Introduction
Acute myeloid leukemia (AML) is a clonal expansion
disorder of myeloid precursors, which is characterized by the
deregulation of important regulators in hematopoiesis (1,2). The
mechanisms underlying the formation and development of AML remain
to be fully elucidated. Therefore, further investigations on the
molecules and signaling pathways, which predict the metastasis and
recurrence of AML, are essential.
In previous years, microRNAs (miRNAs) have emerged
as novel regulators in myelopoiesis and monocytic leukemia. The
overexpression of miRNA (miR)-22, which modulates PU.1, contributes
to monocyte/macrophage differentiation and acute monocytic leukemia
(3). The enforced expression of
miR-34a in T-cell immunoglobulin and mucin domain-containing
protein-3-positive leukemia stem cells inhibits its clonogenic
expansion and metastasis (4),
whereas miR-142-3p and miR-29a promote myeloid differentiation
(5). The abnormal expression of
miRNAs is involved in AML, serving as useful diagnostic and
prognostic indicators (6–8).
Studies have suggested that miR-21 is involved in
the initiation and progression of several types of cancer in
previous years. In a glioblastoma animal model, the anticancer
effect of the R3V6 peptide was mediated by the delivery of an
anti-miR-21 antisense-oligodeoxynucleotide (9). The serum expression level of miR-21
is a potential diagnostic marker in colorectal cancer (10) and esophageal carcinoma (11). In addition, miR-21 in peripheral
blood mononuclear cells can serve as a novel biomarker in the
diagnosis and prognosis of prostate cancer (12). The genetic deletion of miR-21
suppresses the proliferation, migration and invasion in colorectal
cancer cells (13), gastric cancer
cells (14) and breast cancer
cells (15).
In tumors, cancer cells recruit new blood vessels
for their growth, maintenance and metastasis. Angiogenesis is a
complex process, which depends on the interaction between growth
factors, cytokines and a number of components of the extracellular
matrix (16). Of note, miR-21 can
act as either a negative modulator or a positive modulator in
different pathways. As a negative modulator of angiogenesis, the
overexpression of miR-21 reduces human umbilical vein endothelial
cell (HUVEC) proliferation, migration and angiogenic capacity
(17). By contrast, miR-21 has
also been reported to promote angiogenesis in critical limb
ischemia by targeting Hsc70-interacting protein to enhance the
activity of hypoxia-inducible factor-1α (18).
In the present study, the potential function of
miR-21 in AML was investigated, and the results indicated that
miR-21 was overexpressed in the peripheral blood monocytes of
patients with AML. The abnormal expression of miR-21 promoted
angiogenesis by repressing the release of IL-12, and these results
assist in elucidating the underlying mechanism by which miR-21
promotes the development of AML.
Patients and methods
Patients
A total of 26 newly diagnosed patients with AML were
recruited between July 2013 and September 2015 from the Military
General Hospital of Beijing PLA (Beijing, China). A total of 28
age- and sex-matched healthy individuals were included as controls.
The exclusion criteria included individuals with hypertension and
diabetes, and those who have received any other surgery. The
present study was approved by the Medical Ethics Committee of the
Military General Hospital of Beijing PLA and every patient provided
written informed consent. The characteristics of the 26 patients
with AML and the 28 healthy individuals are listed in Table I.
 | Table I.Demographic and clinical data of
patients with AML and control subjects. |
Table I.
Demographic and clinical data of
patients with AML and control subjects.
| Variable | AML | Healthy subject |
|---|
| Age (years) | 47±7 | 50±5 |
| Sex
(male/female) | 15/11 | 18/10 |
| FAB
classification |
|
|
| M4
(n) | 16 | – |
| M5
(n) | 10 | – |
| WBC
(×109/l) | 4.48±3.83 | 5.60±4.22 |
| PB blast
(%) | 51.75±30.65 | 18.00±6.75 |
| PB
monocyte (%) | 21.01±20.24 | 3.65±0.67 |
| RBC
(×1012/l) | 3.05±0.86 | 4.15±0.43 |
| Hb
(g/l) | 96±21.90 | 145±36.21 |
| PLT
(×109/l) | 75.23±50.45 | 174.58±55.47 |
| CRP
(mg/dl) | 2.84±0.63 | 0.51±0.14 |
Peripheral blood monocyte
isolation
Samples of ~0.5 ml blood were harvested from the
patients and were dissolved in PBS (1:1). The samples were added to
1 ml gradient centrifugation liquid 10771 (Sigma; Merck Millipore,
Darmstadt, Germany), and centrifuged at 300 × g at 4°C for 25 min.
The monocytes were present in a layer between the PBS and 10771,
and this cell layer was harvested. The primary monocytes were
incubated with a rat anti-human CD14 antibody (1 µg/ml; cat. no.
367116; BioLegend, Inc., San Diego, USA) at 37°C for 30 min. The
monocytes were identified as CD14-positive cells using flow
cytometry, and the purity of the cells was ≥90%.
