Introduction
Developmental dysplasia of the hip (DDH) is one of
the most common malformations affecting the lower extremities in
children. If a stable and concentric reduction is maintained after
close reduction, the acetabulum has the potential to remodel and
resume normal growth and development. Furthermore, if younger
patients are at the onset of treatment, this results in a greater
potential for acetabular remodeling (1–5). The
use of magnetic resonance imaging has resulted in enhanced
consideration of the importance of acetabular cartilage in hip
remodeling (6,7). Chondrocytes, one of the important
components of cartilage, serve a critical role in maintaining the
function and biological features of cartilage. However, it is
difficult to obtain hip cartilage from DDH patients due to ethical
constraints. Consequently, the function and pathophysiology of
chondrocytes in the hips of patients with DDH cannot be in
investigated in vivo. In addition, it is not possible to
obtain cultured human chondrocytes from patients with DDH. Due to
these constraints, animal models are used to improve our
understanding of chondrocytes in DDH in vivo. A rat model of
unilateral DDH was established by Sijbrandij (8), and since then various experimental
animals have been used to show that remodeling of the acetabulum is
possible after the removal of fixation (9,10).
However, previous studies mainly focused on the morphological and
histological alterations of abnormal hips and the corresponding
cartilage (11–13). Furthermore, since the swaddle
position in infants is considered to be an important risk for the
development of DDH, the model designed by the principle is thought
to be an accurate model of cartilage in human DDH and may be
suitable for further investigations (14). The authors previously developed a
successful neonatal rat model of DDH corresponding to the swaddling
position of the hip and early cartilage degeneration in DDH
(10).
The aim of the present study was to assess the
features of chondrocytes in DDH cartilage via primary cell culture
in vitro. DDH models of neonatal Wistar rats were prepared
in the present study and serial sections of hip cartilage were
isolated and incubated primarily to investigate the cellular
characteristics after the removal of fixation.
Materials and methods
Experimental animal models
All experimental protocols were approved by the
Animal Ethical Committee of Fudan University (Shanghai, China). A
total of 80 male specific pathogen free neonatal Wistar rats (~5 g)
were purchased from the Animal Research Institute of Medical
College of Fudan University. Feeding environment was as follows:
Temperature, 21–26°C, relative humidity 45–65%, ventilation for
8–12 times/h, 12-h light/dark cycle. The rats were fed with sterile
pure water and adequate feed (HFK bio-technology, Beijing, China).
Rats in the experimental DDH group (n=40) were immobilized, with
the hip and knee fixed in an extended position with medical tapes
for 10 days as described in our previous study (10,12).
Following the removal of the fixation, rats were allowed to move
freely in their cage for 2, 4, 6 or 8 weeks. Rats were sacrificed
and the hips were isolated for macro-morphological examination and
primary cell culture of the articular cartilage. Rats in the
control group (n=40) were allowed to move freely throughout the
study period.
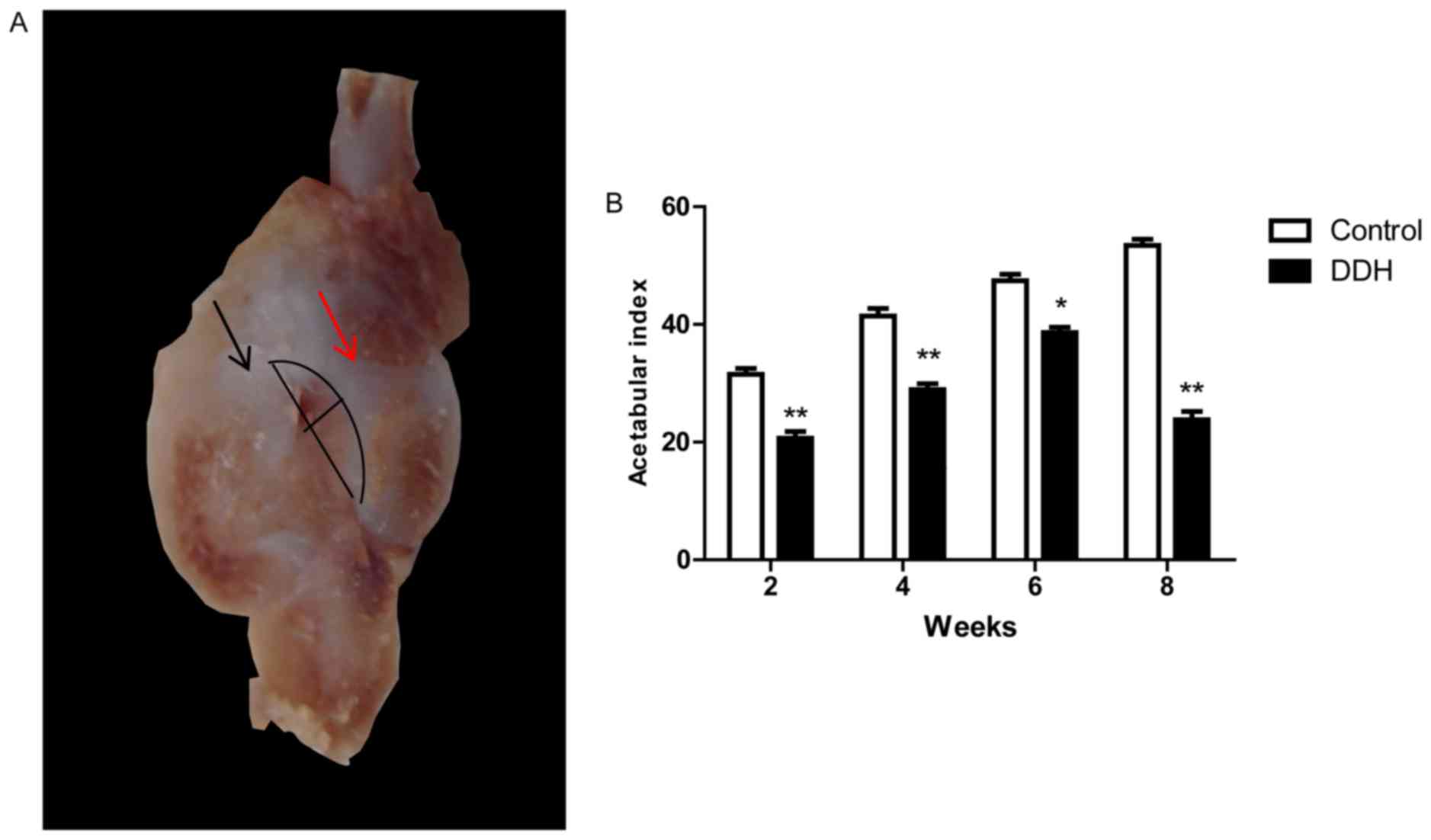
Coronal histology and morphometry
Hips were isolated and fixed in 4% paraformaldehyde
for 24 h in room temperature and decalcified with 10% EDTA,
following which they were dissected through the longitudinal line
from the ilium to the ischium of acetabulum. An abnormal
association between the acetabulum and femoral head was observed in
the experimental DDH group. The largest coronal that was selected
from sections (5-µm) and acetabular index was measured. The
acetabular depth ratio (ADR=depth/width ×100%) was measured to
assess changes in the acetabulum (Fig.
