Introduction
Glioma is a common tumor of the central nervous
system that is derived from glial cells. Classification of glioma
is primarily based on the tissue source, tumor site and invasive
ability. Astrocytoma is histologically close to astrocytes.
According to the classification criteria of the World Health
Organization, astrocytoma may be divided into grades I–IV. Grade I
and II astrocytomas are low-grade gliomas with a weak degree of
malignancy, while grade III and IV astrocytomas are classified as
malignant gliomas. Grade III astrocytoma is also termed anaplastic
astrocytoma and grade IV astrocytoma is usually glioblastoma (GBM),
which has the strongest invasive potential.
Unfortunately, GBM is the most common type of
glioma. Its annual incidence rate is 3.19/10,000 in the United
States (1,2). Despite advances in the equipment and
technology used for surgery and radiation therapy, the recurrence
of glioma cannot be prevented. Glioma cells are highly invasive and
infiltrate through normal tissue gaps to metastasize (3). Targeted therapy is the next frontier
in glioma treatment.
Anoikis is a type of programmed cell death that is
induced following disengagement between cells and the extracellular
matrix (ECM) (4). Anoikis has
important roles in development, organization balance, disease
occurrence and tumor metastasis (5). Anoikis prevents epithelial cells from
cloning in other sites. Cells that adhere to ECM proteins do not
undergo anoikis and resistance to anoikis is essential for
successful tumor metastasis. Once the adhesion of tumor cells to
the surrounding cells and ECM is reduced, they escape from primary
foci. During the process of transfer and final colonization, tumor
cells must be able to survive at ectopic sites (6), and resistance to anoikis aids this
process. In both normal and tumor cells, studies have demonstrated
that ECM is an inhibitor of anoikis, and integrins have a profound
impact on cell survival (7,8). The
most important factors involved in the transduction pathways
regulated by integrins include focal adhesion kinase,
integrin-linked kinase, tyrosine kinase, phosphatidylinositol
3-kinase (PI3K), extracellular signal-regulated kinase and the
connexin Src homology 2 domain-containing-transforming protein 1
(9).
Motor neuron and pancreas homeobox (MNX1) is a
homologous box transcription factor that promotes motor neuron
differentiation and pancreatic development. MNX1 knockout in mice
indicated that only the early expression of MNX1 is associated with
the formation of pancreatic buds, and MNX1 is expressed in late β
cells and associated with β cell maturation (10). MNX1 has the ability to induce
endocrine progenitor cells to differentiate into β cells and
inhibit their ability to differentiate into α cells, thereby
maintaining the balance between α and β cells (11). In addition, the association between
MNX1 gene mutations and Currarino syndrome has also been
demonstrated. The Currarino syndrome phenotype caused by a MNX1
mutations was demonstrated to be a result of haploinsufficiency
(12). A recent study also
indicated that MNX1 may be an oncogene in prostate cancer (13).
Tyrosine kinase receptors (Trks) are neurotrophin
(NT) receptors that are encoded by proto-oncogenes, which are key
regulators of cell proliferation, differentiation and apoptosis
signaling pathways (14). The TrkB
signal transduction pathway has an important role in tumor
progression and may lead to the malignant transformation of
fibroblasts (15). Brain-derived
neurotrophic factor (BDNF) is a ligand of TrkB, and the BDNF/TrkB
ligand-receptor interaction induces receptor dimerization. The
receptor Trk region activates the autophosphorylation of tyrosine
residues in this region, and phosphorylation of Y484 and Y785 in
the surrounding area also occurs. This phosphorylation subsequently
triggers the binding of the receptor to the SHC, which is the
protein adapter of the phosphotyrosine-binding region binding to
the phospholipase C (PLC)γ protein, and promotes signal
transmission. Downstream signaling pathways include the
Ras/mitogen-activated protein kinase pathway,
PI3K/3-phosphoinositide dependent protein kinase 1/Akt pathway,
increased Ca2+ release and activation of the PLCγ
pathway (16).
The present study demonstrated that MNX1 was
ectopically expressed in glioma cells and glioma cell lines with a
higher malignancy were associated with higher MNX1 expression
compared with a glioma cell line with lower malignancy.
Furthermore, MNX1 contributed to cell adhesion and reduced the
level of anoikis during detachment from adhesion. The expression
pattern of TrkB in glioma cell lines was similar to that of MNX1,
and TrkB expression was altered with the regulation of MNX1. It was
also confirmed that MNX1 was able to bind directly to the TrkB gene
and regulate its expression. Thus, TrkB, as a downstream signaling
pathway of MNX1, may increase the ability of glioma cells to escape
anoikis.
Materials and methods
Cell culture
HUVEC-C, U-87 MG, T98G and M059K cell lines were
obtained from the American Type Culture Collection (Manassas, VA,
USA). Concerning the U-87 MG cell line, this cell line is not the
original GBM cell line established in 1968 at the University of
Uppsala, but is most probably also a GBM cell line whose origin is
unknown (17). HUVEC-C cells were
maintained in EGM™-2 BulletKit™ (Lonza Group, Ltd., Basel,
Switzerland). U-87 MG cells were maintained in Dulbecco's modified
Eagle's/F12 supplemented with 20 ng/ml rh-b-FGF, 20 ng/ml rh-EGF,
2% B27 and 2 mg/l L-glutamine (all Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA). T98G and M059K cells were maintained in
Eagle's minimum essential medium (Gibco; Thermo Fisher Scientific,
Inc.) supplemented with 10% FBS. Cells were maintained in an
incubator at 37°C in a humidified atmosphere containing 5%
CO2.
RNA isolation and cDNA synthesis
For RNA isolation, ~1×106 cells were
lysed with 1 ml TRIzol (Invitrogen; Thermo Fisher Scientific,
Inc.). Cell lysates were added to 200 µl chloroform and centrifuged
at 12,000 × g for 15 min at 4°C. The upper phase was extracted,
added to an equal volume of isopropanol, incubated for 10 min at
room temperature and centrifuged at 12,000 × g for 10 min at 4°C.
The supernatant was discarded and the precipitate was washed with
75% ethanol and centrifuged at 7,500 × g for 10 min at 4°C. The
supernatant was again discarded, the RNA precipitate was allowed to
dry and it was subsequently dissolved in RNase-free
H2O.
A PrimeScript RT reagent kit (Takara Biotechnology
Co., Ltd., Dalian, China) was used to conduct reverse transcription
(RT) reactions. cDNA synthesis was performed for 1 h at 42°C and 10
min at 72°C.