Cell culture and transfection
The human leukemia THP-1 cells were purchased from
the Cell Bank of the Institute of Biochemistry and Cell Biology,
China Academy of Sciences (Shanghai, China). The cells were
maintained in medium containing 10% fetal bovine serum (FBS; Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) and cultured at
37°C in humidified air containing 5% CO2. miR-21 mimics,
inhibitor and negative control were purchased from Guangzhou
RiboBio Co., Ltd. (Guangzhou, China). For transfection, the THP-1
cells were cultured to 80% confluence and transfected with either
50 nM miR-21 mimic (sequence unavailable), 100 nM inhibitor
(sequence unavailable), 100 nM negative control (sequence
unavailable), 50 µM small interfering RNA targeting VEGF (siVEGF;
5′-GGAGUACCCUGAUGAGAUCdTdT-3′; Sangon Biotech Co., Ltd., Shanghai,
China) or 150 mM recombinant human (rh) IL-12 (PrimeGene; R&D
Systems China Co., Ltd., Shanghai, China) using a Ribo FECT™ CP
Transfection kit (Guangzhou RiboBio Co, Ltd.) according to the
manufacturer's protocol.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
The expression of miR-21 was determined using a 7900
Real-Time PCR system (Applied Biosystems; Thermo Fisher Scientific,
Inc.). Total cellular RNA and miRNAs were isolated from the
peripheral blood monocytes and cell lines using an RNeasy/miRNeasy
Mini kit (Qiagen AB, Limburg, The Netherlands) according to the
manufacturer's protocol. The RT reactions were performed using a
RevertAid™ First Strand cDNA Synthesis kit (Fermentas; Thermo
Fisher Scientific, Inc.). The qPCR analysis was performed using
SYBR-Green PCR Master mix (Applied Biosystems; Thermo Fisher
Scientific, Inc.) in a final volume of 20 µl, comprising of 2 µl
cDNA, 10 µl master mix, 1 µl ROX and primers (0.4 pmol/µl each; 0.8
µl) and 6.2 µl ddH2O. qPCR cycling conditions were as
follows: 95°C for 5 sec, followed by 40 cycles of 60°C for 30 sec,
95°C for 15 sec and 60°C for 15 sec, and 95°C for 15 sec. The
expression levels of miRNAs and RNAs were calculated using the
2−ΔΔCq method (19)
using U6 as an internal reference. The primer sequences were as
follows: miR-21 forward, 5′-TAGCTTATCAGACTGATGTTGA-3′ and reverse,
5′-AACGCTTCACGAATTTGCGT-3′; U6 forward, 5′-CTCGCTTCGGCAGCACA-3 and
reverse, 5′-AACGCTTCACGAATTTGCGT-3′; PDCD4 forward,
5′-AGGCCGAGGTGGGCGGATCACTTGA-3′ and reverse,
5′-GCCACCATGCCTGGCTACT-3′. The RT and PCR primers used for miR-21
and U6 were purchased from Guangzhou RiboBio Co., Ltd.
ELISA for IL-12 and VEGF
The protein levels of IL-12 and VEGF in the serum
and in the supernatants of the transfected cells were measured
using ELISA kits. ELISA was performed using the human IL-12 and
VEGF Quantikine kits (R&D Systems, Inc., Minneapolis, MN, USA)
according to manufacturer's protocol. The final concentration was
calculated according to the standard curve.
Luciferase assay
The wild-type 3′untranslated region (3′UTR) of IL-12
was cloned into the Renilla luciferase gene. (Shanghai GeneChem
Co., Ltd, Shanghai, China). The THP-1 cells were cotransfected with
vectors carrying wild-type 3′UTR and miR-21 mimic or negative
control. The cells were collected 48 h following transfection and
analyzed using the Luciferase Reporter Assay system (Promega
Corporation, Madison, WI, USA). The luciferase activity values were
normalized relative to that of the Renilla luciferase internal
control.
Tube formation assay
The THP-1 cells were transfected with either 100 nM
negative control, 50 nM miR-21 mimic or 100 nM inhibitor, or were
co-transfected with 50 nM miR-21 and 50 µM siVEGF for 24 h.
Following transfection, the supernatants were harvested. HUVECs
