Introduction
Keratosis pilaris (KP; OMIM #604093), also known as
lichen pilaris, is a benign genodermatosis that is estimated to
effect ~40% of the population (1).
KP is characterized by the presence of symmetric, asymptomatic and
grouped keratotic follicular papules with varying degrees of
perifollicular erythema. KP lesions often involve the proximal and
extended parts of extremities, the cheeks and the buttocks
(2). Cases may be generalized or
unilateral (2). Most patients
develop KP in their childhood, with a peak in incidence during
adolescence (3). The incidence of
KP subsequently decreases with age in 35% of those affected, but
can persist in adulthood within 43%, and worsen in 22% of patients
(4). KP can exist as an
independent condition or in association with other diseases, such
as xerosis, ichthyosis, atopic dermatitis and various conditions,
including keratitis-ichthyosiss-deafness syndrome, Noonan syndrome
and cardiofaciocutaneous syndrome (4–6).
Genetic epidemiology surveys have indicated that KP is an autosomal
dominant-inherited disease (4). A
study reported a deletion of the short arm of chromosome 18 may be
associated with KP (7). Brown
et al (8), Mevorah et
al (9) and Sandilands et
al (10) identified mutations
in the filaggrin (FLG) gene associated with atopic
dermatitis and ichthyosis vulgaris in KP. Gruber et al
(3) revealed that only 35% of
patients with KP have mutations in FLG, suggesting that
FLG mutations only partially result in the KP phenotype.
Other genes may be the causative genetic factor contributing to the
development of KP; however, further investigation is required.
Nevus comedonicus (NC) is a rare skin disorder
caused by abnormal development of the pilosebaceous unit. The
prevalence of NC has been estimated to occur in 1/45,000 to
1/100,000 individuals, with no gender or racial association
(11). The pathogenesis of NC is
yet to be investigated. Genetic studies have revealed that the
fibroblast growth factor (FGF) and FGF receptor-2 signaling
pathways, and somatic mutations of tyrosine kinase receptors or
never in mitosis gene A-related kinase 9 (12) may be associated with the
development of NC (13). The
clinical features of NC include grouped, dilated hair follicles
with a dark, firm hyperkeratotic plug at the center. NC can present
with open or closed comedo, which primarily occur on the face, neck
and upper trunk, and may be linear, interrupted, unilateral,
bilateral or segmental (14).
Clinically, there are two types of NC: Noninflammatory and
inflammatory, with the formation of cysts, fistulas, and pustules
(15).
In the present study, a novel heterozygous missense
mutation was identified in ATP-binding cassette sub-family A member
12 (ABCA12) within a family afflicted with KP. In addition,
upregulated ABCA12 expression levels in the sebaceous glands
of patients with NC were investigated.
Materials and methods
High-throughput sequencing
Written informed consent was obtained from patients
and individuals involved in the present study. The present study
was approved by the ethics committee of Nanfang Hospital of
Southern Medical University (Guangzhou, China). A total of 5 ml of
peripheral blood was obtained from one KP and two NC probands,
family members and 100 unrelated healthy controls (aged 18–28 years
old, 44 males and 56 females, recruited in December 2015). The KP
proband was a 12-year-old female (recruited August 2013). The NC
probands were an 8-year-old male (recruited May 2014) and a
17-year-old male (recruited December 2015). Genomic DNA was
extracted using the Blood DNA Mini kit (cat. no. 3001050; Hangzhou
Simgen Biotechnology Co., Ltd., Hangzhou, China) according to the
manufacturer's protocol. The genomic sequences of 147 genes
associated with 143 skin diseases were initially analyzed in the KP
proband using a custom-designed GeneChip [Skin single-gene genetic
disease detection package, Illumina High-throughput sequencing
platform (Illumina, Inc., San Diego, CA, USA), Beijing Genomics
Institute, Guangdong, China] (P<0.05). The details of the gene
and skin disease analysis were not presented.