Construction of overexpression and
knockdown vectors
Total RNA was extracted from HUVEC-C cells using
TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.) and reverse
transcribed according to the aforementioned protocol. HUVEC-C cDNA
was used to amplify the full-length MNX1 open reading frame (ORF)
and TrkB ORF via polymerase chain reaction (PCR) using
Platinum® Taq DNA Polymerase (Invitrogen; Thermo Fisher
Scientific, Inc.). BamHI and XhoI restriction sites were ligated to
both ends of the amplified fragments, which were subsequently
cloned into pCDH expression vectors (Addgene, Inc., Cambridge, MA,
USA). The sequences of the primers used for amplification of MNX1
ORF were forward, 5′-CGCGGATCCATGGGGGGACTCTCAACAGT-3′ and reverse,
5′-CCGCTCGAGCTACTGGGGCGCGGGCTGG-3′. The sequences of the primers
used for amplification of TrkB ORF were forward,
5′-CGCGGATCCATGTCGTCCTGGATAAGGTGGCA-3′ and reverse,
5′-CCGCTCGAGCTAGCCTAGAATGTCCAGGTAG-3′. The thermoycling conditions
used for PCR were as follows: Initial denaturation for 5 min at
95°C; followed by 35 cycles of 30 sec at 95°C, 30 sec at 55°C and 2
min at 72°C; followed by a final extension for 5 min at 72°C. Empty
pCDH vectors were used as a control for overexpression vectors.
pLKO.1 vectors containing short hairpin (sh)RNA for MNX1 and TrkB
knockdown were purchased from Shenzhen Zhonghong Boyuan Biological
Technology Co., Ltd. (Shenzhen, China). The following shRNA
sequences were employed, MNX1 shRNA-1, GCCCGACTTCAACTCCCAGGC; MNX1
shRNA-2, GCTCCTCGGAGGACGACTCGC; TrkB shRNA-1,
TGAAAGATTTCTCATGGTTTG; and TrkB shRNA-2, TGGCGTCTGCGTGGAGGGCGA.
Empty PLKO.1 vectors were used as control. HEK293T cells were used
for lentiviral packaging. A total of 12 ng target plasmids were
transfected into HEK293T cells at a density of 80% in 100 mm
dishes. The amount of transfection reagent-polyethylenimine
(Polysciences, Inc., Warrington, PA, USA) is one third of the
plasmid. Following 24 h, lentiviruses were collected in order to
transfect corresponding target cells. Prior to transfection, a
total of 1.2×106 cells were incubated for 24 h in
six-well plates at 37°C. MNX1 and TrkB pCDH overexpression vectors
were used for transfection of HUVECs and U-87 MG cells. MNX1/TrkB
shRNA pLKO.1 vectors were used for transfection of T98G and M059K
cells. The mass of transfected MNX1 and TrkB pCDH overexpression
vectors, as well as MNX1/TrkB shRNA pLKO.1 vectors, were the
same.
Adhesion assay
For the adhesion assay 500 µl fibronectin (10 g/ml;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was added to 6-well
plates and incubated at 37°C for 24 h. Subsequently, excess liquid
was discarded and 500 µl bovine serum albumin (BSA; 0.2%) (Sangon
Biotech, Co., Ltd., Shanghai, China) was added to block each well
at room temperature for 1 h. A total of 24 h post-transfection,
1×105 transfected cells were placed into each well and
incubated for 30 min. Medium was subsequently discarded and 5%
(w/v) crystal violet was used to stain adhered cells for 10 min at
room temperature, and the results were observed using a light
microscope (magnification, ×100).
Cell death detection ELISA
A total of 24 post-transfection, 1×105
cells were seeded in normal and low-attachment surface 24-well
plates. Following incubation for 24 h at 37°C, a Cell Death
Detection ELISA PLUS kit (cat. no. 11774425001; Sigma Aldrich;
Merck KGaA, Darmstadt, Germany) was used to detect apoptosis,
according to the manufacturer's protocol.
RT-semi-quantitative (sq)PCR and
RT-qPCR analyses
RT-sqPCR was performed with Platinum® Taq
DNA Polymerase (Invitrogen; Thermo Fisher Scientific, Inc.).
Cycling conditions were as follows: Initial denaturation for 5 min
at 95°C, followed by 32 cycles of 30 sec at 95°C, 30 sec at 57°C
and 1 min at 72°C; followed by a final extension for 5 min at 72°C.
Primer sequences used for RT-sqPCR were as follows: GAPDH forward,
5′-GATTCCACCCATGGCAAATTC-3′ and reverse,
5′-GTCATGAGTCCTTCCACGATAC-3′; MNX1 forward,
5′-CTAAGATGCCCGACTTCAACT-3′ and reverse,
5′-CTTCTGTTTCTCCGCTTCCT-3′; TrkB forward,
5′-GACACCACGAACAGAAGTAATG-3′ and reverse,
5′-CTGCTCAGGACAGAGGTTATAG-3′. DNA products were run on a 1.5%
agarose gel and then visualized using 0.5 µg/ml ethidium bromide.
ImageJ 1.8.0 software (National Institutes of Health, Bethesda, MD,
USA) was used for densitometry.
RT-qPCR was performed using SYBR-Green qPCR Master
Mix (Takara Biotechnology Co., Ltd.). The thermocycling conditions
used were as follows: Initial denaturation for 3 min at 95°C;
followed by 15 sec at 95°C, 15 sec at 57°C and 20 sec at 72°C;
followed by a final extension for 3 min at 72°C. The sequences of
primers used for RT-qPCR were as follows: MNX1 forward,
5′-CGAGACCCAGGTGAAGATTT-3′ and reverse, 5′-CTTCTGTTTCTCCGCTTCCT-3′;
TrkB forward, 5′-GGACACCACGAACAGAAGTAA-3′ and reverse,
5′-CAATCACCACCACAGCATAGA-3′; and GAPDH forward,
5′-GGTGTGAACCATGAGAAGTATGA-3′ and reverse,
5′-GAGTCCTTCCACGATACCAAAG-3′. Protein levels were normalized
against GAPDH, and the 2−∆∆Cq method was used for
protein quantification (18).
Protein extraction and western blot
analysis
For protein extraction, 2×106 cells were
incubated in lysis buffer that was composed of 100 µl 50 mM
Tris-HCl (pH 8.0) containing 150 mM NaCl, 0.1% SDS, 1% NP-40 and
100 µg/ml phenylmethylsulfonyl fluoride. Lysis was performed on ice
and lysates were subsequently denatured at 100°C for 10 min. A
Pierce BCA Protein assay kit (Invitrogen; Thermo Fisher Scientific,
Inc.) was used to measure the protein concentration and 20 µg
protein was loaded into each well. Proteins were separated by 10%
SDS-PAGE and transferred to nitrocellulose membranes. The membranes
were blocked in 5% (w/v) milk for 1 h at room temperature. The
following primary antibodies were employed: Rabbit MNX1 polyclonal
antibody in 5% (w/v) milk (cat. no. SAB2101494; 1:2,000;
Sigma-Aldrich; Merck KGaA), rabbit TrkB polyclonal antibody in 5%
(w/v) milk (cat. no. ab18987; 1:2,000; Abcam, Cambridge, UK) and
mouse GAPDH monoclonal antibody in 5% (w/v) milk (cat. no. ab8245;
1:5,000; Abcam). The primary antibodies were incubated with the
membranes at 4°C overnight. Goat anti-rabbit IgG H&L in 3%
(w/v) BSA (cat. no. ab6721; 1:2,000; Abcam) and goat anti-mouse IgG
H&L in 5% (w/v) milk (cat. no. ab6789; 1:2,000; Abcam)
horseradish peroxidase (HRP)-conjugated antibodies were used as
secondary antibodies. The secondary antibodies were incubated with
the blots at room temperature for 4 h. Bands were visualized with
Chemiluminescent HRP Substrate (EMD Millipore, Billerica, MA, USA)
and images were captured by X-OMAT BT Film (Carestream Health,
Inc., Rochester, NY, USA).