were purchased from the Cell Bank of the Institute of Biochemistry
and Cell Biology, Chinese Academy of Sciences (Shanghai, China).
The cells were incubated at 1×105 cells/well at 37°C
with these supernatants for 24 h, respectively. The angiogenic
ability of the HUVECs were detected. Matrigel was used to determine
the effects on in vitro vascular tube formation. The HUVECs
were respectively seeded at 5×104 cells per well in a
48-well plate on Matrigel (BD Biosciences, Franklin Lakes, NJ,
USA). The cells were incubated for 6 h at 37°C and evaluated by
phase-contrast microscopy, and images were captured. Tubes were
defined as straight cellular extensions joining cycle, and were
counted in three random digital images (magnification, ×200) for
each well.
Statistical analysis
Data are presented as the mean ± standard deviation.
Graphs were drawn using GraphPad Prism v5.0 (GraphPad Software,
Inc., La Jolla, CA, USA). Data were analyzed using one-way analysis
of variance followed by Student's t-test to assess significant
differences between groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
miR-21 and VEFG are significantly
increased in patients with AML
To examine the changes in the expression miR-21 in
cases of AML, the expression levels of miR-21 were examined in
peripheral blood monocyte specimens from 26 patients with AML and
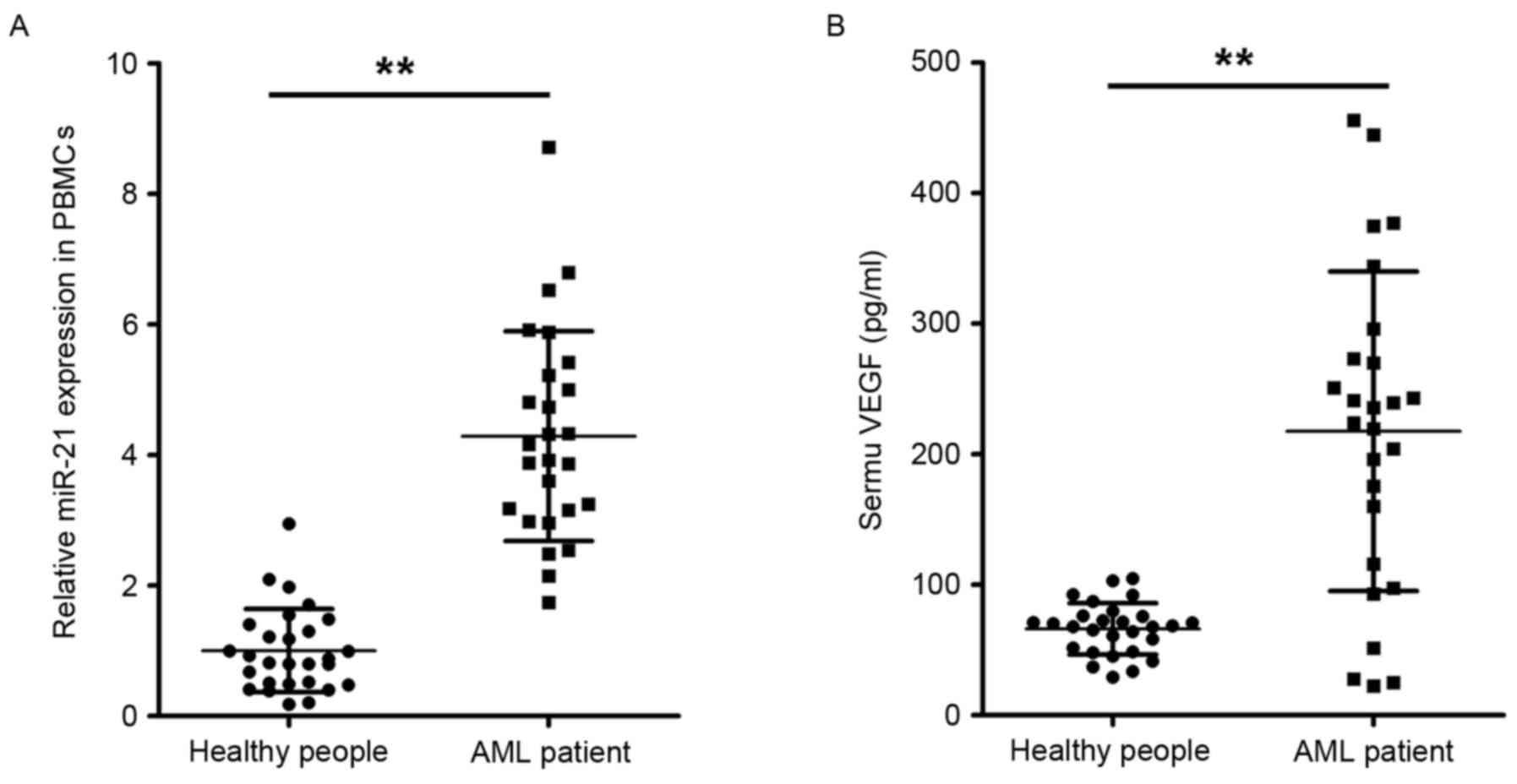
28 healthy individuals. As shown in Fig. 1A, the expression levels of miR-21
were significantly increased in the peripheral blood monocytes of
patients with AML, compared with those of healthy controls. These
results of suggested an association between miR-21 and AML. The
results of the present study confirmed that the serum levels of
VEGF were also significantly elevated in patients with AML,
compared with those in the healthy controls, as shown in Fig. 1B. It was hypothesized that miR-21
functionally regulated AML; therefore, subsequent experiments were
performed focusing on the role of miR-21 in the regulation of
angiogenesis.
miR-21 promotes the angiogenic ability
of HUVECs
To determine whether AML cells or normal monocytes
overexpress miR-21, the present study used peripheral blood
monocytes of healthy individuals as a control to compare the
expression of miR-21 in different AML cell lines. It was found that
the expression levels of miR-21 were relatively higher in the AML
cell lines, particularly in THP-1 cells, as shown in Fig. 2A. Based on this expression pattern,
the present study selected the THP-1 cell line for the following
experiments. Subsequently, the miR-21 mimic or inhibitor was used
to confirm whether miR-21 affects the release of angiogenic
factors. It was found that the levels of VEGF in the supernatant of
the miR-21 mimic-transfected cells were increased, compared with
levels in the negative control (Fig.
2B). However, miR-21 inhibitor transfection led to the level of
VEGF being significantly decreased, as shown in Fig. 2C. In addition, pretreatment of the
HUVECs with these respective supernatants altered the angiogenic
ability of the HUVECs. As shown in Fig. 2D and E, tube formation in the
HUVECs pretreated with the supernatant from THP-1 cells transfected
with miR-21 mimic was higher, compared with that in the negative
control, and was lower in the miR-21 inhibitor-transfected cells.
The angiogenic ability of the HUVECs pretreated with supernatant
from the THP-1 cells co-transfected with miR-21 mimic and siVEGF
were decreased, compared with those in the miR-21 mimic-transfected
cells. These data suggested that miR-21 stimulated the monocyte
release of VEGF to promote angiogenesis.
