Introduction
Maternal immune activation (MIA) during pregnancy
increases the risk of developing several mental disorders,
including autism and schizophrenia (1). Epidemiological studies have provided
compelling evidence that perinatal factors such as MIA increase the
risk of schizophrenia and associated disorders following prenatal
maternal exposure to infection or inflammation (2,3). To
replicate such risks in animal models, fetuses can be exposed to
MIA by administering viral mimetic double-stranded RNA
polyriboinosinic-polyribocytidylic acid [poly(I:C)], a
pro-inflammatory cytokine inducer, to pregnant females (2,4).
Notably, a broad range of behavioral impairments in rodent
offspring due to gestational immune activation have been commonly
described in schizophrenia patients, including deficits in prepulse
inhibition (PPI) of acoustic startle response (ASR) (5,6) and
social interaction (6), as well as
brain abnormalities (7). Among the
behavioral outcomes investigated following prenatal immune
treatment, PPI is considered as an operational measure of
sensorimotor gating. Its deficit has been observed in acute/chronic
patients with schizophrenia (8,9) and
PPI deficit in schizophrenia patients has been well documented
(9,10).
Through experimental investigation, the MIA model
has provided substantial evidence of psychosis-associated
dysfunction in schizophrenia. Psychosis-associated behavioral
abnormalities in adult life have etiological significance in the
neurodevelopmental perspective of schizophrenia (5,11,12).
A previous study demonstrated that treatment with the typical
antipsychotic medication clozapine and the atypical antipsychotic
haloperidol reverse cognitive impairment in MIA offspring (6). The effects of maternal treatment with
certain drugs on MIA offspring have also been observed; clozapine,
haloperidol, risperidone, paliperidone and fluoxetine have
exhibited protective effects against the emergence of behavioral
and structural abnormalities in MIA offspring (13,14).
It has been reported that long-term administration of clozapine
improves cognitive performance in poly(I:C)-induced MIA mice
(14). In addition, treatment with
risperidone was reported to prevent the majority of
neuropathological alterations in a peri-adolescent MIA mouse model
(13). Results from our previous
study also demonstrated that MIA induces abnormal behavior
associated with alterations in neurodevelopmental protein
expression level in the offspring (15).
Panax ginseng C.A. Meyer (PG) is one of the
most important medicinal plants in Asia (16). In an experimental brain injury
model, PG can suppress microglial activation induced by
lipopolysaccharide and improve the recovery of motor function after
spinal cord injury, thus providing neuroprotection by alleviating
post-traumatic inflammatory responses (16,17).
Experimental evidence has suggested that it can improve
neurotransmission in the brain as an important pharmacological
effect of PG (16,17). Ginseng and its constituents are
known to have beneficial effects on cognitive performance, memory,
and neurodegenerative disease (18). A number of previous studies have
focused on the effects of ginseng on central nervous system
diseases such as Alzheimer's disease, Parkinson's disease and
depression (19,20). However, few studies have
investigated the effects of ginseng on schizophrenia. Chen and Hui
(21) observed that ginseng has
the therapeutic potential to treat various neurological diseases
including schizophrenia. Our previous study has also suggested that
oral administrated PG can reduces the incidence of
schizophrenia-like symptoms in a neurodevelopmental animal model
induced by prenatal stress (22).
The aim of the present study was to examine the
effects of PG on behavioral abnormalities relevant to schizophrenia
in MIA-induced mouse model offspring. It focused on two aspects:
First, the manifestation of behavioral symptoms in a MIA model.
Second, the influence of PG on expression levels of
neurodevelopmental proteins in medial prefrontal cortex (mPFC) of a
MIA model. The potential beneficial effects of PG on MIA offspring
were examined based on schizophrenia-like behavior and protein
levels in mPFC.
Materials and methods
Preparation of PG extracts
Dried roots of PG were purchased from a local farm
in Yunpung (Chungbuk, Republic of Korea). The root specimens were
taxonomically identified by an Oriental medicine physician at the
National Institute of Horticultural & Herbal Science, Rural
Development Administration (Jeonju, Korea). A voucher specimen
(HPR-207) was deposited in the herbarium of Herbal Crop Research
Institute (Eumsung, Korea). The majority of traditional Oriental
herbal materials are decocted with boiling water. Therefore, water
extraction was used in the present study. In addition, ginsenosides
are more soluble in water than in organic solvents (17). Briefly, crushed PG materials (200
g) were extracted under reflux with distilled water three times.
Water extracts were then combined and lyophilized. The yield was
18.3% (wt/wt) for PG in a dried state. Extracts were stored at
−20°C until use.
Animals
A total of 27 C57BL6/J mice (9 male and 18, female;
18–25 g) were purchased from Daehan BioLink Co., Ltd. (Eumseong,
Korea). At the age of 8 weeks, the mice were mated in groups of one
male with two females. When a vaginal plug was observed during
daily checks, female mice were considered pregnant and mice were
separated. All animals were housed under standard conditions
(21±3°C and 40% humidity) in a 12-h light/dark cycle and had free
access to food and water. All animal procedures were performed in
accordance with the Guide for the Care and Use of Laboratory
Animals of the National Institutes of Health (23). Experimental procedures were
approved by the Institutional Animal Care and Use Committee of
Soonchunhyang University (approval no. SCH16-0063; Cheonan).
MIA and drug administration
Pregnant female mouse were single injected on
gestation day 9 (GD9) about 200 µl of poly(I:C) (n=12; potassium
salt; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) or saline
(n=6) solution via intravenous route at the tail vein under mild
physical constraint. Poly(I:C) was dissolved in isotonic 0.9% NaCl
solution to obtain desired dosage [5 mg/kg; calculated based on
pure form poly(I:C)] (15). All
animals were returned to their cages immediately following the
injection and left undisturbed until the weaning of the offspring.
Powdered PG extracts were dissolved in water and orally
administered (300 mg/kg/day) on postnatal day 35 for ~4 weeks,
until postnatal day 65 (24). All
groups contained litters of 8 to 15 pups with similar numbers of
males and females. Extremely large or small litters were
eliminated. The offspring were weaned at 21 days and group-housed.
Male offspring (n=41) were selected and used for further
experiments. Three experimental groups were tested as adults: i)
‘Control’ (n=14) group animals were offspring of unstressed
mothers; ii) Poly I:C injected group ‘MIA’ (n=13 male offspring)
animal were offspring of mothers subjected to stress before
parturition; and iii) PG group (n=14 male offspring) animals were
those subjected to injection of Poly I:C followed by PG
administration.
Behavioral tests
Behavioral testing was performed when offspring
reached 10 weeks of age (n=10–12/group; ≥2 pups/litter from each
group). Only male subjects were included. The number of subjects in
each of the three experimental groups was 10 (at least two pups per
litter).
PPI
An automated Startle Reflex System (SR-LAB; San
Diego Instruments, Inc., San Diego, CA, USA) was used to measure
PPI (n=10–12 animals/group). This system consisted of a startle
chamber housed in a sound attenuated isolation cabinet equipped
with an internal fan and light. A cylindrical transparent acrylic
holding apparatus resting on a four-pegged platform within the
isolation chamber was used to hold each subject throughout the
testing session. Background noise and acoustic stimuli were
controlled through the SR-LAB microcomputer and interface assembly.
