finally, hippocampal
neuron cells images were captured with a ZEISS LSM 510 confocal
microscope at 488 nm.
Statistical analysis
All data are presented as the mean ± standard error
of the mean of triplicate experiments. Comparisons between multiple
groups were made by one-way analysis of variance followed by
Dunnett's t test. P<0.05 was considered to indicate a
statistically significant difference.
Results
HGF expression, blood glucose, blood
lipid levels and body weight in mice with chronic renal
failure
In order to investigate the role of HGF in chronic
renal failure, the present study analyzed the expression levels of
HGF in renal cells from mice with chronic renal failure, and
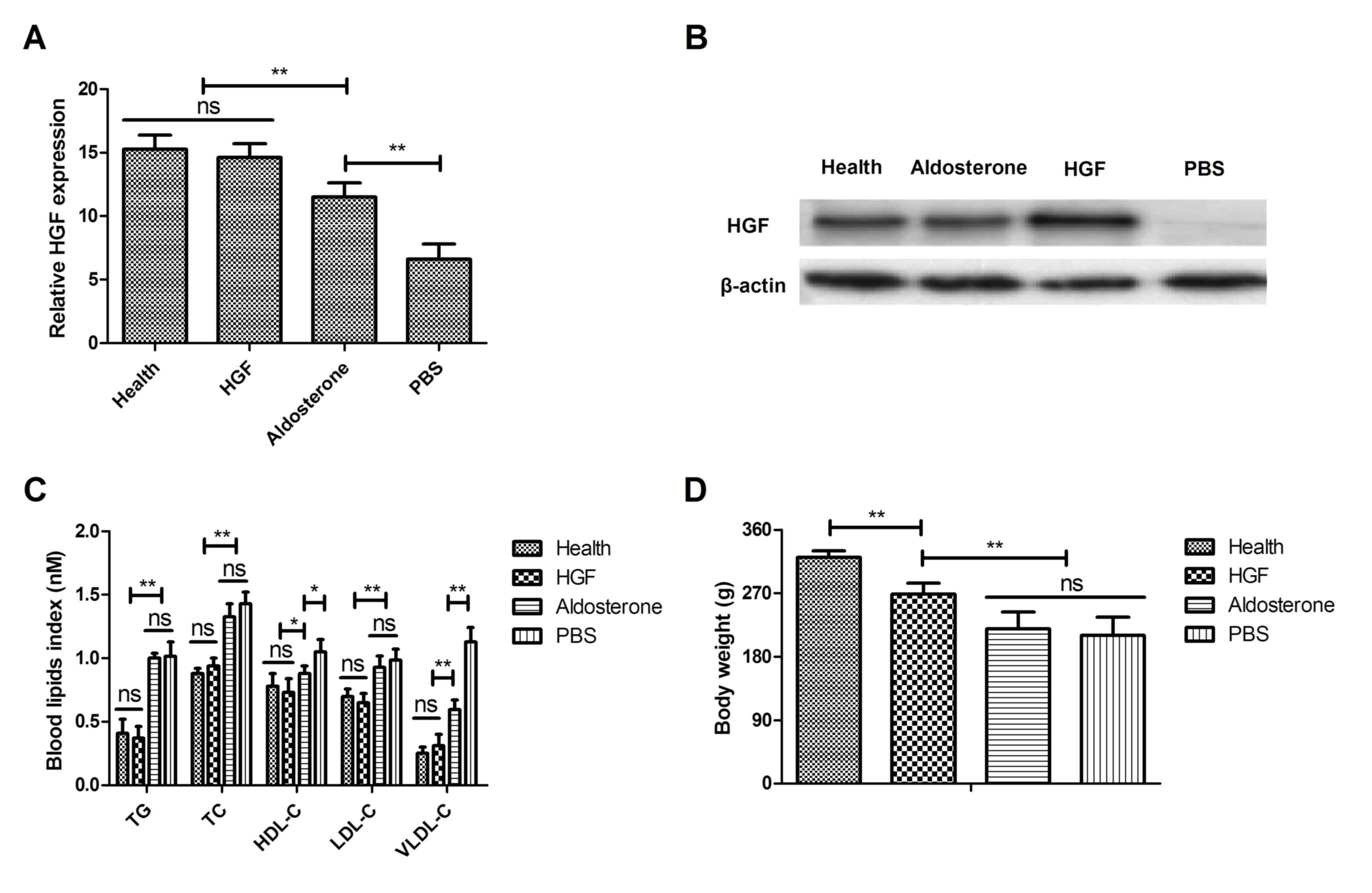
compared them with those in healthy mice. As shown in Fig. 1A, the expression levels of HGF were
downregulated compared with the healthy mice. However, treatment
with HGF upregulated HGF expression, as determined by western
blotting. Furthermore, protein expression of HGF was decreased in
chronic renal failure mice compared with healthy mice (Fig. 1B). Blood lipid levels were improved
in mice with type II diabetes-induced chronic renal failure treated
with HGF compared with in those treated with PBS (Fig. 1C). The present study also aimed to
determine the therapeutic effects of HGF on body weight in a mouse
model of type II diabetes-induced chronic renal failure. The
results indicated that HGF markedly inhibited body weight compared
with in the aldosterone-treated and control mice on day 30
(Fig. 1D). Collectively, these
data suggested that HGF was downregulated in a mouse model of type
II diabetes-induced chronic renal failure, whereas restoration of
HGF exerted beneficial effects on blood lipid levels and body
weight in mice with type II diabetes-induced chronic renal
failure.