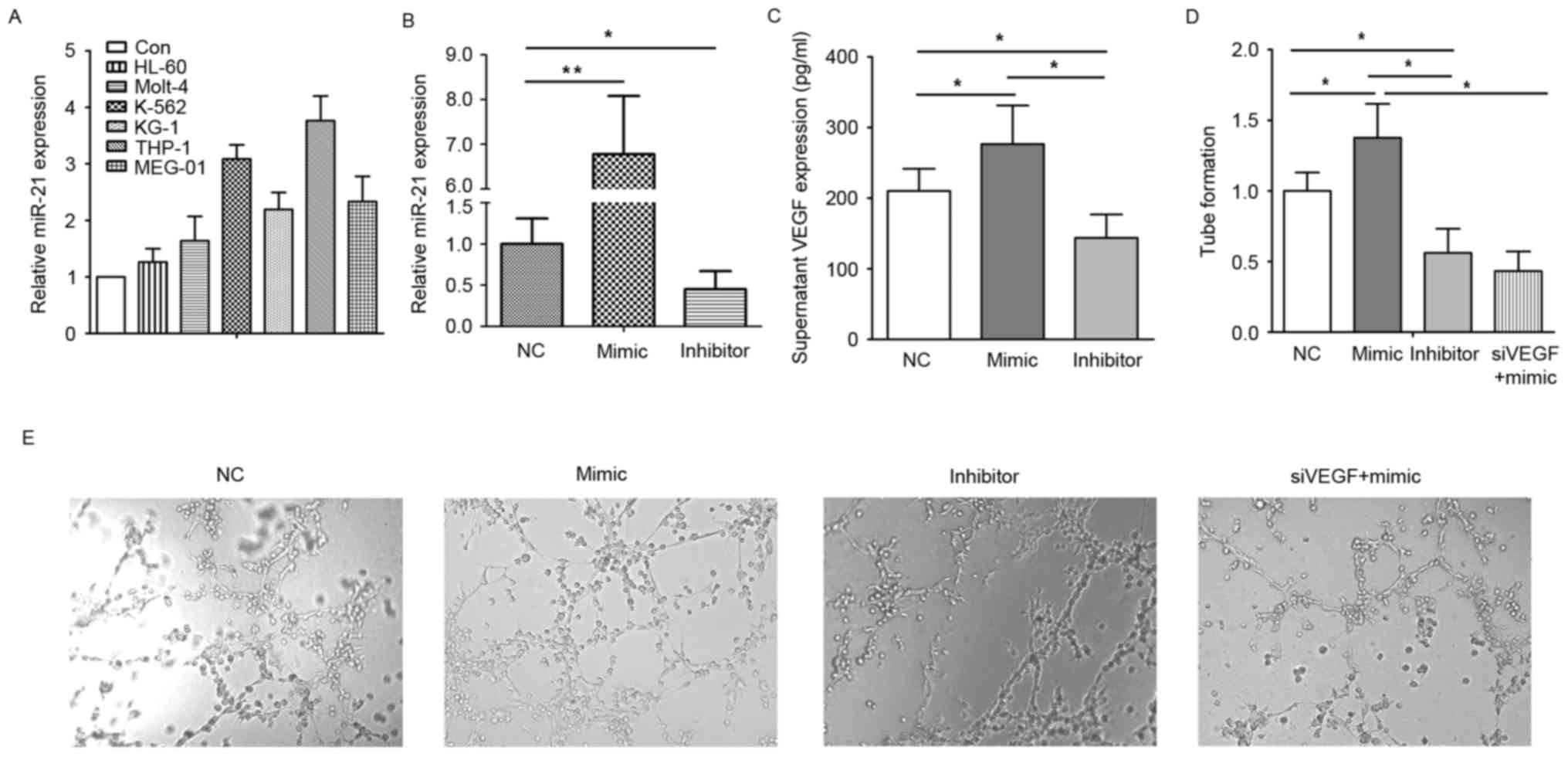 | Figure 2.miR-21 promotes the angiogenic ability
of HUVECs. (A) Expression of miR-21 in different types of cell. (B)
Expression of miR-21 in THP-1 cells following transfection with NC,
miR-21 mimic or miR-21 inhibitor. (C) VEGF in the supernatants of
THP-1 cells transfected with NC, miR-21 mimic or miR-21 inhibitor.
(D) Tube formation of HUVECs pretreated with the supernatant from
THP-1 cells transfected with NC, miR-21 mimic, miR-21 inhibitor or
siVEGF and miR-21 mimic. (E) Images of tube formation.
Magnification, ×200. *P<0.05; **P<0.01. miR, microRNA; Con,
control; VEGF, vascular endothelial growth factor; AML, acute
monocytic leukemia; PBMCs, peripheral blood mononuclear cells;
HUVECs, human umbilical vein endothelial cells; NC, negative
control; siVEGF, small interfering RNA targeting VEGF. |
miR-21 upregulates angiogenesis by
targeting IL-12
To obtain further insight into the molecular
