Introduction
Sepsis is one of the most frequent causes of
mortality among hospitalized patients. The mortality rate of sepsis
is similar to that of myocardial infarction (1). In North America, the number of
hospitalized patients (per 100,000 patients) suffering from
septicemia or sepsis increased by 70% between 2001 (2) and 2008 (3), and the incidence of severe
postoperative sepsis has increased by three times (from 0.3 to
0.9%) (2,3). In addition, the incidence of sepsis
is particularly high among older people and tends to increase with
advancing age (1). In North
America, ~90% of the population are unfamiliar with the medical
term ‘sepsis’, whereas the majority of those who understand this
term do not know that sepsis is the biggest contributor to
mortality (4).
Among the Toll-like receptors (TLRs), TLR4 serves an
important role in a variety of inflammatory disorders, including
asthma, sepsis and chronic obstructive pulmonary disease.
Therefore, the TLR4 signaling pathway may be very important in the
regulation of these disorders. It was reported that TAK-242, a new
small-molecule cyclohexene derivative, may selectively suppress
TLR4 signaling and may lead to beneficial outcomes in a mouse model
of endotoxins (5). TLR4 acts as a
receptor for a variety of endogenous ligands, including heat shock
proteins, hyaluronic acid, fibrinogen and high mobility group box
1, and the interaction between endogenous ligands and TLR4 is
involved in a number of human diseases, including sepsis.
Interleukin (IL)-6 is a multifunctional cytokine
released by a variety of cells; however, the level of IL-6 in
healthy populations is undetectable (6). In trauma patients, an early increase
in IL-6 may be associated with the magnitude of trauma, the
severity of proinflammatory reactions and the incidence of
complications (sepsis, multiple organ failure and mortality)
(7,8). Similarly, IL-10 is a major
anti-inflammatory factor and its expression levels are undetectable
within healthy individuals. The expression level of IL-10 is
increased in patients with sepsis, and correlates with the
magnitude of sepsis and the likelihood of mortality (9). The ratio of IL-6/IL-10 has long been
used to evaluate the immune system in intensive care unit patients,
as such a ratio may reveal the balance between proinflammatory and
anti-inflammatory responses (systemic inflammatory syndrome and
compensatory anti-inflammatory response) (10). A reduction in the IL-6/IL-10 ratio
may affect patient prognosis and the occurrence of postoperative
sepsis (11).
As transcripts of >200 bp in length, long
noncoding (lnc)RNAs do not demonstrate any significant
protein-coding capability (12).
At present, an increasing amount of data has demonstrated that
lncRNAs participate in a variety of pathological and physiological
processes, and hence may impact various cellular functions
(13). It has been recognized that
nuclear factor (NF)-κB serves as a bridge between cancer and
inflammation (14). A recent study
demonstrated the critical role of NF-κB in the maintenance and
spread of cancer stem cells (CSCs) (15). In addition, it has been reported
that lnc-deleted in liver cancer 1 (DILC) may induce the
interaction between the autocrine IL-6/signal transducer and
activator of transcription 3 (STAT3) cascade and tumor necrosis
factor (TNF)-α/NF-κB signaling in liver CSCs (LCSCs), thus linking
liver inflammation to the dissemination of LCSCs (16). It has been demonstrated that the
expression of IL-6 may be inhibited by DILC, whereas the expression
of STAT3, a downstream component of the IL-6 signaling pathway, may
be enhanced by IL-6 stimulation (16). Furthermore, IL-6 signaling has been
demonstrated to modulate TLR4-dependent inflammatory responses via
STAT3 (17). In the present study,
the role of DILC, IL-6, STAT3 and TLR4 in the pathogenesis of
sepsis was investigated.
Materials and methods
Sample collection
A total of 36 patients, including 18 patients with
sepsis and 18 healthy participants, were involved in the present
study. The mean age of all subjects was 56 years old (42–78 years),
and the ratio of female to male subjects was 1.0:1.5. Peripheral
blood samples from 18 participants diagnosed with sepsis and 18
people free of any health problems, were collected using Lymphocyte
Separation Medium (Human) (Applygen Technologies Inc., Beijing,
China), and stored at −80°C. Written informed consent was obtained
from all patients. All data processing and sample collection were
approved by the Ethics Committee of Guangdong Academy of Medical
Sciences. The present study was performed according to the
Declaration of Helsinki.
Peripheral blood mononuclear cell
(PMBC) isolation
PBMCs were maintained at −80°C until use. A 70-mm
strainer (Falcon; Corning, Inc., Corning, NY, USA) was utilized for
cell passaging to yield single cell suspensions, according to the
manufacturer's protocol. Dulbecco's modified Eagle's medium (DMEM;
Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) containing
10% fetal bovine serum (FBS; Thermo Fisher Scientific, Inc.), 100
mg/ml streptomycin sulfate and 100 U/ml penicillin sodium was
utilized to culture the cells into 48-well plates with 5%
CO2 at 37°C. Growth medium was changed every 2 days
until the cells were passaged, then transferred to a new dish; at
80% confluence, the PMBCs were passaged again, and cells at passage
2–3 were utilized for analysis.
Cell culture and transfection
DMEM (Gibco; Thermo Fisher Scientific, Inc.)
containing 10% FBS (Thermo Fisher Scientific, Inc.), 100 mg/ml
streptomycin sulfate and 100 U/ml penicillin sodium was utilized to
maintain THP-1 cells under a humidified atmosphere with 5%
CO2 at 37°C. DILC mimic
(5′-AATATCGTCAATGGCTATGGCCGTGAAGCGGGCATGCCCCTGGCCACTAGCCGCCGCATCGCCAAAATCGCCTTTACCGGCTCCACCTCCACCGGCCGCGTGATTGCCCAGGCCGCAGCCAACAATTTGATCCCCGCCACGCTGGAACTCGGCGGCAAATCGCCCAACATTTTCTTCGCCGACGTGATGGACAAAGATGATGGCTTCCTG-3′)
and its negative control were synthesized by Guangzhou RiboBio Co.,
Ltd. (Guangzhou, China). When the cells reached 80% confluence,
Lipofectamine® 2000 (Thermo Fisher Scientific, Inc.) was
used to transfect the DILC mimic or NC into THP-1 cells according
to the manufacturer's protocol. The time interval between the
completion of transfection and the subsequent experiments was 4 h.
The culture cells were also treated with LPS (1 mg/ml) for 30 min.
A total of three independent experiments were carried out.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis
TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) was used to extract total RNA from THP-1 cells
and PBMCs, according to the manufacturer's protocol. A TaqMan micro
(mi)RNA reverse transcription kit (Applied Biosystems; Thermo
Fisher Scientific, Inc.) was used to reverse transcribe the RNA to
DNA (cDNA) with designed primers including primers for DILC
(forward: 5′-ATGGCAAAAGATGCCGGTCTAATTG-3′; reverse:
5′-TCGTCCTCGTCATTCTCG-3′), IL-6 (forward:
5′-ATGAACTCCTTCTCCACAAGC-3′; reverse:
5′-CTACATTTGCCGAAGAGCCCTCAGGCTGGACTG-3′), STAT3 (forward:
5′-ACTCCATCGCTGACAAAA-3′; reverse: 5′-CAGTGACCAGGCAGAAGA-3′), and
TLR4 (forward: 5′-AGCCACCTCTCTACCTTAATATTGA-3′; reverse:
5′-CCGAGTGTTAGAATAGGTTAGAAAG-3′) synthesized by Applied Biosystems
(Thermo Fisher Scientific, Inc.), according to the manufacturer's
protocol. The Applied Biosystems 7500 Real-Time PCR system (Applied
Biosystems; Thermo Fisher Scientific, Inc.) was used to perform
RT-qPCR, with a 20-µl mixture containing 10 µl SYBR advantage qPCR
Premix (Clontech Laboratories, Inc., Mountainview, CA, USA), 1 µl
cDNA, 7 µl H2O and 1 µl each forward and reverse primer.
