Introduction
Reprogramming, a widely used technique in
regenerative medicine, is the direct transformation of fully
differentiated somatic cell into another type of fully
differentiated cell in response to the induction of one or more
transcription factors without requiring the induced pluripotent
stem cell (iPSC) stage (1). In the
laboratory, this technique is generally performed by using
fibroblasts as the initial cells to be reprogramed into induced
neurons (2), cardiomyocytes
(3) and haematopoietic progenitor
cells (4).
Using reprogramming technology, Hu et al
(5) successfully reprogrammed
T-box18 (TBX18)-transfected cardiomyocytes directly to induced
sinoatrial node (iSAN) cells in adult pig hearts with a complete
heart block. Cells in the vicinity of the injection site expressed
higher levels of SAN-specific genes and lower levels of
chamber-specific genes. TBX18, a transcription factor, is required
for the embryonic development of the head area of the SAN but is
undetectable after birth and in adulthood (6). In addition, Tbx18 is the only
transcription factor that has converted working myocytes into SAN
cells and has caused an increase in the spontaneous beating rate
(7). In addition, cardiac
fibroblasts (CFs), the most important non-cardiomyocyte cell type
in the heart, can electrically couple with cardiomyocytes to affect
their electrophysiological properties (8). So, it is unclear what changes might
occur with fibroblasts when TBX18 is injected directly into the
heart and what effect these changes might have on surrounding
cardiomyocytes.
In this study, we explored the reprogramming effect
of TBX18 on in vitro neonatal rat CF cell cultures and
observed the effect of these changes on beating rates when
TBX18-CFs were co-cultured with neonatal rat ventricular
cardiomyocytes (NRVMs) and TBX18-NRVMs. These data will help us
understand the contributions of fibroblasts to the development of
biological pacemakers when TBX18 is directly injected into the
heart.
Materials and methods
Materials
Dulbecco's modified Eagle's medium (DMEM)/F12 (1:1)
and foetal bovine serum were purchased from Gibco (Thermo Fisher
Scientific, Inc., Waltham, MA, USA). Trypsin, type II collagenase
and 5-bromodeoxyuridine were purchased from Sigma-Aldrich (Merck
KGaA, Darmstadt, Germany). DH5α competent cells were purchased from
Tiangen (Beijing, China). Specific rabbit monoclonal antibodies
against hyperpolarization-activated cyclic nucleotide-gated cation
channel 4 (HCN4), which is a marker for SAN, connexin 43 (COX43),
which is a common connexion between cardiac cells, cardiac troponin
I (cTnI), which is a marker for NRVMs, α-striated actin (α-SA),
which is a marker for NRVMs, and GAPDH were purchased from Abcam
(Cambridge, MA, USA); antibodies for vimentin, which is a marker
for CFs, and COX-45, which is another common connexion between
cardiac cells, were purchased from Cell Signaling Technology, Inc.
(Danvers, MA, USA) and Santa Cruz Biotechnology, Inc. (Dallas, TX,
USA), respectively; and antibodies for myosin heavy chain (MHC),
which is a marker for NRVMs, and α-striated muscle actin (α-SMA),
which is a marker for cardiac myofibroblasts (CMFs) were purchased
from Wuhan Sanying Biotechnology (Wuhan, China) and Wuhan Tiandeyue
Biotechnology (Wuhan, China), respectively.
Construction of the TBX18 lentiviral
vector
pHBAd-MCMV-GFP (Ad-GFP) (Hanbio, Shanghai, China)
was digested using BamHI and NotI. The ORF sequence
of the human TBX18 gene (GenScript, Nanjing, China) was amplified
using polymerase chain reaction (PCR). After the restriction enzyme
digestion, gel extraction was performed. The digested fragment and
vector were ligated to form pHBAd-MCMV-GFP-TBX18 (Ad-TBX18), which
was then transformed into competent DH5α cells (Tiangen). Positive
clones were identified by liquid sequencing. Bacteria in the
logarithmic growth phase were incubated at 37°C overnight in LB
culture medium while shaking at 300 × g. The large-scale
preparation of recombinant plasmids was conducted using a Plasmid
Midi Preparation kit (Beijing CW Biotech Co., Ltd., Beijing,
China). Cells were transfected with Ad-TBX18 and the backbone
vector pHBAd-BHG using LipofilterTM (both from Hanbio).
The supernatant was harvested after viral amplification. Ad-GFP and
Ad-TBX18 were adjusted to 1×1010 PFU/ml and stored at
−80°C.
Isolation and culture of CFs and
NRVMs
The present study was approved by the Ethical Board
of The Renmin Hospital of Wuhan University (Wuhan, China). Hearts
were dissected from 16 neonatal (1- to 2-day-old) rat heart
ventricles, minced and washed in solution A (0.02% phenol red, 137
mM NaCl, 5.4 mM KCl, 0.34 mM Na2HPO4, 0.44 mM
KH2PO4, 5.6 mM D-glucose and 20 mM HEPES, pH
7.3) at room temperature. The heart tissue was dissociated using a
digestion solution containing 450 U collagenase and 14 U DNase per
ml of solution A in an Erlenmeyer flask containing glass beads.
