Introduction
Diabetes has become a major public health issue in
the world, associating with increased micro- or macro-vascular
disease, disability and premature mortality (1–4).
Type 2 diabetes mellitus (T2DM), also known as
non-insulin-dependent diabetes mellitus, mainly disrupts the normal
action and secretion of insulin in the body (5). This can lead to a series of severe
syndromes, including hypertension, cardiovascular disease and
increased risk of cancer (6). β
cells apoptosis and insulin secretory dysfunction are happened in
T2DM (7–11), resulting in an inability to
compensate for insulin resistance. Therefore, it is necessary to
discover a strategy to facilitate β cell proliferation and increase
insulin secretory.
Nuclear receptor-binding SET domain protein 2
(NSD2), also known as Wolf-Hirschhorn syndrome candidate 1 (WHSC1)
or multiple myeloma SET domain (MMSET), together with NSD1 and
NSD3, belongs to the SET histone methyltransferase family (12,13).
As a transcription factor, NSD2 mediates H3K36 dimethylation and
trimethylation, or H4K20 dimethylation (14,15).
Previous studies have shown that NSD2 is up-regulated in multiple
types of human cancers, including small-cell lung cancers, stomach
cancer, neuroblastoma, colon cancer, hepatocellular carcinoma,
ovarian carcinoma and prostate cancer (16–22).
Furthermore, NSD2 has been demonstrated to support the
proliferation and/or survival of many cancer cell lines, such as
prostate cancer (22–24), myeloma cell lines with t(4;14)
translocations (25–28) and leukemia cell lines carrying the
E1099K mutation (29), Wang et
al (30) has found that NSD2
regulates glucose metabolism; however, whether NSD2 plays roles in
diabetes has not been explored.
Here, we found that NSD2 was down-regulated in T2DM.
Down-regulation of NSD2 was associated with elevated glucose level.
Moreover, NSD2 promoted β cell lines proliferation and insulin
secretion. NSD2 as a transcription factor, we found that it
transcriptionally regulated PDX1 expression.
Materials and methods
Cell culture
INS-1 and MIN6 cells were purchased from ATCC
(Manassas, VA, USA) and cultured in 5 mmol/l glucose DMEM (HyClone;
GE Healthcare Life Sciences, Logan, UT, USA) supplemented with 10%
horse serum (Invitrogen; Thermo Fisher Scientific, Inc., Waltham,
MA, USA), 5.5 mM 2-mercaptoethanol, 100 U/ml penicillin and 0.1
mg/ml streptomycin (Invitrogen; Thermo Fisher Scientific, Inc.) at
37°C with 5% CO2.
Blood sample collection
All healthy volunteers and T2DM patients have known
and written informed consent before experiments. All human sample
experiments have been approved by the Ethics Committee of The First
Affiliated Hospital of Zhengzhou University. Blood samples were
collected from The First Affiliated Hospital of Zhengzhou
University during 2013 to 2017 and stored at −80°C immediately
before using.
Cell transfection
The INS-1 and MIN6 cells were placed into a 6-well
plate (a density of 4×105 cells/well) and grown to
70–80% confluence. The cells were then transfected with 2.5 µg
plasmid or 50 nM siRNAs using Lipofectamine 2000 according to
manufacturer's instruction (Invitrogen; Thermo Fisher Scientific,
Inc.). After transfection for 48 h, cells were collected and used
to further experiments. The sequences of siRNA as followed:
Scramble siRNA (SCR): 5′-UUCUCCGAACGUGUCACGU-3′; NSD2 siRNA#1:
5′-TGGAGCACACGAAGCACCA-3′; NSD2 siRNA#2: 5′-TGTCCAGGAACGCTGAGCT-3′;
PDX siRNA: 5′-TCCAAAACCGCCGCATGAAGTGG-3′. The vector plasmid
(pcDNA3.1), pcDNA3.1-NSD2 and pcDNA3.1-PDX1 were purchased from
Vigene Biosciences Co., Ltd., (Shandong, China).
Reverse transcription- quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from human blood samples and
transfected cells using TRIzol (Invitrogen; Thermo Fisher
Scientific, Inc.). cDNA was prepared using a PrimeScript TM RT
reagent kit (Takara Bio, Inc., Otsu, Japan) according to
manufacturer's instruction. Subsequently, SYBR green PCR mix (Roche
Diagnostics GmbH, Mannheim, Germany) was used to perform qRT-PCR
assay according to the manufacturer's protocol. GAPDH was used as
an endogenous control. The primer sequences were as follows: NSD2
forward primer: 5′-TGTAAACCACTGAAGAAGCGA-3′, reverse primer:
5′-GTCCGAGACCTCATTCTCAG-3′; PDX1 forward primer:
5′-CCTTTCCCATGGATGAAGTC-3′ and reverse primer:
5′-AACCAGATCTTGATGTGTCTC-3′; GAPDH forward primer:
5′-TCTCTGATTTGGTCGTATTGG-3′ and reverse primer:
5′-CATGTAAACCATGTAGTTGAGGTC-3′. The relative mRNA expression was
normalized to GAPDH and measured using 2−ΔΔCt (31). Each independent experiment was
performed in triplicate.
Western blotting
Whole protein was prepared from transfected cells
using RIPA lysis buffer (Beyotime Institute of Biotechnology,
Haimen, China) with 1% cocktail (Roche Diagnostics GmbH). The
concentration of protein was measured using a BCA kit (Pierce;
Thermo Fisher Scientific, Inc.) according to manufacturer's
instruction. Approximately 40 µg proteins were separated on a 10%
SDS-PAGE and transferred to a nitrocellulose (NC) membrane.