The temperature conditions were: 95°C for 3 min, followed by 30
cycles of 94°C for 40 sec, 56°C for 35 sec and final extension at
72°C for 60 sec. The relative expression levels of DILC, IL-6,
STAT3 and TLR4 were quantified using the standard curve method. U6
(primer sequence forward: 5′-ATGCACTATCATATGCTTACCGTA-3′; reverse:
5′-AGGCGATTAAGTTGGGTA-3′) and NADPH (primer sequence forward:
5′-CCGAGAAGTTTCAGCACATCC-3′; reverse: 5′-TGGCAGTGATAGCGAAGGCT-3′)
were used internal control for mRNAs, respectively. Relative
expression levels of DILC, IL-6, STAT3 and TLR4 were presented
using the 2−ΔΔCq method (18). All reactions were run in
triplicate.
Luciferase assay (promoter)
A luciferase assay was performed to examine whether
DILC alters the transcriptional ability of the promoter of IL-6.
qPCR was performed according to the protocol described above to
amplify the full promoter segment from DNA templates collected from
PBMCs. IL-6 promoter-luciferase reporter plasmids containing either
a wild type (WT) or mutant (MUT) DILC binding site were generated
accordingly. Sequencing was performed to confirm the reporters with
WT or MUT DILC binding sites. Endonuclease (New England BioLabs,
Inc., Ipswich, MA, USA) was used to digest the pcDNA3-control
vector and amplified fragments. Subsequently the qPCR products were
inserted into the pcDNA3-control vector (Ambion; Thermo Fisher
Scientific, Inc.). THP-1 cells were seeded into 96-well plates at a
final concentration of 3×104 cells/ml, and transfected
with 50 ng pcDNA3-DILC (WT or MUT) via electrophoresis (Lonza
Group. Ltd., Basel, Switzerland). A dual-luciferase reporter assay
system (Promega Corporation, Madison, WI, USA) was used to
determine the firefly and Renilla luciferase activity 48 h
post-transfection. All tests were repeated in triplicate.
Western blot analysis
Ice-cold PBS was used to wash the THP-1 cells twice,
and radioimmunoprecipitation buffer (Pierce; Thermo Fisher
Scientific, Inc.) was used to lyse cells and tissue sample to
isolate protein for 30 min on ice, according to the manufacturer's
protocols. The lysates were subjected to centrifugation for 20 min
at 12,000 × g at 4°C. A bicinchoninic acid protein assay kit
(Boster Biological Technology, Pleasanton, CA, USA) was used to
quantify proteins. SDS-PAGE on a 10% gel was used to separate 30-µg
protein samples, and electroblotting was employed to transfer the
target protein onto a polyvinylidene difluoride (PerkinElmer, Inc.,
Waltham, MA, USA) membrane for 90 min. To block the membrane, 5%
non-fat milk was applied to the membrane to avoid nonspecific
binding for 2 h at room temperature. The primary antibody anti-IL-6
(1:5,000; cat. no. sc-130326; Santa Cruz Biotechnology Inc.,
Dallas, TX, USA), primary antibody anti-STAT3 (1:5,000; cat. no.
sc-8019; Santa Cruz Biotechnology, Inc.), primary antibody
anti-TLR4 (1:5,000; cat. no. sc-52962, Santa Cruz Biotechnology
Inc.) and anti-β-actin (1:10,000; cat. no. sc-47778, Santa Cruz
Biotechnology Inc.) were used to incubate the membrane at 4°C for
12 h. Horseradish peroxidase (HRP)-labeled anti-mouse goat
secondary antibody was used to treat the membrane at 37°C for 1 h
(1:3,000; cat. no. 7074; Cell Signaling Technology, Inc.). A
chemiluminescence detection system (Pierce; Thermo Fisher
Scientific, Inc.) was used to determine the protein bands, and the
Bio-Rad Chemi-DocXRS (Bio-Rad Laboratories, Inc., Hercules, CA, US)
was utilized to visualize the bands of the target proteins. Each
test was performed in triplicate.
ELISA analysis
A total of 50 mM carbonate-bicarbonate buffer
supplemented with mouse polyclonal immunoglobulin G (IgG)
anti-TNF-α (cat. no. 6945; Cell Signaling Technology, Inc.)/CCL5
(cat. no. 2988; Cell Signaling Technology, Inc.) /E-selectin (cat.
no. 14-0627-82; Thermo Fisher Scientific, Inc., Waltham, MA,
USA)/CXCR1 antibodies (1:1,500; cat. no. MA1-201; Thermo Fisher
Scientific, Inc.) were used to treat THP-1 cells on 96-well plates
at a final concentration of 3×104 cells/ml, and
maintained at 4°C for 12 h. PBS supplemented with 4% bovine serum
albumin (BSA, LI-COR Biosciences, Lincoln, NE, USA) was used to
treat for another 60 min and PBS with 0.1% Tween-20 was used to
wash the plates. A total of 50 µl purified
TNF-α/CCL5/E-selectin/CXCR1 antigens and clinical samples were
distributed into 96 wells, incubated for 120 min at 37°C, and
washed and cultured for 60 min at 37°C in 100 µl PBS, containing l%
BSA and rabbit monoclonal IgG anti-TNF-α (cat. no. 6945; Cell
Signaling Technology, Inc.) /CCL5 (cat. no. 2988; Cell Signaling
Technology, Inc.) /E-selectin (cat. no. 14-0627-82; Thermo Fisher
Scientific, Inc.) /CXCR1 (cat.no.MA1-201; Thermo Fisher Scientific,
Inc.) at a dilution of 1:1,500. HRP-conjugated secondary antibodies
(1:3,000; cat. no. 7074; Cell Signaling Technology, Inc.) and 1%
BSA (LI-COR Biosciences) were used to maintain the plate. A
microplate reader was used to determine the HRP activity with the
use of O-phenylenediamine based on the absorbance at 492 nm, and
H2O2 served as the enzyme substrates. All
tests were performed in triplicate.