Stimuli were delivered through a speaker that was mounted above the
cylindrical holding apparatus. All test chambers were located in a
sound attenuated experimental room to minimize external noise, as
previously described (25).
Background noise was present throughout the test session at 68 dB.
Following a 5 min acclimation period to background noise, trials
were presented in a pseudorandom order, including: i) 14
pulse-alone trials, in which a 40 msec, 120 dB broadband noise
burst was presented; ii) 30 prepulse + 30 pulse trials, in which a
20 msec broadband noise prepulse at intensities of 3, 6 and 12 dB
were applied above the background noise (10 trials at each
intensity) preceded the onset of the 120 dB pulse for 100 msec; and
iii) 8 non-stimulus trials comprising background noise only. The
prepulse intensities used in the protocol did not induce a startle
reaction. All trials were presented with an average intertrial
interval of 22 sec (range, 15–30 sec). Additionally, four 120 dB
pulse trials were presented at the beginning and the end of the
test session, with a series of 60 acoustic stimuli trials. However,
they were not used for the calculation of PPI values. Holding
chambers were cleaned with 75% ethanol between test sessions. PPI
values were calculated as a percentage score for each prepulse
using the following formula: PPI (%)=100-[(ASR for prepulse+pulse
trial)/(ASR for pulse alone trial)] ×100 (25). Mean %PPI was used as an overall
measure of the observed treatment for which percentage PPI data
were averaged for three prepulses (14,15).
Forced-swim test (FST)
FST was performed as described previously (26,27).
Mice (n=10–12 animals/group) were gently placed in a large
transparent cylinder filled with fresh tap water (25±2°C) for 5
min; water was changed between mice. The swimming, climbing and
immobility behaviors were recorded with a video camera and by an
observer with a stopwatch. The predominant behaviors were counted
every 5 sec. Test scores for swimming (horizontal movement
throughout the chamber and crossing quadrants), climbing
(upward-directed movements up the side of the chamber and jump-ups
from the bottom of the chamber) and immobility (no additional
activity other than keeping the head above water or tiny whip
kicks) behaviors were recorded.
Open field test (OFT)
OFT was conducted to assess exploratory activity and
reactivity to a novel environment. Mice (n=10–12 animals/group)
were removed from their cages and placed individually in the center
zone (17×17 cm) of the open field arena (50×50 cm) for 20 min; The
apparatus was constructed of opaque Polygal. No background noise
was provided. The duration in the central zone and the frequency of
entry into the central zone were used as indices for anxiety-like
behaviors. The box was cleaned with 70% ethanol between tests to
eliminate odors of other mice. The experimenter exited the room and
the behavior of the mouse was recorded as described previously
(28).
Social interaction test (SIT)
SIT was adapted from previous studies (n=10–12
animals/group) (29,30). Their social interaction partners
were the same-sex siblings with approximately the same body weight
that resided in the same cage after weaning. The room in which the
chamber was located was darkened during testing and the chamber was
illuminated with a single 25 W red light bulb placed ~100 cm above
the base of the chamber. Each session lasted 20 min. Total duration
of social play and the number and types of interactions were
scored.
Western blot
mPFC tissues (n=5–6 animals/group; ~0.5 mg/ml) were
lysed in radioimmunoprecipitation assay buffer containing protease
inhibitors (cat. no. EBA-1149; ELPIS-Biotech, Inc., Daejeon, Korea)
and centrifuged at 18,341 × g for 10 min at 4°C. The Bradford assay
was used to determine protein concentration. Proteins (80 µg/lane)
were subjected to 10 and 12% SDS-PAGE and subsequently transferred
to polyvinylidene difluoride membranes (Merck KGaA, Darmstadt,
Germany). Membranes were blocked with 5% skimmed milk at room
temperature for 1 h and subsequently probed with
anti-dihydropyrimidinase-related 2 (Dpysl2; 1:1,000; cat. no. 9393;
Cell Signaling Technology, Inc., Danvers, MA, USA), anti-LIM and
SH3 domain 1 (Lasp1; 1:2,000; cat. no. MAB8991; Merck KGaA),
anti-neurofilament medium (Nefm; 1:1,000; cat. no. 2838; Cell
Signaling Technology, Inc.), anti-discs large homolog 4 (Dlg4;
1:1,000; cat. no. 3450; Cell Signaling Technology, Inc.) or
anti-β-actin (Actb; 1:1,000; cat. no. sc-81178; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) antibodies overnight at 4°C.
Following washing with 1× TBST, these membranes were incubated with
horseradish peroxidase-conjugated secondary anti-mouse (1:10,000;
cat. no. A9044; Sigma-Aldrich; Merck KGaA) or anti-rabbit (1:5,000;
cat. no. LF-SA8002; Abfrontier; Adipogen AG, Liestal, Switzerland)
for 1 h at room temperature. Immunoreactive bands were detected
using an Enhanced Chemiluminescence kit (ELPIS-Biotech, Inc.).
Quantitative measurements of Dpysl2, Lasp1, Nefm, Dlg4 and Actb
protein expression levels were made using ImageJ software version
1.51k (National Institutes of Health, Bethesda, MD, USA; http://imagej.nih.gov/ij).
Immunohistochemistry
Mice were deeply anesthetized with ethyl ether and
perfused with 4% paraformaldehyde at room temperature for ≥30 min.
Fixed brains were removed, frozen and sectioned (30 µm; n=4–5
animals/group). Frozen mPFC sections were blocked with normal horse
serum (cat. no. S-2000; Vector Laboratories, Inc., Burlingame, CA,
USA) for 1 h at room temperature and subsequently incubated with
anti-Dpysl2 (1:700; cat. no. HPA002381; Atlas Antibodies AB,
Stockholm, Sweden), anti-Nefm (1:100; cat. no. 2838; Cell Signaling
Technology, Inc.) or anti-neuronal nuclei (NeuN; 1:100; cat. no.
MAB377; Merck KGaA) antibodies overnight at 4°C. Following
incubation, sections were incubated with Cy3-conjugated anti-rabbit
(1:500; cat. no. 715-545-151; Jackson ImmunoResearch Laboratories,
Inc., West Grove, PA, USA) and anti-mouse (1:800; cat. no.
111-165-003, Jackson ImmunoResearch Laboratories, Inc.) secondary
antibodies at room temperature for 1 h. Fluorescent images were
captured using a confocal laser-scanning microscope (FV10i; Olympus
Corporation, Tokyo, Japan). Images were quantified with ImageJ
software (National Institutes of Health, Bethesda, MD, USA) version
1.51k using a previously described protocol (31).
High-performance liquid chromatography
(HPLC) analysis of ginsenosides
Analyses of ginsenosides Rb1, Rc, Rd, Re and Rf on
10 mg extract were conducted using a reverse-phase HPLC system in
room temperature. HPLC chromatograms were recorded with a Waters
1525 Binary HPLC pump equipped with a Waters 2489 UV/VIS detector
(Waters, Miami, FL, USA). Chromatographic separation was performed
using a SunFire C-18 column (2.1×50 mm; 5 µm; Waters) with a mobile
phase consisting of water (solvent A) and acetonitrile (solvent B).