Effects of HGF on inflammatory factor
expression in renal cells from experimental mice
The present study further analyzed the effects of
HGF on inflammatory factor expression in renal cells from
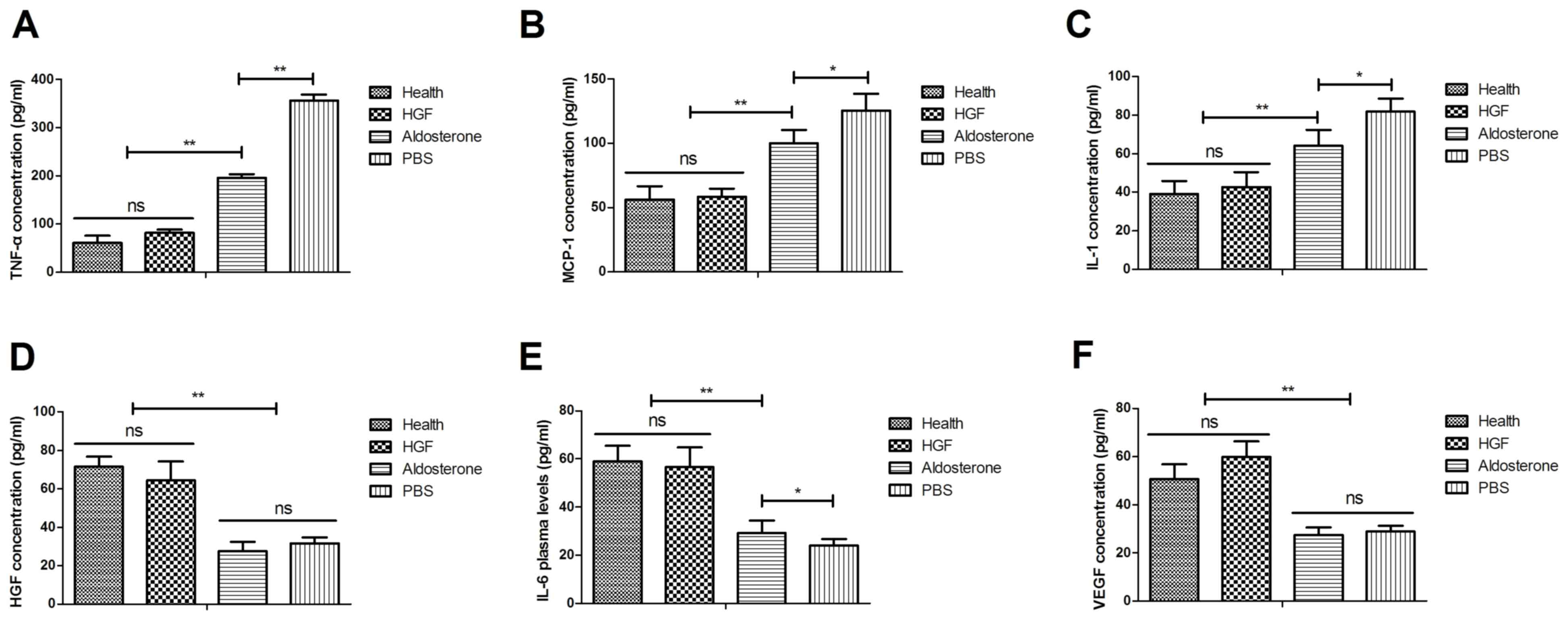
experimental mice. As presented in Fig. 2A, the results indicated that TNF-α
plasma concentration levels were decreased following treatment with
HGF compared with aldosterone. In addition, MCP-1 levels were
downregulated in HGF-treated mice (Fig. 2B). IL-1 concentration levels were
also decreased in mice with chronic renal failure following HGF
treatment (Fig. 2C). Notably, HGF
expression was recovered to normal levels following HGF treatment
(Fig. 2D). Furthermore, the
concentration levels of IL-6 and VEGF were increased in renal cells
from experimental mice following HGF treatment, which may
contribute to improved functioning of renal cells (Fig. 2E and F). These results suggested
that treatment with HGF was beneficial for the treatment of mice
with chronic renal failure.