mechanism by which miR-21 affect AML cells, the present study
performed a search for genes targeted by miR-21 using the
biological target prediction website (http://www.microrna.org/microrna/home.do), which drew
attention to IL-12. To verify the prediction, a luciferase reporter
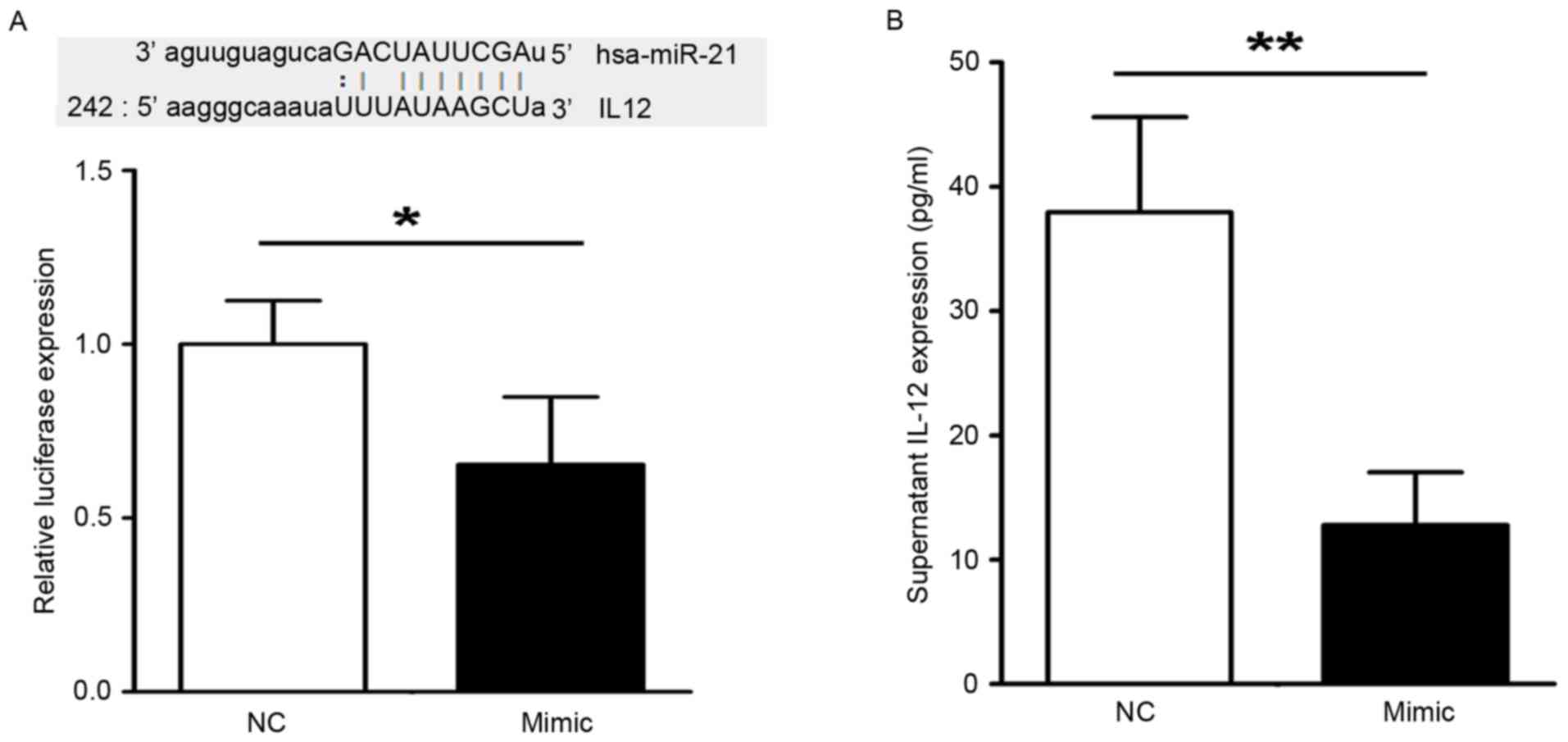
vector was constructed containing the 3′UTR of IL-12. As shown in
Fig. 3A, the miR-21 mimic
decreased the luciferase activity of the vector carrying the 3′UTR
of IL-12. It was also found that the levels of IL-12 in
supernatants from the THP-1 cells transfected with miR-21 mimic
were decreased, compared with that in the negative control, as
shown in Fig. 3B. The present
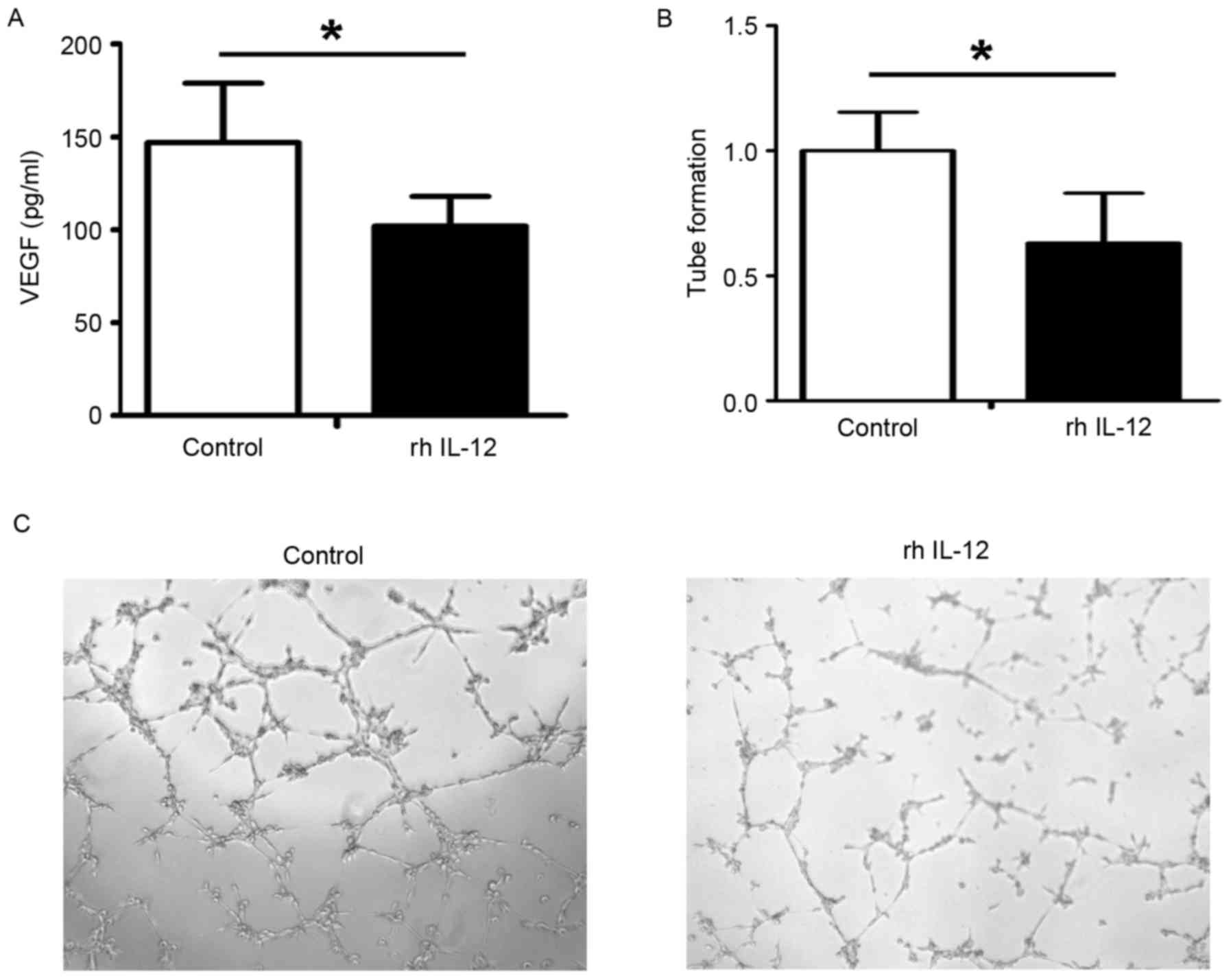
study then used IL-12 to pretreat HUVECs, and found that the level
of VEGF in the supernatants was decreased following rh IL-12
pretreatment (Fig. 4A) and the
angiogenic ability of the HUVECs was reduced following IL-12
pretreatment (Fig. 4B and C).