Then, the flask was placed in a shaking water bath at 37°C. We
pooled cell suspensions from two dissociations and centrifuged the
mixture 1,000 × g for 15 min. We then resuspended the cells in
Ham's F10 medium supplemented with 10% fetal bovine serum (FBS) and
10% horse serum (HS) and plated the cells onto 12 Primaria-coated 6
cm culture dishes. The cells were plated for 45 min to allow
fibroblasts to preferentially attach to the bottom of the culture
dishes. The non-adherent cells (cardiomyocytes) were collected, and
the adherent cells (mainly fibroblasts) were supplemented with DMEM
containing 10% FBS and antibiotics (100 U/ml penicillin and 0.1 g/l
streptomycin). Fibroblasts were grown to confluence and then
passaged and plated onto 10 collagen I-coated 6-well stretch
plates. The collected cardiomyocytes were directly plated at a
density of 1×105 cells/cm2 onto 8 collagen
I-coated 6-well stretch plates and cultured in Ham's F10 and DMEM
(1:1) supplemented with 8% HS and antibiotics. The cardiomyocytes
and fibroblasts were incubated at 37°C in 5% CO2 in a
humidified incubator. The culture medium used to incubate both cell
types was refreshed every 2–3 days.
CFs and NRVMs transfected with
TBX18
CFs in passages 3–5 were removed from the culture
dishes by digestion. A cell suspension was prepared and inoculated
onto 6-well plates. When cell confluence reached 70–80%, Ad-TBX18
in DMEM/F12 was added to the cells at a multiplicity of infection
(MOI) of 100, and these cells were used as the experimental group.
CFs that were treated with Ad-GFP were used as the control group.
After a 2-h incubation period, the medium was replaced with fresh
complete medium. Following the same method, we transfected NRVMs
with Ad-TBX18 at an MOI of 100. After a 2-h incubation in an
incubator, the medium was replaced, and the cells were cultured for
an additional 2 to 5 days. The cells were observed under light and
fluorescence microscopy. The percentage of green fluorescent
protein-positive cells was determined using flow cytometry (BD
Biosciences, Franklin Lakes, NJ, USA).
Western blot assays
TBX18-CFs and GFP-CFs were seeded into 6-well
culture dishes. The cells were harvested using RIPA lysis buffer.
Equal amounts of protein were loaded onto a gel for sodium dodecyl
sulphate-polyacrylamide gel electrophoresis (SDS-PAGE), and the
proteins were separated and transferred to nitrocellulose
membranes. Then, the membranes were incubated with primary
antibodies against HCN4, α-SMA, COX43, COX45, cTnI, α-SMA and MHC
overnight at 4°C. The bound primary antibodies were detected by
incubating the membranes with horseradish peroxidase
(HRP)-conjugated secondary antibodies that were raised in the
appropriate species, and the results were then detected using
enhanced chemiluminescence. The level of GAPDH was used to
normalize the signal intensities.
Immunofluorescence staining
After the cells were pre-plated, we calculated the
purity of the obtained cell populations, and the CFs and NRVMs were
cultured on gelatine-coated coverslips in 6-well culture dishes.
The cells were washed with phosphate-buffered saline (PBS) and
fixed with 4% paraformaldehyde. Following permeabilization with
0.1% Triton X-100, the CFs and NRVMs were incubated with primary
anti-vimentin and anti-cTnI antibodies overnight at 4°C. Secondary
antibodies (HRP-Goat anti-Rat IgG and HRP-Goat anti-Rabbit IgG)
were then used to detect cTnI. We used
4′,6-diamidino-2-phenylindole (DAPI) to visualize the nuclei. The
cells were observed under a fluorescence microscope. We randomly
selected three visual fields in each of three different cell
isolates to calculate the percentage of fluorescent cells in the
total number of cells, and the mean value was determined. In
addition, the same method was used to incubate TBX18-CFs and
GFP-CFs with antibodies against HCN4, vimentin and α-SMA to observe
expression of these proteins.
Co-culture conditions
In the co-culture experiments, CFs and NRCMs were
mixed and plated at a ratio of 1:4 (20% CFs) on 60-cm2
culture dishes. Two groups of co-cultures were established
according to the experimental strategy, as follows. The first group
consisted of co-cultures of NRVMs, NRVMs+CFs, NRVMs+GFP-CFs and
NRVMs+TBX18-CFs; the second group consisted of co-cultures of
NRVMs, TBX18-NRVMs, TBX18-NRVMs+CFs, TBX18-NRVMs+GFP-CFs and
TBX18-NRVMs+TBX18-CFs. The combinations in the first group were
co-cultured for 14 days as isotropic monolayers, and the medium was
changed after 24 h. Fluorescence mapping was performed after 2
days, the cells were observed once every two days, and the beating
frequency was determined in each group by observing red
fluorescence using a BX41 microscope. In addition, the mean, min
and max number of spontaneous beats were determined in the second
group at the time of the highest value observed in the first group.
The co-culture experiments were performed three times to validate
the results.
Statistical analysis
All data are presented as the mean ± standard error
of the mean. Statistical comparisons among multiple groups were
analysed using one-way analysis of variance with Dennett's T3 test
in SPSS 19.0 software (IBM Corp., Armonk, NY, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Changes in FCs following TBX18
transfection-induced reprogramming
Protein expression in TBX18-CFs was
characteristic of iSAN cells
After the cells were pre-plated, we obtained CFs
with a purity of 96%. The transfection efficiency in the CFs was
76.2±6.2% at an MOI of 100.