A gradient elution was used and the ratio of these solvents was as
follows: 95:5 (A:B) at 0 min, 65:35 (A:B) at 35 min, and 20:80
(A:B) at 40 min. UV detection was set at 204 nm. All injections
were conducted three times. The injection volume was 10 µl and the
flow rate was set at 1.0 ml/min.
Statistical analysis
All data were expressed as the mean ± standard error
of the mean. Data were assessed with one-way analysis of variance
(ANOVA) followed by Tukey's post-hoc test. All statistical analyses
were performed using SPSS software version 21 (IBM Corp., Armonk,
NY, USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
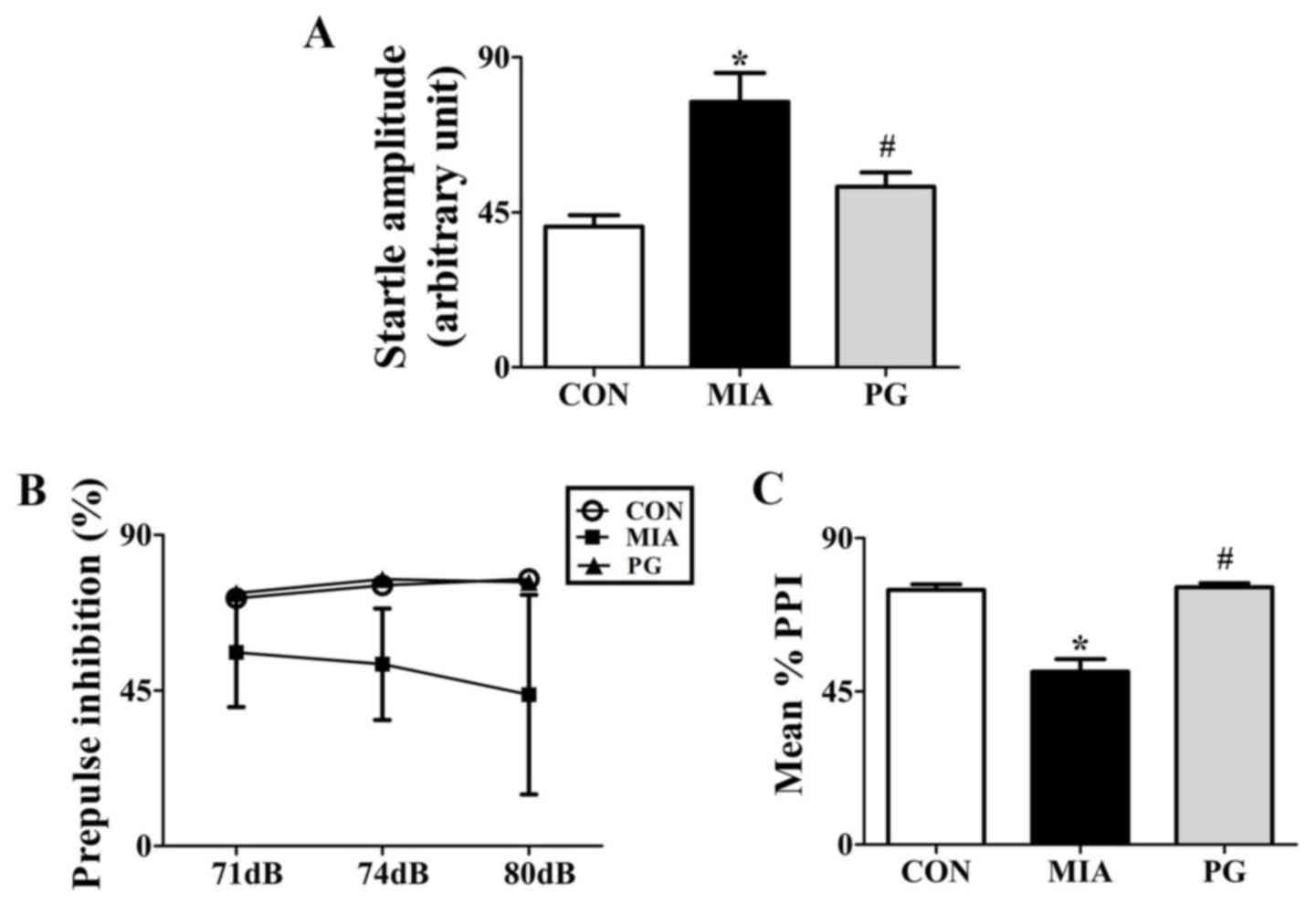
PPI
We tested whether PG treatment to effective in
preventing emergence of sensorimotor gating deficiency following
MIA during adulthood. To determine the effects of PG on
sensorimotor gating function, we measured acoustic startle response
(ASR) and performed PPI analysis for Control, MIA-induced PPI
disrupted mice and PG treated MIA mice. One-way analysis of
variance showed significant effects of PG. It decreased ASR and
reversed MIA-induced PPI disruption as shown in Fig. 1. MIA significantly (P<0.05)
increased the acoustic startle amplitude at 120 dB compared with
control group mice (Fig. 1A). PPI
values were not significantly reduced (P>0.05) at 71, 74 and 80
dB following MIA alone compared to those in the control (Fig. 1B). The MIA-induced PPI disruption
in 120 dB pulse stimulus trials (Fig.
1A) was consistently seen across all prepulse levels, leading
to significant (P<0.05) decreases in the mean percent PPI in MIA
offspring compared with the control group (Fig. 1C). This indicated that MIA induced
sensorimotor gating deficits. Moreover, MIA-induced PPI disruption
was significantly (P<0.05) improved by treatment with PG. This
indicated that PG treatment group animals recovered sensorimotor
gating deficits from MIA-induced PPI disruption (P<0.05).
Forced swim test
There were significant differences in result of FST
among Control, MIA and PG treated MIA groups (Table I). Total duration of immobility in
MIA offspring was significantly (P<0.05) increased compared to
that in the control. Changed Behavior were significantly
(P<0.05) recovered after PG-treated MIA mice, compared with the
untreated MIA group mice (P<0.05). Each behavior shown that
total duration of swimming and climbing decrease in MIA group mice
compared with the control and PG treatment groups (P<0.05).
 | Table I.Behavior of CON, MIA and PG mice in
the forced swim test. |
Table I.
Behavior of CON, MIA and PG mice in
the forced swim test.
| Behavior | CON | MIA | PG |
|---|
| Swimming (n) | 33.86±1.35 |
21.57±1.31a |
33.71±0.71b |
| Climbing (n) | 18.57±1.70 |
10.43±0.48a |
14.86±0.96b |
| Immobility (n) |
7.57±1.39 |
28.00±1.00a |
11.43±0.90b |
Open-field test
The number of central entries, line crossings and
grooming, and number and duration of cage sniffing behaviors in the
MIA group were significantly decreased (P<0.05) compared with
those in the control. However, these scores were reversed
(P<0.05) to normal levels following PG treatment (Table II). The number and duration of
immobility behaviors in the MIA group significantly increased
(P<0.05) compared with the control. These scores were also
recovered (P<0.05) to normal levels following PG treatment and
indicated a recovery from depression behavior (Table II).
 | Table II.Behavior of CON, MIA and PG mice in
the open field test. |
Table II.