Following by incubation with 5% skimmed milk at room temperature
for 1 h, the membranes were incubated with primary antibodies at
4°C overnight. The antibodies as followed: NSD2 (1:1,000; cat. no.
75359), PDX1 (1:2,000; cat. no. 47267), β-actin (1:5,000; cat. no.
8226; all from Abcam, Cambridge, MA, USA). The membranes were
washed with TBST three times, and incubated with secondary
horseradish peroxidase (HRP)-conjugated antibodies (1:5,000; cat.
no. 6789 and 6721; Abcam, Cambridge, MA, USA) at room temperature
for 1 h. After washing with TBST three times, the blots were
visualized using ECL kit. Each independent experiment was performed
in triplicate.
CCK-8 cell viability assay
NSD2 was overexpressed or knocked down in INS-1 and
MIN6 cells, 24 h after transfection, approximately 2×103
cells were placed into a 96-well with three wells for each group.
The cells were maintained in an incubator with a 5% CO2
atmosphere at 37°C. At 0, 24, 48 and 72 h, 20 µl CCK-8 solution was
added into medium, and incubated with cells at 37°C for 1 h. The
absorbance was measured at 450 nm with a microplate reader (BioTek
Instruments, Inc., Winooski, VT, USA). The effect of NSD2 on cell
growth and cell viability was determined. Each independent
experiment was performed in triplicate.
Colony formation assay
After transfection for 48 h, approximately
5×104 INS-1 or MINE6 cells were placed into 6-well
plates, and cultured with serum-free DMEM. After incubating for two
weeks, cells were fixed with 4% paraformaldehyde solution at room
temperature for 15 min and stained with 0.1% crystal violet
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) at room temperature
for 15 min. The number of colonies were counted under a light
microscope. Each independent experiment was performed in
triplicate.
Insulin secretion and insulin content
measurements
NSD2 was overexpressed or knocked down in INS-1 and
MIN6, after transfection for 48 h, approximately 3.5×105
cells were placed in a 24-well plate. Cells were washed with SAB
(secretion assay buffer, 0.2% BSA, 2.5 mM CaCl2, 25.5 mM
NaHCO3, 4.7 mM KCl, 20 mM HEPES (pH 7.2), 1.2 mM
KH2PO4, 1.16 mM MgSO4, 2.8 mM
glucose and 114 mM NaCl) for three times. Next, cells were
incubated with 1.5 ml SAB at 37°C with 5% CO2.
Immediately after incubation, a Coat-A-Count insulin RIA kit
(Diagnostic Products Corp., Los Angeles, CA, USA) was used to
measure insulin secretion according to the manufacturer's
instruction. Subsequently, cells were lysed with RIPA buffer (50 mM
Tris HCl pH 8, 150 mM NaCl, 1% NP-40/Triton X, 0.1% SDS, 0.5%
sodium deoxycholate, 2 mM EDTA and 50 mM NaF). The supernatant of
lysate was collected and the protein concentration was measured.
The insulin secretion was normalized by protein concentration. Each
independent experiment was performed in triplicate.
ChIP and qChIP assay
A ChIP assay was performed with 3 µg goat
anti-rabbit IgG (cat. no. ab171870; Abcam), anti-NSD2 (cat. no.
ab75359; Abcam) and anti-H3K36me2 (cat. no. ab9043; Abcam) using a
ChIP assay kit (Beyotime Institute of Biotechnology) according to
the manufacturer's instructions. The antibody-bound DNA was used to
perform qChIP. SYBR green Mix (Roche Diagnostics GmbH) was used to
perform RT-qPCR. The thermocycling conditions were as follows: 30
cycles of denaturation at 95°C for 5 min, annealing at 57°C for 30
sec followed by extension at 72°C for 5 min. The following primers
were used: PDX1 forward primer: 5′-AGGGTCTCATTCTGTCGTTC-3′ and
reverse primer: 5′-GCCTGTAATCCCAGCTACTC-3′. The fold of enrichment
was normalized to that of IgG and quantified using the
2−ΔΔCt method. Each independent experiment was performed
in triplicate.
Luciferase reporter assay
The promoter region (−2000 to +200) of PDX1 were
cloned into pGL3 luciferase vector (Invitrogen; Thermo Fisher
Scientific, Inc.). For the luciferase assay, INS-1 and MIN6 cells
were co-transfected with pGL3-slug, renilla and NSD2 or vector.
After transfection for 24 h, luciferase reporter assay was
performed using a dual-luciferase reporter assay kit according to
manuscript's protocol (Promega Corporation, Madison, WI, USA). The
Renilla luciferase activity was used to normalize firefly
luciferase activity. Each independent experiment was performed in
triplicate.
Statistical analysis
Each independent experiment was performed in
triplicate for statistical analysis. The data are presented as the
mean ± SD. All data were analyzed using GraphPad Prism v.5.01
(GraphPad Software, Inc., La Jolla, CA, USA). The correlation
between the clinic pathological features of T2DM patient and NSD2
expression was analyzed by χ2 test. One-way ANOVA and
Tukey test were used to analyze the difference between multiple
comparisons. Two-tailed Student t-test was used to analyze the
difference between two groups. The correlation of blood glucose
levels and NSD2 expression was determined by Pearson's correlation
coefficient. P<0.05 was considered to indicate a statistically
significant difference.