Effects of HGF on the NF-κB signaling
pathway
A previous study demonstrated that NF-κB serves an
essential regulatory role in inflammation, via its control of the
expression of numerous genes involved in cellular activity
(24). Therefore, the present
study analyzed the association between HGF and the NF-κB signaling
pathway in mice with type II diabetes-induced chronic renal
failure. Treatment with HGF blocked the nuclear import of activated
NF-κB (p65) in renal cells from experimental mice (Fig. 3A). In addition, HGF treatment
markedly suppressed activation of NF-κB in renal cells from mice on
day 60 (Fig. 3B). The expression
levels of IκB kinase (IKK)α, IKKβ and IκB were downregulated in
renal cells from mice treated with HGF (Fig. 3C). Furthermore, the present study
indicated that HGF treatment decreased the expression levels of
proinflammatory genes involved in the NF-κB signaling pathway,
including C-C motif chemokine ligand (Ccl)2, Ccl5, intercellular
adhesion molecule 1 (Icam1) and TNF-α, as determined by RT-qPCR
(Fig. 3D). The present data also
demonstrated that p53 and BH3 interacting-domain death agonist
expression levels were decreased in renal cells following HGF
treatment (Fig. 3E). Notably, the
number of TUNEL-positive renal cells was markedly decreased
following treatment with HGF (Fig.
3F). These results indicated that HGF may regulate the
physiological functions of renal cells by controlling the NF-κB
signaling pathway.
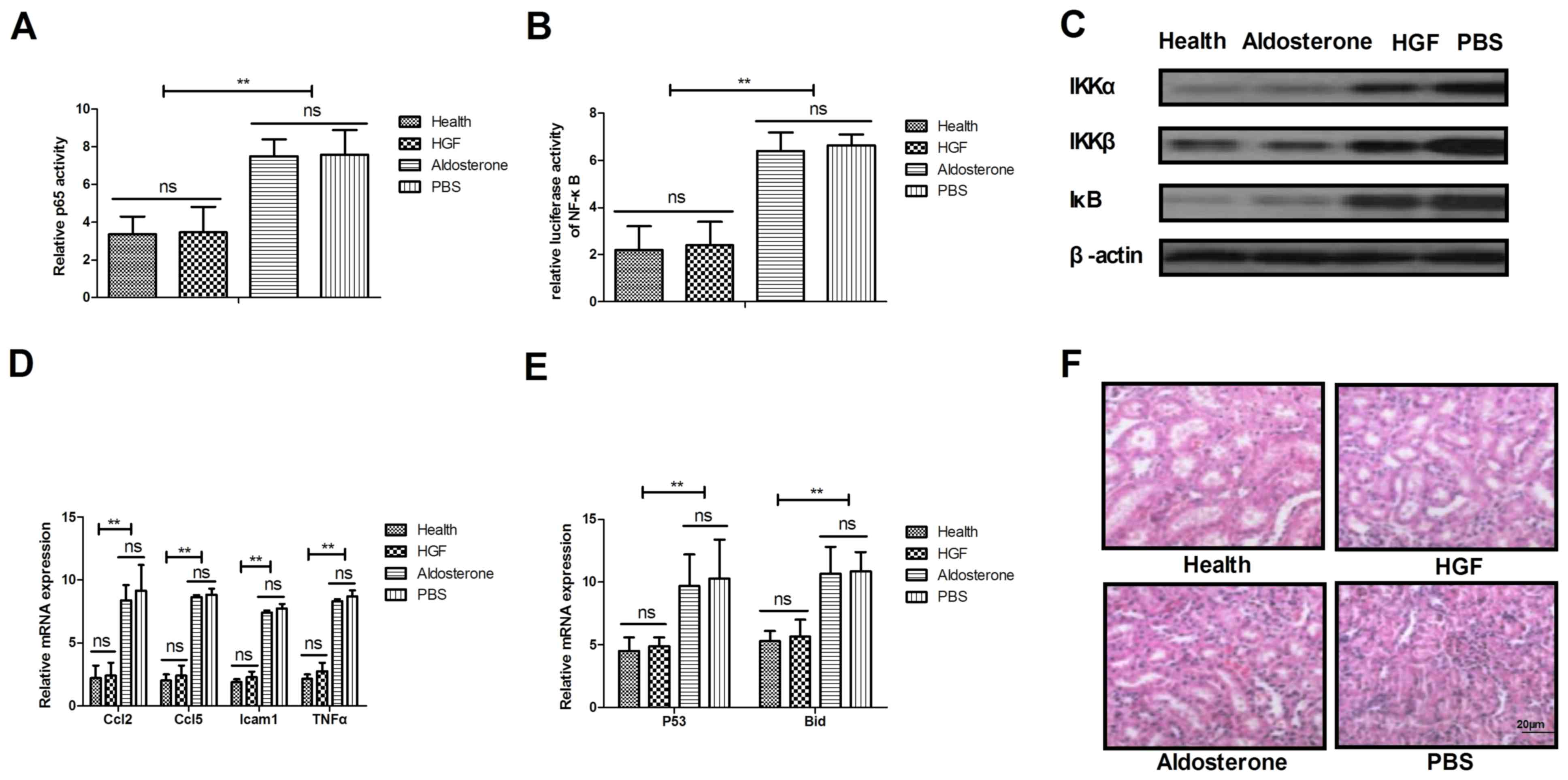 | Figure 3.Mechanism underlying the HGF-mediated
benefits against chronic renal failure. (A) Expression of p65 in
renal cells from experimental mice following treatment with HGF,
aldosterone and PBS. (B) Activation of NF-κB in renal cells from
experimental mice. (C) IKKα, IKKβ and IκB expression levels in
renal cells from mice treated with HGF, aldosterone and PBS. (D)
Ccl2, Ccl5, Icam1 and TNF-α expression levels in renal cells
following 30-day treatment. (E) Analysis of p53 and Bid expression
levels in renal cells following treatment of mice with HGF,
aldosterone and PBS. (F) Analysis of TUNEL-positive renal cells
following treatment of mice with HGF, aldosterone and PBS. Data are
presented as the mean ± standard error of the mean. **P<0.01.