1A). The acetabular index (AI) was defined as the ratio of
depth: Width measured using the reference line presented in
Fig. 1A. The long line is
identified as the width diameter of the longitudinal acetabulum
from the upper edge to the distal border, excluding the rim of
labrum. The short line is the perpendicular line to the width
diameter of the acetabulum, and the curved line represents the
acetabular shape following the removal of fixation.
Primary cell culture protocol
Primary cell culture was performed using the
modified Manning method (15).
Cartilage was obtained from the hips under sterile conditions,
minced into 1 mm3 pieces with scissors and digested
using 0.25% Trypsin-EDTA and 2% collagenase II (Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA). Tissues were passed
through a 0.25 µm molecular filter and the cell suspension was
centrifuged at 240 × g for 5 min in room temperature. The resulting
pellet was resuspended in fresh Dulbecco's modified Eagle's medium
(DMEM; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), supplemented
with 10% fetal bovine serum (Sigma-Aldrich; Merck KGaA), 5 µg/ml
penicillin and 5 µg/ml streptomycin. The final cell density was
5×106 cells/ml and the suspension was incubated at 37°C
in an atmosphere containing 5% CO2 overnight, to allow
primary chondrocytes to adhere. The medium was replaced with fresh
DMEM every two days. Cells were then identified using collagen II
immunofluorescence staining.
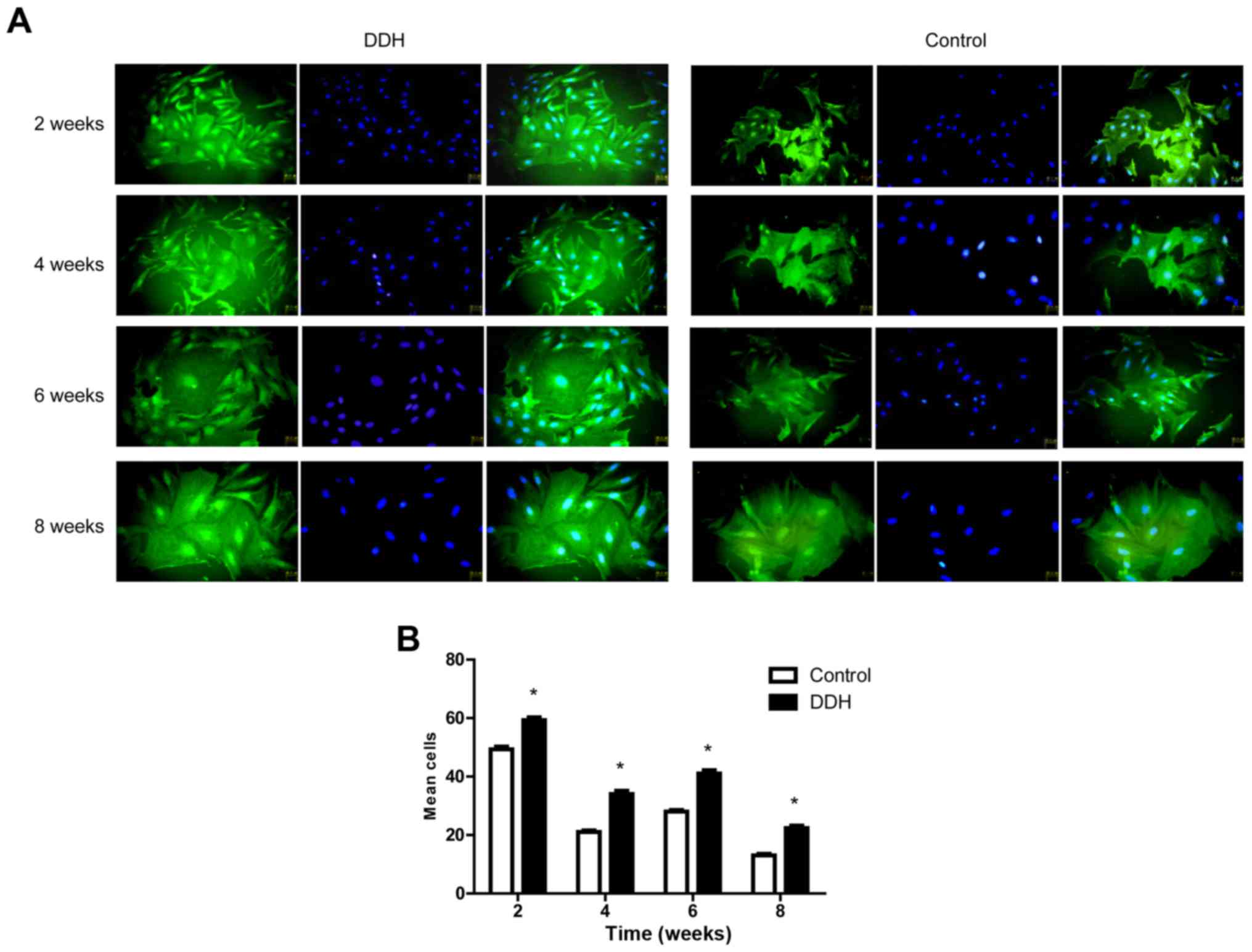
Chondrocyte identification using
collagen II immunofluorescence
The medium was discarded and cells were rinsed three
times with PBS for 5 min. Cell samples were fixed with 4%
paraformaldehyde for 30 min at room temperature, following which,
they were blocked in 0.2% Triton X-100 (PBST; Sigma-Aldrich; Merck
KGaA) mixed with goat serum (Sigma-Aldrich; Merck KGaA) for 30 min
at 37°C. Cells were immunoblotted and incubated overnight at 4°C
with primary antibodies against collagen II (cat. no. ab34712;
Abcam, Cambridge, UK; 1:100). The specimens were incubated for 1 h
with secondary antibodies goat anti-rabbit IgG Alexa
Fluor® 488 (cat. no. ab150077 Abcam; 1:200) at room
temperature in the dark. Finally, specimens were stained with DAPI
at room temperature and then viewed under an inverted fluorescence
microscope (excitation wavelength 488 nm, magnification 200X) and
images were captured. Collagen II staining at different time (2, 4,
6 and 8 weeks) was assessed and the number of cells was counted
using Image Plus Pro 6.0 (Media Cybernetics, Inc., Rockville, MD,
USA).