Polymerase chain reaction (PCR) and
Sanger sequencing
Specific primers for the ABCA12 gene were
designed using the web-based tool Primer 3 (http://primer3.ut.ee/; ABCA12 primer sequences
available on reasonable request). All exons and exon-intron
boundaries of the ABCA12 gene were amplified using PCR with
a 20 µl total reaction volume containing 1 µl genomic DNA with a
final concentration of 30 ng/µl, 10 µl 2X Taq PCR StarMix (Taq DNA
polymerase, Mg2+, dNTPs and reaction buffer solution;
cat. no. A112-05, GenStar Biosolutions Co., Ltd.), 1 µl (10 µM)
forward and reverse primers, and 7 µl ddH2O. The
thermocycling conditions were as follows: Initial denaturation at
94°C for 5 min, 35 cycles of 94°C for 30 sec, annealing at 57°C for
30 sec and 72°C for 15 sec, followed by a final extension at 72°C
for 15 sec. PCR products were confirmed using 1% agarose gel
electrophoresis and sequenced. The sequencing results were analyzed
on an ABI 3130 genetic analyzer (Applied Biosystems; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). The functional alterations in
the protein were predicted by web-based tools PolyPhen-2
(genetics.bwh.harvard.edu/pph2/) and SIFT release 63
(sift.jcvi.org/). Sequence conservation
surrounding the mutated region was verified using ClustalX version
2.1 (http://www.clustal.org/).
Immunofluorescence
Skin lesions from two patients with NC and the
patient with KP were collected (one per patient; ~0.8×1 cm). The
lesion from the KP proband was obtained from the left leg. NC
samples were obtained from the waist and neck. Samples were fixed
at room temperature by immersion in 10% formalin solution and,
after 12 h the samples were processed into paraffin blocks.
Sections (~3–4 µm) were obtained, and the prepared paraffin
sections were placed on adhesive slides and incubated overnight at
37°C. Immunofluorescence staining was performed as described below.
The slides were de-waxed in 100% dimethylbenzene twice and
subsequently dehydrated via a descending alcohol (100, 95, 85 and
70%) gradient. The antigen was retrieved via incubation in a water
bath (95°C) for 6 min and then cooled to room temperature. The
samples were blocked with 3% peroxide-methanol for 20 min at room
temperature to quench endogenous peroxidase activity and then
rinsed in PBS three times. The following steps were conducted in a
moisture chamber: i) Samples were incubated with rabbit anti-ABCA12
antibody (cat. no. bs-11906R; BIOSS, Beijing, China) diluted 1:50
in antibody diluent (cat. no. IH0340; Beijing Leagene Biotech Co.,
Ltd., Beijing, China) at 37°C for 1 h and then 4°C overnight. ii)
Following rinsing with PBS three times, DyLight goat anti-rabbit
green fluorescent protein (cat. no. 4412; CST Biological Reagents
Co., Ltd., Shanghai, China) diluted 1:500 in antibody diluent was
applied; the samples were then incubated at room temperature
without light for 90 min. iii) Following three rinses with PBS, the
slides were stained with DAPI at room temperature for 10 min. iv)
After washing with PBS for 5 min, the slides were sealed with
glycerin. v) The slides were allowed to dry naturally and then
visualized under a fluorescence microscope at 360 and 488 nm
(magnification, ×200). A total of 5 samples of normal skin were
also analyzed which was obtained from a normal skin biopsy.
Immunohistochemistry was conducted in the Central Laboratory of
Nanfang Hospital of the Southern Medical University.
Results
Familial background
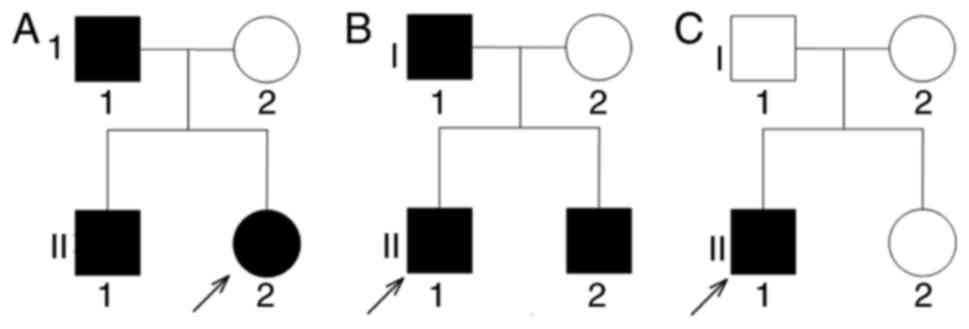
The familial association between the proband in a
family with KP was presented in Fig.
1A; that of the two NC families were presented in Fig. 1B and C.