Bid, BH3 interacting-domain death agonist; Ccl, C-C motif chemokine
ligand; HGF, hepatocyte growth factor; Icam1, intercellular
adhesion molecule 1; IKK, IκB kinase; NF-κB, nuclear factor-κB;
TNF-α, tumor necrosis factor-α. |
Effects of HGF on histopathological
alterations of renal cells from mice with type II diabetes-induced
chronic renal failure
To further determine the beneficial effects of HGF
on the morphology and function of renal cells, histopathological
alterations were analyzed by H&E and immunohistochemical
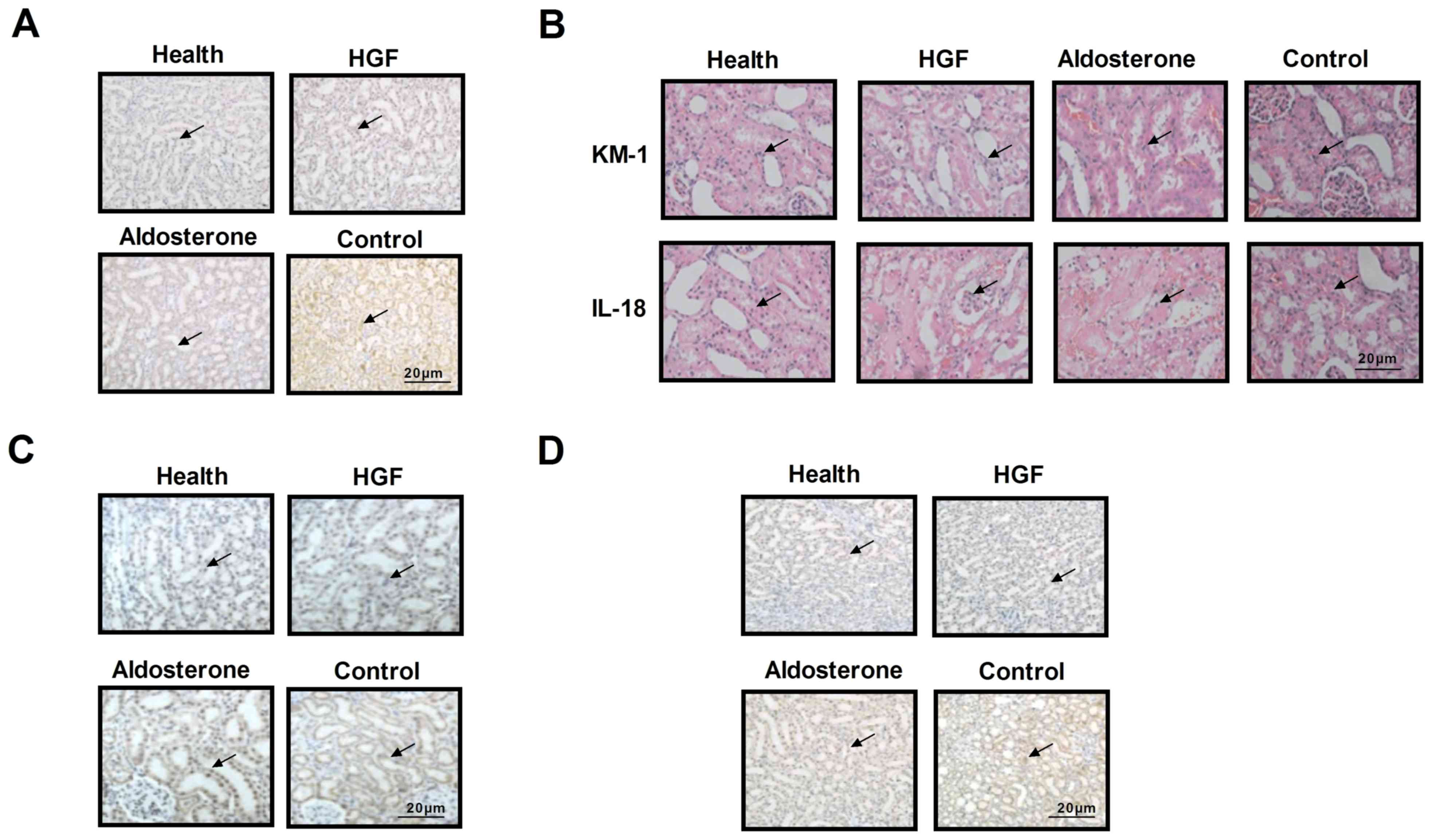
staining of renal cells. As shown in Fig. 4A, cellular morphology was improved
following treatment with HGF, presenting a pebble-like shape that
is typical renal cell morphology. In addition, KM-1 and IL-18
levels were downregulated following HGF treatment (Fig. 4B), and the number of immune cells
was decreased in renal sections obtained from HGF-treated mice
(Fig. 4C). Furthermore, reduced
CD86 expression was detected in HGF-treated mice, whereas CD86
expression was much higher in the control group (Fig. 4D). These data indicated that the
physiology of renal cells was improved in HGF-treated experimental
mice.