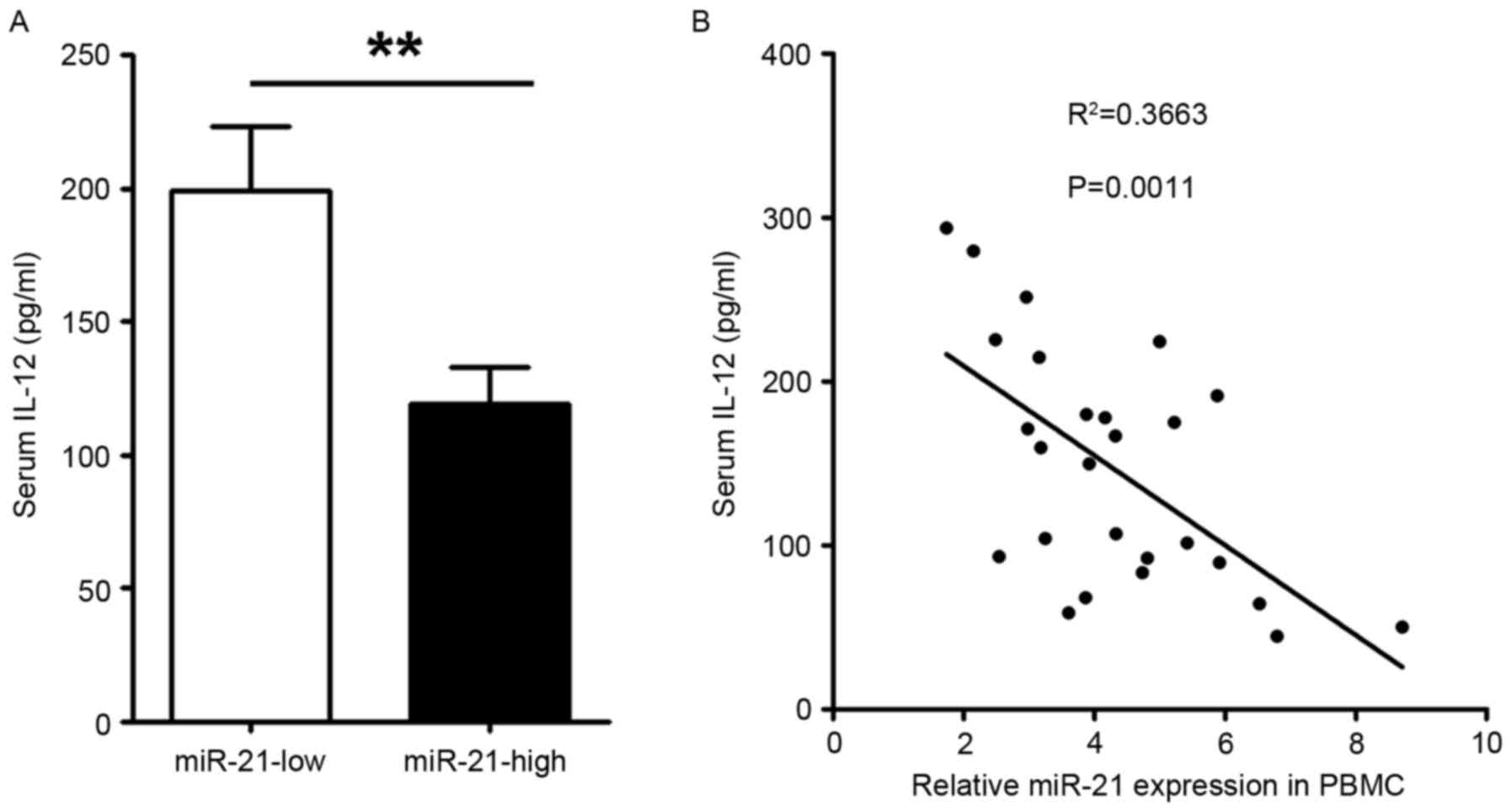
Patients with <3.5 relative expression of miR-21 were defined as
having a low expression of miR-21, and the present study found that
serum levels of IL-12 were significantly decreased in patients with
AML with a high level of miR-21, compared with those with a low
level of miR-21 (Fig. 5A). To
clarify the association between IL-12 and miR-21, the present study
further analyzed the levels of serum IL-12 and miR-21, which
revealed a negative correlation between the two (Fig. 5B).
Discussion
AML is the most common type of acute leukemia
affecting adults, and its incidence increases with age. Although
AML is a relatively rare disease, accounting for ~1.2% of cases of
cancer-associated mortality, its incidence is expected to increase
as the population ages (20).
Several miRNAs are located inside or close to chromosomal fragile
sites, which are frequently lost or amplified in cancer, and the
correlation between miRNAs and cancer has become a focus for the
diagnosis and therapy of cancer (21,22).
In the present study, it was demonstrated that the expression of
miR-21 was significantly increased in peripheral blood monocytes of
patients with AML, compared with healthy controls. The peripheral
blood monocytes of healthy individuals were then used as a control
to compare the expression of miR-21 in different AML cell lines. It
was found that the expression levels of miR-21 were relatively
higher in the AML cell lines. These data indicated that the
excessive expression of miR-21 was from AML cells, not from the
normal monocytes.
miRNAs can act as oncogenes or tumor suppressors and
are involved in numerous cellular processes, being key in
tumorigenesis by regulating angiogenic processes (23,24).
The genetic deletion of miR-21 suppresses the proliferation,
migration and invasion in colorectal cancer cells (13), gastric cancer cells (14) and breast cancer cells (15). Furthermore, the expression of
miR-21 has been associated with angiogenesis (17,18).