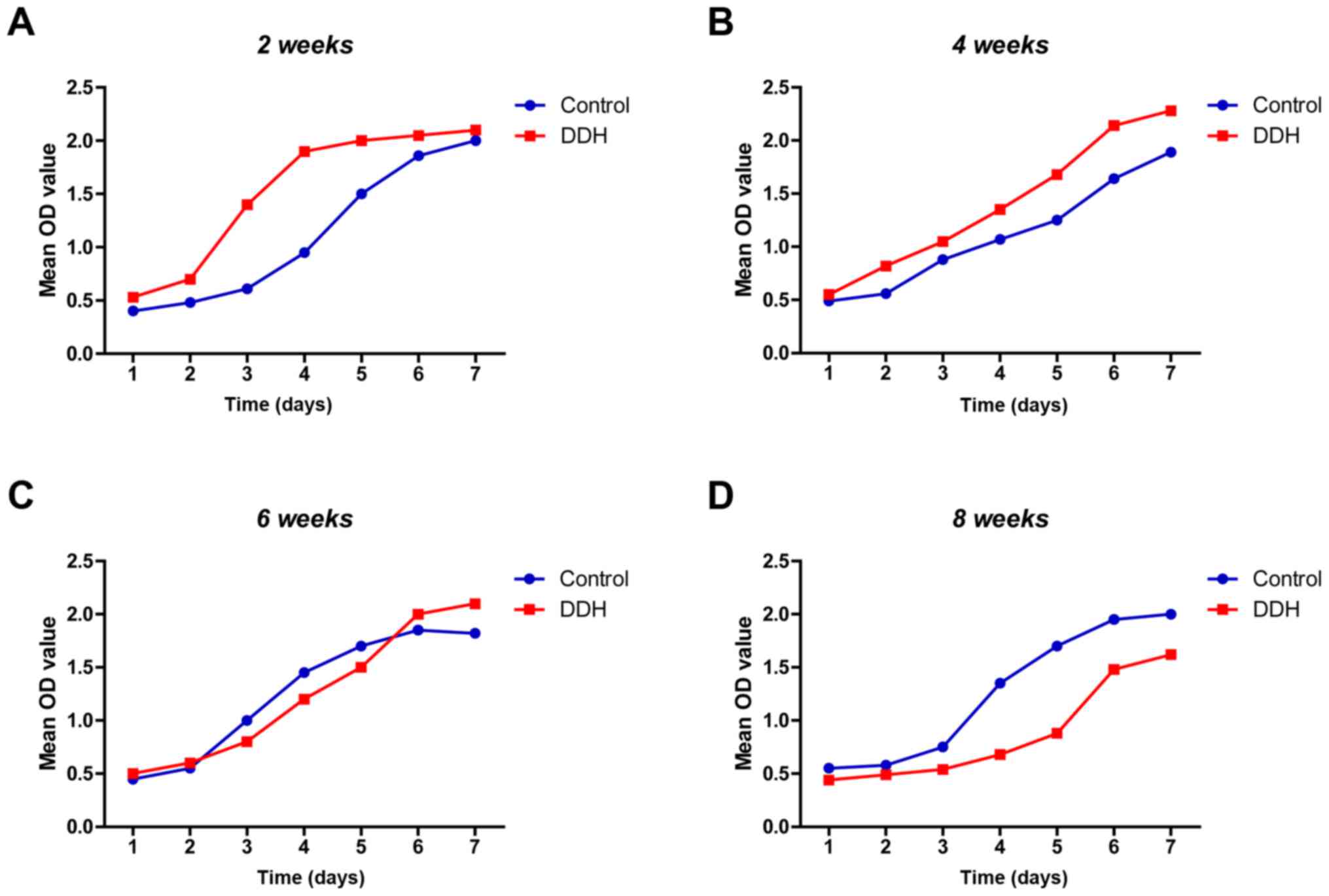
Cell proliferation kinetics and growth
curve
Primary cells were seeded in a 96-well plate at a
density of 4,000 cells/200 µl and the number of chondrocytes was
assessed using a Cell Counting Kit (CCK)-8 (DJDB4000X; Dojindo
Molecular Technologies, Inc., Kumamoto, Japan) each day for 1 week.
The optical density (OD) value of CCK-8 absorbance was measured at
a wavelength of 450 nm using an ELISA reader and used to construct
the growth curve.
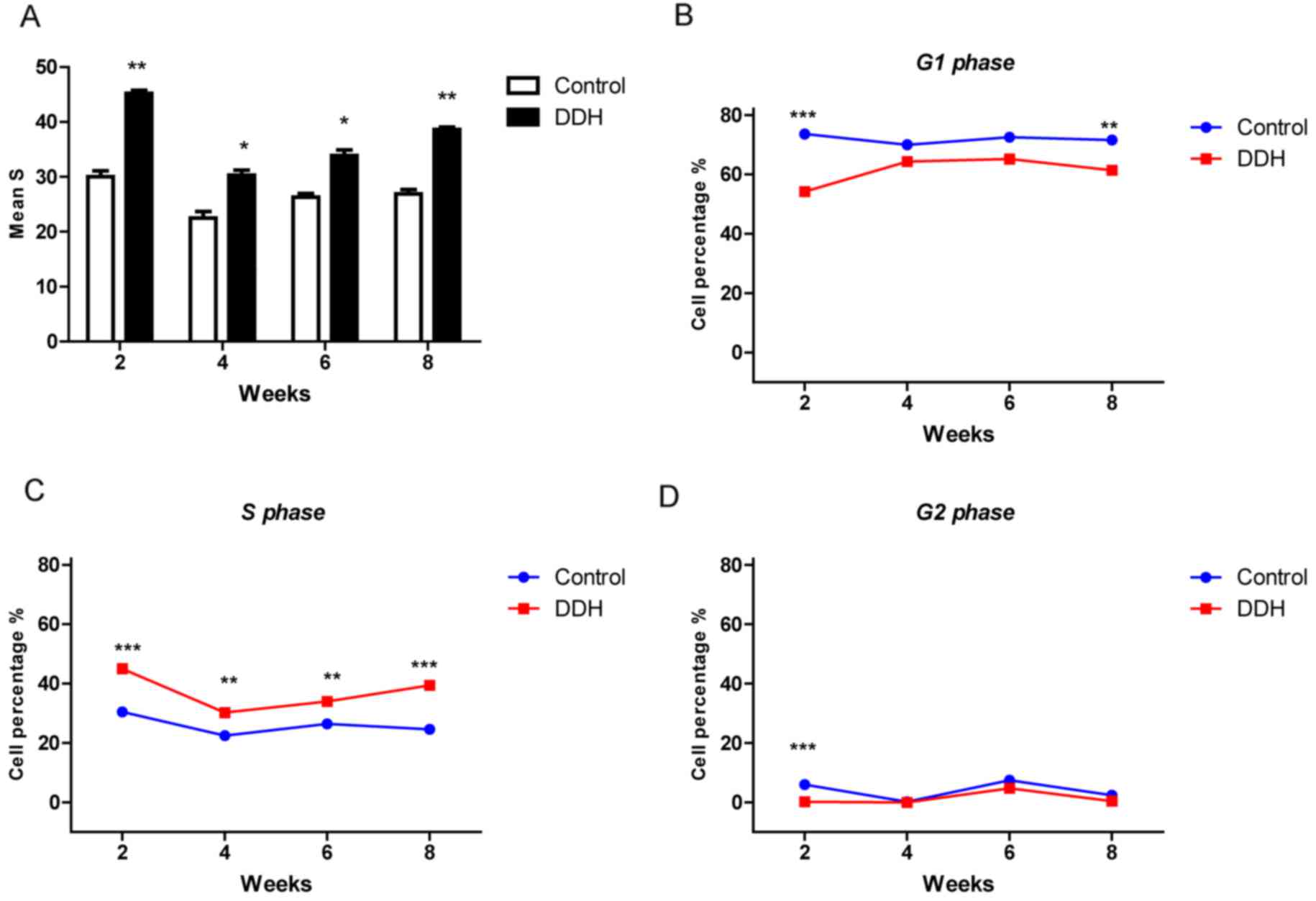
Cell cycle analysis
Samples were centrifuged at 300 × g for 3 min at
4°C, then collected and rinsed with cold PBS. Cells were then fixed
with 70% cold ethanol at 4°C overnight, then the cell were treated
with Cell Cycle Detection kit (KGA512, Nanjing KeyGen Biotech Co.,
Ltd., Nanjing, China). Cell cycle analysis was performed using flow
cytometry equipment (FACSAria II; BD Biosciences, Franklin Lakes,
NJ, USA) and the data were collected using FlowJo analysis software
(FlowJo-10.5.0; Flowjo LLC, Ashland, OR, USA). The number of cells
in each phase of the cell cycle was recorded and the proportion of
cells in S-phase was taken to be representative of proliferative
activity.
mRNA expression levels of collagen II,
aggrecan, matrix metallopeptidase (MMP)-13 and ADAM
metallopeptidase with thrombospondin type 1 motif 5 (ADAMTS-5)
Total RNA was extracted from the monolayer confluent
chondrocytes using TRIzol® (Invitrogen; Thermo Fisher
Scientific, Inc.) and the purity and integrity were assayed using
spectrophotometry and 10% agarose-gel electrophoresis respectively.
The OD values of these mRNAs were detected between 1.8 and 2.0. A
total of 1 µg RNA was transcribed to produce cDNA using a ReverTra
Ace qPCR RT kit (Toyobo, Life Science, Osaka, Japan) according to
the manufacturer's protocol. The yield was quantified
spectrophotometrically.
Reverse transcription-quantitative polymerase chain
reaction (RT-qPCR) (Denaturing 94°C, 60 sec; 40 cycles (Denaturing
94°C, 10 sec; annealing and extension 60°C, 30 sec) was performed
using 5 µl cDNA (100 ng), 2 µl each primer (10 µM), 25 µl SYBR
Green Real-time PCR Master Mix (Toyobo, Life Science) and 16 µl
water to give a total volume of 50 µl. The RT-qPCR was programmed
to an initial step of 10 min at 95°C for polymerase activity,
followed by 40 cycles of 15 sec denaturation at 95°C, 15 sec
annealing at 60°C, and 45 sec extension at 72°C. The expression