Results
NSD2 expression is down-regulated in
T2DM
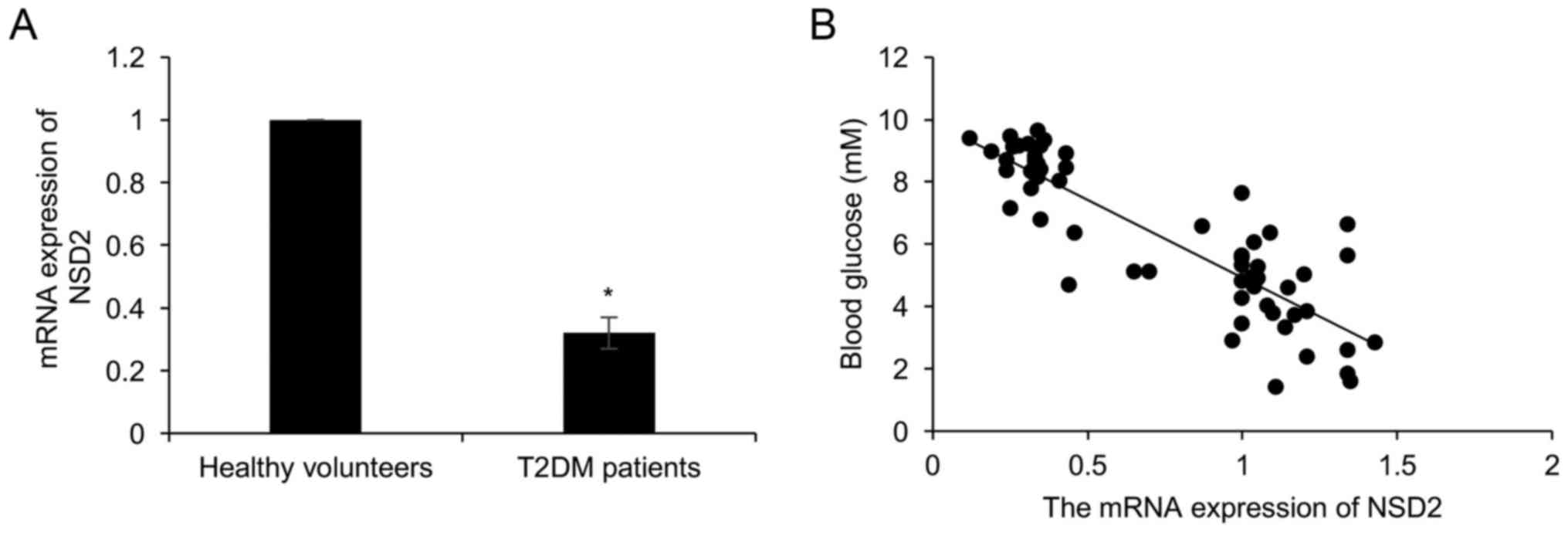
To explore the underlying mechanism of NSD2 in T2DM,
we collected the plasma from 58 T2DM patients and 58 healthy
volunteers, and detected the expression of NSD2. As shown in
Fig. 1A, the expression of NSD2 in
T2DM patients was less than that in healthy volunteers group
(Fig. 1A). Moreover, the
association between NSD2 expression and patient clinical
information was analyzed, we found that the expression NSD2 had no
correlation with age and gender (Table
I). Furthermore, we found that the NSD2 expression was
negatively associated with the glucose concentration (r=0.81;
Fig. 1B).
 | Table I.Clinicopathologic variables in 58
patients with T2DM. |
Table I.
Clinicopathologic variables in 58
patients with T2DM.
|
|
| NSD2 protein
expression |
|
|---|
|
|
|
|
|
|---|
| Variables | No. (n=58) | Low (n=40) | High (n=18) | P-value |
|---|
| Age |
|
|
| 0.509 |
|
<35 | 35 | 23 | 12 |
|
| ≥35 | 23 | 17 | 6 |
|
| Sex |
|
|
| 0.724 |
|
Male | 27 | 18 | 9 |
|
|
Female | 31 | 22 | 9 |
|
| Glucose
concentration |
|
|
| 0.040 |
| <7.0
mM | 21 | 11 | 10 |
|
| >7.0
mM | 37 | 29 | 8 |
|
| PDX1
expression |
|
|
| 0.008 |
|
Low | 37 | 30 | 7 |
|
|
High | 21 | 10 | 11 |
|
NSD2 promotes the proliferation of
pancreatic β cell lines and the insulin secretion
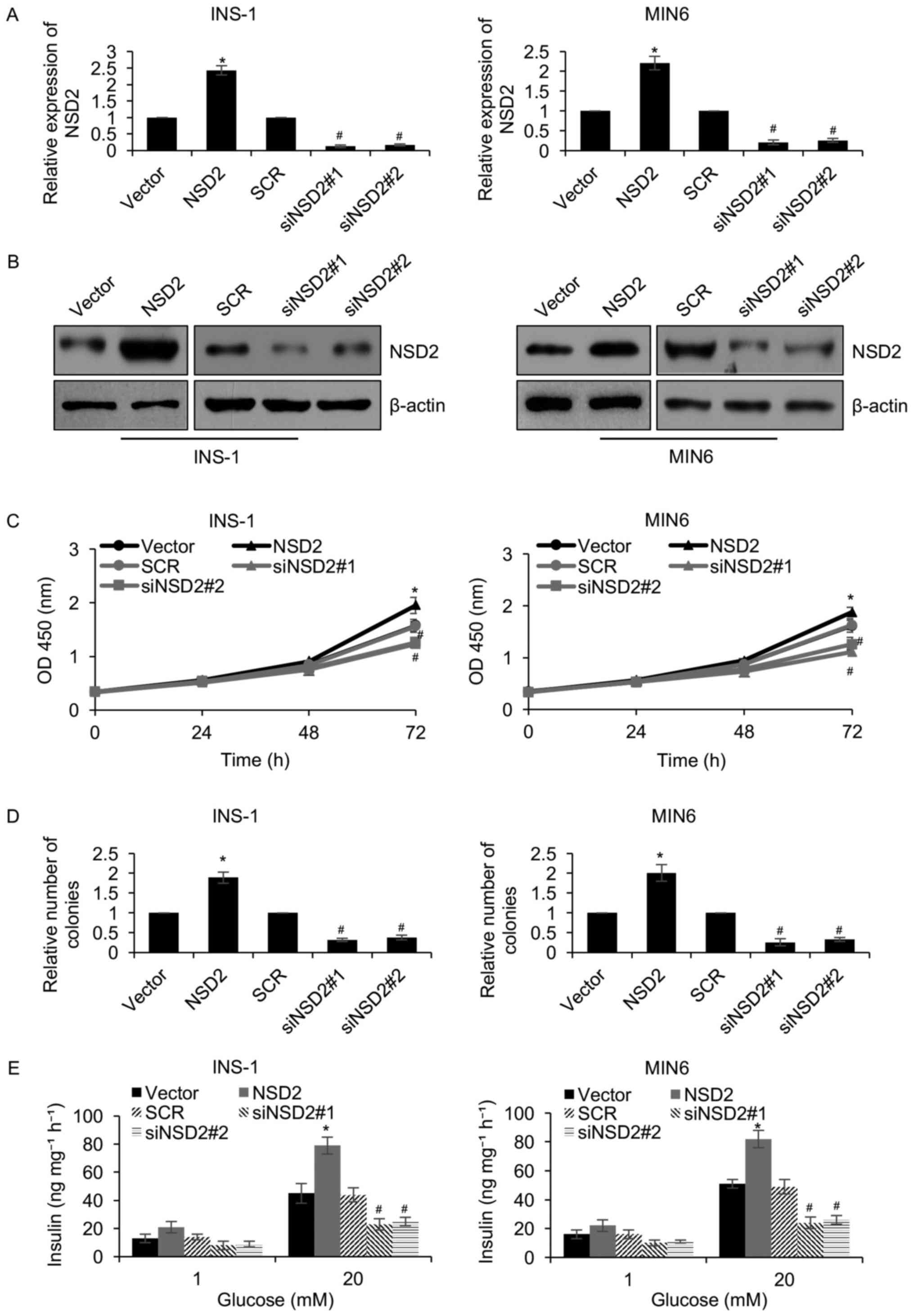
In order to further decipher the role of NSD2 in
diabetes, we overexpressed or knocked down NSD2 in two pancreatic β