Biochemical analysis of the
therapeutic effects of HGF in mice with type II diabetes-induced
chronic renal failure
Following detection of the histopathological
alterations of renal cells from mice with type II diabetes-induced
chronic renal failure, the plasma biochemical indexes of mice were
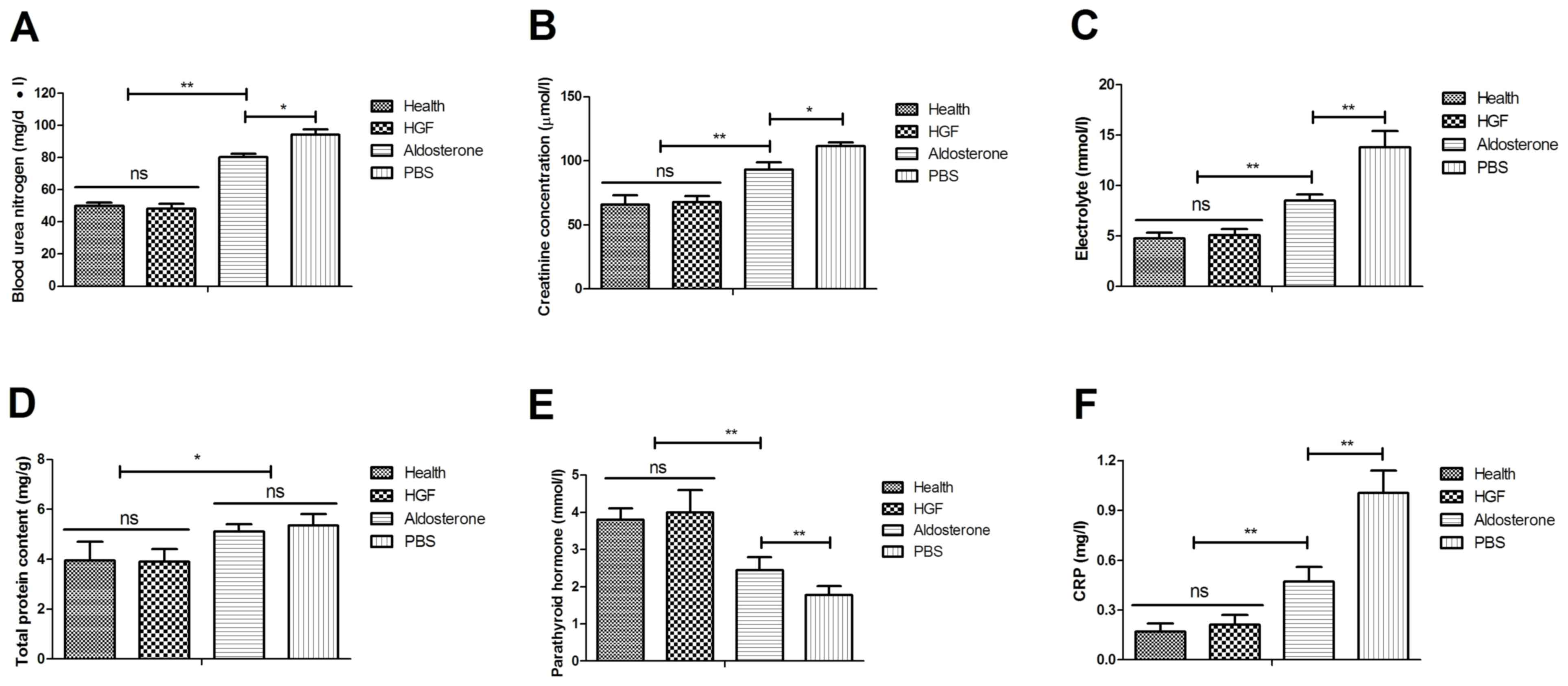
determined following treatment with HGF. As shown in Fig. 5A, blood urea nitrogen levels were
decreased following HGF treatment. Creatinine plasma concentration
levels also were downregulated in HGF-treated mice (Fig. 5B). In addition, electrolyte
concentration was increased in renal cells obtained from the HGF
group (Fig. 5C). Serum total
protein content was also recovered to normal levels in HGF-treated
mice (Fig. 5D). Furthermore,
parathyroid hormone levels were upregulated and C-reactive protein
(CRP) expression levels were downregulated in HGF-treated mice
(Fig. 5E and F). Taken together,
these data suggested that HGF not only improves renal cell
histopathology, but also regulates the biochemical indexes of mice
with type II diabetes-induced chronic renal failure.