Statistical analysis
SPSS 13.0 software (SPSS, Inc. Chicago, IL, USA) was
used to perform all statistical analyses. All results are presented
as the mean ± standard deviation. Unpaired t-tests and one-way
analysis of variance followed by Mann-Whitney U test were used to
analyze the significance comparisons among more than two groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Lipopolysaccharide (LPS) alters the
production of inflammatory mediators
LPS, a widely recognized stimulator of inflammation,
was used in the present study to treat THP-1 cells. RT-qPCR was
performed to determine whether LPS affected the expression of
relevant inflammatory cytokines. In particular, the expression
levels of DILC, IL-6, STAT3 and TLR4 in the cells treated with or
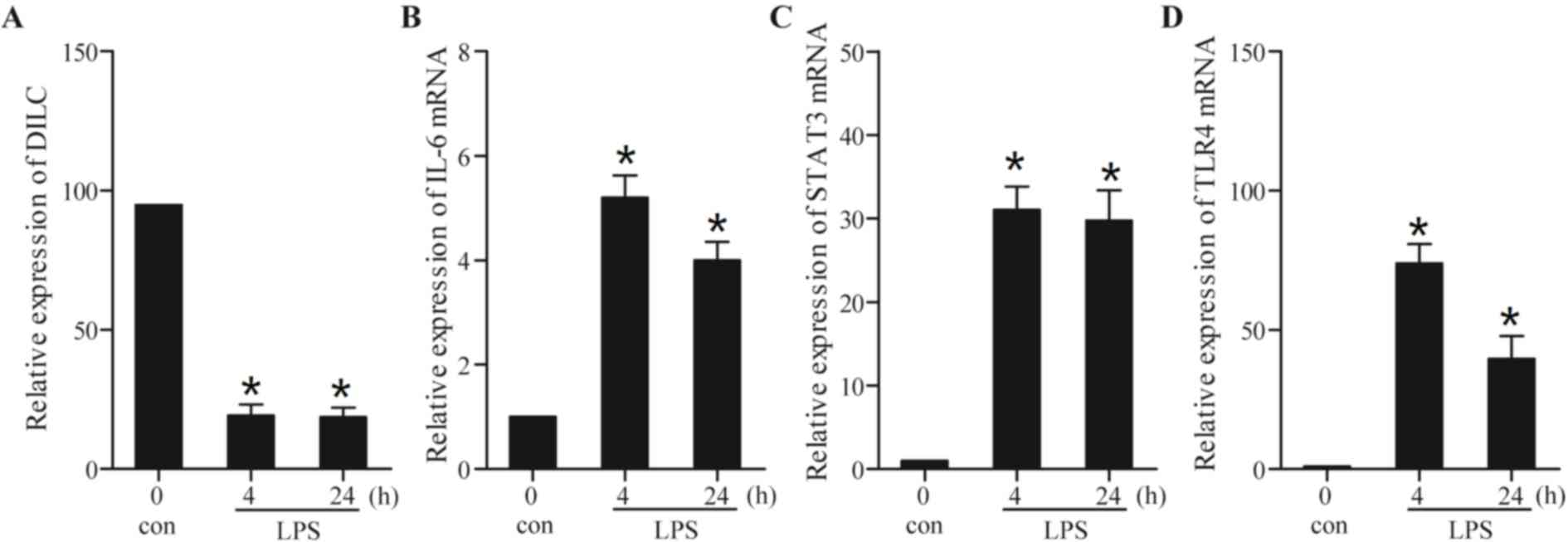
without LPS was measured. As presented in Fig. 1A, the expression of DILC reached a
stable state at 4 h following treatment with LPS; however, the
levels of DILC expression were decreased in LPS-treated THP-1 cells
compared with control cells. Conversely, the expression levels of
IL-6 (Fig. 1B), STAT3 (Fig. 1C) and TLR4 (Fig. 1D) were increased in LPS-treated
cells. Consistent with the observation that treatment with LPS
upregulated the protein expression levels of STAT3 (19), these results suggested that LPS
inhibited the expression of DILC and enhanced the expression of
IL-6, STAT3 and TLR4.
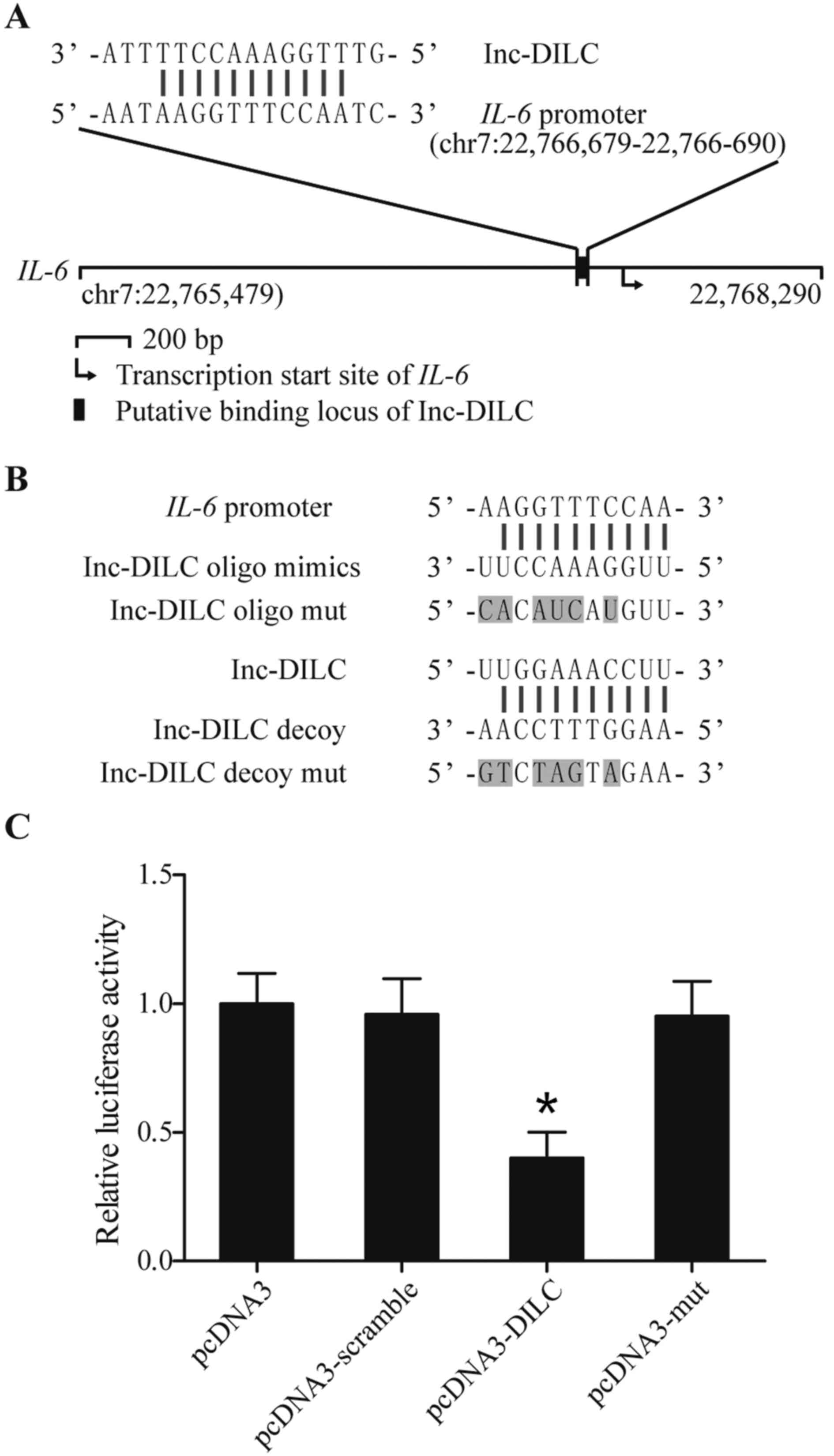
Lnc-DILC binds to the promoter of IL-6
and suppresses the transcription of IL-6
As presented in Fig.
2A, a putative complementary binding site of lnc-DILC was
located within the promoter region of IL-6. To further verify the
regulatory association between lnc-DILC and the IL-6 promoter, an
oligo mimic (oligoribonucleotide) and its mutated control (oligo
mut), in addition to an oligodeoxynucleotide (ODN) decoy and its
mutated control (ODN mut), were synthesized based on the sequence
of the binding locus (Fig. 2B).