Chromatin immunoprecipitation
(ChIP)
The DNA products following RT-qPCR and RT-sqPCR
analysis were subjected to ChIP analysis. The MNX1 binding motif
was predicted using the JASPAR database (http://jaspar.genereg.net/). Following the
identification of DNase I hypersensitive sites using the
Encyclopedia of DNA Elements at the University of California, Santa
Cruz database (http://genome.ucsc.edu/ENCODE/), ChIP primers were
designed as follows: TrkB-1F, 5′-TTCCACCTCCCAGGTTCAAG-3′ and
TrkB-1R, 5′-GTCAAGAGATCGAGACCATCC-3′; TrkB-2F,
5′-ATTCTGTTCCTTCCACTGAC-3′ and TrkB-2R,
5′-GAGGTAAGAGGAGAAAAGACC-3′; TrkB-3F, 5′-TAAAGGAACTCCAGCAGAAC-3′
and TrkB-3R, 5′-TGGATTGTGGTGATGGTTTC-3′; TrkB-4F,
5′-TCTTCACAAAGAACCAGCTC-3′ and TrkB-4R 5′-CCACCTTAAGAAATCAGAGG-3′;
TrkB-5F, 5′-ACTGCGGTGTATTTTCCCCGTT-3′ and TrkB-5R,
5′-TAGGGACAAATTAGGCGATCCG-3′; TrkB-6F, 5′-GACAGTCTCTCCCTTAACAT-3′
and TrkB-6R, 5′-CACTCAGAACTTCTCCTCTT-3′; TrkB-7F,
5′-TTGACCCTACTTTCTTCCAC-3′ and TrkB-7R,
5′-AAACTCCACACTAGGGAACTGG-3′; TrkB-8F, 5′-CCAGTGAGCAAACTTCAGGA-3′
and TrkB-8R 5′-AGGGTGCTTTTCCTGGAAAG-3′. A Pierce Agarose ChIP kit
(Thermo Fisher Scientific, Inc.) was used to conduct ChIP according
to the manufacturer's protocol. During the process, the MNX1
primary antibody (cat. no. SAB2101494; Sigma-Aldrich; Merck KGaA)
was used at a 1:100 dilution.
Luciferase reporter gene
technology
T98G and M059K cells (1×105) were plated
in 24-well plates and tested at a cell density of ~80% after
approximately 24 h. Fragments of different lengths upstream of TrkB
were cloned into pGL3 vectors containing firefly luciferase
(Promega Corporation), which were considered to be the target
plasmids. The primers used to generate each of the 7 fragments were
as follows: Position −2,665 bp to position −2,368 bp,
5′-TTCCACCTCCCAGGTTCAAG-3′; −2,665 bp to position −2368 bp,
5′-TCAACGTTGTATGTTTTA-3′; −2,665 bp to position −2,368 bp,
5′-GGTCAGCAGATTGAGACCAT-3′; −2,665 bp to position −2,368 bp,
5′-TGGAGGTTCTAAAGGAACTCCA-3′; −2,368 bp reverse primer,
5′-TATAGCACTAAATTCCTGTATCAG-3′; −3,425 bp to position −2,666 bp,
5′-TCAACGTTGTATGTTTTA-3′; −4,216 bp to position −2,666 bp,
5′-GGTCAGCAGATTGAGACCAT-3′; −5,002 bp to position-2,666 bp,
5′-TGGAGGTTCTAAAGGAACTCCA-3′; −2,666 bp reverse primer
5′-ACACTGAATATTTCATAG-3′. The cells in each well were
co-transfected with 800 ng target plasmid and 15 ng control
Renilla reniformis luciferase plasmid (pRL-TK; cat. no.
E2231; Promega Corporation) using 1 µl Lipofectamine®
(Invitrogen; Thermo Fisher Scientific, Inc.). After 24 h, the
Dual-Luciferase® Reporter assay system was used to
measure the luciferase activity in a Promega GloMax 20/20
Luminometer following cell lysis (all Promega Corporation). Firefly
luciferase activity was normalized to Renilla luciferase
activity.
Statistical analysis
Statistical analyses were performed using SPSS 19.0
software (IBM Corp., Armonk, NY, USA). Data are presented as the
mean + standard deviation for continuous data. One-way analysis of
variance followed by Dunnett's post-hoc test was performed for
multiple comparisons. All experimental groups were compared with
the control groups. P<0.05 was considered to indicate a
statistically significant difference. Each experiment was performed
in triplicate.
Results
Positive association between MNX1 and
TrkB expression in normal human HUVEC-C cells and glioma cell
lines
The present study investigated the expression of
MNX1 in the HUVEC-C normal human cell line, U-87 MG (low
malignancy) human glioma cell line, and T98G and M059K (high
malignancy) human glioma cell lines. RT-sqPCR (Fig. 1A) and RT-qPCR (Fig. 1B and C) were used to detect the
expression of MNX1 and TrkB at the transcriptional level. Western
blotting was performed to detect the expression of MNX1 and TrkB at
the protein level (Fig. 1D). The
results demonstrated that the expression of MNX1 was higher at both
the transcriptional and translational levels in high malignancy
T98G and M059K cells compared with HUVEC-C and low malignancy U-87
MG cells (Fig. 1A-D).