levels of MMP-13, Collagen 2a1, ADAMTS-4 and ADAMTS-5 were
normalized to β-actin. All primers used are listed in Table I. The results were quantified using
the 2_∆∆Cq method (16).
 | Table I.Primers used for amplification of
target genes and β-actin. |
Table I.
Primers used for amplification of
target genes and β-actin.
| Gene | Sequence
(5′-3) |
|---|
| Collagen II |
|
| F |
ACGCTCAAGTCGCTGAACAA |
| R |
TCAATCCAGTAGTCTCCGCTCT |
| Aggrecan |
|
| F |
TCCAAACCAACCCGACAAT |
| R |
TCTCATAGCGATCTTTCTTCTGC |
| MMP-13 |
|
| F |
TACGAGCATCCATCCCGAGACC |
| R |
AACCGCAGCACTGAGCCTTTTC |
| ADAMTS-5 |
|
| F |
GGCTGTGGTGTGCTGTG |
| R |
CTGGTCTTTGGCTTTGAAC |
| β-actin |
|
| F |
GGAGATTACTGCCCTGGCTCCTA |
| R |
GACTCATCGTACTCCTGCTTGCTG |
Statistical analysis
Data are expressed as the mean ± standard error of
the mean. Statistical significance was determined using one-way
analysis of variance and paired t-tests. The bonferroni method was
used for post hoc tests. Data were analyzed using SPSS software,
version 16.0 (SPSS, Inc., Chicago, IL, USA). Each experiment was
repeated three times. P<0.05 was considered to indicate a
statistically significant difference.
Results
Model and morphometry
Gross observations of the coronal dissection
morphology of the hip are presented in Fig. 1A. A distinct DDH model was
identified in the experimental DDH group, with dislocational hips
observed (Fig. 1A). The acetabular
index (AI) was significantly lower in the DDH rats compared with
the control group at all time points (P<0.001; Fig. 1B).
Immunofluorescence staining of
collagen II and evaluation of cell proliferation
The expression of collagen II was used to identify
chondrocytes. No apparent differences in cell morphology were
observed between the control and experimental DDH groups (Fig. 2A). Cell morphology varied, with
cobblestone, ellipse and polygonal shapes observed after the
primary cells had adhered (Fig.
2A). In addition, cell proliferation was assessed by counting
cells following staining with DAPI (Fig. 2B). The results revealed increased
proliferation in the experimental DDH group compared with the
control group at all time points (P<0.001).
Cell growth curve
At 2 weeks, the growth curve for the experimental
DDH group began to enter the linear phase, the slope of which was
greater than the control group, which indicated an increased trend
of proliferation (Fig. 3A).