Subsequently, the luciferase activity of IL-6 was detected. As
presented in Fig. 2C, the
luciferase activity in cells transfected with the WT DILC vectors
was markedly decreased compared with the control cells, whereas the
IL-6 luciferase activity in cells transfected with the mutant DILC
vectors remained unchanged, indicating that lnc-DILC bound to the
promoter of IL-6 and suppressed its production.
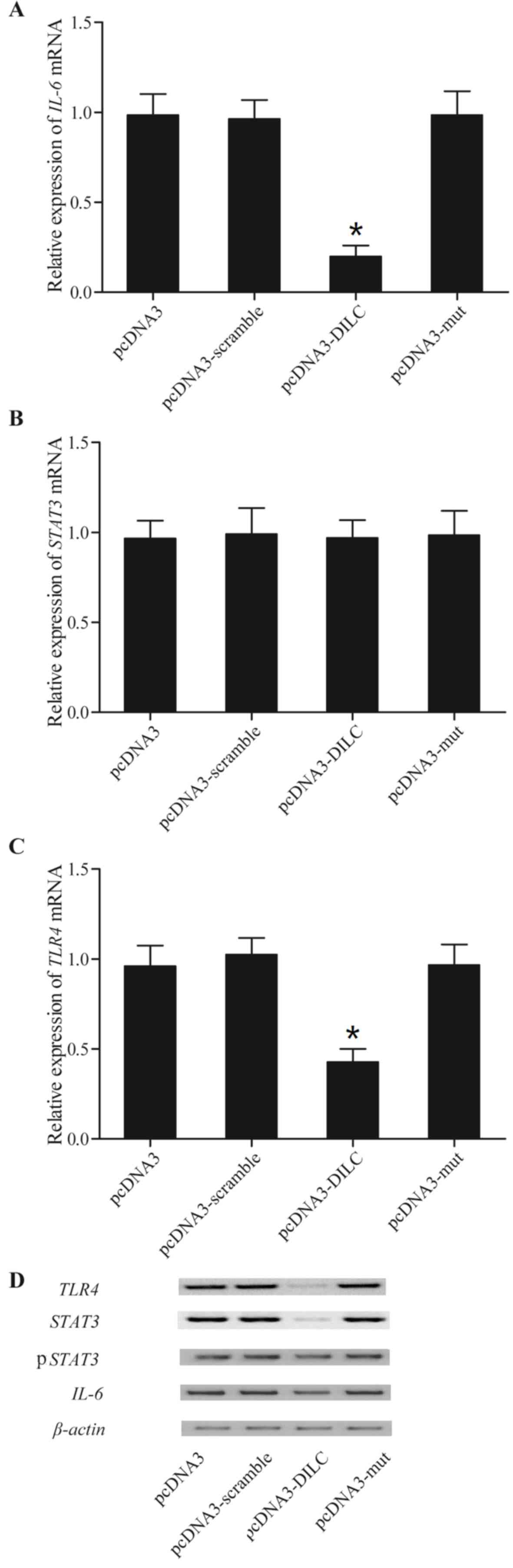
Lnc-DILC alters the expression of
IL-6, STAT3 and TLR4 in LPS-treated THP-1 cells
To further understand whether DILC affects the
productions of inflammatory cytokines involved in sepsis,
LPS-treated THP-1 cells were transfected with WT or MUT DILC
vectors, and the mRNA expression level of IL-6, STAT3 and TLR4 in
these cells was measured using RT-qPCR and western blot analyses.
Transfection with WT DILC significantly reduced the mRNA levels of
IL-6 and TLR4, whereas transfection with mutant DILC did not alter
the mRNA expression levels of IL-6 and TLR4 (Fig. 3). In addition, it was observed that
the WT and MUT DILC vectors demonstrated a minimal effect on the
mRNA expression levels of STAT3 (Fig.
3). Furthermore, the protein expression levels of IL-6, STAT3,
phosphorylated (p)-STAT3 and TLR4 in these cells was measured and
it was observed that transfection with WT DILC reduced the protein
expression levels of IL-6, STAT3, p-STAT3 and TLR4, whereas
transfection with MUT DILC did not alter the protein expression
levels of these genes (Fig.
3).
lnc-DILC alters the levels of STAT3
and TLR4 by regulating the expression of IL-6
To investigate the effects of DILC on the expression
of IL-6, STAT3 and TLR4, the mRNA and protein expression levels of
IL-6, STAT3 and TLR4 in THP-1 cells transfected with DILC and IL-6
siRNAs were measured. As presented in Fig. 4, transfection with DILC siRNA
significantly upregulated the mRNA levels of IL-6 and TLR4, whereas
transfection with IL-6 siRNA significantly reduced the mRNA level
of IL-6 and TLR4. Conversely, transfection with DILC and IL-6
siRNAs did not alter the mRNA expression of STAT3 (Fig. 4B). In addition, transfection with
DILC siRNA upregulated the protein expression levels of IL-6,
STAT3, p-STAT3 and TLR4; however, transfection with IL-6 siRNA
downregulated the protein expression levels of IL-6, STAT3, p-STAT3
and TLR4 (Fig. 4D).
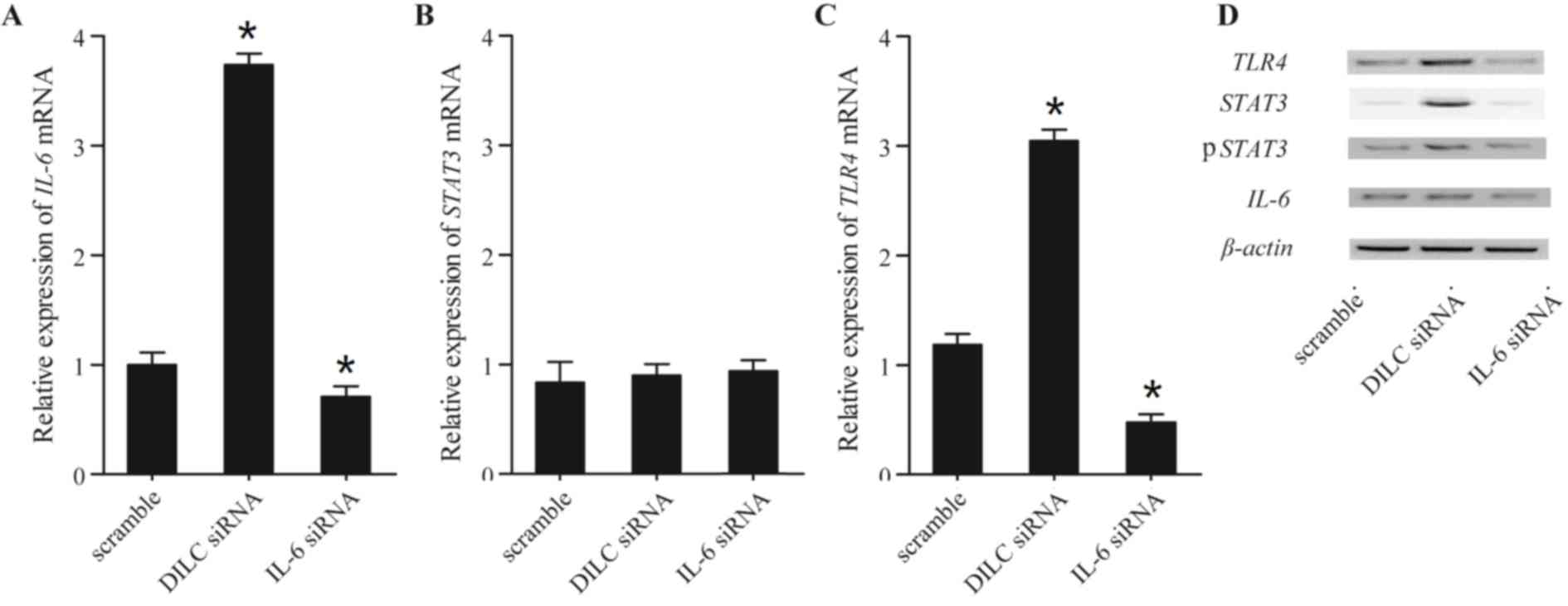 | Figure 4.IL-6, STAT3 and TLR4 expression
levels in cells transfected with DILC siRNA and IL-6 siRNA. (A)
DILC siRNA increased, IL-6 siRNA decreased IL-6 mRNA expression
levels. (B) STAT3 mRNA expression levels in the DILC siRNA group
were much higher, but much lower in IL-6 siRNA. (C) TLR4 mRNA
expression levels were upregulated in the DILC siRNA-treated group,
but downregulated in the IL-6 siRNA group. *P<0.05 vs. scramble.