Clinical and histopathological
findings
KP family
A Chinese pedigree family with three members
afflicted with KP across two generations (Fig. 1A) were employed in the present
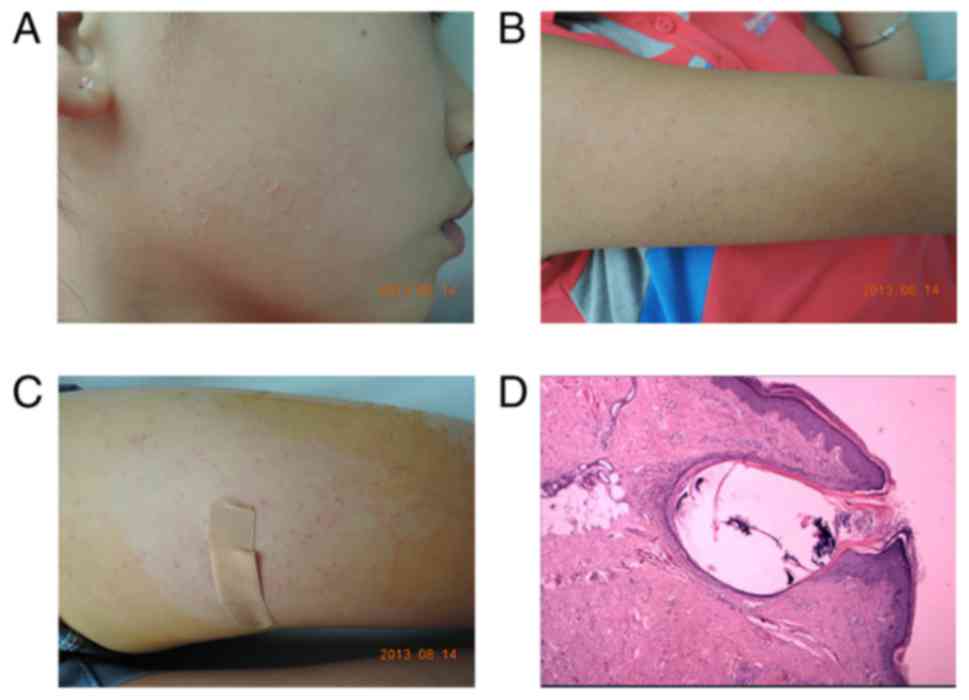
study. The proband, a 12-year-old female, presented symmetric,
asymptomatic and rough keratotic follicular papules, and mild
perifollicular erythema at the cheek. The extensor of the upper
arms and anterior thighs (Fig.
2A-C) were analyzed. The patient had no notable medical or
family history and no mental retardation. The father and younger
brother of the proband presented similar phenotypes.
Histopathology of skin biopsies
Samples were fixed at room temperature by immersion
in 10% formalin solution (at room temperature) and after 12 h the
samples were processed into paraffin blocks. Sections (~3–4 µm)
were obtained, and the prepared paraffin sections were placed on
adhesive slides and incubated overnight at 37°C. The slides were
de-waxed in 100% dimethylbenzene twice and subsequently dehydrated
via a descending alcohol (100, 95, 85 and 70%) gradient. Then the
slides were stained with hematoxylin (cat. no. DH0001; Beijing
Leagene Biotech Co., Ltd., Beijing, China) for 8 min, then wash in
pure water for 3 sec and finally stained with eosin (cat. no.
DH0050; Beijing Leagene Biotech Co., Ltd.) for 1 min at room
temperature. Finally the slides were visualized under a light
microscope (magnification, ×200). The samples were obtained from
the left thigh of the proband and revealed that the follicular
orifice was distended by a keratin plug; mild infiltration of
mononuclear cells in the superficial dermis was observed (Fig. 2D), supporting a diagnosis of
KP.