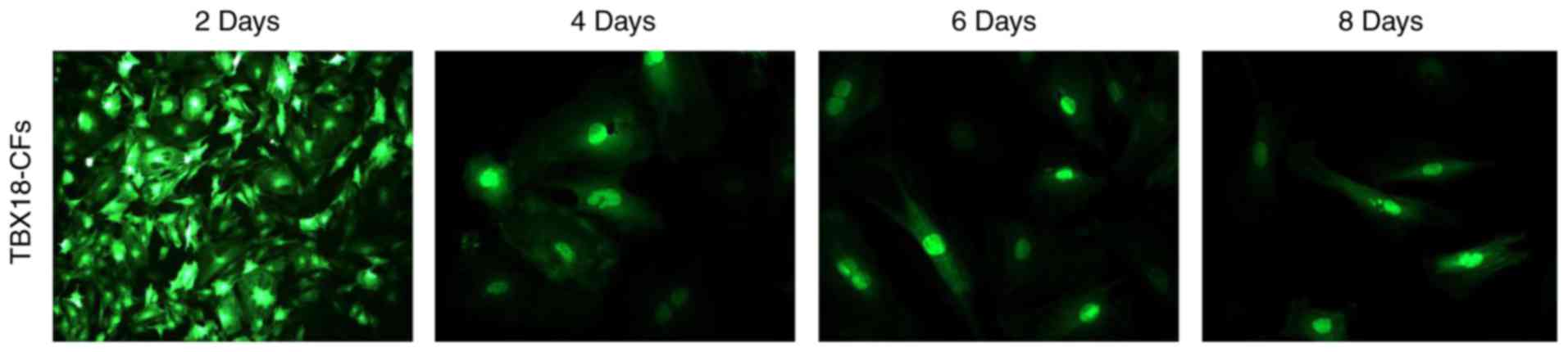
On the 2nd day after cells were transfected with
Ad-TBX18, a few cells became round (indicative of dead cells), but
the remaining cells were morphologically radial and flaky. There
were vacuoles in the cytoplasm of some cells, and cell
proliferation slowed down. No spontaneous beating was observed in
any of the cultures. After 4–6 days of culture, the CFs
proliferated, and their synapses were partially extended without
fusiform, triangular or conical changes, which are the
morphological characteristics of SANs. In addition, spontaneous
beatings were not observed until the 8th day of culture (Fig. 1). These results indicate that the
TBX18-CFs did not transform into iSAN cells.
HCN4 is an important channel protein that functions
as a pacemaker current (If) channel and is highly expressed in SAN
cells (3). The HCN4 protein was
expressed at significantly higher levels in TBX18-CFs but was
rarely observed in GFP-CFs, which perhaps reflects the immature
state of fibroblasts in neonatal rats. The expression levels of
COX43 and COX45 were higher in GFP-CFs than in TBX18-CFs
(P<0.05), and the expression levels of COX proteins were lower
in TBX18-CFs than in GFP-CFs (Fig.
2), indicating that TBX18 reduced the connections between
CFs.