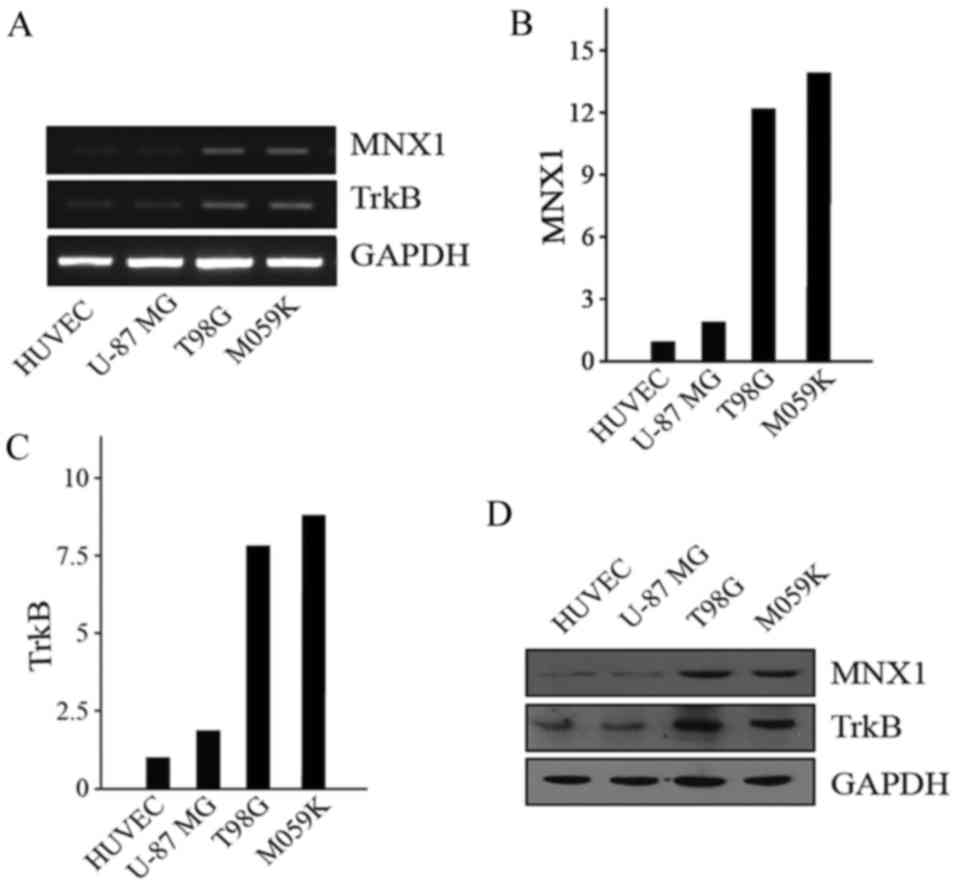 | Figure 1.Expression of MNX1 and TrkB in normal
human HUVEC-C cells and glioma cell lines. (A) Representative gel
presenting mRNA expression of MNX1 and TrkB in in HUVEC-C, U-87 MG,
T98G and M059K cells. Quantified mRNA levels of (B) MNX1 and (C)
TrkB in HUVEC-C, U-87 MG, T98G and M059K cells were determined via
reverse transcription-quantitative polymerase chain reaction. (D)
Protein levels of MNX1 and TrkB were measured by western blotting
in HUVEC-C, U-87 MG, T98G and M059K cells. P<0.05 vs. HUVEC-C
cells. MNX1, motor neuron and pancreas homeobox 1; TrkB, tyrosine
kinase receptor B; HUVECs, human umbilical vein endothelial cells;
NS, not significant. |
It has been reported that mature BDNF and its
receptor, TrkB, are upregulated in human glioma tissues (19); however, its specific mechanism of
action has not been clearly explained. Thus, the present study also
measured the expression of TrkB in the aforementioned cell lines.
The results demonstrated that TrkB expression was similar to that
of MNX1; TrkB levels were higher in T98G and M059K cells compared
with HUVEC-C and U-87 MG cells at the transcriptional and
translational levels (Fig. 1A, C and
D).
MNX1 and TrkB promote cell detachment
and anchorage independence
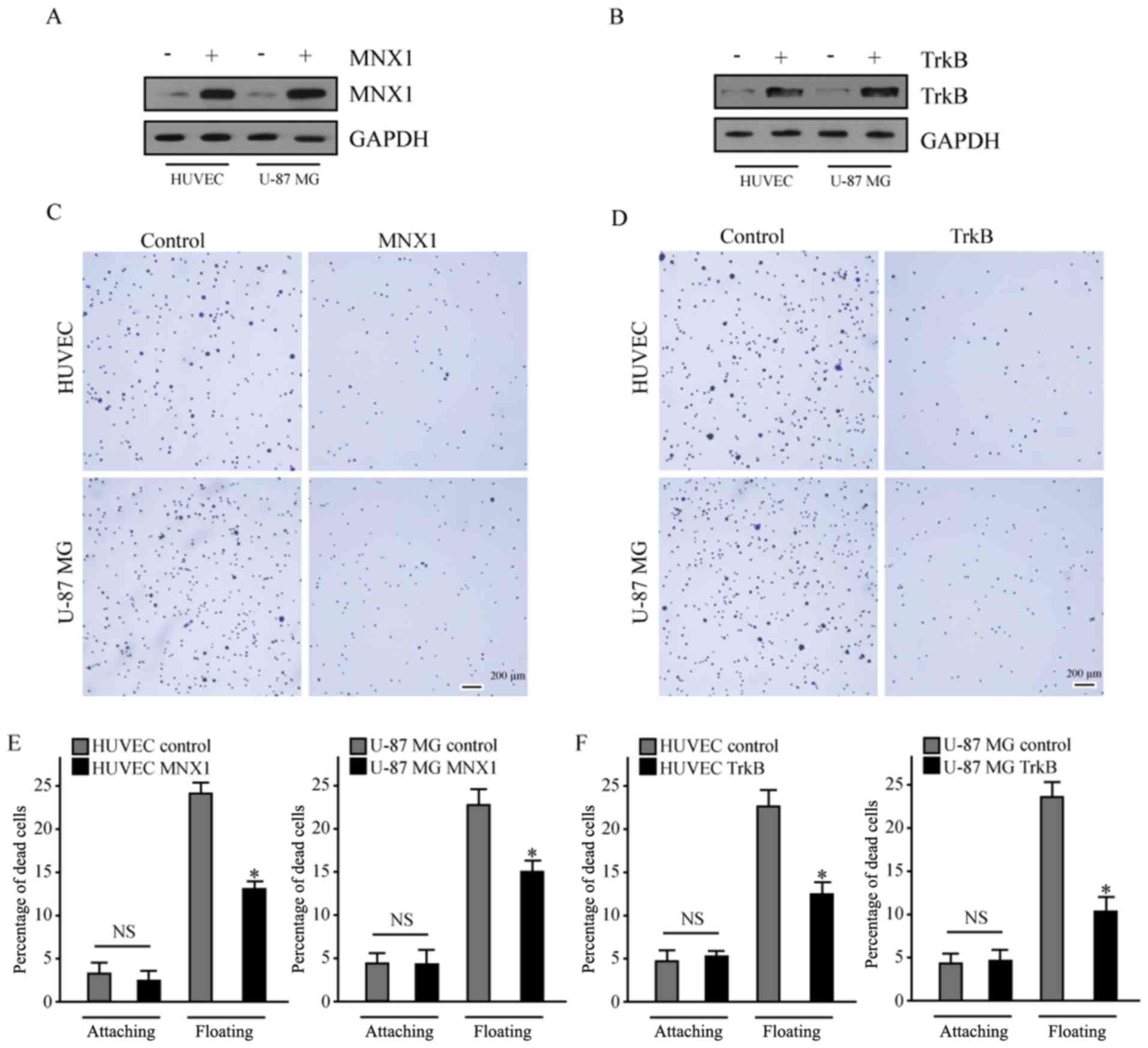
Western blot analysis confirmed that MNX1 and TrkB
were successfully overexpressed in the HUVEC-C and U-87 MG cells
(Fig. 2A and B). Fibronectin is an
important constituent of the ECM, therefore, the present study
employed cell culture dishes that were coated with fibronectin to
detect changes in adhesion between cells and the ECM. This method
is termed the adhesion assay. The results of the adhesion assay
demonstrated that MNX1 and TrkB overexpression led to reduced
adhesion between cells and fibronectin compared with the respective
control groups (Fig. 2C and D),
which is considered to be an early event in tumor cell metastasis
(20).