NC patients
A total of two NC pedigrees were employed in the
present study. The proband of the first family was an 8-year-old
male with a 4-year medical history. The clinical manifestations of
the proband constituted numerous keratotic papules and comedo-like
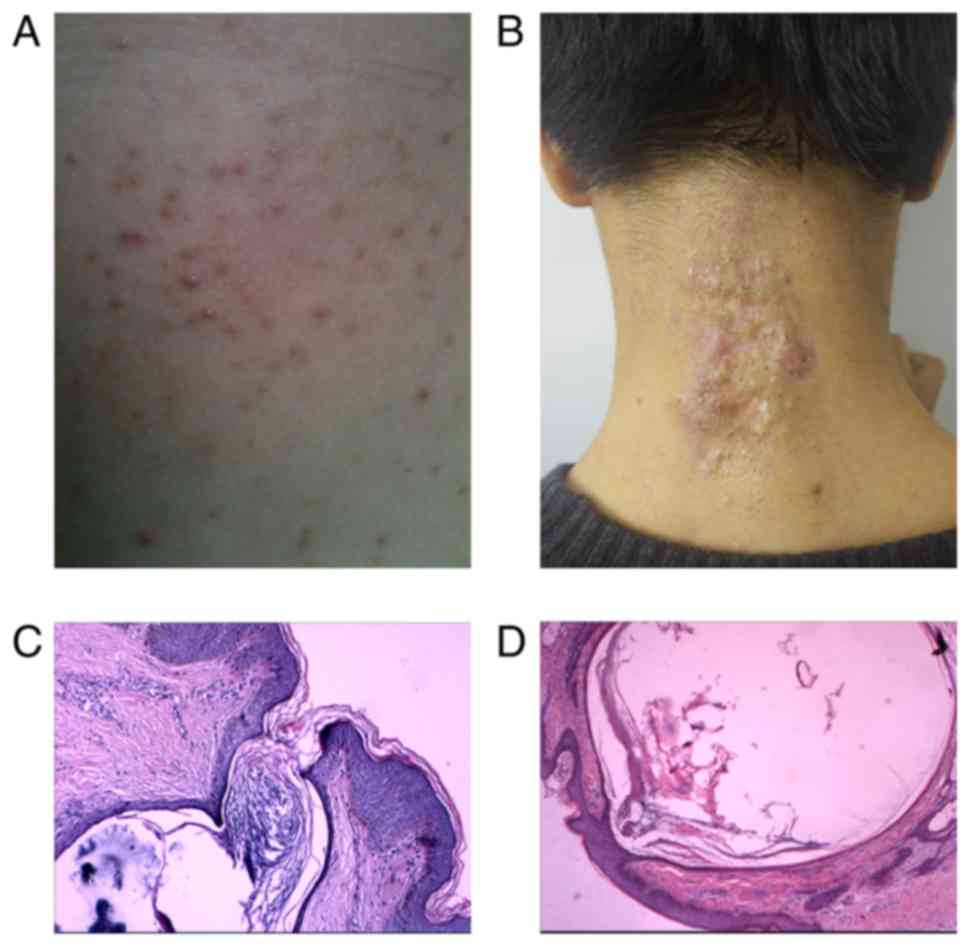
lesions on the waist, buttocks and legs (data not shown). Physical
examination revealed symmetric, light brown-colored papules 2–5 mm
in size (Fig. 3A). The father and
younger brother of the proband (Fig.
1B) exhibited the same symptoms. The second patient, a
17-year-old male, presented aggregated, dilated hair follicles,
blackheads with cysts, fistulas and abscesses on the neck (Fig. 3B). Family disease history was not
positive for the second NC family (Fig. 1C). The pathology of both patients
demonstrated dilated follicular ostia filled with keratin layers
(Fig. 3C and D).
Mutation screening for the ABCA12
genomic sequence
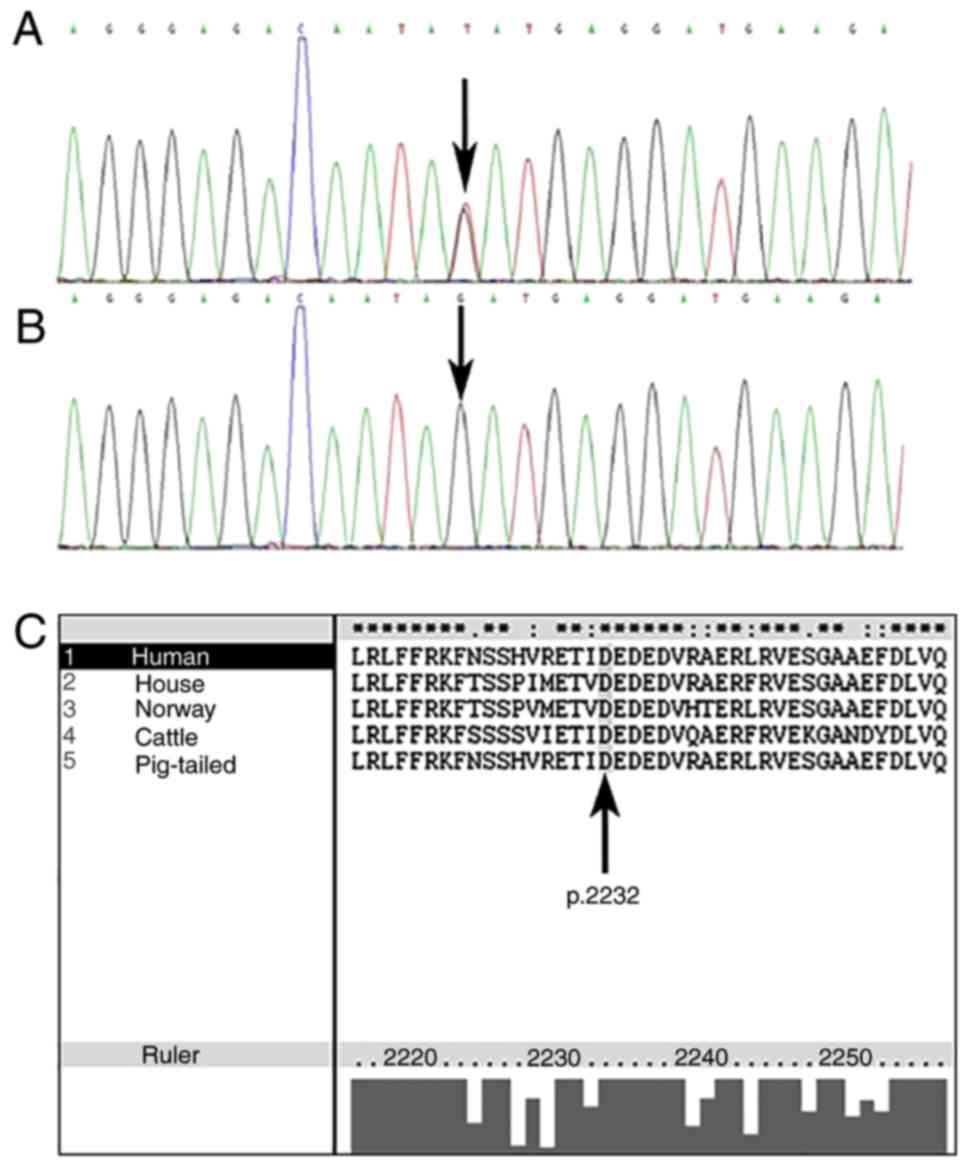
High-throughput sequencing revealed a heterozygous
missense c.6694G>T (p.Asp2232Tyr) mutation in ABCA12 in
the KP proband (Fig. 4A), and