However, no significant differences in the linear phase slope were
observed between groups at 4 weeks. This suggested that
proliferation in the experimental DDH group slowed over time
(Fig. 3B). Furthermore, at week 6
the slope of the linear phase was reversed (Fig. 3C); this was maintained until 8
weeks with the experimental DDH group exhibiting a decreased slope
(Fig. 3D).
Number of cells in S phase increases
during DDH
Flow cytometry was performed to measure the
proportion of cells in each phase of the cell cycle at different
time points (Fig. 4). S-phase is
when DNA synthesis occurs, and is therefore reflective of cell
proliferation. At all time points, a greater number of cells in the
experimental DDH group were in S-phase compared with the control
group (P<0.001; Fig. 4A).
Following the 2 week period, a gradual increase in the proportion
of cells in S-phase was observed in the experimental DDH group over
time (Fig. 4C). A reduced number
of cells were observed in G1-phase in the experimental DDH group
compared with the control, particularly at 2 and 8 weeks (Fig. 4B). The number of experimental DDH
cells in G2-phase was also lower compared with the control group,
particularly at 2 weeks (Fig. 4D).
A significant increase in experimental DDH cells in the S-phase was
observed at 2 and 8 weeks (Fig.
4C).
mRNA expression levels of collagen II,
aggrecan, MMP-13 and ADAMTS-5 are varied at different time-points
in DDH
The mRNA expression levels were investigated using
RT-qPCR (Fig. 5). Collagen II mRNA
expression in the experimental DDH group was upregulated at 2 weeks
after the removal of fixation, and downregulated at 4 weeks.
However, a significant reversal was present at 6 weeks followed by
a downregulation again at 8 weeks (Fig. 5A). The expression of MMP-13 mRNA
was overexpressed in DDH cells compared with the control group at 2
weeks, decreased at 4 weeks and then this expression gradually
increased during the following weeks (Fig. 5C). Aggrecan expression levels were
significantly different between the experimental DDH and control
groups at weeks 4 and 8; however, no significant differences were
observed at weeks 2 and 6 (Fig.
5B). Conversely ADAMTS-5 mRNA in the DDH group was
significantly different at weeks 2, 6 and 8 compared with the
control (Fig. 5D).
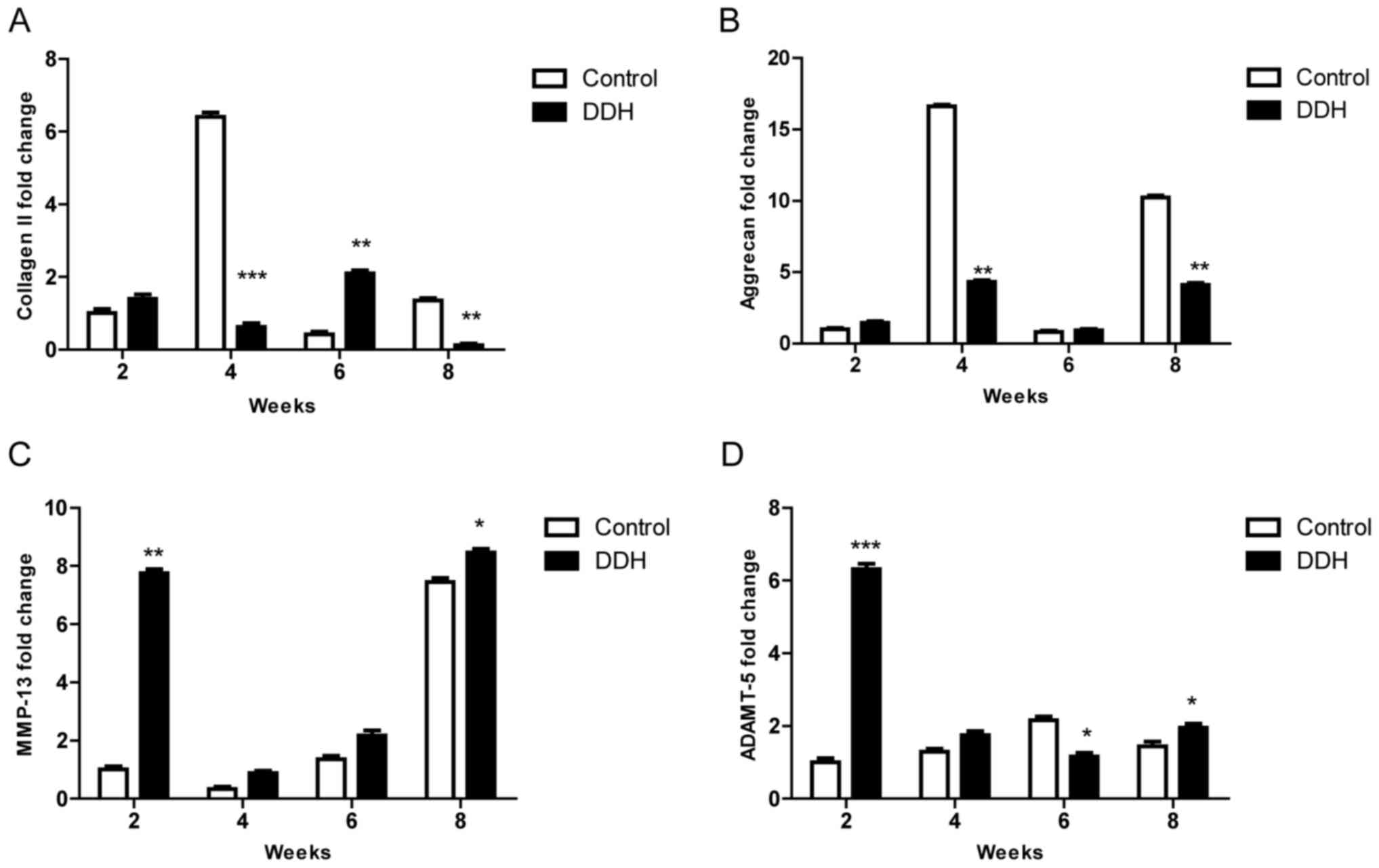 | Figure 5.mRNA expression levels of collagen
II, aggrecan, MMP-13 and ADAMTS-5 are varied at different
time-points from 2 to 8 weeks in DDH. (A) The expression of
collagen II mRNA was upregulated in the DDH group at 2 weeks after
the removal of fixation, whereas it was downregulated at 4 weeks.
This effect was reversed at 6 weeks followed by a further
downregulation of expression at 8 weeks. (B) The expression of
aggrecan mRNA was markedly different at 4 weeks compared with 8
weeks, whereas there was no great difference between 2 weeks and 6
weeks. (C) MMP-13 mRNA was overexpressed in the experimental DDH
group compared with the control group from 2 to 8 weeks, and the
expression in DDH was gradually upregulated during the times. (D)
ADAMTS-5 mRNA expression in the experimental DDH group was markedly
different compared with the control group at weeks 2, 6 and 8.