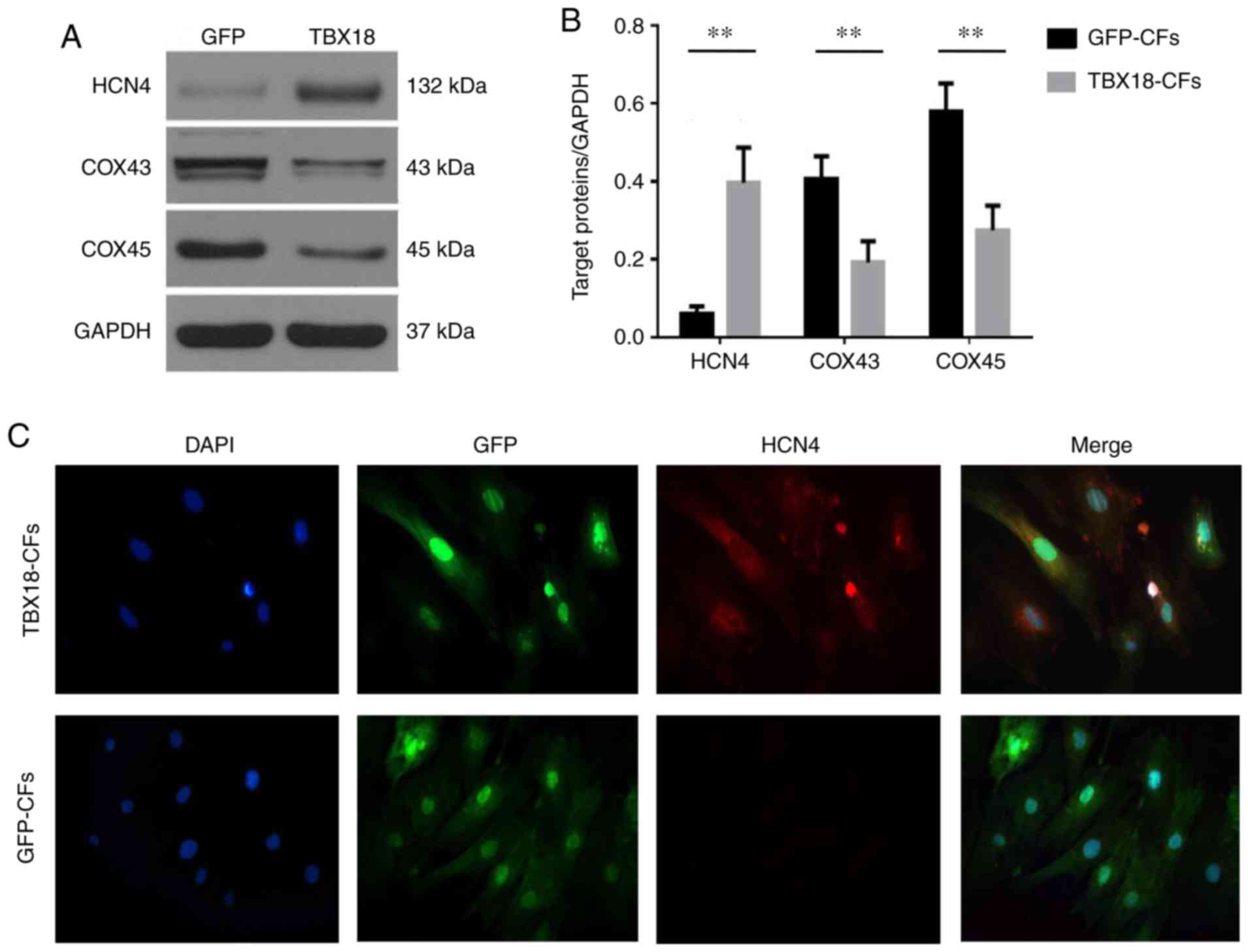 | Figure 2.Expression of sinoatrial node specific
proteins in fibroblasts transfected with TBX18. (A) The protein
expression levels of HCN4, COX43 and COX45 were observed in
TBX18-CFs and GFP-CFs. (B) The expression of HCN4 protein in
TBX18-CFs was significantly increased; however, the expression
levels of COX43 and COX45 were decreased. (C) The expression of
HCN4 protein was observed in the TBX18-CF and GFP-CFs groups
(magnification, ×200). Blue fluorescence indicates nuclear
staining, green fluorescence indicates GFP staining and red
fluorescence indicates HCN4 protein. **P<0.01, as indicated.
TBX18, T-box18; CFs, cardiac fibroblasts; HCN4,
hyperpolarization-activated cyclic nucleotide-gated cation channel
4; COX, connexin; GFP, green fluorescence protein. |
TBX18-CFs were not converted into
cardiomyocytes
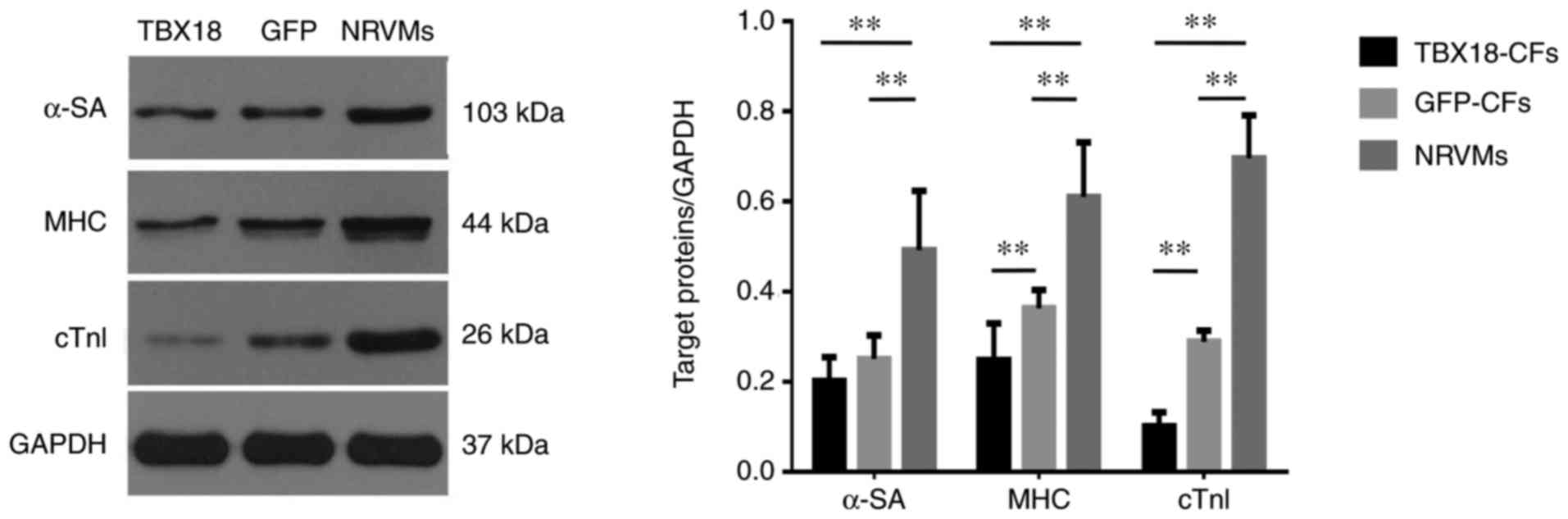
cTnI, α-SA and MHC are cardiomyocyte-specific
proteins. All three proteins were expressed at much lower levels in
TBX18-CFs and GFP-CFs than in NRVMs, which were used as the
positive control group. In addition, the difference in cTn1
expression was much more conspicuous in each group (Fig. 3). These results indicate that CFs
did not exhibit cardiomyocyte-like phenotypes after they were
transfected with TBX18.