cell lines INS-1 and MIN6. The NSD2 expression was detected by
western blotting and qRT-PCR, respectively. The results revealed
transfection with plasmids expressing NSD2 increased two-fold NSD2
levels in INS-1 and MIN6 cells, and NSD2 expression was decreased
followed by transfected with NSD2 siRNA (siNSD2) (Fig. 2A and B). Subsequently, CCK-8
analysis was performed to determine the function of NSD2 on
cellular proliferation in pancreatic β cell lines, suggesting that
ectopic expression of NSD2 obviously facilitated the proliferation
rate of INS-1 and MIN6 cells, compared to the control group;
however, inhibition of NSD2 significantly led to a decrease of
proliferation rate of INS-1 and MIN6 cells, compared to the control
group (Fig. 2C). Moreover, similar
results were observed in colony formation assay, suggesting that
compared to the control groups, ectopic regulation of NSD2 resulted
in an elevated number of colonies in INS-1 and MIN6 cells (Fig. 2D). Meanwhile, NSD2 inhibition
decreased the number of colonies in INS-1 and MIN6 cells, compared
to the control groups (Fig. 2D).
In addition, in response to glucose stimulation, we found that
ectopic expression of NSD2 remarkably improved the insulin
secretion (Fig. 2E); however,
inhibition of NSD2 significantly suppressed insulin secretion
(Fig. 2E). Our findings suggest
that NSD2 facilitates the proliferation of pancreatic β cell lines
and the insulin secretion.