*P<0.05, **P<0.01 and ***P<0.001 vs. control. DDH,
developmental dysplasia of the hip; MMP-13, matrix
metallopeptidase; ADAMTS-5, ADAM metallopeptidase with
thrombospondin type 1 motif 5. |
Discussion
The DDH model of neonatal rats shows that the
swaddling position is a mechanical risk factor that plays an
important role in the pathogenesis of DDH. Previous studies of DDH
models have revealed that the hip may be remodeled after the
removal of fixation, which resembles close reduction treatment in
human infants with DDH (17).
Furthermore, Yamamoto (2) reported
that shorter fixation duration gave better results. It has also
been reported that the DDH is able to be completely reversed to
prevent degeneration (18).
Nevertheless, the results of the present study were not consistent
with previous reports due to the failure to achieve close
reduction; although remodeling of the macro-morphology was observed
in the early stage, degeneration increased irreversibly with
skeletal maturity (10,12). However, changes may be associated
with cartilage content and resulting differences in mechanical
features at different ages. In addition, the maintenance of
subluxation resulted in no complete reduction, as the long duration
of immobilization made the cartilage suffer from more abnormal
weight-bearing. These kinds of changes leading to cartilage
degeneration can be observed in clinical DDH X rays.
The histological and gross observation results
revealed that remodeling occurred with proliferation at an early
stage. Few studies focus on cellular proliferation of the
chondrocytes, no matter whether or not the cells are loaded with
abnormal mechanical forces. It is therefore important to
investigate the proliferative ability at a cellular level (19). Chondrocytes in patients with DDH
suffer from a variety of stresses, including shear, compression and
tension loading, and so it is difficult to analyze the relationship
between cell proliferation and mechanical loading. A number of
studies have investigated the association between simple stress
in vitro and cell proliferation and demonstrated that
proliferation is associated with the type, intensity and mode of
stress (20–25). In addition, a novel method for
assessing articular cartilage chondrocytes in vivo has been
described (26). However, the
effects of different types of stress loading on the cartilage
cannot be examined in vivo in a rat model, as the hip volume
is too small. In the present study, cell proliferation was assessed
using cell cycle analysis, and CCK-8 assays.
Cell cycle progression is the predominant means of
regulating cell proliferation and differentiation, and so
increasing our understanding of cell cycle progression in DDH
chondrocytes may be beneficial. Changes in the number of cells in
S-phase at 2 weeks may be due to an increase in proliferative
ability following the transient removal of compress loading. The
changes at weeks 4 and 6 may occur as a result of sustained complex
loading due to mobilization, and accumulation of the proliferative
cells may be a compensational reaction to maintain cartilage
function. However, it is unclear why at 8 weeks cell numbers
decreased while the proportion of cells in S-phase increased.
Although an increase in S-phase cells indicated early proliferative
activity, the proportion of cells in G0/G1-phase was significantly
lower in the experimental DDH group compared with the control
group, suggesting that proliferation and differentiation may be
elevated in DDH chondrocytes. Nevertheless, the proliferation index
regarding the S-phase accounted for the cell proliferative ability
at the actual time points, while CCK-8 results indicated
proliferative kinetics according to the slope of the growth curve.
The results revealed that proliferation occurred faster soon after
the removal of fixation in DDH chondrocytes.
The relationship between cartilage remodeling and
cell proliferation has recently been reported by assessing the
spatial reorganization of superficial chondrocytes in the early
stages of osteoarthritis (27).
Furthermore, a clear association has been reported between cell
proliferation and ECM metabolism (28). Further investigation is required to
discover further details regarding changes in ECM metabolism in DDH
chondrocytes in response to changes in loading.
Collagen II and aggrecan provide the cartilage with
tensile and compressive strength by forming a meshwork of collagen
II in which the interstices are filled with aggrecans (29,30).
MMPs and ADAMTSs secreted by chondrocytes are the two main groups
of proteases in the ECM that mediate the degradation of collagen II
and aggrecan. mRNA was extracted from primary cells and the
expression of Col2a1 and aggrecan were assessed along with MMP-13
and ADAMTS-5 expression.
Homeostasis of the cellular environment is important
for the function of the cartilage, maintaining a balance between
the structural components and their proteolytic enzymes in response
to dynamic loading (31). If
chondrocyte metabolism is disrupted due to abnormal mechanical
stresses and degradation of the ECM, the chondrocytes will initiate
a compensational mechanism to counteract the inappropriate
mechanical loading. Studies have revealed that cartilage
regeneration and degeneration are dependent on the duration,
quality and strength of abnormal loading (32,33).
However, results have indicated that the metabolism of collagen in
response to abnormal loading is different to that of aggrecan
(20). As such, the upregulation
of collagen II independent of aggrecan is considered to be a marker
for early degeneration in DDH experiments as well as early
osteoarthritis (11,34–36).
In the present study, no significant differences in expression were
observed between collagen II and aggrecan at 2 weeks. However, both
were upregulated during the period after the transient removal of
fixation (37). Collagen II rather
than aggrecan demonstrated a difference between DDH and control
group, while collagen II expression was downregulated at 8 weeks
after modeling, which followed a gradual elevation until 6 weeks.
Conversely, aggrecan mRNA expression was downregulated until 8
weeks post-modeling. The expression levels of MMP-13 and ADAMTS-5
were significantly affected by ECM synthesis and increased at 2
weeks, which was associated with the compression loading being
released. Furthermore, following re-mobilization of the hip, the
expression of both proteases was downregulated. MMP-13 and ADAMTS-5
have been reported to have an important effect in early
degeneration during loading stress (38). Furthermore, ADAMTS-5 expression is
higher than collagen II and aggrecan during the early stages of DDH
and lower in the later stages, suggesting that ADAMTS-5 is more
sensitive to changes in loading stress and serves a predominant
role in hip remodeling in the early stages following load removal.
If complete reduction is achieved, the expression of ADAMS-5 mRNA
is reversible and cartilage degeneration may be prevented, as
reported by Karsdal et al (39). A study by Breckon et al
(40) involving a 14-year-old
patient with DDH suggested that MMP-13 was not essential for the
remodeling of cartilage growth and chondrocyte proliferation.
Conversely, other studies have reported that MMP-13 serves an
important role in cartilage development and ECM remodeling.