However, the effect of miR-21 on the development and metastasis of
AML remains to be fully elucidated. Studies have shown that VEGF is
a growth factor and an angiogenic inducer, and suppression of the
secretion of VEGF and/or inhibition of the activity of VEGF can
attenuate the tumor-induced development of novel blood vessels
(25). In the present study, it
was found that supernatant levels of VEGF from the miR-21
mimic-transfected cells were increased, compared with those in the
negative control. Following miR-21 inhibitor-transfection, the
level of VEGF was significantly decreased. The present study also
compared tube formation in HUVECs pretreated with supernatant from
THP-1 cells transfected with the miR-21 mimic. The tube formation
was increased, compared with that in the negative control and was
decreased by miR-21 inhibitor transfection. Following
neutralization of the effect of VEGF with siVEGF, the angiogenic
promotion of miR-21 was eliminated. These results indicated that
aberrant increases in the level of miR-21 may have the ability to
stimulate the secretion of VEGF to promote angiogenesis.
Certain miRNAs coordinate large numbers of target
genes (26). Several miR-21
targets have been reported, including phosphatase and tensin
homolog (27), programmed cell
death protein 4 (28) and small
mothers against decapentaplegic 7 (29). To investigate the molecular
mechanism by which miR-21 affects AML cells, the present study
performed a search for possible mRNA targets using biological the
target prediction website (http://www.microrna.org/microrna/home.do), which
identified IL-12. IL-12 is a potent immunostimulatory cytokine,
which exhibits antitumoral activity. Intratumoral IL-12 gene
therapy can stimulate the immune system and decreases angiogenesis
in dogs with spontaneous cancer (30). In the present study, it was found
that the serum levels of IL-12 were significantly decreased in the
patients with AML and high expression levels of miR-21, compared
with those with low expression levels of miR-21, and there was a
negative correlation between serum IL-12 and miR-21. The miR-21
mimic also decreased the luciferase activity of the vector carrying
the 3′UTR of IL-12, and the level of IL-12 in supernatants from
THP-1 cells transfected with the miR-21 mimic was increased,
compared with that in the negative control. These data indicated
that IL-12 was a direct target of miR-21. In a previously reported
murine model of breast cancer, following 7 days of IL-12 treatment,
the protein levels of VEGF in the tumor decreased markedly and were
undetectable at 14 days (31). In
the present study, it was also found that supernatant levels of
VEGF were decreased, and that the angiogenic ability of the HUVECs
expanded following rh IL-12 pretreatment. These results suggested
that IL-12 regulated angiogenesis by inhibiting the pro-angiogenic
VEGF release.
In conclusion, the present study demonstrated that
miR-21 was upregulated in patients with AML. It was also found that
miR-21 regulated angiogenesis targeting IL-12. Therefore, the
inactivation of miR-21 or activation of its target gene may be a
potential therapy for human AML.
Acknowledgements
Not applicable.
Funding
Not applicable.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
X-PH designed the experiment and was a major
contributor in conducting the experiments and writing the
manuscript. PC and KY collected and analyzed the PCR data, and
contributed to cell culture and transfection. BL and YZ analyzed
and interpreted the patient data. FW detected VEGF levels in serum.
ZG, X-DL, J-XL and H-RC performed the molecular assays. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Medical Ethics
Committee of the Military General Hospital of Beijing PLA and every
patient provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Medina J, Kantarjian H, Talpaz M, O'Brien
S, Garcia-Manero G, Giles F, Rios MB, Hayes K and Cortes J:
Chromosomal abnormalities in Philadelphia chromosome-negative
metaphases appearing during imatinib mesylate therapy in patients
with Philadelphia chromosome-positive chronic myelogenous leukemia
in chronic phase. Cancer. 98:1905–1911. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sive JI and Gottgens B: Transcriptional
network control of normal and leukaemic haematopoiesis. Exp Cell
Res. 329:255–264. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shen C, Chen MT, Zhang XH, Yin XL, Ning
HM, Su R, Lin HS, Song L, Wang F, Ma YN, et al: The PU.1-modulated
MicroRNA-22 is a regulator of monocyte/macrophage differentiation
and acute myeloid leukemia. PLoS Genet. 12:e10062592016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang S, Wang T, Li MZ, Cheng XL and Li XL:
Expression of microRNA miR-34a inhibits leukemia stem cells and its
metastasis. Eur Rev Med Pharmacol Sci. 20:2878–2883.
2016.PubMed/NCBI
|
|
5
|
Wang XS, Gong JN, Yu J, Wang F, Zhang XH,
Yin XL, Tan ZQ, Luo ZM, Yang GH, Shen C and Zhang JW: MicroRNA-29a
and microRNA-142-3p are regulators of myeloid differentiation and
acute myeloid leukemia. Blood. 119:4992–5004. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang TJ, Wang YX, Yang DQ, Yao DM, Yang
L, Zhou JD, Deng ZQ, Wen XM, Guo H, Ma JC, et al: Down-regulation
of miR-186 correlates with poor survival in de novo acute myeloid
leukemia. Clin Lab. 62:113–120. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lin X, Wang Z, Wang Y and Feng W: Serum
MicroRNA-370 as a potential diagnostic and prognostic biomarker for
pediatric acute myeloid leukemia. Int J Clin Exp Pathol.
8:14658–14666. 2015.PubMed/NCBI
|
|
8
|
Wang YX, Zhang TJ, Yang DQ, Yao DM, Yang
L, Zhou JD, Deng ZQ, Ma JC, Guo H, Wen XM, et al: Reduced miR-215
expression predicts poor prognosis in patients with acute myeloid
leukemia. Jpn J Clin Oncol. 46:350–356. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Oh B, Song H, Lee D, Oh J, Kim G, Ihm SH
and Lee M: Anti-cancer effect of R3V6 peptide-mediated delivery of
an anti-microRNA-21 antisense-oligodeoxynucleotide in a
glioblastoma animal model. J Drug Target. 25:132–139. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bastaminejad S, Taherikalani M, Ghanbari
R, Akbari A, Shabab N and Saidijam M: Investigation of MicroRNA-21
expression levels in serum and stool as a potential non-invasive
biomarker for diagnosis of colorectal cancer. Iran Biomed J.