Behavior of CON, MIA and PG mice in
the open field test.
| Behavior | CON | MIA | PG |
|---|
| Central entered
(n) | 42.29±6.11 |
15.57±1.31a |
29.57±4.72b |
| Line cross (n) | 22.00±4.04 |
5.00±1.02a |
12.29±4.86b |
| Run (n) | 0.00±0.00 | 0.00±0.00 | 0.43±0.79 |
| Run (sec) | 0.00±0.00 | 0.00±0.00 | 0.43±0.79 |
| Rear (n) | 96.14±12.47 | 74.71±6.15 | 69.14±23.41 |
| Rear (sec) | 149.57±9.97 | 126.86±8.84 | 122.86±35.59 |
| Grooming (n) | 11.43±2.86 |
3.43±0.57a |
15.71±3.64b |
| Grooming (sec) | 63.14±2.73 | 43.00±11.01 | 47.86±10.57 |
| Cage sniff (n) | 106.43±18.76 |
62.86±4.97a |
181.71±30.34b |
| Cage sniff
(sec) | 221.57±36.15 |
123.71±13.79a |
381.86±77.10b |
| Immobile (n) | 3.14±1.06 |
25.57±3.14a |
0.29±0.76b |
| Immobile (sec) | 12.86±6.61 |
89.43±11.72a |
0.43±1.13b |
SIT
Severe social deficits were induced in the MIA
offspring (Table III). The
number and duration of cage sniffing and following, in the MIA
group were significantly decreased (P<0.05) compared with the
control (Table III). These
scores were increased to control levels following treatment with
PG. However, aggressive behaviors (for example, fighting and
aggressive grooming, including grooming to the neck), excluding
biting, during SIT were significantly increased (P<0.05) in the
MIA group compared with the control group (Table III). These scores were decreased
to levels observed in the control group following treatment with
PG. These results indicated that MIA-induced social interaction
impairment was reversed by PG treatment.
 | Table III.Behavior of CON, MIA and PG mice in
social interaction test. |
Table III.
Behavior of CON, MIA and PG mice in
social interaction test.
| Behavior | CON | MIA | PG |
|---|
| Sniffing (n) | 50.43±6.28 |
21.86±2.12a |
43.71±1.96b |
| Sniffing (sec) | 81.29±8.20 |
38.43±5.06a |
80.86±8.04b |
| Following (n) | 7.86±1.28 |
4.57±0.61a |
11.71±2.21b |
| Following
(sec) | 16.86±2.34 |
8.00±0.90a |
28.00±6.11b |
| Grooming (n) | 1.71±0.36 | 1.00±0.65 |