TBX18-CFs were transformed into
CMFs
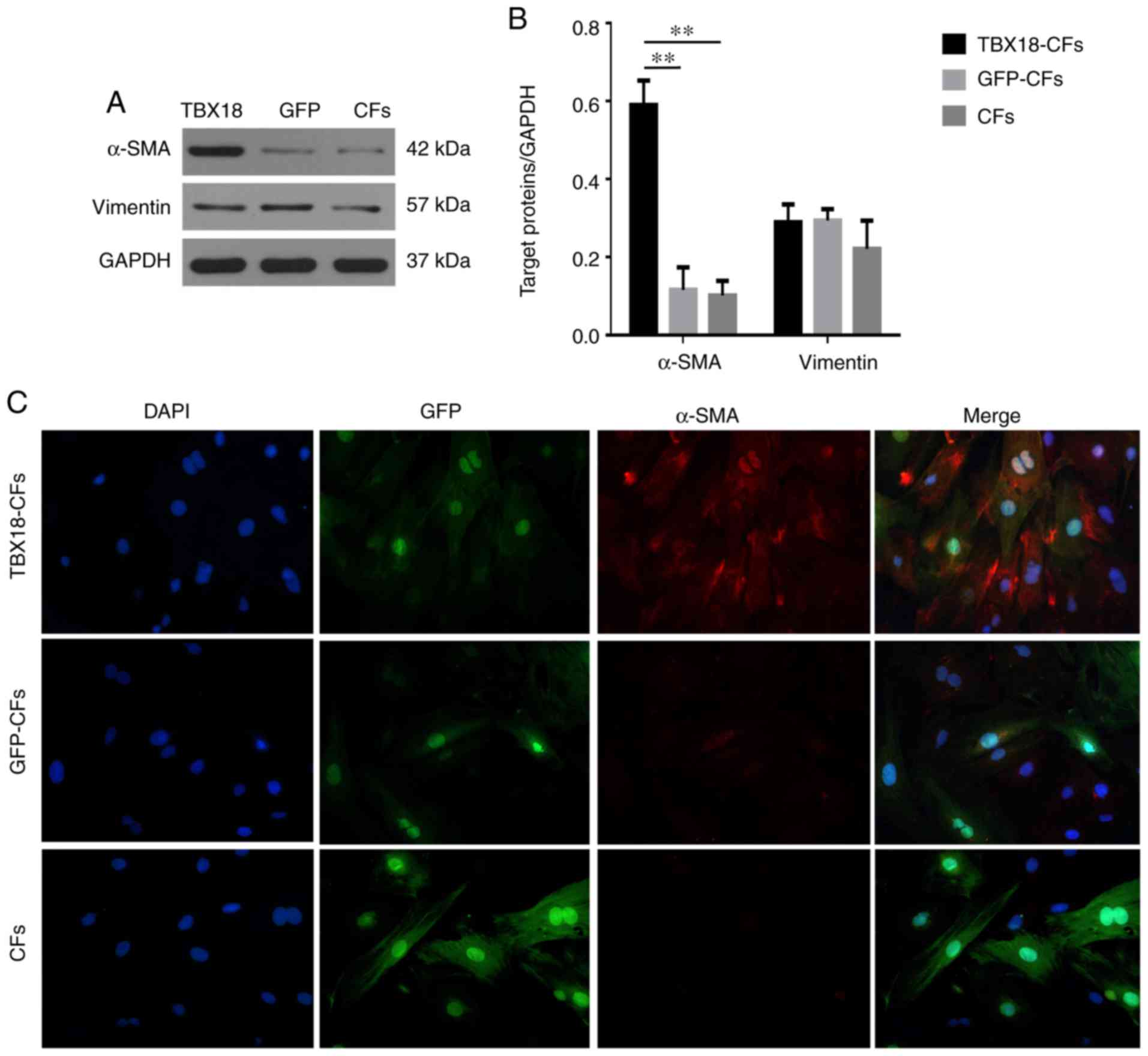
Vimentin is a fibroblast-specific protein. Our
results showed that under fluorescence microscopy, TBX18-CFs and
GFP-CFs exhibited red fluorescence and a filamentous arrangement in
the cytoplasm, indicating that these two groups of cells expressed
a fibroblast-specific protein and maintained the histological
characteristics of fibroblasts (Fig.
4A and B). α-SMA is a CMF-specific protein (8). Western blot and immunofluorescence
analyses showed that α-SMA was expressed at higher levels in
TBX18-CFs but at much lower levels in GFP-CFs and CFs, both of
which served as negative controls (Fig. 4), indicating that TBX18 induced
fibroblasts to transform into CMFs.
TBX18-transfected CFs improved the
pulsation rate of co-cultured NRVMs
Spontaneous beating was analysed in the groups of
co-cultured cells for 14 days, and the results are shown in
Fig. 5A. On the 8th day of
co-culture, the rate of spontaneous beating in the NRVM+TBX18-CF
cultures was higher (86±10 b.p.m.) than the rate in the NRVM
culture (65±9 b.p.m.) and significantly higher than the rate in the
NRVM+GFP-CF and NRVM+CF cultures (24±7 and 21±6 b.p.m.,
respectively). There was no difference in the rate of spontaneous
beating between the NRVM+GFP-CF and NRVM+CF cultures. Spontaneous
beating began to decrease in the NRVMs after 8 days in co-culture
and then gradually slowed until it stopped on the 14th day.