TrkB has an important role in resistance to anoikis
(21). The current study aimed to
determine whether MNX1 and TrkB may increase the ability of HUVEC-C
and U-87 MG cells to bypass anoikis. Cells in the control and
experimental groups were cultured in the normal adherent state and
low-attachment plates for 24 h. A Roche Cell Death Detection ELISA
kit was used to detect cell apoptosis levels. The results
demonstrated that MNX1 and TrkB overexpression both reduced the
mortality of cells in suspension culture, compared with the control
group (Fig. 2E and F).
Knockdown of MNX1 and TrkB increases
cell adhesion and induces anoikis in highly malignant glioma cell
lines
To further verify the role of MNX1 and TrkB in the
development of glioma, shRNA was employed in the current study to
knock down their expression in T98G and M059K cells. To prevent
off-target effects, two shRNAs each were designed for MNX1 and
TrkB. Western blot analysis demonstrated that MNX1 and TrkB were
successfully downregulated in T98G and M059K cells following
transfection with shRNA (Fig. 3A and
B). Furthermore, downregulation of MNX1 and TrkB markedly
increased the adhesion of T98G and M059K cells to fibronectin
(Fig. 3C and D); this was
consistent with the above experimental results in HUVEC-C and U-87
MG cells, and confirmed that MNX1 and TrkB may allow cells to
reduce their association with the ECM. Additionally, downregulation
of MNX1 and TrkB led to increases in the apoptosis of T98G and
M059K cells in suspension (Fig. 3E and
F), further indicating that MNX1 and TrkB may contribute to
anoikis resistance in cells.
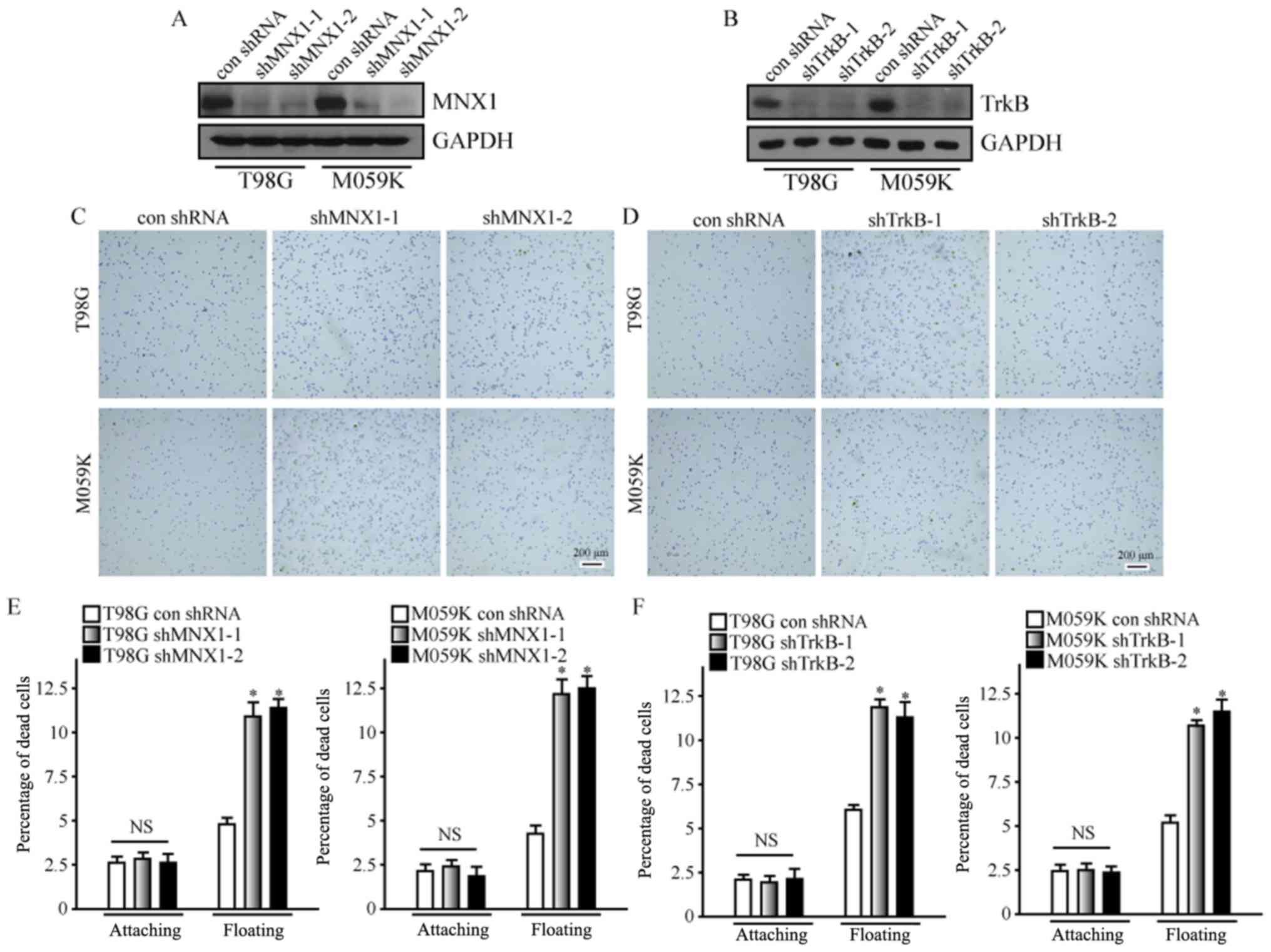 | Figure 3.Knockdown of MNX1 and TrkB increases
cell adhesion and induces anoikis in highly malignant glioma cell
lines. Western blot analysis confirmed successful knockdown of (A)
MNX1 and (B) TrkB in T98G and M059K cells. Knockdown of (C) MNX1
and (D) TrkB increased the adhesion ability of T98G and M059K
cells. Scale bars, 200 µm. The ratio of suspended T98G and M059K
cells undergoing anoikis was increased following knockdown of (E)
MNX1 and (F) TrkB. *P<0.05 vs. con shRNA. MNX1, motor neuron and
pancreas homeobox 1; TrkB, tyrosine kinase receptor B; shRNA, short
hairpin RNA; con shRNA, control shRNA; NS, not significant;
attaching, cells attached to the extracellular matrix; floating,
cells suspended without the extracellular matrix; A405 nm,
absorbance at 405 nm; A490 nm, absorbance at 490 nm. |
MNX1 confers anoikis resistance
through TrkB
To verify the association between MNX1 and TrkB, a
series of experiments were performed. Western blot analysis
demonstrated that overexpression of MNX1 and TrkB in HUVEC-C and
U-87 MG cells both led to the upregulation of TrkB (Fig. 4A), and decreased levels of
apoptosis in cells in suspension culture were also observed
(Fig. 4B). However, when cells
were co-transfected with MNX1 overexpression vector and TrkB
shRNA-1 to restore normal TrkB levels (Fig. 4A), the anoikis levels of cells were
also partially restored (Fig. 4B).