NSD2 transcriptionally regulates PDX1
through its histone methylation activity in diabetes
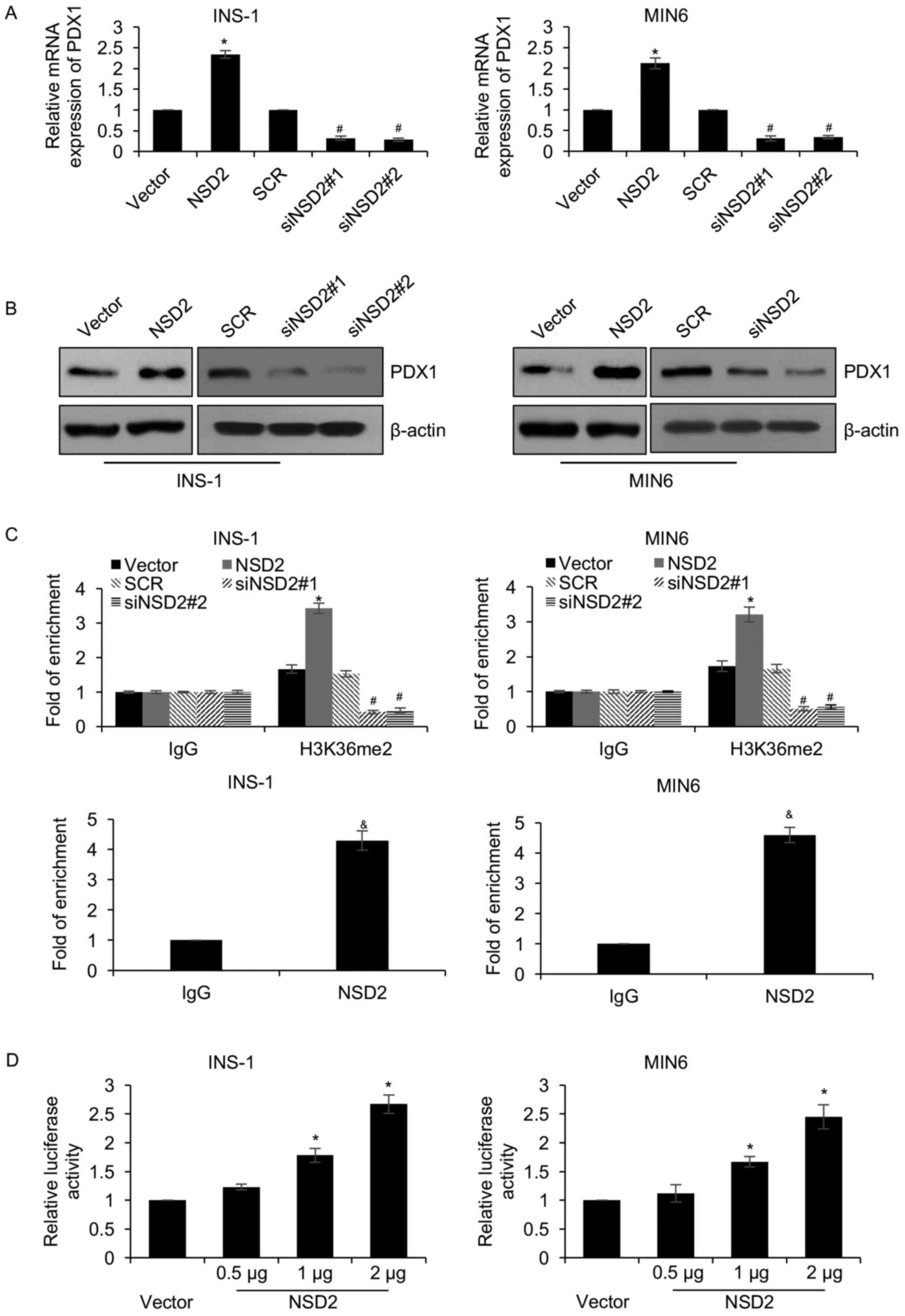
As a histone methylase, NSD2 has been reported to
regulate gene expression. Moreover, PDX1 has been reported to play
key roles in diabetes (32).
Because we found NSD2 regulated pancreatic β cells proliferation
and insulin secretion, we assumed that NSD2 might regulate PDX1
expression in diabetes. To verify our hypothesis, we detected the
expression of PDX1 after overexpression or knockdown NSD2 in INS-1
and MIN6 cells. The results of western blotting and qRT-PCR
analyses demonstrated that ectopic expression of NSD2 significantly
increased PDX1 expression, and knockdown of NSD2 obviously
decreased PDX1 expression (Fig. 3A and
B). That both protein and mRNA levels of PDX1 were regulated by
NSD2 indicated that PDX1 might be regulated by NSD2 at
transcription level. Subsequently, ChIP and qChIP assay was
performed to determine whether NSD2 transcriptionally regulated
PDX1 expression through methylating H3K36, showing that the
occupation of H3K36me2 at the promoter region of PDX1 was
obviously increased while NSD2 was overexpressed (Fig. 3C); however, the occupation of
H3K36me2 at the promoter region of PDX1 was obviously
decreased while NSD2 was knocked down (Fig. 3C). Additionally, we found that NSD2
could bind the promoter region of PDX1 (Fig. 3C). The similar results were
observed in MIN6 cells (Fig. 3C).
Moreover, dual luciferase reporter assay comfirmed that NSD2
transcriptionally activated PDX1 (Fig.
3D). In addition, we found PDX1 expression in the plasma from
T2DM patients was less than that in healthy volunteers, positively
correlated with NSD2 expression (Table
I). These results reveal that NSD2 transcriptionally regulates
PDX1 through its histone methylation activity in diabetes.