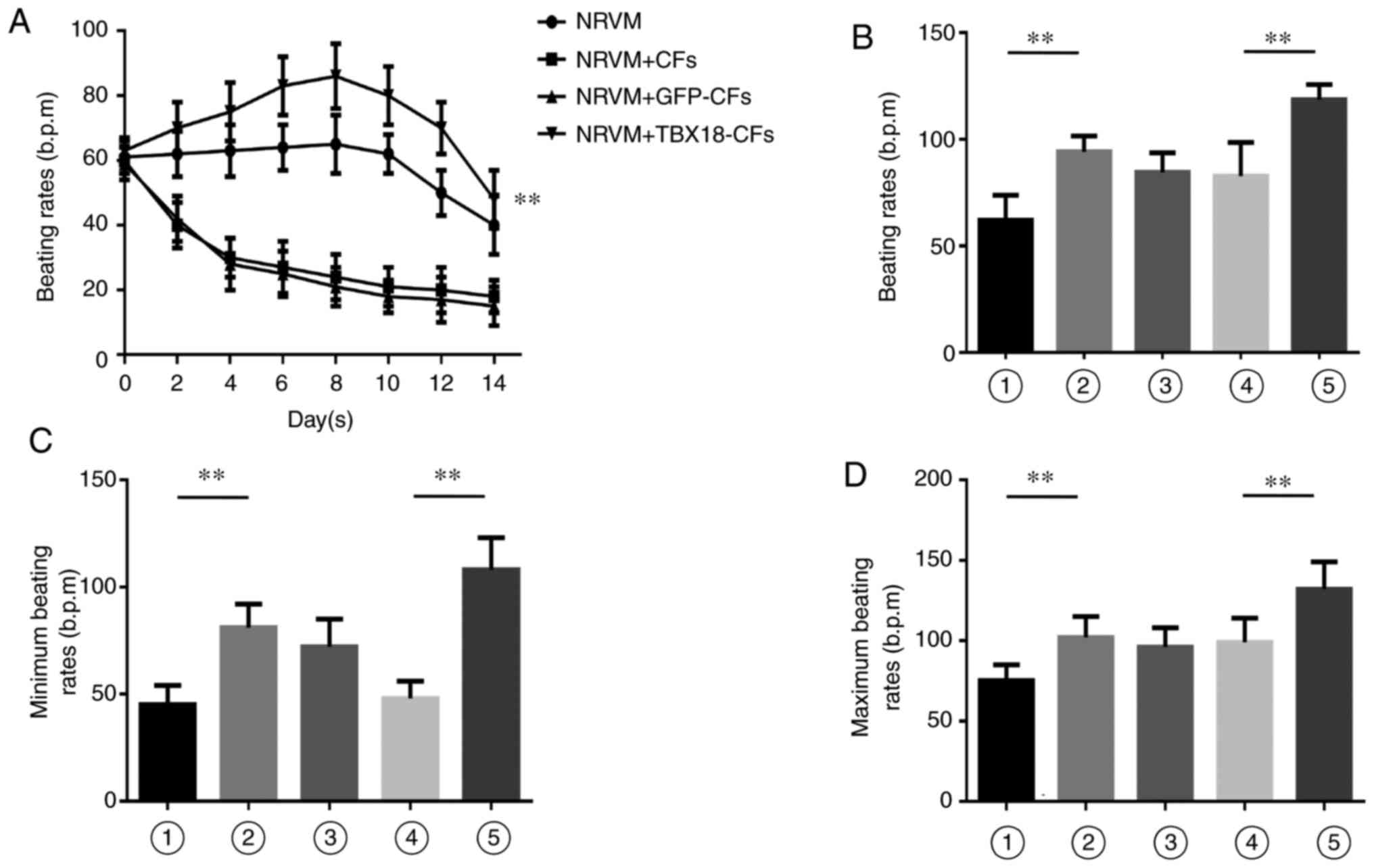 | Figure 5.Differences in pulsation frequency
were observed in the co-culture system. (A) TBX18-CFs improved the
beating rate of NRVMs in the co-culture system. The frequency of
spontaneous beating of TBX18-CFs+NRVMs was significantly higher
than that of the other groups. The (B) beating rates, and the (C)
minimum and (D) maximum beating rates were compared in each group.
The beating frequency of TBX18-NRVMs increased by ~40 to 132 bpm
(depending on the conditions) when TBX18-NRVMs were co-cultured
with TBX18-CFs. Bar 1, NRVMs; bar 2, TBX18-NRVMs; bar 3,
TBX18-NRVMs+CFs; bar 4, TBX18-NRVMs+GFP-CFs; and bar 5,
TBX18-NRVMs+TBX18-CFs. **P<0.01, as indicated. TBX18, T-box18;
CFs, cardiac fibroblasts; NRVMs, neonatal rat ventricular
cardiomyocytes; GFP, green fluorescence protein; bpm, beats per
minute. |
TBX18-transfected CFs improved
spontaneous beating in co-cultured NRVMs that were transfected with
TBX18
On the 8th day of co-culture, spontaneous beating
was observed in each of the groups (Fig. 5B-D). The cells in the TBX18-NRVM
group were beating significantly faster than the cells in the NRVM
group (94.20±7.38 vs. 62.10±11.67 b.p.m., respectively;
P<0.001), indicating that TBX18 significantly improved
myocardial cell pulsation. There was no significant difference in
the rates between the CF and GFP-CF co-culture groups (P=0.71),
suggesting that transfecting GFP did not affect spontaneous beating
in the co-culture system. There was also no significant difference
between the CF co-culture group and the TBX18-NRVM group (P=0.52),
but co-culturing cells with CFs tended to decrease the spontaneous
beating rates. The rate of spontaneous beating in the TBX18-NRVMs
was increased by approximately 40 b.p.m. (118.50±7.25 b.p.m.) and
reached as high as 132 b.p.m. in the TBX18-NRVMs that were
co-cultured with TBX18-CFs. These results indicate that TBX18 could
abolish the inhibitory effect of CFs on the co-culture system.