Similarly, knockdown of MNX1 and TrkB was performed in T98G and
M059K cells to verify these results. Western blot analysis and
apoptosis analysis demonstrated that knockdown of MNX1 resulted
reduced TrkB protein expression levels and increased apoptosis in
suspension culture (Fig. 4C and
D). Furthermore, overexpression of TrkB combined with MNX1
knockdown restored the levels of TrkB protein and anoikis (Fig. 4C and D). These data indicate that
MNX1 may act by regulating the expression of the downstream TrkB
gene.
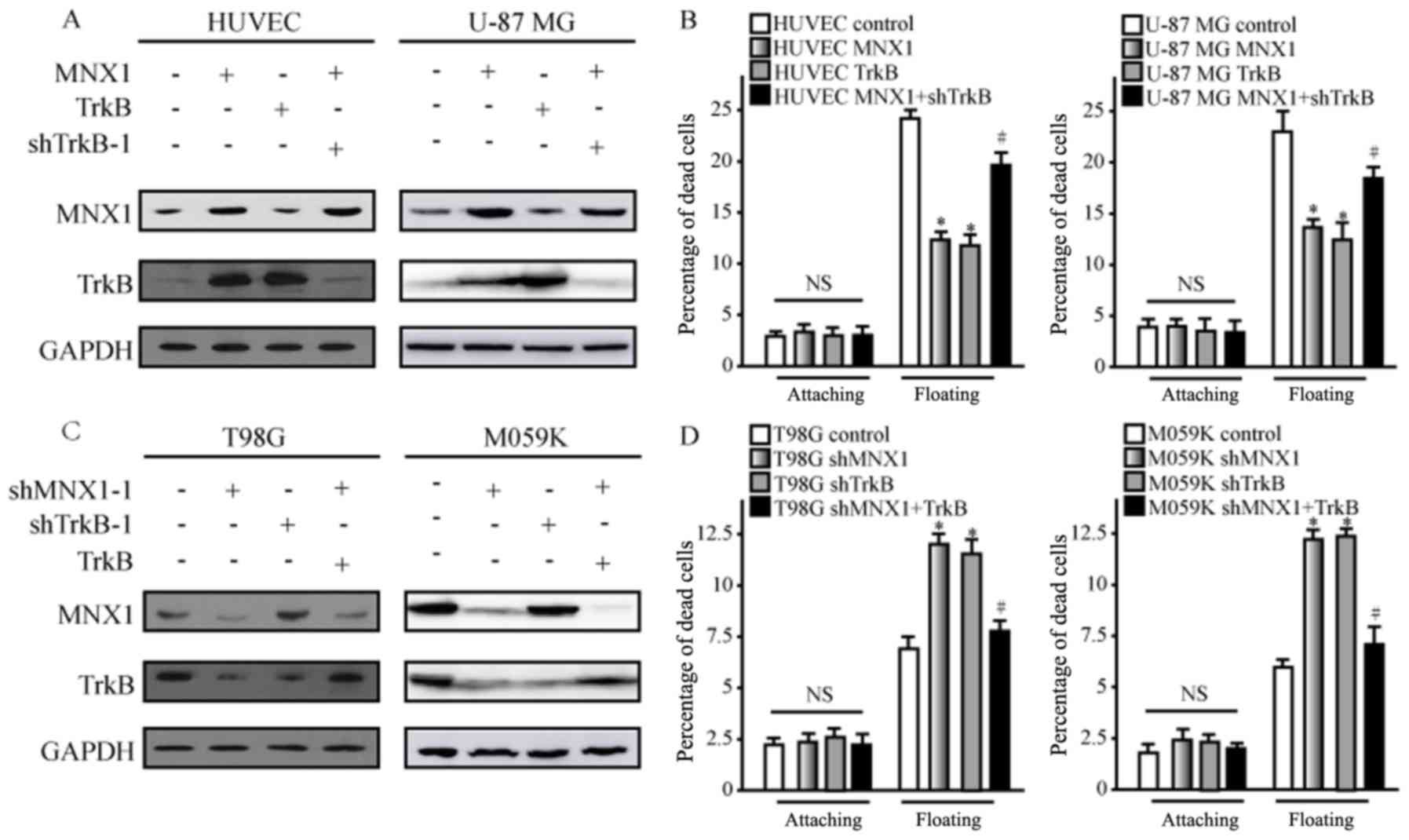 | Figure 4.MNX1 confers anoikis resistance
through TrkB. HUVECs were transfected with MNX1 or TrkB
overexpression vector, or a combination of MNX1 overexpression
vector and shTrkB-1. (A) Western blot analysis was performed on
HUVEC-C and U-87 MG cells to determine the effects on MNX1 and TrkB
protein expression. (B) Anoikis was measured in attached and
suspended U-87 MG cells and HUVECs following transfection. T98G
cells were transfected with shMNX1-1, shTrkB-1 or a combination of
shMNX1-1 and TrkB overexpression vector. (C) Western blot analysis
was performed on T98G and M059K cells to determine the effects on
MNX1 and TrkB protein expression. (D) Anoikis was measured in
attached and suspended T98G and M059K cells following transfection.
For anoikis, the rate of cell death was assessed after 24 h.