3.86±0.70b |
| Grooming (sec) | 7.00±1.72 | 3.29±2.15 |
15.14±2.63b |
| Fight (n) | 0.29±0.18 |
1.29±0.18a |
0.14±0.14b |
| Fight (sec) | 0.43±0.30 |
4.57±1.00a |
0.57±0.57b |
| Aggressive (n) | 0.29±0.18 |
1.71±0.42a |
0.14±0.14b |
| Aggressive
(sec) | 1.14±0.77 |
6.29±2.01a |
0.29±0.29b |
| Biting (n) | 0.29±0.18 | 1.00±0.49 | 1.29±0.52 |
| Biting (sec) | 0.29±0.18 | 2.43±1.17 | 1.57±0.57 |
Western blot and
immunohistochemistry
The expression of neurodevelopmental proteins was
altered in the mPFCs of mice prenatally exposed to poly(I:C). To
determine whether neurodevelopmental proteins such as Nefm, Lasp1,
Dpysl2 and Dlg4 were downregulated in MIA offspring, western blot
analyses were performed. The results revealed that the expression
levels of these four proteins in the mPFC were significantly
decreased in the MIA group, compared with Control mice (Fig. 2). The MIA-induced reduction in
protein expression levels was significantly restored (P<0.05)
following PG treatment. (Fig. 2).
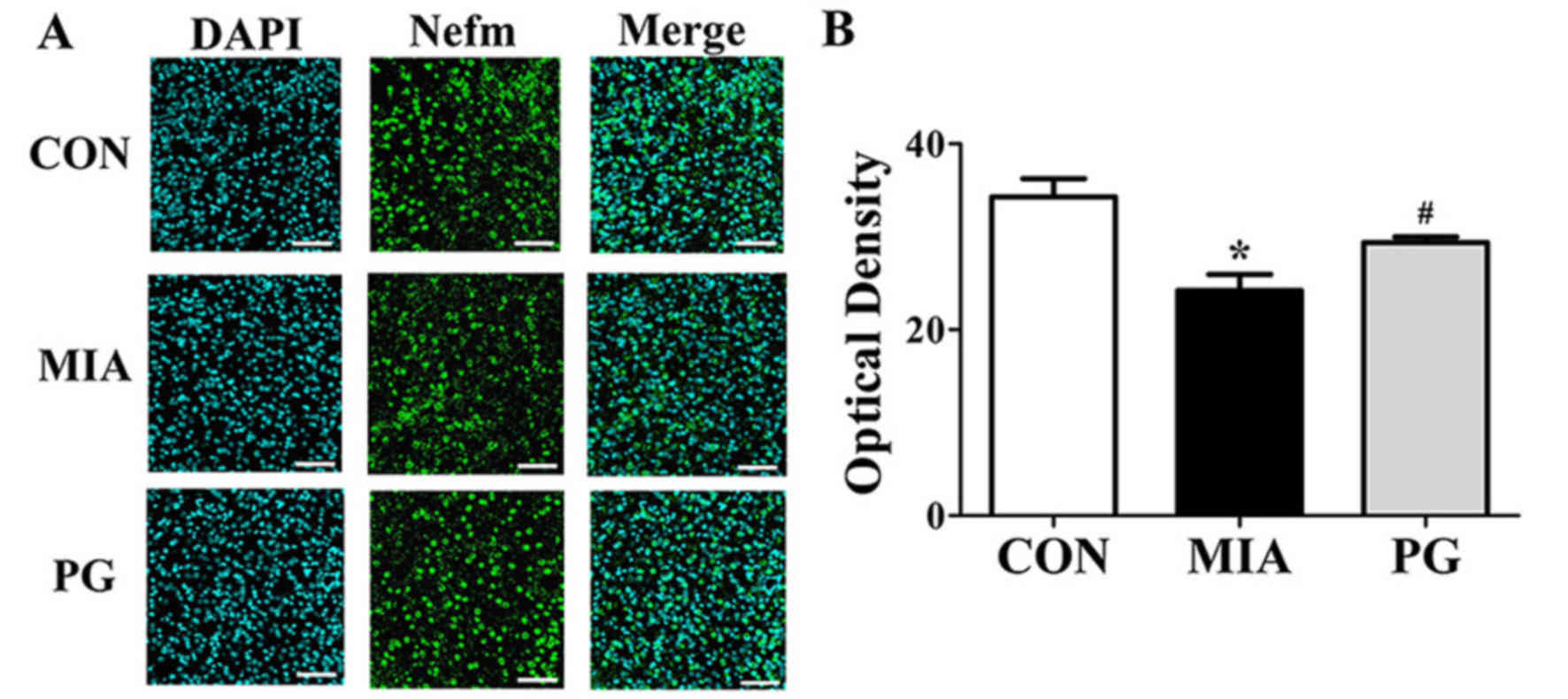
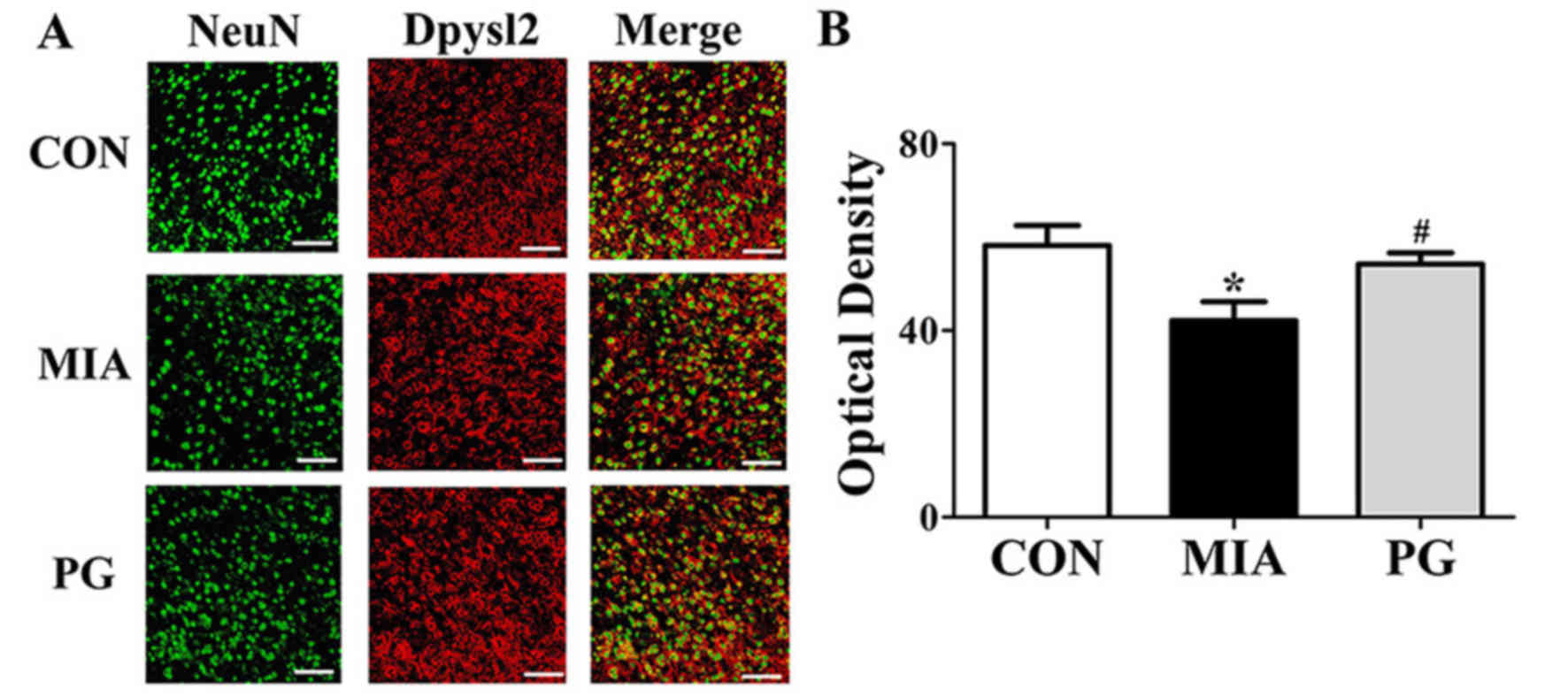
Immunohistochemical analyses of mPFC sections revealed that Nefm
and Dpysl2 protein expressions were significantly reduced
(P<0.05) in MIA mice compared with mice in the control group
(Figs. 3 and 4, respectively), and treatment with PG
significantly increased Nefm and Dpysl2 expression compared with
untreated MIA mice (both P<0.05). These results indicated that
PG treatment significantly altered the expression of
neurodevelopmental proteins in MIA offspring.
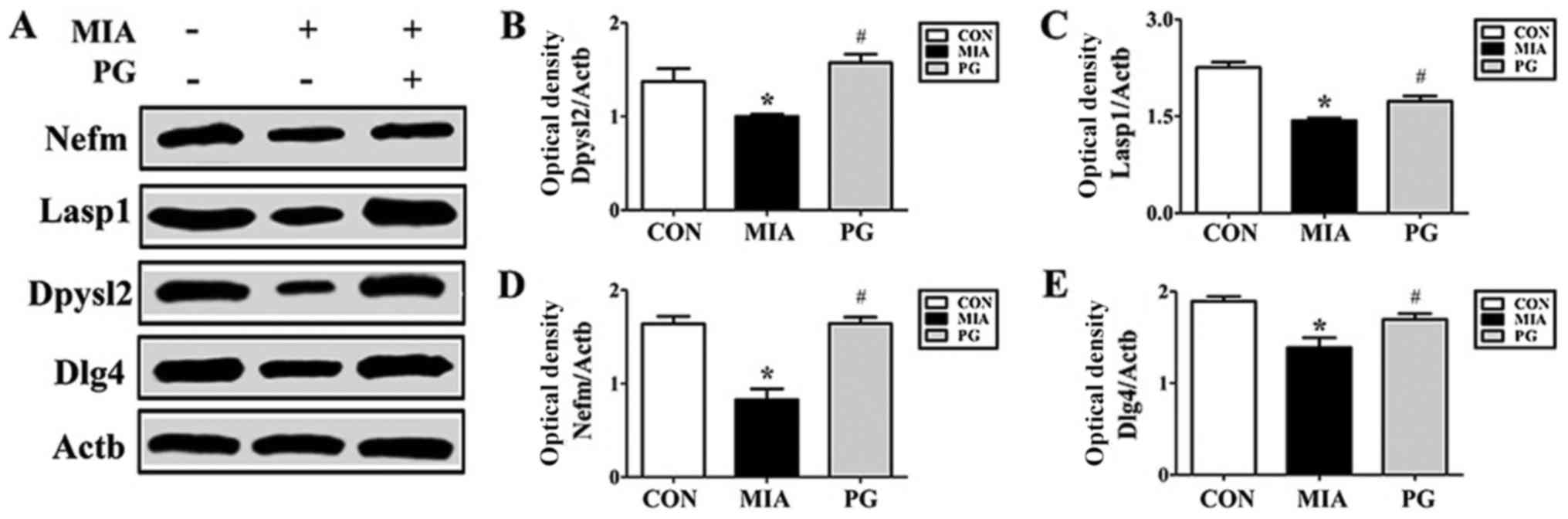 | Figure 2.Protein expression levels of Nefm,
Lasp1, Dpysl2 and Dlg4 in the medial prefrontal cortex of
MIA-induced mice. (A) Protein expression levels were determined by
western blot analysis; Actb was used as an internal control. Nefm,
Lasp1, Dpysl2 and Dlg4 expressions were decreased in the MIA group
compared with CON, and treatment with PG inhibited this decrease.