PDX1 is involved in the NSD2-mediated
insulin secretion and pancreatic β cells proliferation
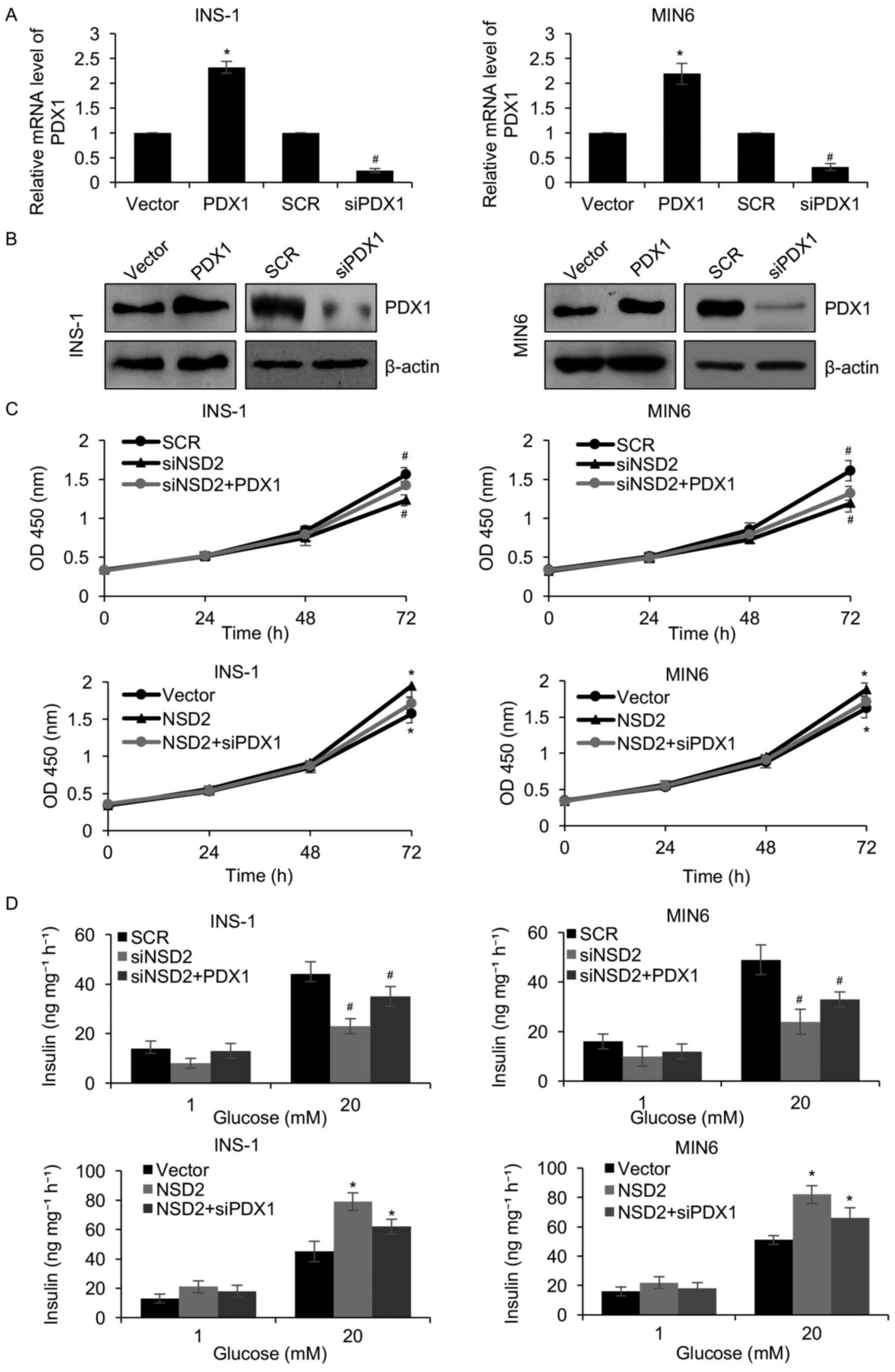
Because PDX1 was transcriptionally regulated by
NSD2, so we hypothesized that NSD2 facilitated pancreatic β cells
proliferation and insulin secretion through regulation of PDX1. In
order to verify our hypothesis, we overexpressed or knocked down of
PDX1 in INS-1 and MIN6 cells. Western blotting and qRT-PCR analyses
were used to determine the protein and mRNA levels of PDX1,
respectively. The results of western blotting and qRT-PCR analyses
revealed that both protein and mRNA levels of PDX1 were obviously
increased approximately 2.4-fold in the INS-1 and MIN6 cells
transfected with FLAG-PDX1 plasmid, and decreased following PDX1
siRNA (Fig. 4A and B).
Subsequently, we explored whether PDX1 involved in the
NSD2-mediated pancreatic β cell proliferation and insulin
secretion. As shown in Fig. 4C,
the CCK-8 assay revealed that ectopic expression of PDX1
significantly rescued the effect of the NSD2 inhibition on
pancreatic β cells proliferation (Fig.
4C). Meanwhile, inhibition of PDX1 significantly impaired the
effect of the NSD2 overexpression on pancreatic β cells
proliferation (Fig. 4C).
Similarly, overexpression of PDX1 also rescued the effect of NSD2
inhibition on glucose-stimulated insulin secretion, and inhibition
of PDX1 significantly impaired the effect of the NSD2
overexpression on glucose-stimulated insulin secretion (Fig. 4D). To sum up, our work show that
PDX1 is a downstream target of NSD2, and NSD2 promotes the
proliferation of pancreatic β cell lines and the insulin secretion
through regulation of PDX1.
Discussion
Previous studies have shown that NSD2 was the
up-regulated in multiple types of human cancers (16–22).
However, the role of NSD2 in T2DM has still not been
elucidated.
Here, we found that NSD2 was down-regulated in T2DM
patients, compared with healthy volunteers group. Moreover, the
expression of NSD2 was negatively associated with high blood
glucose levels, suggesting NSD2 might play a key role in T2DM.
Previous work has found that NSD2 regulates glucose metabolism
(30). To further consider the
effect of NSD2 in T2DM, we used two pancreatic β cell lines, such
as INS-1 and MIN6. NSD2 has been reported to promote cell
proliferation in several cancers (22–24).
Here, CCK8 assay and colony formation assay demonstrated that
ectopic expression of NSD2 facilitated the proliferation of INS-1
and MIN6 cells. Knockdown of NSD2 suppressed the proliferation of
INS-1 and MIN6 cells. Furthermore, the secretion of insulin also
regulated by NSD2.