Discussion
Previous studies have demonstrated that chronic
renal failure may induce the formation of complex symptoms and
reduce quality of life in patients (25,26).
In addition, patients with chronic renal failure frequently develop
uremia, resulting in glomerular cell dysfunction and loss of the
biological activity of kidney cells (27). Dialysis is the most effective
treatment for multiple organ failure and deterioration of the
quality of the life. In addition, the pathogenesis of chronic renal
failure is genetic and multifactorial (28,29).
A previous study indicated that age has an important role in
survival, limitation of physical activity and quality of life in
patients with chronic renal failure (30). The present study investigated
whether the expression and restoration of HGF affects inflammation,
histopathological alterations and biochemical indexes in a mouse
model of type II diabetes-induced chronic renal failure.
Inflammation is one of the most common
characteristics of patients with type II diabetes (31). Temelkova-Kurktschiev et al
(32) reported that subclinical
inflammation was upregulated in patients with type II diabetes,
thus indicating that inflammatory responses may be associated with
the occurrence, degree and prognosis of diabetes. Varughese and Lip
reported that inflammation was associated with hypertension and
urinary albumin excretion in patients with type II diabetes
(3). In addition, a previous study
demonstrated that vascular inflammation serves an important role in
drug-induced rapid and persistent reduction of urinary albumin
excretion, endothelial function and inflammation in patients with
hypertension and type II diabetes (2). Furthermore, Bitar et al
reported that inflammation was associated with the degree of
pathogenesis in aortic tissues from aged patients with type II
diabetes via the phosphatidylinositol 3-kinase/protein kinase
B-dependent signaling pathway (33). In the present study, the expression
levels of inflammatory factors, including IL-6 IL-1, TNF-α, MCP-1
and VEGF, were detected in mice with type II diabetes-induced
chronic renal failure. The results indicated that these
inflammatory factors were downregulated in response to HGF, and the
NF-κB signaling pathway was involved in HGF-mediated improvement of
type II diabetes-induced chronic renal failure.
The present study indicated that treatment with HGF
may regulate biochemical metabolism of renal cells via inactivation
of the NF-κB signaling pathway. A previous study reported that
NF-κB-induced oxidative stress contributes to mitochondrial and
cardiac dysfunction in type II diabetes (34). In addition, receptor activator of
NF-κB ligand exerted beneficial effects for patients with chronic
renal failure via inhibition of the NF-κB signaling pathway
(35). Biochemical indexes,
including blood urea nitrogen, plasma creatinine concentration,
electrolyte, serum protein, parathyroid hormone and CRP levels are
crucial for normal kidney function. In the present study, these
biochemical indexes were analyzed in mice with type II
diabetes-induced chronic renal failure following treatment with HGF
(36). The results revealed that
HGF may be considered an efficient drug for the treatment of type
II diabetes-induced chronic renal failure.
Although a previous study reported that inflammation
is associated with hypertension in patients with type II diabetes,
correlations between inflammatory factors and type II diabetes have
not been clinically analyzed (37). To determine the association between
HGF and serum inflammatory factors, the present preclinical study
analyzed mice with type II diabetes-induced chronic renal failure.
The results indicated that HGF treatment decreased the accumulation
of immune cells in renal tissues in mice with type II
diabetes-induced chronic renal failure.
In conclusion, based on these preclinical data,
serum concentration levels of IL-1, TNF-α and MCP-1 may be
increased in mice with type II diabetes-induced chronic renal
failure. However, HGF treatment was revealed to decrease the
expression of inflammatory factors in mice with type II
diabetes-induced chronic renal failure. In addition, HGF was
downregulated in mice with chronic renal failure, whereas
restoration of HGF exerted beneficial effects on blood lipid levels
and body weight in mice with type II diabetes-induced chronic renal
failure. Furthermore, HGF regulated the physiological function and
biochemical indexes of renal cells via regulation of the NF-κB
signaling pathway. Overall, the present study researched the
function of HGF in a mouse model of type II diabetes-induced
chronic renal failure; however, the role of HGF in patients with
renal failure induced by type II diabetes requires further
study.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
GC and XT performed the experiments and JZ designed
the experiments and analyzed the data. All the authors have read
the manuscript and have approved this submission.