(B-E) Quantitative analysis of (B) Dpysl2, (C) Lasp1, (D) Nefm and
(E) Dlg4 expression. Data are presented as the mean ± standard
error of the mean. *P<0.05 vs. CON; #P<0.05 vs.
MIA. Actb, β-actin; CON, control group; Dlg4, discs large MAGUK
scaffold protein 4; Dpysl2, dihydropyrimidinase like 2; Lasp1, LIM
and SH3 protein; MIA, maternal induced activation group; Nefm,
neurofilament M; PG, Panax ginseng-treated MIA group. |
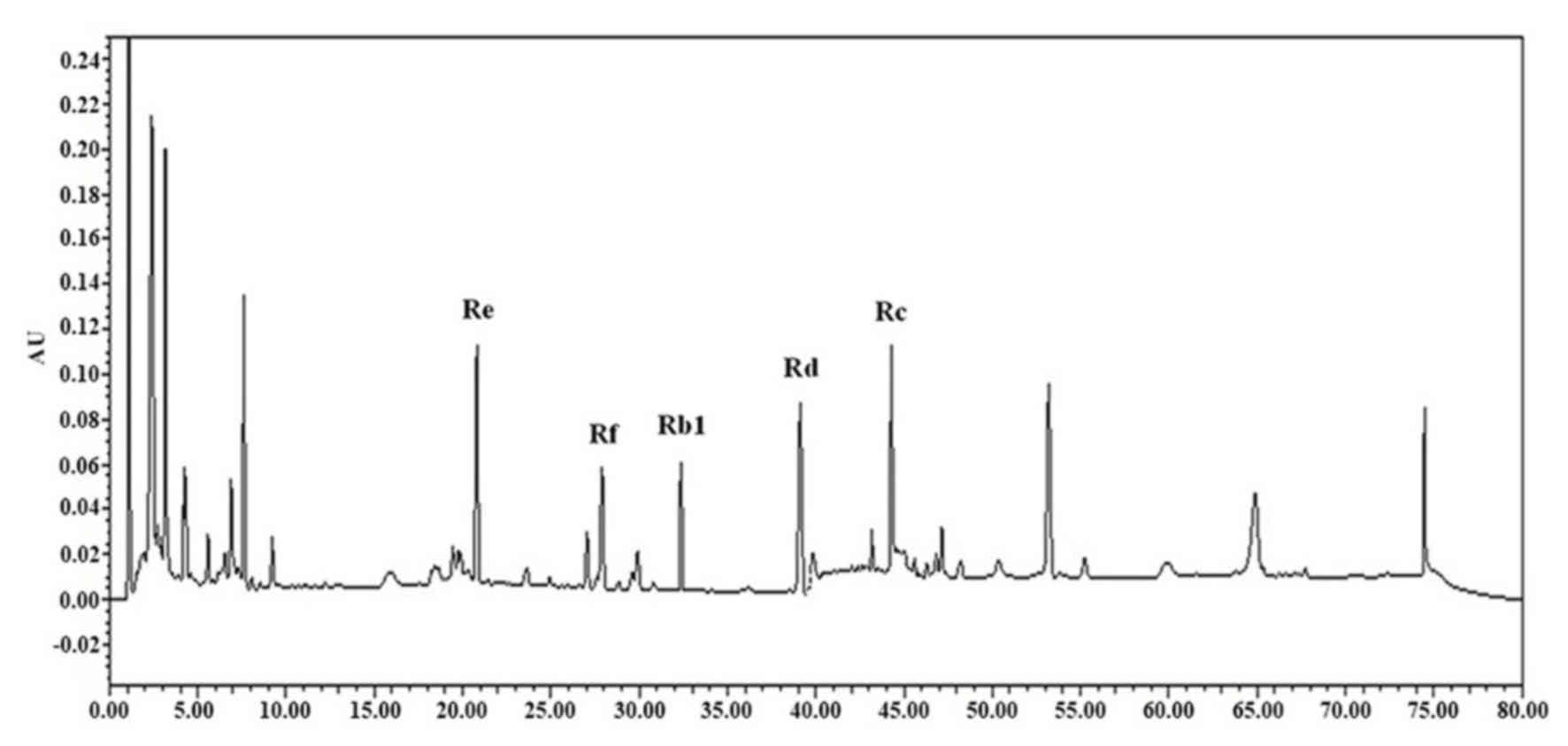
Detection of major ginsenosides
Major ginsenosides, including Rb1, Rc, Rd, Re and Rf
of ginseng were detected by HPLC (Fig.
5). Among the major ginsenosides, ginsenoside Rc was the most
abundant in ginseng samples. Ginsenoside Rc is considered the main
ginsenoside that contributes to the antioxidant activities of
ginseng. Ginsenoside Rc was shown to induce the overexpression of
catalase, which inhibited the production of reactive oxygen species
in human embryonic kidney 293T (HEK293T) cells (32).
Discussion
In the present study, it was determined that
exposure to MIA resulted in schizophrenia-like behaviors, including
impaired sensorimotor gating, decreased PPI and increased startle
sensitivity in adult mouse offspring. MIA-induced behavioral
impairments were reversed by PG treatment. Western blot and
immunohistochemical analyses of the mPFC revealed that several
neurodevelopmental proteins were downregulated in MIA offspring
compared with the control group, with PG treatment inhibiting this
downregulation. To the best of our knowledge, this is the first
report to demonstrate that PG treatment reduced behavioral
alterations and increased neurodevelopmental protein expression in
an MIA-induced animal model of schizophrenia.
A limited number of studies have demonstrated the
potential efficacy of the antipsychotics haloperidol and clozapine,
or the antidepressant fluoxetine in preventing the onset of
psychotic symptoms in a MIA-induced animal model of schizophrenia
at GD9 (14). It has been reported
that PG exerts similar effects to antipsychotic and antidepressant
drugs (22,33).
Ginseng has been demonstrated to have complex
pharmacological activities against significant psychiatric
symptoms, including sleep disorder, pressure of speech, euphoria,
agitation, psychosis, confusion and depression (34,35).
Panax vietnamensis has significant and potentially useful
psychopharmacological effects: It increases spontaneous locomotor
activity, enhances stress endurance, reduces anxiety-like behavior
and ameliorates scopolamine-induced memory impairments in mice
(36).