PDX1 has been reported to play fundamental roles in
diabetes mellitus, abnormal expression of PDX1 suppressed
pancreatic β cell proliferation (32,33).
NSD2 as transcription factor, we assumed NSD2 might
transcriptionally regulated PDX1 in pancreatic β cell lines. ChIP
and qChIP assay as well as luciferase assay suggested PDX1 was
transcriptionally activated by NSD2 through its H3K36me2
methylation activity. Furthermore, we found that NSD2 promoted
pancreatic β cell lines proliferation and insulin secretion through
regulation of PDX1.
Here, our study still has some limitation, we admire
that it is better to detect the expression of NSD2 in T2DM
patients' and health volunteers' pancreatic β cells, but we don't
have enough samples to do it. Moreover, it is necessary to specific
overexpression of NSD2 in mice pancreatic tissues and further
determine the roles of NSD2 in vivo. Furthermore, more
downstream target of NSD2 need be detected using ChIP-seq assay, it
might further uncover the function of NSD2.
Collectively, the current study revealed the
mechanisms of NSD2 on pancreatic β cell proliferation and insulin
secretion in T2DM. We suggested NSD2 as a novel molecular therapy
target of T2DM.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
LLZ and SS conceived and designed the work. SS and
LZ constructed expression plasmids, prepared proteins, and
performed experiments. SS and LZ analyzed the data. SS and LLZ
wrote the paper.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of The First Affiliated Hospital of Zhengzhou University,
and written informed consent was provided from patients and healthy
volunteers prior to enrolment.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gustafson B: Adipose tissue, inflammation
and atherosclerosis. J Atheroscler Thromb. 17:332–341. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Donath MY, Storling J, Berchtold LA,
Billestrup N and Mandrup-Poulsen T: Cytokines and beta-cell
biology: From concept to clinical translation. Endocr Rev.
29:334–350. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Berk BC, Weintraub WS and Alexander RW:
Elevation of C-reactive protein in ‘active’ coronary artery
disease. Am J Cardiol. 65:168–172. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ridker PM, Cushman M, Stampfer MJ, Tracy
RP and Hennekens CH: Inflammation, aspirin and the risk of
cardiovascular disease in apparently healthy men. N Engl J Med.
336:973–979. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Inzucchi SE, Bergenstal RM, Buse JB,
Diamant M, Ferrannini E, Nauck M, Peters AL, Tsapas A, Wender R,
Matthews DR, et al: Management of hyperglycemia in type 2 diabetes:
A patient-centered approach: Position statement of the American
Diabetes Association (ADA) and the European Association for the
Study of Diabetes (EASD). Diabetes Care. 35:1364–1379. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yerlikaya O, Acu M and Kinik O: Importance
of dairy products in cardiovascular diseases and type 2 diabetes.
Crit Rev Food Sci Nutr. 53:902–908. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Stoffers DA: The development of beta-cell
mass: Recent progress and potential role of GLP-1. Horm Metab Res.
36:811–821. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tourrel C, Bailbe D, Lacorne M, Meile MJ,
Kergoat M and Portha B: Persistent improvement of type 2 diabetes
in the Goto-Kakizaki rat model by expansion of the beta-cell mass
during the prediabetic period with glucagon-like peptide-1 or
exendin-4. Diabetes. 51:1443–1452. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sakuraba H, Mizukami H, Yagihashi N, Wada
R, Hanyu C and Yagihashi S: Reduced beta-cell mass and expression
of oxidative stress-related DNA damage in the islet of Japanese
Type II diabetic patients. Diabetologia. 45:85–96. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Marchetti P, Del Guerra S, Marselli L,
Lupi R, Masini M, Pollera M, Bugliani M, Boggi U, Vistoli F, Mosca
F and Del Prato S: Pancreatic islets from type 2 diabetic patients
have functional defects and increased apoptosis that are
ameliorated by metformin. J Clin Endocrinol Metab. 89:5535–5541.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cozar-Castellano I, Fiaschi-Taesch N,
Bigatel TA, Takane KK, Garcia-Ocaña A, Vasavada R and Stewart AF:
Molecular control of cell cycle progression in the pancreatic
beta-cell. Endocr Rev. 27:356–370. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jablecka A, Bogdanski P, Balcer N,
Cieslewicz A, Skoluda A and Musialik K: The effect of oral
L-arginine supplementation on fasting glucose, HbA1c, nitric oxide
and total antioxidant status in diabetic patients with
atherosclerotic peripheral arterial disease of lower extremities.
Eur Rev Med Pharmacol Sci. 16:342–350. 2012.PubMed/NCBI
|
|
13
|
Hollink IH, van den Heuvel-Eibrink MM,
Arentsen-Peters ST, Pratcorona M, Abbas S, Kuipers JE, van Galen
JF, Beverloo HB, Sonneveld E, Kaspers GJ, et al: NUP98/NSD1
characterizes a novel poor prognostic group in acute myeloid
leukemia with a distinct HOX gene expression pattern. Blood.
118:3645–3656. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li Y, Trojer P, Xu CF, Cheung P, Kuo A,
Drury WJ III, Qiao Q, Neubert TA, Xu RM, Gozani O and Reinberg D:
The target of the NSD family of histone lysine methyltransferases
depends on the nature of the substrate. J Biol Chem.