Ethics approval and consent to
participate
The present study was carried out in strict
accordance with the recommendations in the Guide for the Care and
Use of Laboratory Animals of Southern Medical University
(Guangzhou, China). The study was approved by the ethics committee
of Zhujiang Hospital, Southern Medical University. All surgical
procedures and methods of euthanasia were conducted to minimize
suffering.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Finlay E: Review: Most interventions for
preventing bone disease in chronic renal failure improved
biochemical outcomes. Arch Dis Child Educ Pract Ed. 97:402012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Takase H, Nakazawa A, Yamashita S,
Toriyama T, Sato K, Ueda R and Dohi Y: Pioglitazone produces rapid
and persistent reduction of vascular inflammation in patients with
hypertension and type 2 diabetes mellitus who are receiving
angiotensin II receptor blockers. Metabolism. 56:559–564. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Varughese GI and Lip GY: Hypertension in
patients with type-II diabetes: Relation to urinary albumin
excretion, endothelial function and inflammation. J Hum Hypertens.
19:421–424. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yamamoto S, Okada Y, Mori H, Nishida K,
Uriu K and Tanaka Y: Type 2 diabetes mellitus complicated by
hypertension in Japanese patients: Switching treatment from
high-dose angiotensin II receptor blockers to losartan plus
hydrochlorothiazide. Intern Med. 53:1283–1289. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kalaitzidis R and Bakris G: Management of
hypertension in patients with diabetes: The place of angiotensin-II
receptor blockers. Diabetes Obes Metab. 11:757–769. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hasvold LP, Bodegård J, Thuresson M,
Stålhammar J, Hammar N, Sundström J, Russell D and Kjeldsen SE:
Diabetes and CVD risk during angiotensin-converting enzyme
inhibitor or angiotensin II receptor blocker treatment in
hypertension: A study of 15,990 patients. J Hum Hypertens.
28:663–669. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Daimon M, Kamba A, Murakami H, Takahashi
K, Otaka H, Makita K, Yanagimachi M, Terui K, Kageyama K, Nigawara
T, et al: Association between pituitary-adrenal axis dominance over
the renin-angiotensin-aldosterone system and hypertension. J Clin
Endocrinol Metab. 101:889–897. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ficek J, Malyszko J and Chudek J: Renalase
and its role in the development of hypertension in patients with
chronic renal failure. Przegl Lek. 72:306–308. 2015.(In Chinese).
PubMed/NCBI
|
|
9
|
Kasacka I: Review article-involvement of
gastric APUD cells in chronic renal failure. Acta Histochem.
105:319–327. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bos-Touwen I, Schuurmans M, Monninkhof EM,
Korpershoek Y, Spruit-Bentvelzen L, Ertugrul-van der Graaf I, de
Wit N and Trappenburg J: Patient and disease characteristics
associated with activation for self-management in patients with
diabetes, chronic obstructive pulmonary disease, chronic heart
failure and chronic renal disease: A cross-sectional survey study.
PLoS One. 10:e01264002015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nair PA, Jivani NB and Diwan NG: Kyrle's
disease in a patient of diabetes mellitus and chronic renal failure
on dialysis. J Family Med Prim Care. 4:284–286. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Guntani A, Yamaoka T, Okadome J, Kawakubo
E, Kyuragi R, Homma K, Iwasa K, Matsumoto T, Okazaki J and Maehara
Y: Evaluation of the paramalleolar bypass for critical limb
ischemia patients on hemodialysis with diabetes mellitus and
chronic renal failure. Ann Vasc Dis. 6:596–600. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Guerra-Martin MD, Amador-Marin B and
Martinez-Montil JM: Health problems of family caregivers of people
over 65 suffering from chronic renal failure: A systematic review.
An Sist Sanit Navar. 38:425–438. 2015.(In Chinese). PubMed/NCBI
|
|
14
|
Ramezani A, Nägga K, Hansson O, Lönn J,
Sjöwall J, Katoozian F, Mansouri S and Nayeri F: Hepatocyte growth
factor in cerebrospinal fluid differentiates community-acquired or
nosocomial septic meningitis from other causes of pleocytosis.
Fluids Barriers CNS. 12:222015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Faletto DL, Kaplan DR, Halverson DO, Rosen
EM and Vande Woude GF: Signal transduction in c-met mediated
motogenesis. EXS. 65:107–130. 1993.PubMed/NCBI
|
|
16
|
Baum E, Pawlaczyk K, Maćkowiak B, Sosinska
P, Matecka M, Kolodziejczak B, Musielak M and Breborowicz A: Levels
of hepatocyte growth factor in serum correlate with quality of life
in hemodialysis patients. Int J Clin Exp Pathol. 8:13477–13482.
2015.PubMed/NCBI
|
|
17
|
Mizuno S and Nakamura T: Molecular basis
for HGF-mediated regression of renal fibrosis. Nihon Rinsho. 64
Suppl 2:S312–S321. 2006.(In Chinese).
|
|
18
|
Zhang SH, Wen KM, Wu W, Li WY and Zhao JN:
Efficacy of HGF carried by ultrasound microbubble-cationic
nano-liposomes complex for treating hepatic fibrosis in a bile duct
ligation rat model, and its relationship with the
diffusion-weighted MRI parameters. Clin Res Hepatol Gastroenterol.