direct sequencing of all coding and exon-intron boundaries
confirmed that this mutation was present in all of the affected KP
family members, but not present in healthy family members, patients
with NC or 100 population-matched controls (Fig. 4B). PolyPhen-2 and SIFT-based
investigations suggested that this mutation may produce a damaged
protein. The region surrounding this mutation is highly conserved
among other species, according to ClustalX (Fig. 4C). The aforementioned results
support the hypothesis that the p.Asp2232Tyr mutation in
ABCA12 may be one of the pathological factors that
contribute to KP.
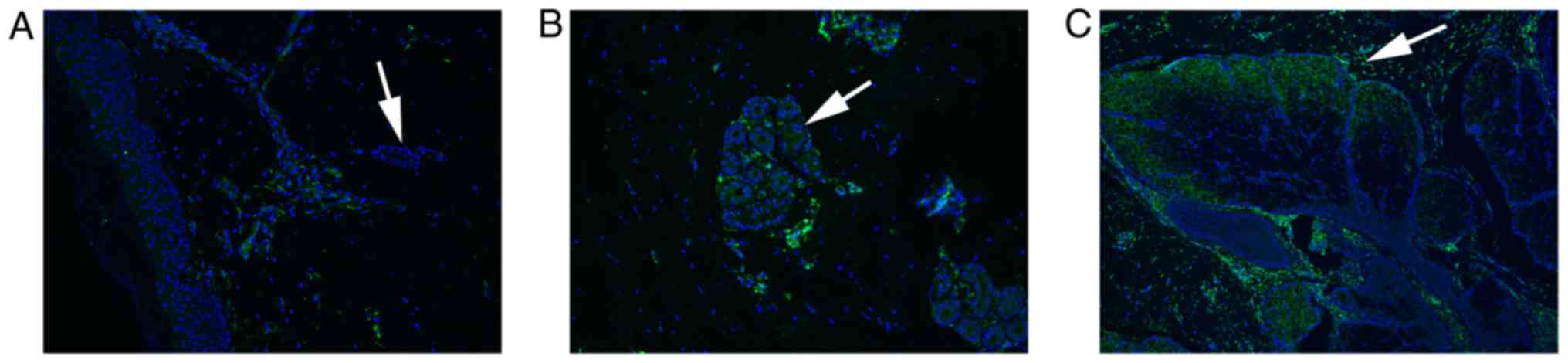
ABCA12 is highly expressed in the
sebaceous gland
To understand whether ABCA12, which regulates lipid
transport, is involved in the development of NC, the expression
levels of ABCA12 were examined using immunofluorescence. The
results demonstrated that ABCA12 expression was upregulated in the
sebaceous glands of patients with NC (Fig. 5B and C) compared with in normal
controls (Fig. 5A). Therefore,
high expression levels of ABCA12 in the sebaceous gland may be
associated with the development of NC.