Discussion
In this study, TBX18-CFs were not transformed into
iSAN cells, but the protein expression levels of HCN4, COX43 and
COX45 were increased. TBX18-CFs also did not undergo a
cardiomyocyte-like transformation, but they overexpressed α-SMA,
indicating that they had transformed into CMFs. In addition,
TBX18-CFs could gradually increase the rate of spontaneous beating
when they were co-cultured with NRVMs or TBX18-NRVMs. However,
GFP-transfected CFs and CFs showed the opposite effect, indicating
that the TBX18 gene could induce CFs to transform, which
contributed to the increase of the beating rates of NRVMs and
TBX18-NRVMs.
In 2006, Takahashi and Yamanaka et al
(9) found that multiple
transcription factors can be combined to reverse the embryonic
fibroblasts into iPSCs; these iPSCs can be transformed into other
cells if exposed to a certain inductive environment. However, many
studies have found that transfecting certain single transcription
factors could cause cells to undergo cross-line reprogramming. For
example, myogenic differentiation (MyoD) can directly reprogram
fibroblasts into skeletal muscle cells (10). The transcription factor
octamer-binding factor 4 (OCT4) directly reprogrammed blood cells
into induced neural progenitor cell (iNPCs) (11) without requiring the intermediate
step of forming iPSCs. This means the technology to directly
transform one cell into another cell is possible, which would
require a short cycle and a simple process and potentially reduce
teratoma formation and the risk of mutation (12). Direct reprogramming has also been
used in biological pacing. Kapoor et al (7) found that cardiomyocytes induced by
TBX18 could directly transform them into pacing iSAN cells both
in vivo and in vitro. Hu et al (5) reported similar results in large
animal experiments. Transfecting TBX18 into neonatal rat hearts
could decrease the expression of COX43, increase the expression of
inward rectifier potassium channels and HCN4, and then increase the
spontaneous beating rates of heart (7). Additionally, transfection with TBX18
could increase the regulation of cAMP (e.g., the ‘membrane clock’
and ‘Ca+ clock’) and induced spontaneous local Ca2+ release (LCR)
events (4). These data indicate
that TBX18-reprogrammed cardiomyocytes exhibit stable pacing
functions.
Fibroblasts, as the most numerous non-cardiomyocyte
cell type in heart, account for 45% of the total number of cardiac
cells (13). Under physiological
conditions, fibroblasts continue to synthesize and degrade both
collagen and fibronectin to support the structural integrity of the
heart. Fibroblasts can also directly influence the
electrophysiological activity of the myocardium by forming gap
junctions with myocardium (14).
Fibroblasts are non-excitatory cells that have high membrane
resistance and high resting potential and can produce mechanically
sensitive currents (8). Therefore,
we can predict that when the TBX18 gene is injected into the heart
of animals, both cardiomyocytes and fibroblasts could be
transfected. However, what type of alterations of the
characteristics of CFs and the effect of these alterations to
adjacent cardiomyocytes has never been studied. Here, we observed
that TBX18-CFs could increase the beating rates of NRVMs or
TBX18-NRVMs in a co-culture system, but GFP-CFs and CFs showed the
opposite effect. Previous research indicates that COX43 is mainly
expressed between the myocardium, while COX45 is mainly expressed
in fibroblasts (15), and
fibroblasts in SANs form electrical couplings and exchange
materials with P cells via COX45 (14). In vitro co-cultures of
fibroblasts and cardiomyocytes with an increased density of
fibroblasts exhibited decreased beating rates of the cardiomyocytes
(16), but when COX43 was
inhibited in the fibroblasts, the pulse and conduction rate were
significantly restored (17,18),
indicating that when fibroblasts and cardiomyocytes are adjacent to
each other, the higher resting potential of the fibroblasts induces
peripheral myocardial cell depolarization through COX43, which
reduces their excitability (19).
In this study, we observed that the expression of both COX43 and
COX45 was decreased in TBX18-CFs. Compared with the results of Hu's
research (5), our data show a
significant decrease in COX43 expression (which is similar with
Hu's results), but COX45 expression just decreased to
physiologically normal levels and was still higher than COX43
expression. Therefore, we hypothesised that the TBX18 gene removes
the restraint of CFs on NRVMs by decreasing COX43 expression and
then delivering an inward current (which is generated by HCN4) to
adjacent NRVMs via COX45, therefore increasing the beating rates of
NRVMs in the co-culture system.
At the same time, several researchers have found
that TBX18 can reprogram cardiomyocytes into iSANs (5,7), but
according to our study, TBX18 cannot induce the same activity in
CFs, which were simply transformed to CMFs with the protein
properties of SANs. Therefore, we consider that the effects of
TBX18 on CFs and cardiomyocytes are different. Though many studies
have found that CFs could be directly programmed into other types
of cells (2–4), our study considers that TBX18 alone
was insufficient to reprogram CFs, but it could transform them into
similar cells (CMFs), which are common in heart and belong to same
family as iSANs.