Previous studies have revealed that MMP-13 and ADAMTS-5 expression
levels are closely associated with stress activity (37,41,42),
suggesting that several signal pathways could play complex roles in
mediating chondrocyte metabolism in DDH cartilage. MMP-13 and
ADAMTS-5 were upregulated during the early degeneration of
cartilage and then downregulated sharply. This result suggests that
DDH degeneration may be reversible during DDH degeneration.
It has previously been reported that collagen
expression is associated with the longitudinal and transverse
distribution and intensity of weight-bearing (43–47).
A phenomenon known as dedifferentiation can occur during cell
culture, and in the present study, early alternations in DDH
cartilage at the cellular and molecular levels in vivo and
in vitro resulted in no complete reduction and subluxation
in consequence. This does not influence our results; although
alternative findings in different tests were observed, the
expression of target molecules was unaffected. Changes at the
molecular level, which are the initial promoters of progressive
degeneration, were not observed. Therefore, in future studies it
will be interesting to focus on it whether the early degeneration
occurs after the operations of DDH at molecular level and what time
will be appropriate for the operation so that the degeneration
could be prevented completely.
In conclusion, although complete reduction was not
achieved after the removal of fixation, transient remodeling of the
hip occurred over time, which was indicative of high proliferative
activity in the chondrocytes as well as cell cycle progression at
the early stage. However, degeneration occurred at the later stage.
These results suggested that MMP-13 and ADAMTS-5 serve a dominant
role not only in the remodeling phase but also in the degeneration
stage.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
DW designed the experiments and revised the paper.
BN, RJ and LW performed the experiments and wrote the paper. BN and
LW analyzed the data. BN and DW read and revised the paper.
Ethics approval and consent to
participate
All methods in this study were approved by the
Research Medical Ethics Committee of Fudan University. All
experimental protocols were performed in accordance with the
Institutional Ethics Committee of the Animal Ethical Committee of
Fudan University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare they have no competing
interests.
References
|
1
|
Nelitz M and Reichel H: Nonsurgical
treatment of developmental dysplasia of the hip. Orthopade.
37:550552–555. 2008.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yamamoto N: Changes of the acetabular
cartilage following experimental subluxation of the hip joint in
rabbits. Nihon Seikeigeka Gakkai zasshi. 57:1741–1753. 1983.(In
Japanese). PubMed/NCBI
|
|
3
|
Ibrahim S: Acetabular dysplasia after
treatment for developmental dysplasia of the hip. J Bone Joint Surg
Br. 87:10252005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kim HT, Kim JI and Yoo CI: Acetabular
development after closed reduction of developmental dislocation of
the hip. J Pediatr Orthop. 20:701–708. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ma R, Ji S, Zhou Y, Liu W and Zhang L:
Evolutionary regularity of acetabular dysplasia after reduction of
developmental dislocation of the hip. Chin Med J (Engl).
110:346–348. 1997.PubMed/NCBI
|
|
6
|
Nishii T, Sugano N, Sato Y, Tanaka H, Miki
H and Yoshikawa H: Three-dimensional distribution of acetabular
cartilage thickness in patients with hip dysplasia: A fully
automated computational analysis of MR imaging. Osteoarthritis
Cartilage. 12:650–657. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nishii T, Shiomi T, Tanaka H, Yamazaki Y,
Murase K and Sugano N: Loaded cartilage T2 mapping in patients with
hip dysplasia. Radiology. 256:955–965. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sijbrandij S: Dislocation of the hip in
young rats produced experimentally by prolonged extension. J Bone
Joint Surg Br. 47:792–795. 1965. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Greenhill BJ, Hainau B, Ellis RD and
el-Sayed RM: Acetabular changes in an experimental model of
developmental dysplasia of the hip (DDH). J Pediatr Orthop.
15:789–793. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bo N, Peng W, Xinghong P and Ma R: Early
cartilage degeneration in a rat experimental model of developmental
dysplasia of the hip. Connect Tissue Res. 53:513–520. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Casali PG and Blay JY;
ESMO/CONTICANET/EUROBONET Consensus Panel of Expert, :
Gastrointestinal stromal tumours: ESMO clinical practice guidelines
for diagnosis, treatment and follow-up. Ann Oncol. 21 Suppl
5:v98–v102. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ning B, Sun J, Yuan Y, Yao J, Wang P and
Ma R: Early articular cartilage degeneration in a developmental
dislocation of the hip model results from activation of β-catenin.
Int J Clin Exp Pathol. 7:1369–1378. 2014.PubMed/NCBI
|
|
13
|
da Silva MA, Yamada N, Clarke NM and Roach
HI: Cellular and epigenetic features of a young healthy and a young
osteoarthritic cartilage compared with aged control and OA
cartilage. J Orthop Res. 27:593–601. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kremli MK, Alshahid AH, Khoshhal KI and
Zamzam MM: The pattern of developmental dysplasia of the hip. Saudi
Med J. 24:1118–1120. 2003.PubMed/NCBI
|
|
15
|
Manning WK and Bonner WM Jr: Isolation and
culture of chondrocytes from human adult articular cartilage.
Arthritis Rheum. 10:235–239. 1967. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Raab P, Lohr J and Krauspe R: Remodeling
of the acetabulum after experimental hip joint dislocation-an
animal experiment study of the rabbit. Z Orthop Ihre Grenzgeb.
136:519–524. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ning B, Yuan Y, Yao J, Zhang S and Sun J:
Analyses of outcomes of one-stage operation for treatment of
late-diagnosed developmental dislocation of the hip: 864 hips
followed for 3.2 to 8.9 years. BMC Musculoskelet Disord.
15:4012014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tsuji Y, Takeshita H, Kusuzaki K, Hirasawa
Y, Ueda K and Ashihara T: Cell proliferation and differentiation of
cultured chondrocytes isolated from growth plate cartilage of rat
rib. Nihon Geka Hokan. 64:50–63. 1995.PubMed/NCBI
|
|
20
|
Hunter CJ, Imler SM, Malaviya P, Nerem RM
and Levenston ME: Mechanical compression alters gene expression and
extracellular matrix synthesis by chondrocytes cultured in collagen
I gels. Biomaterials. 23:1249–1259. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Garcia M and Knight MM: Cyclic loading
opens hemichannels to release ATP as part of a chondrocyte
mechanotransduction pathway. J Orthop Res. 28:510–515.
2010.PubMed/NCBI
|
|
22
|
Bougault C, Paumier A, Aubert-Foucher E
and Mallein-Gerin F: Molecular analysis of chondrocytes cultured in
agarose in response to dynamic compression. BMC Biotechnol.