Discussion
The putative pathogenesis of KP suggests a disorder
of keratinization. The papules may arise from the excessive
accumulation of keratin within the follicular orifices (6). Perifollicular erythema results from
mechanical irritation from hyperkeratosis and increased skin pH,
caused by a reduction in moisturizing amino acids (16). Several genetic studies have
reported null mutations in the FLG gene of patients with KP,
which has been associated with ichthyosis vulgaris and atopic
dermatitis (17). FLG, a
multifunctional protein expressed in the epidermis, not only
aggregates and aligns keratin intermediate filaments within
corneocytes, but also facilitates the release of a variety of amino
acids, which contribute to natural moisture levels or reduced skin
surface pH. Loss of function of FLG may lead to reduced
natural moisture, resulting in epithelial barrier abnormalities
(3); however, Gruber et al
(3) reported that mutations of
FLG may only partially result in the KP phenotype.
Therefore, other genes may serve as causative genetic factors
contributing to the development of KP; however, further research is
required.
In the present study, a heterozygous missense
c.6694G>T (p.Asp2232Tyr) mutation within ABCA12 of the KP
proband was identified. At present, no mutations in ABCA12
have been reported to be associated with the onset of KP. Mutations
of ABCA12 have been considered to be a major cause of
harlequin ichthyosis (18,19). ABCA12, located on chromosome
2q35, encodes a protein constituting 2595 amino acids, including
two ATP nucleotide binding sites between residues 1370–1554 and
2282–2467, and two transmembrane domains (amino acid residues
1063–1271 and 1987–2293), each comprising six hydrophobic membrane
spanning helices (20,21). ABCA12 belongs to the ATP-binding
cassette (ABC) transmembrane transporter protein superfamily, which
bind and hydrolyze ATP for the transport of lipids in lamellar
granules to granular layer keratinocytes (22). The main molecular function of
ABCA12 is yet to be investigated. Akiyama (19) demonstrated that a lack of ABCA12
function disrupted glucosylceramide and ganglioside transport,
leading to intracellular accumulation of these substances; this
phenomenon was recovered by in vitro via
ABCA12-corrective gene transfer (19). Investigations conducted by Wang
et al (23) and Sun et
al (24) indicated that
increases in the production of gangliosides may result in
keratinocyte apoptosis rather than the formation of epidermal
stratification. Jiang et al (25) reported that ceramides, the
precursors of glucosylceramides, stimulate ABCA12 expression via
the PPAR-mediated signaling pathway, subsequently regulating this
lipid transporter. Conversely, Zuo et al (26) reported a lack of desquamation of
the skin cells and rapid water loss from the skin in
ABCA12−/− mice rather than enhanced proliferation of
basal layer keratinocytes. Therefore, the mutations of
ABCA12 may affect lipid transport and skin cell
desquamation, which may lead to the development of solid and dry
lesions associated with KP.
NC is an epidermal nevus involving the pilosebaceous
unit (27); the etiology of NC
requires further investigation. Recently, the underlying signaling
pathways associated with acne and the somatic mutations of tyrosine
kinase receptors have been identified, and may serve a role in the
development of NC (11,13). The present study revealed that the
typical histopathology of NC was associated with large dilated
follicular ostia filled with keratinous layers, similar to that of
KP. Therefore, the present study aimed to investigate whether
patients with NC also possessed mutations in ABCA12, which
may affect lipid transport, resulting in the accumulation of
keratin. Following sequencing, no ABCA12 mutations were
identified in the two NC pedigrees employed in the present study;
however, immunofluorescence analysis revealed that the ABCA12
protein was highly expressed in the sebaceous glands of patients
with NC compared with in normal skin. Under normal conditions,
ABCA12 protein is expressed and localized in epidermal
keratinocytes (28). Conversely,
ABCA12 expression was identified within the sebaceous glands of the
two patients NC in the present study. The findings of the present
study were supported by the presence of abnormal sebaceous glands.