37:602–607. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hasmim M, Bruno S, Azzi S, Gallerne C,
Michel JG, Chiabotto G, Lecoz V, Romei C, Spaggiari GM, Pezzolo A,
et al: Isolation and characterization of renal cancer stem cells
from patient-derived xenografts. Oncotarget. 7:15507–15524. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Friedewald WT, Levy RI and Fredrickson DS:
Estimation of the concentration of low-density lipoprotein
cholesterol in plasma, without use of the preparative
ultracentrifuge. Clin Chem. 18:499–502. 1972.PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li M, Shi X, Chen F and Hao F: Daphnetin
inhibits inflammation in the NZB/W F1 systemic lupus erythematosus
murine model via inhibition of NF-κB activity. Exp Ther Med.
13:455–460. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Naganuma Y, Ichii O, Otsuka S, Hashimoto Y
and Kon Y: Analysis of TdT-mediated dUTP nick end labeling
(TUNEL)-positive cells associated with cardiac myogenesis in mouse
embryo. J Vet Med Sci. 75:283–290. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mallavia B, Recio C, Oguiza A, Ortiz-Muñoz
G, Lazaro I, Lopez-Parra V, Lopez-Franco O, Schindler S, Depping R,
Egido J and Gomez-Guerrero C: Peptide inhibitor of NF-κB
translocation ameliorates experimental atherosclerosis. Am J
Pathol. 182:1910–1921. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Akyüz A, Yıldız A, Akıl MA, Bilik MZ, İnci
Ü, Kayan F, Yıldız İ, Yılmaz Z, Yıldırım Y and Ülgen MS: Assessment
of right ventricular systolic function in patients with chronic
renal failure before and after hemodialysis by means of various
echocardiographic modalities. Turk Kardiyol Dern Ars. 42:717–725.
2014.(In Chinese). View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ma HB, Wang R, Yu KZ and Yu C: Dynamic
changes of early-stage aortic lipid deposition in chronic renal
failure rats and effects of decorin gene therapy. Exp Ther Med.
9:591–597. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vahedi M, Malekzadeh H, Haybar H,
Soltanian AR, Abdollahzadeh S, Yoosefi H, Seyedian M, Yazdanpanah
L, Saeid A, Fooladi Shabanpour M and Ghasemi M: The relationship
between salivary beta-2 microglobulin and uremia intensity in men
with chronic renal failure. Cell J. 14:276–281. 2013.PubMed/NCBI
|
|
28
|
Silverberg D, Yalon T, Rimon U, Reinitz
ER, Yakubovitch D, Schneiderman J and Halak M: Endovascular
treatment of lower extremity ischemia in chronic renal failure
patients on dialysis: Early and intermediate term results. Isr Med
Assoc J. 15:734–738. 2013.PubMed/NCBI
|
|
29
|
Berry PA and Thomson SJ: Comment on
O'Brien et al: Prevalence and outcome of cirrhosis patients
admitted to UK intensive care: A comparison against
dialysis-dependent chronic renal failure patients. Intensive Care
Med. 38:1729author reply 1730. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Marcondes JA, Martins TC, Amaral AS and
Nery M: Falsely elevated testosterone in a type 1 diabetes patients
with acne and chronic renal failure on dialysis. Arq Bras
Endocrinol Metabol. 56:319–323. 2012.(In Chinese). View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Finegood DT: Obesity, inflammation and
type II diabetes. Int J Obes Relat Metab Disord. 27 Suppl 3:S4–S5.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Temelkova-Kurktschiev T, Henkel E, Koehler
C, Karrei K and Hanefeld M: Subclinical inflammation in newly
detected Type II diabetes and impaired glucose tolerance.
Diabetologia. 45:1512002.PubMed/NCBI
|
|
33
|
Bitar MS, Ayed AK, Abdel-Halim SM,
Isenovic ER and Al-Mulla F: Inflammation and apoptosis in aortic
tissues of aged type II diabetes: Amelioration with alpha-lipoic
acid through phosphatidylinositol 3-kinase/Akt- dependent
mechanism. Life Sci. 86:844–853. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mariappan N, Elks CM, Sriramula S,
Guggilam A, Liu Z, Borkhsenious O and Francis J: NF-kappaB-induced
oxidative stress contributes to mitochondrial and cardiac
dysfunction in type II diabetes. Cardiovasc Res. 85:473–483. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shaarawy M, Fathy SA, Mehany NL and Hindy
OW: Circulating levels of osteoprotegerin and receptor activator of
NF-kappaB ligand in patients with chronic renal failure. Clin Chem
Lab Med. 45:1498–1503. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shalini B and Sharma JD: Beneficial
effects of emblica officinalis on fluoride-induced toxicity
on brain biochemical indexes and learning-memory in rats. Toxicol
Int. 22:35–39. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cizmeci D and Arkun Y: Regulatory networks
and complex interactions between the insulin and angiotensin II
signalling systems: Models and implications for hypertension and
diabetes. PLoS One. 8:e836402013. View Article : Google Scholar : PubMed/NCBI
|