*P<0.05 vs. control; #P<0.05 vs. MNX1 or shMNX1-1
group. MNX1, motor neuron and pancreas homeobox 1; TrkB, tyrosine
kinase receptor B; HUVECs, human umbilical vein endothelial cells;
sh, short hairpin RNA; NS, not significant; attaching, cells
attached to the extracellular matrix; floating, cells suspended
without the extracellular matrix; A405 nm, absorbance at 405 nm;
A490 nm, absorbance at 490 nm. |
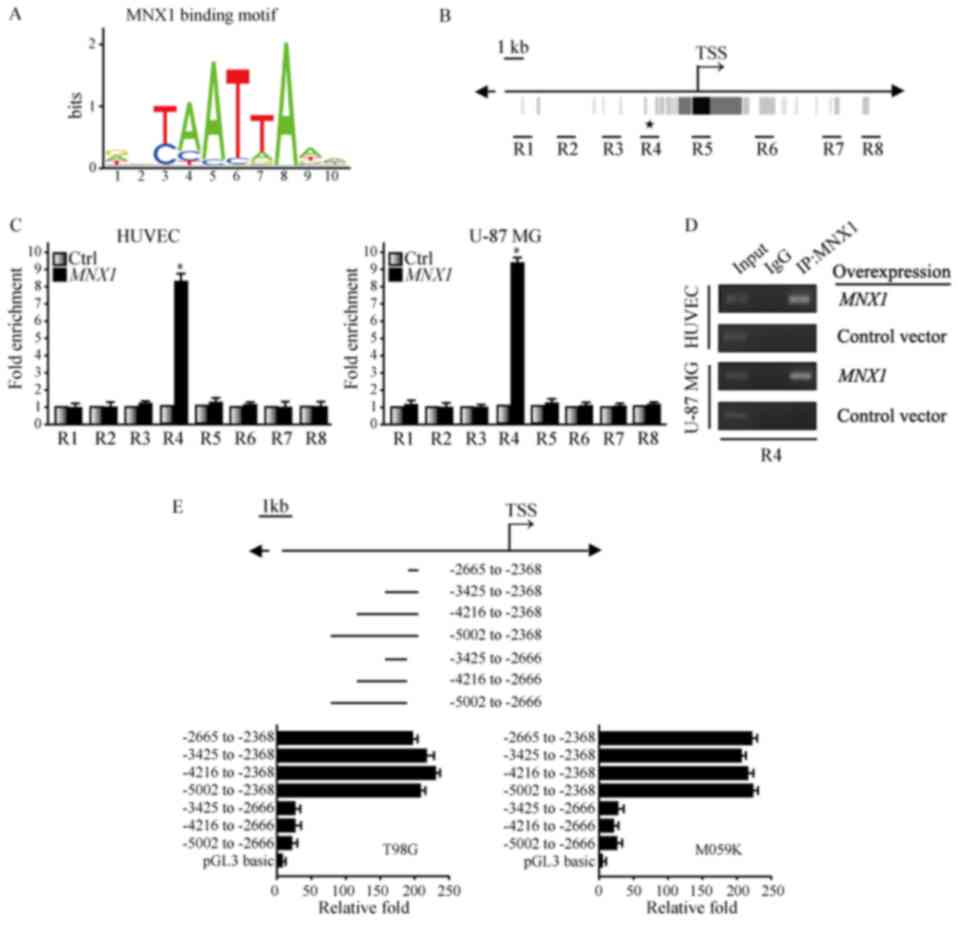
MNX1 binds directly to TrkB to
regulate its expression
The aforementioned experiments clearly demonstrated
an association between MNX1 and TrkB, but the specific mechanism
was yet to be established. For example, it was unclear whether MNX1
binds directly to TrkB as a transcription factor to regulate its
transcription. Initially, the MNX1 binding motif was predicted
using the JASPAR database (Fig.
5A) and a putative MNX1 binding site was identified in the TrkB
gene (Fig. 5B). DNase I
hypersensitive sites were analyzed using the Encyclopedia of DNA
Elements at the UCSC database. Subsequently, ChIP was performed to
analyze whether MNX1 binds to TrkB. In combination with the
analysis of binding sites and DNase I hypersensitive sites, ChIP
primers were designed for eight regions close to the
transcriptional start site of TrkB (R1-8; Fig. 5B). The results of ChIP analysis
demonstrated that R4 of TrkB was pulled down by Pierce Protein A
PLUS Agarose binding with the MNX1 antibody following
overexpression of MNX1 in HUVEC-C and U-87 MG cells, indicating
that MNX1 was bound to the R4 site of TrkB by RT-qPCR (Fig. 5C) and RT-sqPCR (Fig. 5D). A luciferase assay was
subsequently performed. Fragments of different lengths upstream of
TrkB were cloned into a vector containing a luciferase reporter.
For this experiment, T98G and M059K cells that highly express MNX1
were employed to verify the above results. The results demonstrated
that the activity of the luciferase reporter containing the −2665
to −2368 bp fragment was high compared with a number of the other
fragments (Fig. 5E). Furthermore,
the position of this fragment coincided with the position of R4.
The data described here indicated that MNX1 binds directly upstream
of the TrkB gene to regulate its transcription.
Discussion
Glioma is associated with the highest incidence of
intracranial malignant tumors, accounting for ~45% of all cases
(22). The treatment of glioma has
always been a difficult problem in neurosurgery; ~77% of patients
succumb to glioma within 1 year following diagnosis (23). Clinical investigations as well as
experiments on animals and cells have demonstrated the poor
prognosis associated with glioma, and uncontrollable cell
proliferation, reduced apoptosis, tumor cell invasiveness,
neovascularization and other biological behaviors are reported to
contribute to the difficulty of treating glioma (24,25).
With the application and development of molecular biology in tumor
research in recent years, the molecular mechanisms and apoptosis of
glioma have been investigated extensively. Due to its high degree
of hereditary and pathological heterogeneity, even within the same
tumor sample, single molecular abnormalities are not common, and
malignant gliomas usually exhibit multiple gene mutations and
molecular changes (26,27). Therefore, it is difficult to target
and identify a single gene responsible for glioma. In recent years,
the Cancer Genome Atlas has identified three core signaling
pathways in malignant glioma: Trk/Ras/PI3K, p53 and retinoblastoma
protein signal transduction pathways (28). In addition, other typical signaling
pathways, such as the angiogenic pathway, have also been indicated
to be important for the development of gliomas (29,30).
The association between MNX1 and Currarino syndrome
has long been a concern of researchers (31). The role of MNX1 in the development
of β cells has also been previously investigated (10). Furthermore, the role of MNX1 in
cancer development been investigated recently, and MNX1 was
indicated to be an oncogene in prostate cancer (13,32,33).
However, to the best of our knowledge, the expression and function
of MNX1 in gliomas has not been previously reported. The expression
of TrkB has been reported to be closely associated with the
invasion and metastasis of tumor cells; and BDNF/TrkB signaling was
revealed to suppress apoptosis and enhance angiogenesis (34,35).
This provides the basis for growth conditions during the migration
of tumor cells to distant sites and continued proliferation.
However, the association between TrkB and MNX1 has not been
previously investigated.
The present study demonstrated that the expression
of MNX1 and TrkB was higher in highly invasive glioma cell lines
(T98G and M059K) compared with less invasive glioma cells (U-87 MG)
and normal HUVEC-C cells. A positive association was observed
between MNX1 and TrkB expression and the degree of malignancy of
gliomas, and a series of experiments were performed to verify these
results. The results of an adhesion assay demonstrated that MNX1
and TrkB reduced cell adhesion and enhanced the ability to resist
anoikis in HUVEC-C and U-87 MG cells. Furthermore, ChIP and
luciferase assays demonstrated that MNX1 may be an upstream gene
that regulates TrkB expression. Based on these results, it may be
concluded that as a transcription factor, MNX1 may bind directly to
TrkB and promote its transcription.
The present study investigated the expression and
function of the transcription factor MNX1 in glioma. To the best of
our knowledge, the present study is the first to indicate that MNX1
may directly regulate TrkB expression, which may increase their
metastatic potential via suppression of anoikis and enhanced
adhesion to the ECM. The binding site of MNX1 upstream of the TrkB
gene was also identified. However, further validation of pathways
downstream of TrkB that affect the sensitivity of cells to anoikis,
as regulated by MNX1, require further investigation. The results of
the current study may contribute to an improved understanding of
the development of gliomas and provide a theoretical basis for
future therapeutic strategies utilizing MNX1 as a molecular
target.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DZ and LJ conceived and designed the study. LJ, SC,
JY, JC and CY performed the experiments and acquired the data. GZ
analyzed and interpreted the data. DZ wrote, reviewed and revised
the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ostrom QT, Gittleman H, Liao P, Rouse C,
Chen Y, Dowling J, Wolinsky Y, Kruchko C and Barnholtz-Sloan J:
CBTRUS statistical report: Primary brain and central nervous system
tumors diagnosed in the United States in 2007–2011. Neuro Oncol. 16
Suppl 4:iv1–iv63. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Thomas AA, Brennan CW, DeAngelis LM and
Omuro AM: Emerging therapies for glioblastoma. JAMA Neurol.