21:106–113. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lv H, He Z, Wang H, Du T and Pang Z:
Differential expression of miR-21 and miR-75 in esophageal
carcinoma patients and its clinical implication. Am J Transl Res.
8:3288–3298. 2016.PubMed/NCBI
|
|
12
|
Yang B, Liu Z, Ning H, Zhang K, Pan D,
Ding K, Huang W, Kang XL, Wang Y and Chen X: MicroRNA-21 in
peripheral blood mononuclear cells as a novel biomarker in the
diagnosis and prognosis of prostate cancer. Cancer Biomark.
17:223–230. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li C, Zhao L, Chen Y, He T, Chen X, Mao J,
Li C, Lyu J and Meng QH: MicroRNA-21 promotes proliferation,
migration, and invasion of colorectal cancer, and tumor growth
associated with down-regulation of sec23a expression. BMC Cancer.
16:6052016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang XF, Zhang XW, Hua RX, Du YQ, Huang
MZ, Liu Y, Cheng YF and Guo WJ: Mel-18 negatively regulates stem
cell-like properties through downregulation of miR-21 in gastric
cancer. Oncotarget. 7:63352–63361. 2016.PubMed/NCBI
|
|
15
|
Nikiforova ZN, Taipov MA, Kudryavcev IA
and Shevchenko VE: The connection of miR-21 and miR-155 with
regulation of 15-HPGDH mRNA in human breast cancer cells. Biomed
Khim. 62:265–271. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Meehan K and Vella LJ: The contribution of
tumour-derived exosomes to the hallmarks of cancer. Crit Rev Clin
Lab Sci. 53:121–131. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sabatel C, Malvaux L, Bovy N, Deroanne C,
Lambert V, Gonzalez ML, Colige A, Rakic JM, Noël A, Martial JA and
Struman I: MicroRNA-21 exhibits antiangiogenic function by
targeting RhoB expression in endothelial cells. PLoS One.
6:e169792011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhou Y, Zhu Y, Zhang L, Wu T, Wu T, Zhang
W, Decker AM, He J, Liu J, Wu Y, et al: Human stem cells
overexpressing miR-21 promote angiogenesis in critical limb
ischemia by targeting CHIP to enhance HIF-1α activity. Stem Cells.
34:924–934. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jemal A, Thomas A, Murray T and Thun M:
Cancer statistics, 2002. CA Cancer J Clin. 52:23–47. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Calin GA, Sevignani C, Dumitru CD, Hyslop
T, Noch E, Yendamuri S, Shimizu M, Rattan S, Bullrich F, Negrini M
and Croce CM: Human microRNA genes are frequently located at
fragile sites and genomic regions involved in cancers. Proc Natl
Acad Sci USA. 101:pp. 2999–3004. 2004; View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ni Z, Shang XF, Wang YF, Sun YJ and Fu DJ:
Upregulated microRNA-301a in osteosarcoma promotes tumor
progression by targeting CDC14A. Genet Mol Res. 15:2016. View Article : Google Scholar :
|
|
23
|
Li Y, Zhang B, Li W, Wang L, Yan Z, Li H,
Yao Y, Yao R, Xu K and Li Z: MiR-15a/16 regulates the growth of
myeloma cells, angiogenesis and antitumor immunity by inhibiting
Bcl-2, VEGF-A and IL-17 expression in multiple myeloma. Leuk Res.
49:73–79. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang Y, Xing QF, Liu XQ, Guo ZJ, Li CY and
Sun G: MiR-122 targets VEGFC in bladder cancer to inhibit tumor
growth and angiogenesis. Am J Transl Res. 8:3056–3066.
2016.PubMed/NCBI
|
|
25
|
Ji H, Li Y, Jiang F, Wang X, Zhang J, Shen
J and Yang X: Inhibition of transforming growth factor beta/SMAD
signal by MiR-155 is involved in arsenic trioxide-induced
anti-angiogenesis in prostate cancer. Cancer Sci. 105:1541–1591.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lim LP, Lau NC, Garrett-Engele P, Grimson
A, Schelter JM, Castle J, Bartel DP, Linsley PS and Johnson JM:
Microarray analysis shows that some microRNAs downregulate large
numbers of target mRNAs. Nature. 433:769–773. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li H, Yuan SM, Yang M, Zha H, Li XR, Sun
H, Duan L, Gu Y, Li AF, Weng YG, et al: High intensity focused
ultrasound inhibits melanoma cell migration and metastasis through
attenuating microRNA-21-mediated PTEN suppression. Oncotarget.
7:50450–50460. 2016.PubMed/NCBI
|
|
28
|
Xiao J, Pan Y, Li XH, Yang XY, Feng YL,
Tan HH, Jiang L, Feng J and Yu XY: Cardiac progenitor cell-derived
exosomes prevent cardiomyocytes apoptosis through exosomal miR-21
by targeting PDCD4. Cell Death Dis. 7:e22772016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lin L, Tu HB, Wu L, Liu M and Jiang GN:
MicroRNA-21 regulates non-small cell lung cancer cell invasion and
chemo-sensitivity through SMAD7. Cell Physiol Biochem.
38:2152–2162. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cicchelero L, Denies S, Haers H,
Vanderperren K, Stock E, Van Brantegem L, de Rooster H and Sanders
NN: Intratumoural interleukin 12 gene therapy stimulates the immune
system and decreases angiogenesis in dogs with spontaneous cancer.
Vet Comp Oncol. 15:1187–1205. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dias S, Boyd R and Balkwill F: IL-12
regulates VEGF and MMPs in a murine breast cancer model. Int J
Cancer. 78:361–365. 1998. View Article : Google Scholar : PubMed/NCBI
|