(D) IL-6, STAT3/p-STAT3 and TLR4 protein expression levels were
increased following transfection with DILC siRNA, and decreased
subsequent to transfection with IL-6 siRNA. DILC, deleted in liver
cancer 1; IL, interleukin; mut, mutant; p, phosphorylated; STAT3,
signal transducer and activator of transcription 3; siRNA, small
interfering RNA; TLR4, Toll-like receptor 4. |
lnc-DILC alters the levels of TNF-α,
CCL5, E-selectin and CXCR1 by regulating the expression of
IL-6
To investigate the effects of lnc-DILC on the
expression of TNF-α, CCL5, E-selectin and CXCR1, THP-1 cells were
stimulated with LPS and transfected with the WT or MUT DILC vectors
in conjunction with DILC and IL-6 siRNAs. Subsequently, the
expression levels of TNF-α, CCL5, E-selectin and CXCR1 within these
cells was determined using RT-qPCR and western blot analyses. As
presented in Fig. 5, the levels of
TNF-α (Fig. 5A), CCL5 (Fig. 5B), E-selectin (Fig. 5C) and CXCR1 (Fig. 5D) in the cells transfected with the
WT DILC and IL-6 siRNA were significantly reduced, whereas the
levels of these cytokines were significantly increased following
transfection DILC siRNA.
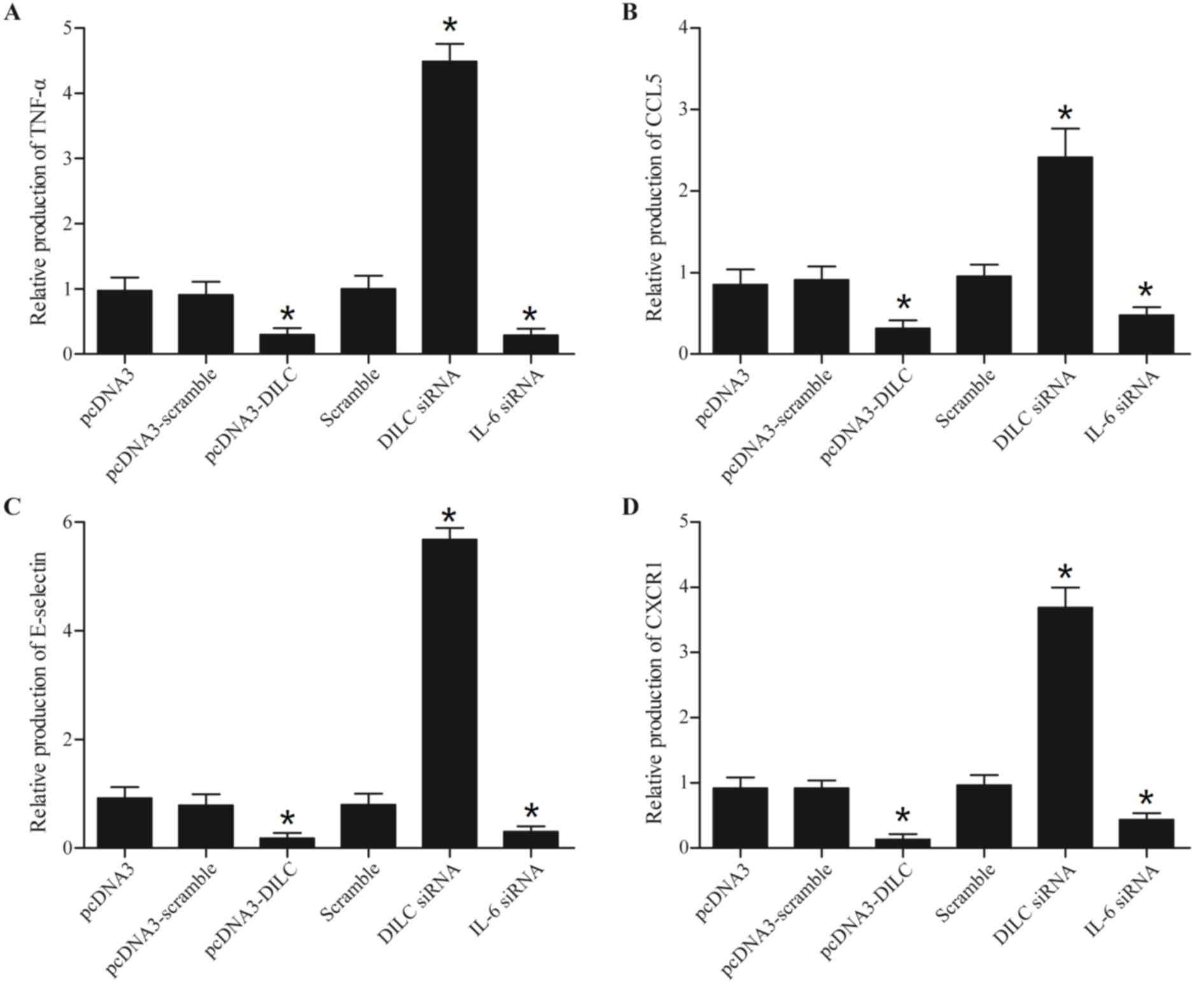 | Figure 5.Alteration in TNF-α, CCL5, E-selectin
and CXCR1 expression in THP-1 cells treated with LPS, and
transfected with plasmids containing DILC, DILC siRNA or IL-6
siRNA. (A) TNF-α mRNA expression levels in the DILC siRNA group
were upregulated, and downregulated in the DILC and IL-6 siRNA
treated groups. (B) DILC siRNA increased, and DILC and IL-6 siRNA
decreased CCL5 expression levels. (C) E-selectin expression was
enhanced in the DILC siRNA group, but inhibited in DILC and IL-6
siRNA groups. (D) CXCR1 mRNA expression levels in the DILC siRNA
group were upregulated, but downregulated in DILC and IL-6 siRNA.