There are some limitations in this paper that should
be noted. First, we used neonatal fibroblasts and cardiomyocytes,
which exhibit phenotypes that are not similar to either mature
cardiomyocytes or mature nodal cells. The effect of TBX18 on
neonatal and mature cells may therefore not be the same as its
effect in mature cells. This limits speculation regarding the
possibility of reprogramming cardiomyocytes and fibroblasts using
TBX18 in adult hearts. Second, because of limitations associated
with the experiment environment, no functional studies were
performed (e.g., an evaluation of ion channels or fura uptake)
apart from an analysis of the beating rate. We therefore could not
determine whether transfected CFs might potentially produce
arrhythmia or other adverse reactions. Further research should be
performed to evaluate these possibilities in the future.
Acknowledgements
The authors would like to thank Hubei Key Laboratory
of Cardiology, Renmin Hospital of Wuhan University (Wuhan, China)
for providing experimental materials and support. The authors are
also grateful to Dr Yu Liu (Department of Cardiology, Renmin
Hospital of Wuhan University) for making detailed adjustments of
the procedures during the experiments.
Funding
The present study was supported by a grant from the
National Natural Science Foundation of China (grant no.
81570306).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HH conceived the study and DJQ performed the
experiments.
Ethics approval and consent to
participate
The present study was approved by the Ethical Board
of The Renmin Hospital of Wuhan University (Hubei, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhou Q and Melton DA: Extreme makeover:
Converting one cell into another. Cell Stem Cell. 3:382–388. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vierbuchen T, Ostermeier A, Pang ZP,
Kokubu Y, Südhof TC and Wernig M: Direct conversion of fibroblasts
to functional neurons by defined factors. Nature. 463:1035–1041.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ieda M, Fu JD, Delgado-Olguin P, Vedantham
V, Hayashi Y, Bruneau BG and Srivastava D: Direct reprogramming of
fibroblasts into functional cardiomyocytes by defined factors.
Cell. 142:375–386. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Szabo E, Rampalli S, Risueño RM, Schnerch
A, Mitchell R, Fiebig-Comyn A, Levadoux-Martin M and Bhatia M:
Direct conversion of human fibroblasts to multilineage blood
progenitors. Nature. 468:521–526. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hu YF, Dawkins JF, Cho HC, Marbán E and
Cingolani E: Biological pacemaker created by minimally invasive
somatic reprogramming in pigs with complete heart block. Sci Transl
Med. 6:245ra942014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wiese C, Grieskamp T, Airik R, Mommersteeg
MT, Gardiwal A, de Gier-de Vries C, Schuster-Gossler K, Moorman AF,
Kispert A and Christoffels VM: Formation of the sinus node head and
differentiation of sinus node myocardium are independently
regulated by Tbx18 and Tbx3. Circ Res. 104:388–397. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kapoor N, Liang W, Marbán E and Cho HC:
Direct conversion of quiescent cardiomyocytes to pacemaker cells by
expression of Tbx18. Nat Biotechnol. 31:54–62. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ongstad E and Kohl P: Fibroblast-myocyte
coupling in the heart: Potential relevance for therapeutic
interventions. J Mol Cell Cardiol. 91:238–246. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Takahashi K and Yamanaka S: Induction of
pluripotent stem cells from mouse embryonic and adult fibroblast
cultures by defined factors. Cell. 126:663–676. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Weintraub H, Tapscott SJ, Davis RL, Thayer
MJ, Adam MA, Lassar AB and Miller AD: Activation of muscle-specific
genes in pigment, nerve, fat, liver, and fibroblast cell lines by
forced expression of MyoD. Proc Natl Acad Sci USA. 86:5434–5438.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Halder G, Callaerts P and Gehring WJ:
Induction of ectopic eyes by targeted expression of the eyeless
gene in Drosophila. Science. 267:1788–1792. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chambers SM and Studer L: Cell fate plug
and play: Direct reprogramming and induced pluripotency. Cell.
145:827–830. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Brown TR, Krogh-Madsen T and Christini DJ:
Computational approaches to understanding the role of
fibroblast-myocyte interactions in cardiac arrhythmogenesis. Biomed
Res Int. 2015:4657142015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Goldsmith EC, Hoffman A, Morales MO, Potts
JD, Price RL, McFadden A, Rice M and Borg TK: Organization of
fibroblasts in the heart. Dev Dyn. 230:787–794. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kizana E, Ginn SL, Allen DG, Ross DL and
Alexander IE: Fibroblasts can be genetically modified to produce
excitable cells capable of electrical coupling. Circulation.
111:394–398. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rosker C, Salvarani N, Schmutz S, Grand T
and Rohr S: Abolishing myofibroblast arrhythmogeneicity by
pharmacological ablation of α-smooth muscle actin containing stress
fibers. Circ Res. 109:1120–1131. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Haraguchi Y, Shimizu T, Yamato M and Okano
T: Electrical interaction between cardiomyocyte sheets separated by
non-cardiomyocyte sheets in heterogeneous tissues. J Tissue Eng
Regen Med. 4:291–299. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fahrenbach JP, Mejia-Alvarez R and Banach
K: The relevance of non-excitable cells for cardiac pacemaker
function. J Physiol. 585:565–578. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Abramochkin DV, Lozinsky IT and Kamkin A:
Influence of mechanical stress on fibroblast-myocyte interactions
in mammalian heart. J Mol Cell Cardiol. 70:27–36. 2014. View Article : Google Scholar : PubMed/NCBI
|