Crucial components of PG have been reported to
possess neuroprotective effects (37) that may decrease psychiatric
symptoms (20). In addition,
previous studies have revealed that component from ginseng extract
may have psychotropic effects and improve agonistic behavior in a
mouse model (38,39). Psychotropic actions, including
aggressive episodes (offensive sideways posture and attack bite)
(38) and maternal aggression
(39) in mice are significantly
suppressed by ginseng. A previous study reported that Panax
quinquefolius is an effective treatment against negative and
cognitive symptoms in both acute and chronic animal models of
psychosis (40). Our previous
study demonstrated that PG extract significantly reduces prenatal
stress-induced psychiatric symptoms in an animal model (22). Results from the present study
suggested that PG may be considered as a candidate to treat several
symptoms of schizophrenia, based on the effects observed in the
adult offspring of mice treated with poly(I:C).
A number of previous studies have suggested that
impaired PPI of the startle response in patients with
schizophrenia, as well as in animal models, may reflect
sensorimotor gating disruption (9,14).
The present study demonstrated that MIA-induced PPI deficits were
markedly reversed by PG. Meyer et al (14) reported the effects of atypical
antipsychotic drug administration on PPI deficit in a mouse model
of schizophrenia. Moreover, impaired social interaction behavior
was observed in MIA mice (14). In
particular, MIA may lead to negative and cognitive symptoms of
schizophrenia (41,42), including social withdrawal,
cognitive dysfunction and anhedonia; in addition, MIA may cause
behavioral deficits in adulthood (41,42).
The results of the present study supported previous findings that
demonstrated that prenatal poly(I:C)-induced MIA in mice is capable
of inducing aggressive behaviors (41). In the present study, the
MIA-induced decrease in non-aggressive behaviors was inhibited by
oral treatment with PG. In addition, certain behavioral patterns
observed in the FST and OFT that indicated depressive behaviors
were prevented by PG treatment.
The expression levels of Nefm, Lasp1, Dlg4 and
Dpysl2 proteins were also determined in the present study. The
results indicated that application of MIA during crucial periods of
fetal brain development resulted in alterations in
neurodevelopmental protein expression, including Nefm, Lasp1, Dlg4
and Dpysl2. Dpysl2, a microtubule-binding protein, has structural
and regulatory roles in cytoskeletal dynamics, vesicle trafficking
and synaptic transmission in the developing brain (43,44).
Furthermore, altered Dpysl2 activity has been associated with
schizophrenia in animal models, and Dpysl2 gene polymorphisms are
associated with schizophrenia susceptibility in humans (45,46).
However, additional research is required to determine the causal
link between the effects of PG and reduced schizophrenia-like
behaviors. Nevertheless, the present study demonstrated that PG has
the potential to be used as a natural alternative remedy for
various psychological disorders.
In conclusion, PG and/or its components may be a
useful treatment option for schizophrenia. Inhibition of psychotic
symptoms may be achieved though the pharmacological effects of
PG.
Acknowledgements
The authors would like to thank Miss Yugyeong Kim
(Soonchunhyang University, Cheonan) for technical assistance.
Funding
The present study was performed with the support of
Basic Science Research Program through the National Research
Foundation of Korea (NRF) funded by the Ministry of Education
(grant no. 2016R1D1A1B03931619).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
H-JK designed and directed the entire project. HW,
JI and HL performed the literature searches and analyses. H-KK and
JK performed the majority of the statistical analyses, YK, SL and
JP edited most of the table and figure data, and assisted with
statistical analyses. H-JK contributed substantially to the first
draft of the manuscript. All authors contributed to, and approved,
the final manuscript.
Ethics approval and consent to
participate
All animal procedures were performed in accordance
with the Guide for the Care and Use of Laboratory Animals of the
National Institutes of Health. Experimental procedures were
approved by the Institutional Animal Care and Use Committee of
Soonchunhyang University (approval no. SCH16-0063; Cheonan).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Brown AS and Patterson PH: Maternal
infection and schizophrenia: Implications for prevention. Schizophr
Bull. 37:284–290. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Brown AS and Derkits EJ: Prenatal
infection and schizophrenia: A review of epidemiologic and
ranslational studies. Am J Psychiatry. 167:261–280. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Clarke MC, Harley M and Cannon M: The role
of obstetric events in schizophrenia. Schizophr Bull. 32:3–8. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Meyer U, Feldon J and Fatemi SH: In vivo
rodent models for the experimental investigation of prenatal immune
activation effects in neurodevelopmental brain disorders. Neurosci
Biobehav Rev. 33:1061–1079. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Meyer U, Feldon J, Schedlowski M and Yee
BK: Towards an immuno-precipitated neurodevelopmental animal model
of schizophrenia. Neurosci Biobehav Rev. 29:913–947. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ozawa K, Hashimoto K, Kishimoto T, Shimizu
E, Ishikura H and Iyo M: Immune activation during pregnancy in mice
leads to dopaminergic hyperfunction and cognitive impairment in the
offspring: A neurodevelopmental animal model of schizophrenia. Biol
Psychiatry. 59:546–554. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Meyer U, Feldon J, Schedlowski M and Yee
BK: Immunological stress at the maternal-foetal interface: A link
between neurodevelopment and adult psychopathology. Brain Behav
Immun. 20:378–388. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Braff DL, Grillon C and Geyer MA: Gating
and habituation of the startle reflex in schizophrenic patients.
Arch Gen Psychiatry. 49:206–215. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Braff DL, Geyer MA and Swerdlow NR: Human
studies of prepulse inhibition of startle: Normal subjects, patient
groups, and pharmacological studies. Psychopharmacology (Berl).
156:234–258. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ludewig K, Geyer MA and Vollenweider FX:
Deficits in prepulse inhibition and habituation in never-medicated,
first-episode schizophrenia. Biol Psychiatry. 54:121–128. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Meyer U, Schwendener S, Feldon J and Yee
BK: Prenatal and postnatal maternal contributions in the infection
model of schizophrenia. Exp Brain Res. 173:243–257. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Meyer U, Nyffeler M, Yee BK, Knuesel I and
Feldon J: Adult brain and behavioral pathological markers of
prenatal immune challenge during early/middle and late fetal
development in mice. Brain Behav Immun. 22:469–486. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Piontkewitz Y, Arad M and Weiner I:
Tracing the development of psychosis and its prevention: What can
be learned from animal models. Neuropharmacology. 62:1273–1289.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Meyer U, Spoerri E, Yee BK, Schwarz MJ and
Feldon J: Evaluating early preventive antipsychotic and
antidepressant drug treatment in an infection-based
neurodevelopmental mouse model of schizophrenia. Schizophr Bull.
36:607–623. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Won H, Kim YO, Lee H, Im J, Lee S, Cho IH,
Lee SW, Park CG, Kim HK, Kwon JT and Kim HJ: Effect of valeriana
fauriei extract on the neurodevelopmental proteins expression and
behavioral patterns in maternal immune activation animal model.