*P<0.05 vs. pcDNA3. CCL5, chemokine ligand 5; CXCR1, C-X-C motif
chemokine receptor 1; DILC, deleted in liver cancer 1; IL,
interleukin; siRNA, short interfering RNA; TNF-α, tumor necrosis
factor-α. |
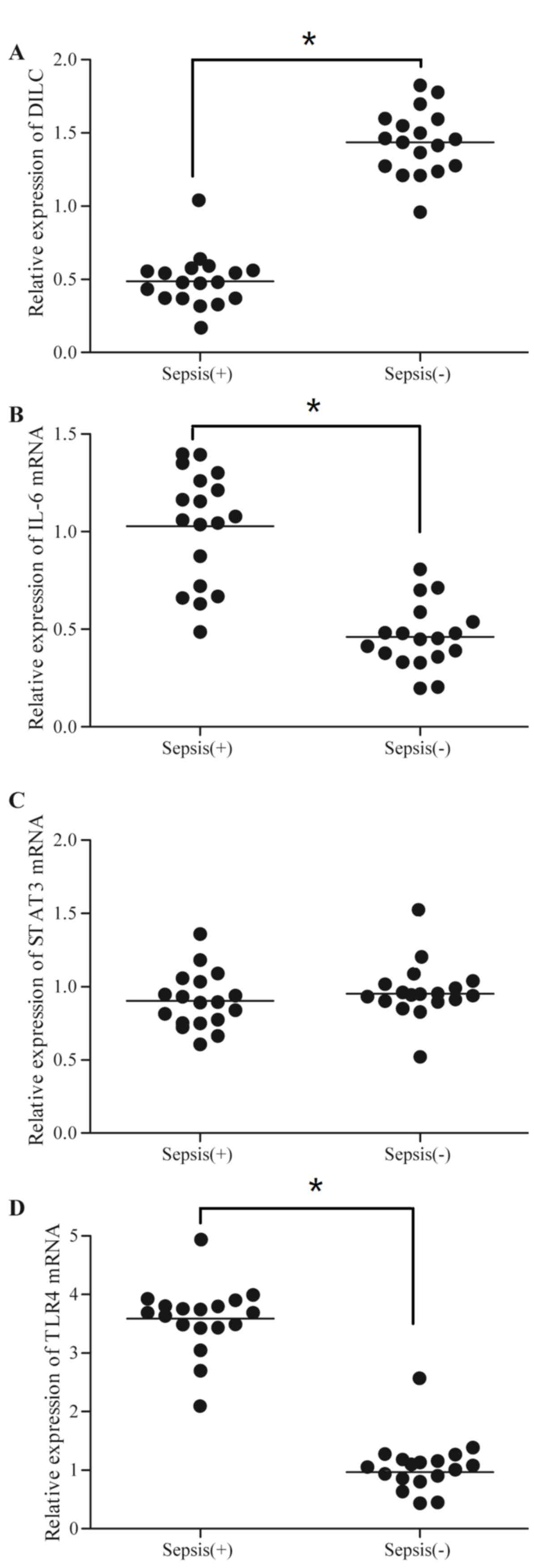
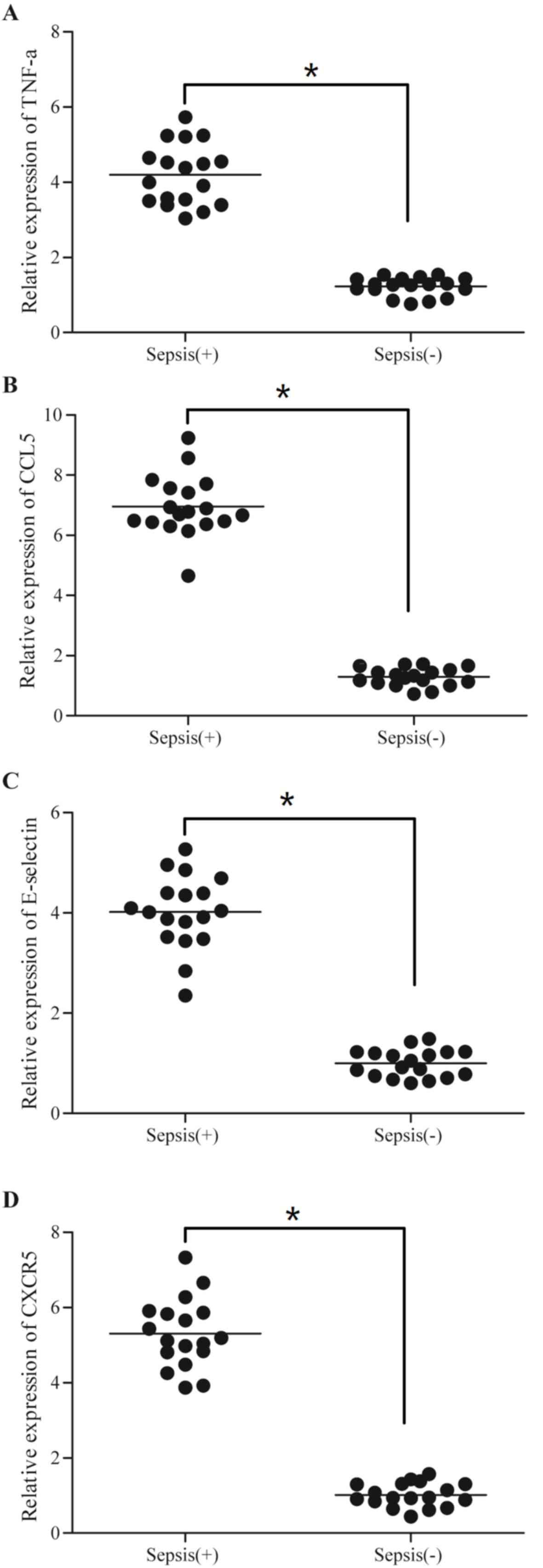
Expression of sepsis-associated genes
varies between septic and healthy groups
In the present study, 36 subjects, including 18
patients with sepsis and 18 healthy control subjects, were
recruited and the expression levels of DILC, IL-6, STAT3, TLR4,
TNF-α, CCL5, E-selectin and CXCR1 in these subjects were measured.
As presented in Fig. 6, the sepsis
group exhibited decreased levels of DILC, and increased levels of
IL-6 and TLR4, compared with the healthy group; however, the levels
of STAT3 between the two groups exhibited no significant
difference. Additionally, the levels of TNF-α (Fig. 7A), CCL5 (Fig. 7B), E-selectin (Fig. 7C) and CXCR1 (Fig. 7D) were higher in the sepsis group
compared with the control group.
Discussion
The interaction between lnc-DILC and the promoter
region of IL-6 was observed in the present study. In addition, the
effects of lnc-DILC overexpression and lnc-DILC knockdown, which
may affect LCSC expansion, STAT3 activation and IL-6 transcription,
were eliminated by the ODN decoy and the oligo mimic of lnc-DILC,
respectively, thus suggesting that the interaction between lnc-DILC
and the promoter of IL-6 exerted an inhibitory effect on LCSC
expansion by suppressing the IL-6/STAT3 axis (15). During the expansion of LCSCs, it
has been suggested that lnc-DILC serves as a bridge linking NF-κB
signaling to the autocrine IL-6/STAT3 cascade (15). It has additionally been
demonstrated that lnc-DILC may inhibit the expansion of LCSCs by
suppressing the IL-6/STAT3 signaling pathway. Additionally, the
elevated expression levels of IL-6 have been revealed to induce the
expansion of CSCs by stimulating the expression of STAT3 in
hepatocellular carcinoma, and colon, lung, breast and prostate
cancers (20–23). In addition, a study demonstrated
the ability of STAT3 to enhance the expression of IL-6 in cancer
cells (24). For example, the mRNA
levels of IL-6 increased in the tumor tissues of gp130 mutant mice
under abnormal stimulation with STAT3 (25). Ogura et al (26) reported that the expression levels
of IL-6 and other cytokines may be lowered by suppressing the
activity of STAT3 in fibroblasts and macrophages (26). In addition, a study suggested that
treatment with STAT3-siRNA may reduce the mRNA expression levels of
vascular endothelial growth factor, IL-10 and IL-6 in human
melanoma cells (27).