In conclusion, a novel missense mutation c.6694G>T in ABCA12 was
identified in a KP family, and it was demonstrated that ABCA12
expression was upregulated in the sebaceous glands of NC patients
without ABCA12 mutation. Therefore, it was inferred that ABCA12
mutations or expression changes may be causative or contributive
factors to disease development of congenital keratinized
dermatoses, such as KP and NC. ABCA12 may be considered as a
therapeutic target for the treatment of NC. There remained
limitations to the present study; for example, further
investigations are needed to clarify the genetic background of the
previous generations of the probands.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from
National Natural Science Foundation of China (grant no. 81371724)
to YHL.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
FL and YY performed the experiments and wrote this
article. YZ collected blood and skin samples. YHL and KZ designed
the experiments. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the ethics
committee of Nanfang Hospital of Southern Medical University
(Guangzhou, China).
Patient consent for publication
Written informed consent was obtained from patients
and individuals involved in the present study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kootiratrakarn T, Kampirapap K and
Chunhasewee C: Epidermal permeability barrier in the treatment of
keratosis pilaris. Dermatol Res Pract. 2015:2050122015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ehsani A, Namazi MR, Barikbin B and Nazemi
MJ: Unilaterally generalized keratosis pilaris. J Eur Acad Dermatol
Venereol. 17:361–362. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gruber R, Sugarman JL, Crumrine D, Hupe M,
Mauro TM, Mauldin EA, Thyssen JP, Brandner JM, Hennies H, Schmuth M
and Elias PM: Sebaceous gland, hair shaft, and epidermal barrier
abnormalities in keratosis pilaris with and without filaggrin
deficiency. Am J Pathol. 185:1012–1021. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Poskitt L and Wilkinson JD: Natural
history of keratosis pilaris. Br J Dermatol. 130:711–713. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Park KY, Son IP, Choi SY, Seo SJ and Hong
CK: Combination peel with incorporated fractional prickle coral
calcium for the treatment of keratosis pilaris: A pilot study. J
Dermatolog Treat. 25:314–318. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Thai K and Sinclair RD: Keratosis pilaris
and hereditary koilonychia without monilethrix. J Am Acad Dermatol.
45:627–629. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nazarenko SA, Ostroverkhova NV, Vasiljeva
EO, Nazarenko LP, Puzyrev VP, Malet P and Nemtseva TA: Keratosis
pilaris and ulerythema ophryogenes associated with an 18p deletion
caused by a Y/18 translocation. Am J Med Genet. 85:179–182. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Brown SJ, Relton CL, Liao H, Zhao Y,
Sandilands A, McLean WH, Cordell HJ and Reynolds NJ: Filaggrin
haploinsufficiency is highly penetrant and is associated with
increased severity of eczema: Further delineation of the skin
phenotype in a prospective epidemiological study of 792 school
children. Br J Dermatol. 161:884–889. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mevorah B, Marazzi A and Frenk E: The
prevalence of accentuated palmoplantar markings and keratosis
pilaris in atopic dermatitis, autosomal dominant ichthyosis and
control dermatological patients. Br J Dermatol. 112:679–685. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sandilands A, O'Regan GM, Liao H, Zhao Y,
Terron-Kwiatkowski A, Watson RM, Cassidy AJ, Goudie DR, Smith FJ,
McLean WH and Irvine AD: Prevalent and rare mutations in the gene
encoding filaggrin cause ichthyosis vulgaris and predispose
individuals to atopic dermatitis. J Invest Dermatol. 126:1770–1775.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tchernev G, Ananiev J, Semkova K,
Dourmishev LA, Schonlebe J and Wollina U: Nevus comedonicus: An
updated review. Dermatol Ther (Heidelb). 3:33–40. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Levinsohn JL, Sugarman JL, McNiff JM,
Antaya RJ and Choate KA: Somatic mutations in NEK9 cause nevus
comedonicus. Am J Hum Genet. 98:1030–1037. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mahajan RS, Shah MM, Ninama KR and
Bilimoria FE: Extensive nevus comedonicus. Indian Dermatol Online
J. 5:520–521. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ito T, Mitamura Y, Tsuji Y, Harada K and
Urabe K: Bilateral nevus comedonicus syndrome. Yonago Acta Med.