284:34283–34295. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Morishita M, Mevius D and di Luccio E: In
vitro histone lysine methylation by NSD1, NSD2/MMSET/WHSC1 and
NSD3/WHSC1L. BMC Struct Biol. 14:252014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Colak H, Uzgur R, Tan E, Hamidi MM, Turkal
M and Colak T: Investigation of prevalence and characteristics of
mesiodens in a non-syndromic 11256 dental outpatients. Eur Rev Med
Pharmacol Sci. 17:2684–2689. 2013.PubMed/NCBI
|
|
17
|
Hudlebusch HR, Skotte J, Santoni-Rugiu E,
Zimling ZG, Lees MJ, Simon R, Sauter G, Rota R, De Ioris MA, Quarto
M, et al: MMSET is highly expressed and associated with
aggressiveness in neuroblastoma. Cancer Res. 71:4226–4235. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Toyokawa G, Cho HS, Masuda K, Yamane Y,
Yoshimatsu M, Hayami S, Takawa M, Iwai Y, Daigo Y, Tsuchiya E, et
al: Histone lysine methyltransferase Wolf-Hirschhorn syndrome
candidate 1 is involved in human carcinogenesis through regulation
of the Wnt pathway. Neoplasia. 13:887–898. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hudlebusch HR, Santoni-Rugiu E, Simon R,
Ralfkiær E, Rossing HH, Johansen JV, Jørgensen M, Sauter G and
Helin K: The histone methyltransferase and putative oncoprotein
MMSET is overexpressed in a large variety of human tumors. Clin
Cancer Res. 17:2919–2933. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhou P, Wu LL, Wu KM, Jiang W, Li JD, Zhou
LD, Li XY, Chang S, Huang Y, Tan H, et al: Overexpression of MMSET
is correlation with poor prognosis in hepatocellular carcinoma.
Pathol Oncol Res. 19:303–309. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang S, Zhang Y, Meng F, Liu Y, Xia B,
Xiao M, Xu Y, Ning X, Li H and Lou G: Overexpression of multiple
myeloma SET domain (MMSET) is associated with advanced tumor
aggressiveness and poor prognosis in serous ovarian carcinoma.
Biomarkers. 18:257–263. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang P, Guo L, Duan ZJ, Tepper CG, Xue L,
Chen X, Kung HJ, Gao AC, Zou JX and Chen HW: Histone
methyltransferase NSD2/MMSET mediates constitutive NF-kappaB
signaling for cancer cell proliferation, survival and tumor growth
via a feed-forward loop. Mol Cell Biol. 32:3121–3131. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ezponda T, Popovic R, Shah MY,
Martinez-Garcia E, Zheng Y, Min DJ, Will C, Neri A, Kelleher NL, Yu
J and Licht JD: The histone methyltransferase MMSET/WHSC1 activates
TWIST1 to promote an epithelial-mesenchymal transition and invasive
properties of prostate cancer. Oncogene. 32:2882–2890. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Asangani IA, Ateeq B, Cao Q, Dodson L,
Pandhi M, Kunju LP, Mehra R, Lonigro RJ, Siddiqui J, Palanisamy N,
et al: Characterization of the EZH2-MMSET histone methyltransferase
regulatory axis in cancer. Mol Cell. 49:80–93. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kuo AJ, Cheung P, Chen K, Zee BM, Kioi M,
Lauring J, Xi Y, Park BH, Shi X, Garcia BA, et al: NSD2 links
dimethylation of histone H3 at lysine 36 to oncogenic programming.
Mol Cell. 44:609–620. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lauring J, Abukhdeir AM, Konishi H, Garay
JP, Gustin JP, Wang Q, Arceci RJ, Matsui W and Park BH: The
multiple myeloma associated MMSET gene contributes to cellular
adhesion, clonogenic growth and tumorigenicity. Blood. 111:856–864.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Brito JL, Walker B, Jenner M, Dickens NJ,
Brown NJ, Ross FM, Avramidou A, Irving JA, Gonzalez D, Davies FE
and Morgan GJ: MMSET deregulation affects cell cycle progression
and adhesion regulons in t(4;14) myeloma plasma cells.
Haematologica. 94:78–86. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Martinez-Garcia E, Popovic R, Min DJ,
Sweet SM, Thomas PM, Zamdborg L, Heffner A, Will C, Lamy L and
Staudt LM: The MMSET histone methyl transferase switches global
histone methylation and alters gene expression in t(4;14) multiple
myeloma cells. Blood. 117:211–220. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jaffe JD, Wang Y, Chan HM, Zhang J,
Huether R, Kryukov GV, Bhang HE, Taylor JE, Hu M, Englund NP, et
al: Global chromatin profiling reveals NSD2 mutations in pediatric
acute lymphoblastic leukemia. Nat Genet. 45:1386–1391. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang J, Duan Z, Nugent Z, Zou JX, Borowsky
AD, Zhang Y, Tepper CG, Li JJ, Fiehn O, Xu J, et al: Reprogramming
metabolism by histone methyltransferase NSD2 drives endocrine
resistance via coordinated activation of pentose phosphate pathway
enzymes. Cancer Lett. 378:69–79. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fujitani Y: Transcriptional regulation of
pancreas development and β-cell function [Review]. Endocr J.
64:477–486. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Spaeth JM, Gupte M, Perelis M, Yang YP,
Cyphert H, Guo S, Liu JH, Guo M, Bass J, Magnuson MA, et al:
Defining a novel role for the PDX1 transcription factor in islet
beta cell maturation and proliferation during weaning. Diabetes.
66:2830–2839. 2017. View Article : Google Scholar : PubMed/NCBI
|