As a proinflammatory cytokine expressed by
fibroblasts, endothelial cells and monocytes, IL-6 may activate T
and B lymphocytes, and induce fever (28). A previous report demonstrated that
IL-6 may serve a critical role in inflammatory responses against
the invasion of microbes (29).
For example, a previous study indicated that a high concentration
of IL-6 was associated with higher mortality and risk of severe
sepsis (30), whereas the critical
role of the IL-6 signaling pathway in systemic inflammation has
been observed within IL-6-deficient mice (31).
As pattern recognition receptors, TLRs serve
important roles in the mediation of innate immunity so as to
provide protection against endotoxins (32). It has been demonstrated that
LPS-induced TLR4 activation may trigger the phosphorylation of
STAT3 primarily via myeloid differentiation primary response gene
MyD88 (MyD88), an adaptor protein of TLR4. In addition, the
suppression of p38, a downstream target of the TLR4 signaling, may
lead to a decreased level of p-STAT3 at the tyrosine site, while
p38 suppression and TLR4 deficiency may inhibit stress-induced
DNA-binding activity and the phosphorylation of STAT3.
Collectively, these data suggested a connection between STAT3 and
TLR4/p38 in chronic stress (33),
and it appears that TLR4/p38/IL-10 activates STAT3 following
chronic stress (33). Previous
studies have revealed that the expression levels of TLR4 are
increased in the monocytes of patients with sepsis and healthy
subjects challenged with LPS (34,35).
In addition, the stimulation of TLR4 may lead to the expression of
anti- and proinflammatory factors by activating two distinct
signaling pathways (36). In
particular, TLR4 is a unique member of the TLRs due to its ability
to mediate the Toll-interleukin receptor
(TIR)-domain-containing-adapter-inducing interferon-β (TRIF)- and
MyD88-dependent pathways (37).
Through TNF receptor-associated factor-6, interferon (IFN)-β and
the TRIF-dependent signaling pathway are involved in the mediation
of NF-κB activation (38). In
addition, IFN-β has been reported to be involved in the regulation
of late-stage hyper-inflammation in sepsis (39).
LPS is secreted by gram-negative bacteria and is a
pathogen-associated molecular pattern. Once recognized by TLR4, LPS
is able to activate a signaling cascade and lead to the upregulated
expression of certain cytokines that are associated with the onset
and progression of sepsis (40).
The majority of sepsis cases (>50%) are triggered by
gram-negative bacteria that are primarily recognized by TLR4
(1). For example, Tsujimoto et
al (41) demonstrated that the
serum levels of TLR4 protein increased markedly in patients with
sepsis upon pathogen infection (41), and it has been suggested that the
protein expression levels of TIR domain-containing adaptor protein
and TLR4, rather than gene polymorphisms, may be associated with
the severity of sepsis (42). LPS
increased STAT3 mRNA expression, whereas DILC or IL-6 did not,
suggesting that LPS did not increase STAT3 mRNA expression via the
DILC/IL-6 signaling pathway (43).
In conclusion, the association between lncRNA DILC
and sepsis was investigated; to the best of our knowledge, the
present study was the first to demonstrate that DILC directly
inhibited the expression of IL-6, which subsequently modulated
TLR4-dependent inflammatory responses via STAT3. In addition, TLR4
may act as an important mediator of inflammatory responses in
sepsis. Therefore, it was hypothesized in the present study that
lnc-DILC may act as a potential prognostic marker of sepsis, and
may additionally be considered to be a potential therapeutic target
for the treatment of sepsis.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Natural
Science Foundation of Guangdong Province (grant no.
S2013010016574), Pilot Projects of Clinical Medical Research and
Transformation Center of Guanzhou City (grant no. 201508020005),
and the National Key Clinical Specialty Construction Foundation
(grant no. 2012-649).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
WH planned the study, collected and analyzed the
data, and prepared the manuscript; LH planned the study, collected
and analyzed the data; MW collected, analyzed and interpreted the
data, and prepared the manuscript; MF collected the literature and
visualized the data; YD collected the data, and prepared the
manuscript; and, HZ planned the study, organized the funding and
prepared the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All data processing and sample collection were
approved by the Ethics Committee of Guangdong Academy of Medical
Sciences. The present study was performed according to the
Declaration of Helsinki.
Patient consent for publication
Written informed consent was obtained from all
patients.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Angus DC, Linde-Zwirble WT, Lidicker J,
Clermont G, Carcillo J and Pinsky MR: Epidemiology of severe sepsis
in the United States: Analysis of incidence, outcome, and
associated costs of care. Crit Care Med. 29:1303–1310. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bateman BT, Schmidt U, Berman MF and
Bittner EA: Temporal trends in the epidemiology of severe
postoperative sepsis after elective surgery: A large, nationwide
sample. Anesthesiology. 112:917–925. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hamano K, Gohra H, Noda H, Katoh T,
Fujimura Y, Zempo N and Esato K: Increased serum interleukin-8:
Correlation with poor prognosis in patients with postoperative
multiple organ failure. World J Surg. 22:1077–1081. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rubulotta FM, Ramsay G, Parker MM,
Dellinger RP, Levy MM and Poeze M: Surviving Sepsis Campaign
Steering Committee; European Society of Intensive Care Medicine;
Society of Critical Care Medicine: An international survey: Public
awareness and perception of sepsis. Crit Care Med. 37:167–170.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kawamoto T, Ii M, Kitazaki T, Iizawa Y and
Kimura H: Tak-242 selectively suppresses toll-like receptor
4-signaling mediated by the intracellular domain. Eur J Pharmacol.
584:40–48. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Biffl WL, Moore EE, Moore FA and Peterson
VM: Interleukin-6 in the injured patient. Marker of injury or
mediator of inflammation? Ann Surg. 224:647–664. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pape HC, Schmidt RE, Rice J, van Griensven
M, das Gupta R, Krettek C and Tscherne H: Biochemical changes after
trauma and skeletal surgery of the lower extremity: Quantification
of the operative burden. Crit Care Med. 28:3441–3418. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Giannoudis PV, Smith RM, Banks RE, Windsor
AC, Dickson RA and Guillou PJ: Stimulation of inflammatory markers
after blunt trauma. Br J Surg. 85:986–990. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stensballe J, Christiansen M, Tønnesen E,
Espersen K, Lippert FK and Rasmussen LS: The early IL-6 and IL-10
response in trauma is correlated with injury severity and
mortality. Acta Anaesthesiol Scand. 53:515–521. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Adib-Conquy M and Cavaillon JM:
Compensatory anti-inflammatory response syndrome. Thromb Haemost.
101:36–47. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sander M, Irwin M, Sinha P, Naumann E, Kox
WJ and Spies CD: Suppression of interleukin-6 to interleukin-10
ratio in chronic alcoholics: association with postoperative
infections. Intensive Care Med. 28:285–292. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mercer TR, Dinger ME and Mattick JS: Long
non-coding RNAs: Insights into functions. Nat Rev Genet.
10:155–159. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Geisler S and Coller J: RNA in unexpected
places: Long non-coding RNA functions in diverse cellular contexts.