8:712008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
De Croos JN, Dhaliwal SS, Grynpas MD,
Pilliar RM and Kandel RA: Cyclic compressive mechanical stimulation
induces sequential catabolic and anabolic gene changes in
chondrocytes resulting in increased extracellular matrix
accumulation. Matrix Biology. 25:323–331. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Villanueva I, Gladem SK, Kessler J and
Bryant SJ: Dynamic loading stimulates chondrocyte biosynthesis when
encapsulated in charged hydrogels prepared from poly (ethylene
glycol) and chondroitin sulfate. Matrix Biol. 29:51–62. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ando K, Imai S, Isoya E, Kubo M, Mimura T,
Shioji S, Ueyama H and Matsusue Y: Effect of dynamic compressive
loading and its combination with a growth factor on the
chondrocytic phenotype of 3-dimensional scaffold-embedded
chondrocytes. Acta Orthop. 80:724–733. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Abusara Z, Seerattan R, Leumann A,
Thompson R and Herzog W: A novel method for determining articular
cartilage chondrocyte mechanics in vivo. J Biomech. 44:930–934.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rolauffs B, Williams JM, Aurich M,
Grodzinsky AJ, Kuettner KE and Cole AA: Proliferative remodeling of
the spatial organization of human superficial chondrocytes distant
from focal early osteoarthritis. Arthritis Rheum. 62:489–498.
2010.PubMed/NCBI
|
|
28
|
Shields KJ, Beckman MJ, Bowlin GL and
Wayne JS: Mechanical properties and cellular proliferation of
electrospun collagen type II. Tissue Eng. 10:1510–1517. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sivan SS, Wachtel E and Roughley P:
Structure, function, aging and turnover of aggrecan in the
intervertebral disc. Biochim Biophys Acta. 1840:3181–3189. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kiani C, Chen L, Wu YJ, Yee AJ and Yang
BB: Structure and function of aggrecan. Cell Res. 12:19–32. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nagase H and Kashiwagi M: Aggrecanases and
cartilage matrix degradation. Arthritis Res Ther. 5:94–103. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
van Meurs JB, van Lent PL, Holthuysen AE,
Singer II, Bayne EK and van den Berg WB: Kinetics of aggrecanase-
and metalloproteinase-induced neoepitopes in various stages of
cartilage destruction in murine arthritis. Arthritis Rheum.
42:1128–1139. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
van Lent PL, Grevers LC, Blom AB, Arntz
OJ, van de Loo FA, Van der Kraan P, Abdollahi-Roodsaz S, Srikrishna
G, Freeze H, Sloetjes A, et al: Stimulation of chondrocyte-mediated
cartilage destruction by S100A8 in experimental murine arthritis.
Arthritis Rheum. 58:3776–3787. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Narmoneva DA, Cheung HS, Wang JY, Howell
DS and Setton LA: Altered swelling behavior of femoral cartilage
following joint immobilization in a canine model. J Orthop Res.
20:83–91. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hagiwara Y, Ando A, Chimoto E, Saijo Y,
Ohmori-Matsuda K and Itoi E: Changes of articular cartilage after
immobilization in a rat knee contracture model. J Orthop Res.
27:236–242. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tchetina EV, Squires G and Poole AR:
Increased type II collagen degradation and very early focal
cartilage degeneration is associated with upregulation of
chondrocyte differentiation related genes in early human articular
cartilage lesions. J Rheumatol. 32:876–886. 2005.PubMed/NCBI
|
|
37
|
Haapala J, Arokoski JP, Hyttinen MM, Lammi
M, Tammi M, Kovanen V, Helminen HJ and Kiviranta I: Remobilization
does not fully restore immobilization induced articular cartilage
atrophy. Clin Orthop Relat Res. 1–229. 1999.PubMed/NCBI
|
|
38
|
Borzi RM, Olivotto E, Pagani S, Vitellozzi
R, Neri S, Battistelli M, Falcieri E, Facchini A, Flamigni F, Penzo
M, et al: Matrix metalloproteinase 13 loss associated with impaired
extracellular matrix remodeling disrupts chondrocyte
differentiation by concerted effects on multiple regulatory
factors. Arthritis Rheum. 62:2370–2381. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Karsdal MA, Madsen SH, Christiansen C,
Henriksen K, Fosang AJ and Sondergaard BC: Cartilage degradation is
fully reversible in the presence of aggrecanase but not matrix
metalloproteinase activity. Arthritis Res Ther. 10:R632008.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Breckon JJ, Hembry RM, Reynolds JJ and
Meikle MC: Regional and temporal changes in the synthesis of matrix
metalloproteinases and TIMP-1 during development of the rabbit
mandibular condyle. J Anat. 184:99–110. 1994.PubMed/NCBI
|
|
41
|
Selvamurugan N, Jefcoat SC, Kwok S,
Kowalewski R, Tamasi JA and Partridge NC: Overexpression of Runx2
directed by the matrix metalloproteinase-13 promoter containing the
AP-1 and Runx/RD/Cbfa sites alters bone remodeling in vivo. J Cell
Biochem. 99:545–557. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tetsunaga T, Nishida K, Furumatsu T,
Naruse K, Hirohata S, Yoshida A, Saito T and Ozaki T: Regulation of
mechanical stress-induced MMP-13 and ADAMTS-5 expression by RUNX-2
transcriptional factor in SW1353 chondrocyte-like cells.
Osteoarthritis Cartilage. 19:222–232. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Aigner T, Stoss H, Weseloh G, Zeiler G and
von der Mark K: Activation of collagen type II expression in
osteoarthritic and rheumatoid cartilage. Virchows Arch B Cell
Pathol Incl Mol Pathol. 62:337–345. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Aigner T, Bertling W, Stöss H, Weseloh G
and von der Mark K: Independent expression of fibril-forming
collagens I, II, and III in chondrocytes of human osteoarthritic
cartilage. J Clin Invest. 91:829–837. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Aigner T, Vornehm SI, Zeiler G, Dudhia J,
von der Mark K and Bayliss MT: Suppression of cartilage matrix gene
expression in upper zone chondrocytes of osteoarthritic cartilage.
Arthritis Rheum. 40:562–569. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hotta H, Yamada H, Takaishi H, Abe T,
Morioka H, Kikuchi T, Fujikawa K and Toyama Y: Type II collagen
synthesis in the articular cartilage of a rabbit model of
osteoarthritis: Expression of type II collagen C-propeptide and
mRNA especially during early-stage osteoarthritis. J Orthop Sci.
10:595–607. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Park K, Min BH, Han DK and Hasty K:
Quantitative analysis of temporal and spatial variations of
chondrocyte behavior in engineered cartilage during long-term
culture. Ann Biomed Eng. 35:419–428. 2007. View Article : Google Scholar : PubMed/NCBI
|