Korean J Med Crop Sci. 24:341–350. 2016. View Article : Google Scholar
|
|
16
|
Kim YO, Kim Y, Lee K, Na SW, Hong SP,
Valan Arasu M, Yoon YW and Kim J: Panax ginseng improves functional
recovery after contusive spinal cord injury by regulating the
inflammatory response in rats: An in vivo study. Evid Based
Complement Alternat Med. 2015:8170962015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Park JS, Park EM, Kim DH, Jung K, Jung JS,
Lee EJ, Hyun JW, Kang JL and Kim HS: Anti-inflammatory mechanism of
ginseng saponins in activated microglia. J Neuroimmunol. 209:40–49.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rokot NT, Kairupan TS, Cheng KC, Runtuwene
J, Kapantow NH, Amitani M, Morinaga A, Amitani H, Asakawa A and
Inui A: A role of ginseng and its constituents in the treatment of
central nervous system disorders. Evid Based Complement Alternat
Med. 2016:26147422016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim HJ, Kim P and Shin CY: A comprehensive
review of the therapeutic and pharmacological effects of ginseng
and ginsenosides in central nervous system. J Ginseng Res. 37:8–29.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee S and Rhee DK: Effects of ginseng on
stress-related depression, anxiety, and the
hypothalamic-pituitary-adrenal axis. J Ginseng Res. 41:589–594.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen EY and Hui CL: HT1001, a proprietary
North American ginseng extract, improves working memory in
schizophrenia: A double-blind, placebo-controlled study. Phytother
Res. 26:1166–1172. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
National Research Council, . Guide for the
care and use of laboratory animals. National Academy Press;
Washington, DC: 1996
|
|
23
|
Kim YO, Lee HY, Won H, Nah SS, Lee HY, Kim
HK, Kwon JT and Kim HJ: Influence of Panax ginseng on the offspring
of adult rats exposed to prenatal stress. Int J Mol Med.
35:103–109. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nozari M, Shabani M, Farhangi AM, Mazhari
S and Atapour N: Sex-specific restoration of MK-801-induced
sensorimotor gating deficit by environmental enrichment.
Neuroscience. 299:28–34. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee HY, Won HS, Im JY, Kim YO, Lee SH, Cho
IH, Kim HK, Kwon JT and Kim HJ: Effect of Valeriana fauriei extract
on the offspring of adult rats exposed to prenatal stress. Int J
Mol Med. 38:251–258. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Renault J and Aubert A: Immunity and
emotions: Lipopolysaccharide increases defensive behaviours and
potentiates despair in mice. Brain Behav Immun. 20:517–526. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schroeder M, Sultany T and Weller A:
Prenatal stress effects on emotion regulation differ by genotype
and sex in prepubertal rats. Dev Psychobiol. 55:176–192. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lee PR, Brady DL, Shapiro RA, Dorsa DM and
Koenig JI: Prenatal stress generates deficits in rat social
behavior: Reversal by oxitocin. Brain Res. 1156:152–167. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shi L, Fatemi SH, Sidwell RW and Patterson
PH: Maternal influenza infection causes marked behavioral and
pharmacological changes in the offspring. J Neurosci. 23:297–302.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhu F, Zheng Y, Liu Y, Zhang X and Zhao J:
Minocycline alleviates behavioral deficits and inhibits microglial
activation in the offspring of pregnant mice after administration
of polyriboinosinic-polyribocytidilic acid. Psychiatry Res.
219:680–686. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Joo J, Lee S, Nah SS, Kim YO, Kim DS, Shim
SH, Hwangbo Y, Kim HK, Kwon JT, Kim JW, et al: Lasp1 is
down-regulated in NMDA receptor antagonist-treated mice and
implicated in human schizophrenia susceptibility. J Psychiatr Res.
47:105–112. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kim DH, Park CH, Park D, Choi YJ, Park MH,
Chung KW, Kim SR, Lee JS and Chung HY: Ginsenoside Rc modulates
Akt/FoxO1 pathways and suppresses oxidative stress. Arch Pharm Res.
37:813–820. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yamada N, Araki H and Yoshimura H:
Identification of antidepressant-like ingredients in ginseng root
(Panax ginseng C.A. Meyer) using a menopausal depressive-like state
in female mice: Participation of 5-HT2A receptors.
Psychopharmacology (Berl). 216:589–599. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen XW, Sneed KB, Pan SY, Cao C, Kanwar
JR, Chew H and Zhou SF: Herb-drug interactions and mechanistic and
clinical considerations. Curr Drug Metab. 13:640–651. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ernst E: Panax ginseng: An overview of the
clinical evidence. J Ginseng Res. 34:259–263. 2010. View Article : Google Scholar
|
|
36
|
Dela Peña IJI, Kim HJ, Botanas CJ, de la
Peña JB, Van Le TH, Nguyen MD, Park JH and Cheong JH: The
psychopharmacological activities of Vietnamese ginseng in mice:
Characterization of its psychomotor, sedative-hypnotic, antistress,
anxiolytic, and cognitive effects. J Ginseng Res. 41:201–208. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Smith I, Williamson EM, Putnam S,
Farrimond J and Whalley BJ: Effects and mechanisms of ginseng and
ginsenosides on cognition. Nutr Rev. 72:319–333. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yoshimura H, Watanabe K and Ogawa N:
Psychotropic effects of ginseng saponins on agonistic behavior
between resident and intruder mice. Eur J Pharmacol. 146:291–297.
1988. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yoshimura H, Watanabe K and Ogawa N: Acute
and chronic effect of ginseng saponins on maternal aggression in
mice. Eur J Pharmacol. 150:319–324. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chatterjee M, Singh S, Kumari R, Verma AK
and Palit G: Evaluation of the antipsychotic potential of Panax
quinquefolium in ketamine induced experimental psychosis model in
mice. Neurochem Res. 37:759–770. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bitanihirwe BK, Peleg-Raibstein D, Mouttet
F, Feldon J and Meyer U: Late prenatal immune activation in mice
leads to behavioral and neurochemical abnormalities relevant to the
negative symptoms of schizophrenia. Neuropsychopharmacology.
35:2462–2478. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Meyer U, Nyffeler M, Engler A, Urwyler A,
Schedlowski M, Knuesel I, Yee BK and Feldon J: The time of prenatal
immune challenge determines the specificity of
inflammation-mediated brain and behavioral pathology. J Neurosci.
26:4752–4762. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Inagaki N, Chihara K, Arimura N, Ménager
C, Kawano Y, Matsuo N, Nishimura T, Amano M and Kaibuchi K: CRMP-2
induces axons in cultured hippocampal neurons. Nat Neurosci.
4:781–782. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
44
|
Charrier E, Reibel S, Rogemond V, Aguera
M, Thomasset N and Honnorat J: Collapsin response mediator proteins
(CRMPs): Involvement in nervous system development and adult
neurodegenerative disorders. Mol Neurobiol. 28:51–64. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lee H, Joo J, Nah SS, Kim JW, Kim HK, Kwon
JT, Lee HY, Kim YO and Kim HJ: Changes in Dpysl2 expression are
associated with prenatally stressed rat offspring and
susceptibility to schizophrenia in humans. Int J Mol Med.
35:1574–1586. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Nakata K, Ujike H, Sakai A, Takaki M,
Imamura T, Tanaka Y and Kuroda S: The human
dihydropyrimidinase-related protein 2 gene on chromosome 8p21 is
associated with paranoid-type schizophrenia. Biol Psychiatry.
53:571–576. 2003. View Article : Google Scholar : PubMed/NCBI
|