Nat Rev Mol Cell Biol. 14:699–712. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ben-Neriah Y and Karin M: Inflammation
meets cancer, with NF-kB as the matchmaker. Nat Immunol.
12:715–723. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kagoya Y, Yoshimi A, Kataoka K, Nakagawa
M, Kumano K, Arai S, Kobayashi H, Saito T, Iwakura Y and Kurokawa
M: Positive feedback between NF-kB and TNF-α promotes
leukemia-initiating cell capacity. J Clin Invest. 124:528–542.
2014. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang X, Sun W, Shen W, Xia M, Chen C,
Xiang D, Ning B, Cui X, Li H, Li X, et al: Long non-coding RNA DILC
regulates liver cancer stem cells via IL-6/STAT3 axis. J Hepatol.
64:1283–1294. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Greenhill CJ, Rose-John S, Lissilaa R,
Ferlin W, Ernst M, Hertzog PJ, Mansell A and Jenkins BJ: IL-6
trans-signaling modulates TLR4-dependent inflammatory responses via
STAT3. J Immunol. 186:1199–1208. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li RJ, Gao CY, Guo C, Zhou MM, Luo J and
Kong LY: The Anti-inflammatory Activities of two major withanolides
from physalisminima via acting on NF-κB, STAT3, and HO-1 in
LPS-stimulated RAW264.7 Cells. Inflammation. 40:401–413. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schroeder A, Herrmann A, Cherryholmes G,
Kowolik C, Buettner R, Pal S, Yu H, Müller-Newen G and Jove R: Loss
of androgen receptor expression promotes a stem-like cell phenotype
in prostate cancer through STAT3 signaling. Cancer Res.
74:1227–1237. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Korkaya H, Kim GI, Davis A, Malik F, Henry
NL, Ithimakin S, Quraishi AA, Tawakkol N, D'Angelo R, Paulson AK,
et al: Activation of an IL6 inflammatory loop mediates trastuzumab
resistance in HER2+ breast cancer by expanding the cancer stem cell
population. Mol Cell. 47:570–584. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jinushi M, Chiba S, Yoshiyama H, Masutomi
K, Kinoshita I, Dosaka-Akita H, Yagita H, Takaoka A and Tahara H:
Tumor-associated macrophages regulate tumorigenicity and anticancer
drug responses of cancer stem/initiating cells. Proc Natl Acad Sci
USA. 108:12425–12430. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
He G, Dhar D, Nakagawa H, Font-Burgada J,
Ogata H, Jiang Y, Shalapour S, Seki E, Yost SE, Jepsen K, et al:
Identification of liver cancer progenitors whose malignant
progression depends on autocrine IL-6 signaling. Cell. 155:384–396.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huang WL, Yeh HH, Lin CC, Lai WW, Chang
JY, Chang WT and Su WC: Signal transducer and activator of
transcription 3 activation up-regulates interleukin-6 autocrine
production: A biochemical and genetic study of established cancer
cell lines and clinical isolated human cancer cells. Mol Cancer.
9:3092010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Judd LM, Bredin K, Kalantzis A, Jenkins
BJ, Ernst M and Giraud AS: STAT3 activation regulates growth,
inflammation, and vascularization in a mouse model of gastric
tumorigenesis. Gastroenterology. 131:1073–1085. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ogura H, Murakami M, Okuyama Y, Tsuruoka
M, Kitabayashi C, Kanamoto M, Nishihara M, Iwakura Y and Hirano T:
Interleukin-17 promotes autoimmunity by triggering a
positive-feedback loop via interleukin-6 induction. Immunity.
29:628–636. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sumimoto H, Imabayashi F, Iwata T and
Kawakami Y: The BRAF-MAPK signaling pathway is essential for
cancer-immune evasion in human melanoma cells. J Exp Med.
203:1651–1656. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gao JW, Zhang AQ, Pan W, Yue CL, Zeng L,
Gu W and Jiang J: Association between IL-6-174G/C polymorphism and
the risk of sepsis and mortality: A systematic review and
meta-analysis. PLoS One. 10:e01188432015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Borden EC and Chin P: Interleukin-6: A
cytokine with potential diagnostic and therapeutic roles. J Lab
Clin Med. 123:824–829. 1994.PubMed/NCBI
|
|
30
|
Uusitalo-Seppälä R, Koskinen P, Leino A,
Peuravuori H, Vahlberg T and Rintala EM: Early detection of severe
sepsis in the emergency room: Diagnostic value of plasma C-reactive
protein, procalcitonin, and interleukin-6. Scand J Infect Dis.
43:883–890. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jin YH, Hou W, Kang HS, Koh CS and Kim BS:
The role of interleukin-6 in the expression of PD-1 and PDL-1 on
central nervous system cells following infection with Theiler's
murine encephalomyelitis virus. J Virol. 87:11538–11551. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Takeda K and Akira S: Toll-like receptors
in innate immunity. Int Immunol. 17:1–14. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hu D, Wan L, Chen M, Caudle Y, LeSage G,
Li Q and Yin D: Essential role of IL-10/STAT3 in chronic
stress-induced immune suppression. Brain Behav Immun. 36:118–127.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wittebole X, Coyle SM, Kumar A, Goshima M,
Lowry SF and Calvano SE: Expression of tumour necrosis factor
receptor and Toll-like receptor 2 and 4 on peripheral blood
leucocytes of human volunteers after endotoxin challenge: A
comparison of flow cytometric light scatter and immunofluorescence
gating. Clin Exp Immunol. 141:99–106. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Härter L, Mica L, Stocker R, Trentz O and
Keel M: Increased expression of toll-like receptor-2 and −4 on
leukocytes from patients with sepsis. Shock. 22:403–409. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hoebe K, Janssen E and Beutler B: The
interface between innate and adaptive immunity. Nat Immunol.
5:971–974. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kawai T and Akira S: The role of
pattern-recognition receptors in innate immunity: Update on
Toll-like receptors. Nat Immunol. 11:373–384. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kumar V and Sharma A: Innate immunity in
sepsis pathogenesis and its modulation: New immunomodulatory
targets revealed. J Chemother. 20:672–683. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Weighardt H, Kaiser-Moore S, Schlautkötter
S, Rossmann-Bloeck T, Schleicher U, Bogdan C and Holzmann B: Type I
IFN modulates host defense and late hyperinflammation in septic
peritonitis. J Immunol. 177:5623–5630. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Namath A and Patterson AJ: Genetic
polymorphisms in sepsis. Crit Care Clin. 25(835–856): x2009.
|
|
41
|
Tsujimoto H, Ono S, Efron PA, Scumpia PO,
Moldawer LL and Mochizuki H: Role of Toll-like receptors in the
development of sepsis. Shock. 29:315–321. 2008.PubMed/NCBI
|
|
42
|
Zhang J, Yang J, Xu X, Liang L, Sun H, Liu
G, Zhang L and Su Y: The influence of genetic polymorphisms in TLR4
and TIRAP, and their expression levels in peripheral blood, on
susceptibility to sepsis. Exp Ther Med. 11:131–139. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Liu X, Su X, Xu S, Wang H, Han D, Li J,
Huang M and Cao X: MicroRNA in vivo precipitation identifies
miR-151-3p as a computational unpredictable miRNA to target Stat3
and inhibits innate IL-6 production. Cell Mol Immunol. 15:99–110.
2018. View Article : Google Scholar : PubMed/NCBI
|