71:1437–1444. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cuddapah VA, Robel S, Watkins S and
Sontheimer H: A neurocentric perspective on glioma invasion. Nat
Rev Neurosci. 15:455–465. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Frisch SM and Francis H: Disruption of
epithelial cell-matrix interactions induces apoptosis. J Cell Biol.
124:619–626. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gilmore AP: Anoikis. Cell Death Differ. 12
Suppl 2:S1473–S1477. 2005. View Article : Google Scholar
|
|
6
|
Chaffer CL and Weinberg RA: A perspective
on cancer cell metastasis. Science. 331:1559–1564. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Thompson EW, Newgreen DF and Tarin D:
Carcinoma invasion and metastasis: A role for
epithelial-mesenchymal transition? Cancer Res. 65:5991–5995. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zanotti S, Gibertini S, Bragato C,
Mantegazza R, Morandi L and Mora M: Fibroblasts from the muscles of
Duchenne muscular dystrophy patients are resistant to cell
detachment apoptosis. Exp Cell Res. 317:2536–2547. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hanks SK, Ryzhova L, Shin NY and Brábek J:
Focal adhesion kinase signaling activities and their implications
in the control of cell survival and motility. Front Biosci.
8:d982–d996. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Harrison KA, Thaler J, Pfaff SL, Gu H and
Kehrl JH: Pancreas dorsal lobe agenesis and abnormal islets of
Langerhans in Hlxb9-deficient mice. Nat Genet. 23:71–75. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dalgin G, Ward AB, le Hao T, Beattie CE,
Nechiporuk A and Prince VE: Zebrafish mnx1 controls cell fate
choice in the developing endocrine pancreas. Development.
138:4597–4608. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Garcia-Barceló MM, Lui VC, So MT, Miao X,
Leon TY, Yuan ZW, Ngan ES, Ehsan T, Chung PH, Khong PL, et al: MNX1
(HLXB9) mutations in Currarino patients. J Pediatr Surg.
44:1892–1898. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Das M: MNX1: A novel prostate cancer
oncogene. Lancet Oncol. 17:e5212016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lamballe F, Klein R and Barbacid M: The
trk family of oncogenes and neurotrophin receptors. Princess
Takamatsu Symp. 22:153–170. 1991.PubMed/NCBI
|
|
15
|
Shen J and Maruyama IN: Brain-derived
neurotrophic factor receptor TrkB exists as a preformed dimer in
living cells. J Mol Signal. 7:22012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li M, Dai FR, Du XP, Yang QD, Zhang X and
Chen Y: Infusion of BDNF into the nucleus accumbens of aged rats
improves cognition and structural synaptic plasticity through
PI3K-ILK-Akt signaling. Behav Brain Res. 231:146–153. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Allen M, Bjerke M, Edlund H, Nelander S
and Westermark B: Origin of the U87MG glioma cell line: Good news
and bad news. Sci Transl Med. 8:354re32016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xiong J, Zhou LI, Lim Y, Yang M, Zhu YH,
Li ZW, Fu DL and Zhou XF: Mature brain-derived neurotrophic factor
and its receptor TrkB are upregulated in human glioma tissues.
Oncol Lett. 10:223–227. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wirtz D, Konstantopoulos K and Searson PC:
The physics of cancer: The role of physical interactions and
mechanical forces in metastasis. Nat Rev Cancer. 11:512–522. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dawson H, Grundmann S, Koelzer VH, Galván
JA, Kirsch R, Karamitopoulou E, Lugli A, Inderbitzin D and Zlobec
I: Tyrosine kinase receptor B (TrkB) expression in colorectal
cancers highlights anoikis resistance as a survival mechanism of
tumour budding cells. Histopathology. 66:715–725. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kornblith PL and Walker M: Chemotherapy
for malignant gliomas. J Neurosurg. 68:1–17. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rao JS: Molecular mechanisms of glioma
invasiveness: The role of proteases. Nat Rev Cancer. 3:489–501.
2003. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wirsching HG, Galanis E and Weller M:
Glioblastoma. Handb Clin Neurol. 134:381–397. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Furnari FB, Cloughesy TF, Cavenee WK and
Mischel PS: Heterogeneity of epidermal growth factor receptor
signalling networks in glioblastoma. Nat Rev Cancer. 15:302–310.
2015. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Reardon DA and Wen PY: Glioma in 2014:
Unravelling tumour heterogeneity-implications for therapy. Nat Rev
Clin Oncol. 12:69–70. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Black PM: Brain tumors. Part 1. N Engl J
Med. 324:1471–1476. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cancer Genome Atlas Research Network:
Comprehensive genomic characterization defines human glioblastoma
genes and core pathways. Nature. 455:1061–1068. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sawamiphak S, Seidel S, Essmann CL,
Wilkinson GA, Pitulescu ME, Acker T and Acker-Palmer A: Ephrin-B2
regulates VEGFR2 function in developmental and tumour angiogenesis.
Nature. 465:487–491. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Brennan CW, Verhaak RG, McKenna A, Campos
B, Noushmehr H, Salama SR, Zheng S, Chakravarty D, Sanborn JZ,
Berman SH, et al: The somatic genomic landscape of glioblastoma.
Cell. 155:462–477. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ross AJ, Ruiz-Perez V, Wang Y, Hagan DM,
Scherer S, Lynch SA, Lindsay S, Custard E, Belloni E, Wilson DI, et
al: A homeobox gene, HLXB9, is the major locus for dominantly
inherited sacral agenesis. Nat Genet. 20:358–361. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang Y, Wang J, Zhang L, Karatas OF, Shao
L, Zhang Y, Castro P, Creighton CJ and Ittmann M: RGS12 is a novel
tumor-suppressor gene in African American prostate cancer that
represses AKT and MNX1 expression. Cancer Res. 77:4247–4257. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang L, Wang J, Wang Y, Zhang Y, Castro
P, Shao L, Sreekumar A, Putluri N, Guha N, Deepak S, et al: MNX1 is
oncogenically upregulated in African-American prostate cancer.
Cancer Res. 76:6290–6298. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li Z, Tan F and Thiele CJ: Inactivation of
glycogen synthase kinase-3beta contributes to brain-derived
neutrophic factor/TrkB-induced resistance to chemotherapy in
neuroblastoma cells. Mol Cancer Ther. 6:3113–3121. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kermani P and Hempstead B: Brain-derived
neurotrophic factor: A newly described mediator of angiogenesis.
Trends Cardiovasc Med. 17:140–143. 2007. View Article : Google Scholar : PubMed/NCBI
|