56:59–61. 2013.PubMed/NCBI
|
|
15
|
Beck MH and Dave VK: Extensive nevus
comedonicus. Arch Dermatol. 116:1048–1050. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee D, Yamasaki K, Rudsil J, Zouboulis CC,
Park GT, Yang J and Gallo RL: Sebocytes express functional
cathelicidin antimicrobial peptides and can act to kill
propionibacterium acnes. J Invest Dermatol. 128:1863–1866. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Brown SJ, Relton CL, Liao H, Zhao Y,
Sandilands A, Wilson IJ, Burn J, Reynolds NJ, McLean WH and Cordell
HJ: Filaggrin null mutations and childhood atopic eczema: A
population-based case-control study. J Allergy Clin Immunol.
121:940–946. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Thomas AC, Cullup T, Norgett EE, Hill T,
Barton S, Dale BA, Sprecher E, Sheridan E, Taylor AE, Wilroy RS, et
al: ABCA12 is the major harlequin ichthyosis gene. J Invest
Dermatol. 126:2408–2413. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Akiyama M: ABCA12 mutations and autosomal
recessive congenital ichthyosis: A review of genotype/phenotype
correlations and of pathogenetic concepts. Hum Mutat. 31:1090–1096.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Annilo T, Shulenin S, Chen ZQ, Arnould I,
Prades C, Lemoine C, Maintoux-Larois C, Devaud C, Dean M, Denefle P
and Rosier M: Identification and characterization of a novel ABCA
subfamily member, ABCA12, located in the lamellar ichthyosis region
on 2q34. Cytogenet Genome Res. 98:169–176. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lefevre C, Audebert S, Jobard F, Bouadjar
B, Lakhdar H, Boughdene-Stambouli O, Blanchet-Bardon C, Heilig R,
Foglio M, Weissenbach J, et al: Mutations in the transporter ABCA12
are associated with lamellar ichthyosis type 2. Hum Mol Genet.
12:2369–2378. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Akiyama M, Sugiyama-Nakagiri Y, Sakai K,
McMillan JR, Goto M, Arita K, Tsuji-Abe Y, Tabata N, Matsuoka K,
Sasaki R, et al: Mutations in lipid transporter ABCA12 in harlequin
ichthyosis and functional recovery by corrective gene transfer. J
Clin Invest. 115:1777–1784. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang XQ, Sun P and Paller AS: Inhibition
of integrin-linked kinase/protein kinase B/Akt signaling: mechanism
for ganglioside-induced apoptosis. J Biol Chem. 276:44504–44511.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sun P, Wang XQ, Lopatka K, Bangash S and
Paller AS: Ganglioside loss promotes survival primarily by
activating integrin-linked kinase/Akt without phosphoinositide 3-OH
kinase signaling. J Invest Dermatol. 119:107–117. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jiang YJ, Uchida Y, Lu B, Kim P, Mao C,
Akiyama M, Elias PM, Holleran WM, Grunfeld C and Feingold KR:
Ceramide stimulates ABCA12 expression via peroxisome
proliferator-activated receptor in human keratinocytes. J Biol
Chem. 284:18942–18952. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zuo Y, Zhuang DZ, Han R, Isaac G, Tobin
JJ, McKee M, Welti R, Brissette JL, Fitzgerald ML and Freeman MW:
ABCA12 maintains the epidermal lipid permeability barrier by
facilitating formation of ceramide linoleic esters. J Biol Chem.
283:36624–36635. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kurokawa I, Nakai Y, Nishimura K, Hakamada
A, Isoda K, Yamanaka K, Mizutani H and Tsubura A: Cytokeratin and
filaggrin expression in nevus comedonicus. J Cutan Pathol.
34:338–341. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yamanaka Y, Akiyama M, Sugiyama-Nakagiri
Y, Sakai K, Goto M, McMillan JR, Ota M, Sawamura D and Shimizu H:
Expression of the keratinocyte lipid transporter ABCA12 in
developing and reconstituted human epidermis. Am J Pathol.
171:43–52. 2007. View Article : Google Scholar : PubMed/NCBI
|